Figure 2 - DE
Estef Vazquez
2025-04-04
Last updated: 2025-04-04
Checks: 7 0
Knit directory: Ulceration_paper_github/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20250330) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 90f2ad0. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: omnipathr-log/
Ignored: output/ulceration_combined_panel.pdf
Untracked files:
Untracked: .Rproj.user/
Untracked: data/clinical_am_prim.csv
Untracked: volcanoplot.pdf
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/figure2_DE.Rmd) and HTML
(docs/figure2_DE.html) files. If you’ve configured a remote
Git repository (see ?wflow_git_remote), click on the
hyperlinks in the table below to view the files as they were in that
past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 90f2ad0 | Estef Vazquez | 2025-04-04 | Add data download system and update gitignore |
| html | 89c14cd | Estef Vazquez | 2025-04-04 | Build site. |
| html | c17335e | Estef Vazquez | 2025-04-04 | Build site. |
| html | 3148fdc | Estef Vazquez | 2025-04-04 | Build site. |
| Rmd | 248524c | Estef Vazquez | 2025-04-04 | Update |
| Rmd | 467227d | Estef Vazquez | 2025-04-04 | wflow_rename("analysis/test_render_figure2.Rmd", "analysis/figure2_DE.Rmd") |
| html | 467227d | Estef Vazquez | 2025-04-04 | wflow_rename("analysis/test_render_figure2.Rmd", "analysis/figure2_DE.Rmd") |
Differential expression analysis
This document incorporates differential expression analysis and PCA, in relation to ulceration status in acral melanoma samples. It generates PCA and volcano plots.
# Load required libraries
library(tidyverse)
library(DESeq2)
library(factoextra)
library(RColorBrewer)
library(ggrepel)
library(plotly)
library(here)# Data Preparation - subset metadata
exp_design <- metadata %>% dplyr::select(sample_id, batch_number, sex, age, ulceration)
# Load raw count data
cts <- readRDS("data/rawcounts_am.rds")
# Scale age
exp_design$age_scaled <- scale(exp_design$age)
# Evaluate ulceration effect while accounting for batch, sex, and age
dds <- DESeqDataSetFromMatrix(
countData = cts,
colData = exp_design,
design = ~ batch_number + age_scaled + sex + ulceration,
tidy = FALSE
)
# Estimate size factors
dds <- estimateSizeFactors(dds)
# Pre-filtering
keep <- rowSums( counts(dds, normalized = TRUE) >= 10 ) >=20
dds <- dds[keep,]
# Set reference level for ulceration
dds$ulceration <- relevel(dds$ulceration, ref = "0")
# Extract normalized counts
normalized_counts_deseq <- counts(dds, normalized=TRUE)
# Run DE
dds_ulc <- DESeq(dds)
dim(dds_ulc)[1] 19319 59# Apply apeglm shrinkage
resLFC <- lfcShrink(dds_ulc, coef="ulceration_1_vs_0", type="apeglm")
# Extract results with ulceration 1 vs 0 contrast
res <- results(dds_ulc, contrast = c("ulceration", "1", "0"), alpha = 0.05)
# Variance stabilizing transformation for visualization
vsd <- vst(dds_ulc, blind=FALSE)
# Processing results - order by LFC
resOrdered <- res[order(res$log2FoldChange),]
ranked_GSEA <- as.data.frame(resOrdered)
#saveRDS(ranked_GSEA, "DE_results_ulceration_ranked.rds")
# Extract significant genes
sig_genes <- subset(res, padj < 0.05)
# Order significant genes by fold change
LFC_ordered <-( sig_genes[ order( sig_genes$log2FoldChange ), ] )
LFC_ordered_df <- as.data.frame(LFC_ordered)
# Prepare results with gene IDs
res_ids <- as.data.frame(res) %>%
rownames_to_column(var = "ENSEMBL_GENE_ID")
# Load gene annotation mapping
gene_ann <- readRDS("data/annotation.rds")
# Join results
res_final <- inner_join(res_ids, gene_ann, by="ENSEMBL_GENE_ID") %>%
relocate(external_gene_name, .after = ENSEMBL_GENE_ID)
# Make unique rownames
names <- make.unique(res_final$external_gene_name)
rownames(res_final) <- names
# Order complete results by LFC
res_final_ordered_LFC <- ( res_final[ order( res_final$log2FoldChange ), ] )
# Process significant genes with gene symbols
significant_genes_ids <- LFC_ordered_df %>%
rownames_to_column(var = "ENSEMBL_GENE_ID")
# Join with gene symbols
significant_genes_final <- inner_join(significant_genes_ids, gene_ann, by="ENSEMBL_GENE_ID") %>%
relocate(external_gene_name, .after = ENSEMBL_GENE_ID)
# Make row names
names <- make.unique(significant_genes_final$external_gene_name)
rownames(significant_genes_final) <- names
# write_csv(significant_genes_final, "DE_results_significant.csv")2A - Principal Component Analysis
# PCA with top 1000 highly variable genes
pcaData <- plotPCA(vsd, intgroup=c("ulceration"),
ntop=1000,
returnData=TRUE)
# Extract variance percentages
percentVar <- round(100 * attr(pcaData, "percentVar"))
# Create plot
ggplot(pcaData, aes(x = PC1, y = PC2, color = group)) +
geom_point(size = 3) +
theme_bw() +
scale_color_manual(
values = c("#730769", "#E8CC03"),
name = "Ulceration Status",
labels = c("No", "Yes")
) +
theme(
legend.position = "right",
panel.grid.minor = element_blank(),
axis.title = element_text(size = 12, face = "bold"),
legend.title = element_text(size = 11, face = "bold"),
legend.text = element_text(size = 10)
) +
ggtitle("PCA Plot of Gene Expression by Ulceration Status") +
xlab(paste0("PC1 (", percentVar[1], "% variance)")) +
ylab(paste0("PC2 (", percentVar[2], "% variance)"))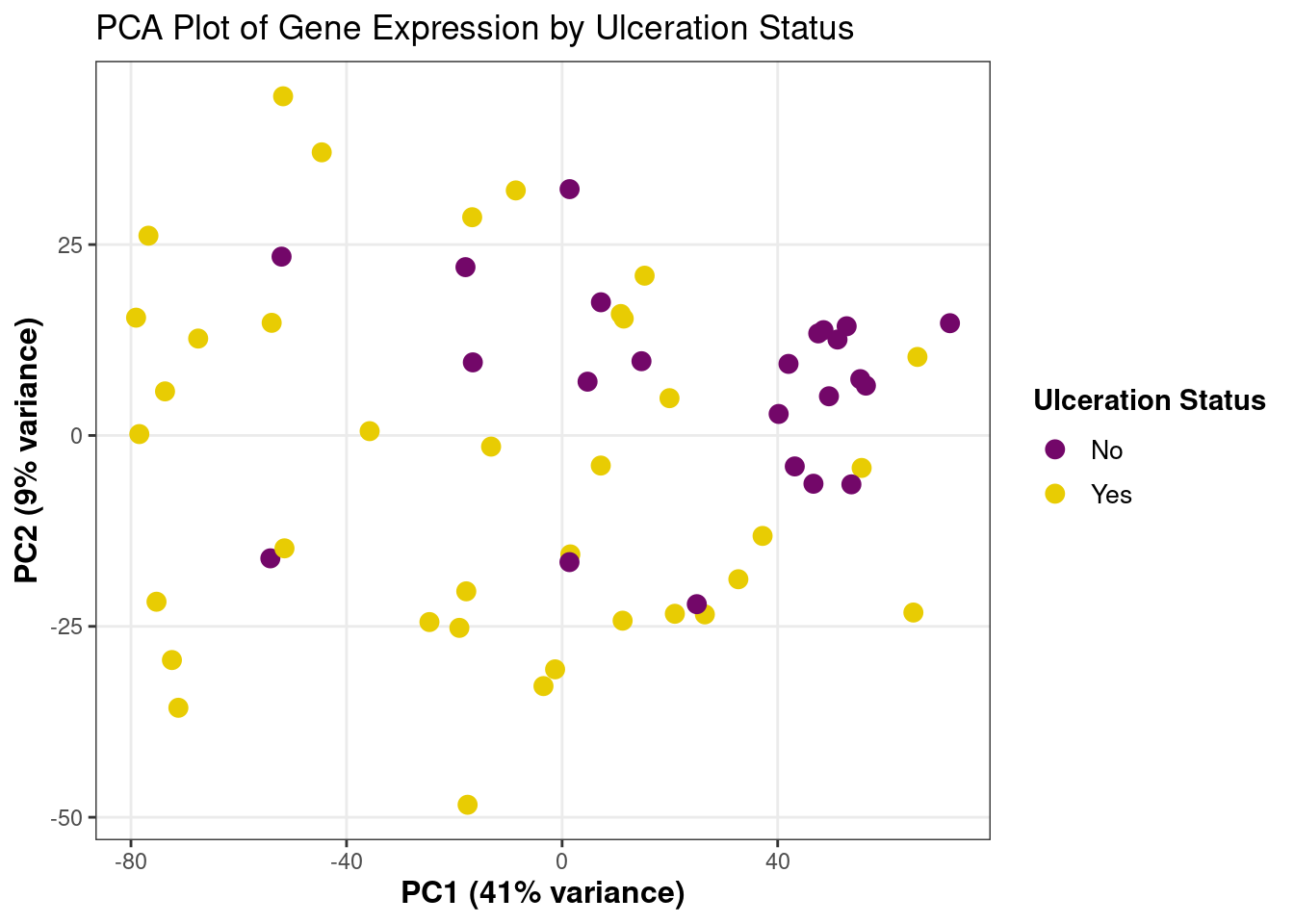
| Version | Author | Date |
|---|---|---|
| 3148fdc | Estef Vazquez | 2025-04-04 |
2B - Volcano Plot
# Theme
theme_set(theme_classic(base_size = 20) +
theme(
axis.title.y = element_text(face = "bold", margin = margin(0, 20, 0, 0), size = rel(1.1), color = 'black'),
axis.title.x = element_text(hjust = 0.5, face = "bold", margin = margin(20, 0, 0, 0), size = rel(1.1), color = 'black'),
plot.title = element_text(hjust = 0.5)
))
# Create and save plot
create_volcano_plot <- function(data,
fc_threshold = 1.5,
padj_threshold = 0.05,
ylim = c(0, 10),
xlim = c(-6, 6),
top_genes = 100,
output_file = NULL,
italic_labels = TRUE) {
# Classify genes by expression
data$diffexpressed <- "NO"
data$diffexpressed[data$log2FoldChange > fc_threshold & data$padj < padj_threshold] <- "UP"
data$diffexpressed[data$log2FoldChange < -fc_threshold & data$padj < padj_threshold] <- "DOWN"
# Label top differentially expressed genes
data$delabel <- ifelse(
data$external_gene_name %in% head(data[order(data$padj), "external_gene_name"], top_genes),
data$external_gene_name,
NA
)
plot <- ggplot(data = data,
aes(x = log2FoldChange,
y = -log10(padj),
col = diffexpressed,
label = delabel)) +
geom_vline(xintercept = c(-fc_threshold, fc_threshold), col = "gray", linetype = 'dashed') +
geom_hline(yintercept = -log10(padj_threshold), col = "gray", linetype = 'dashed') +
geom_point(size = 2.5) +
scale_color_manual(
values = c("#00AFBB", "grey", "#bb0c00"),
labels = c("Downregulated", "Not significant", "Upregulated")
) +
coord_cartesian(ylim = ylim, xlim = xlim) +
labs(
color = 'DE Genes',
x = expression("log"[2]*"FC"),
y = expression("-log"[10]*"padj")
) +
scale_x_continuous(breaks = seq(-10, 10, 2)) +
ggtitle('Ulcerated vs Non-ulcerated Acral Melanoma') +
geom_text_repel(
max.overlaps = Inf,
fontface = if(italic_labels) "italic" else "plain"
)
if (!is.null(output_file)) {
pdf(file = output_file, width = 13, height = 12)
print(plot)
dev.off()
}
return(plot)
}
# Generate final plot
volcanoplot <- create_volcano_plot(
data = res_final_ordered_LFC,
fc_threshold = 1.5,
padj_threshold = 0.05,
ylim = c(0, 10),
xlim = c(-6, 6),
top_genes = 100,
italic_labels = TRUE,
output_file = "volcanoplot.pdf"
)
volcanoplot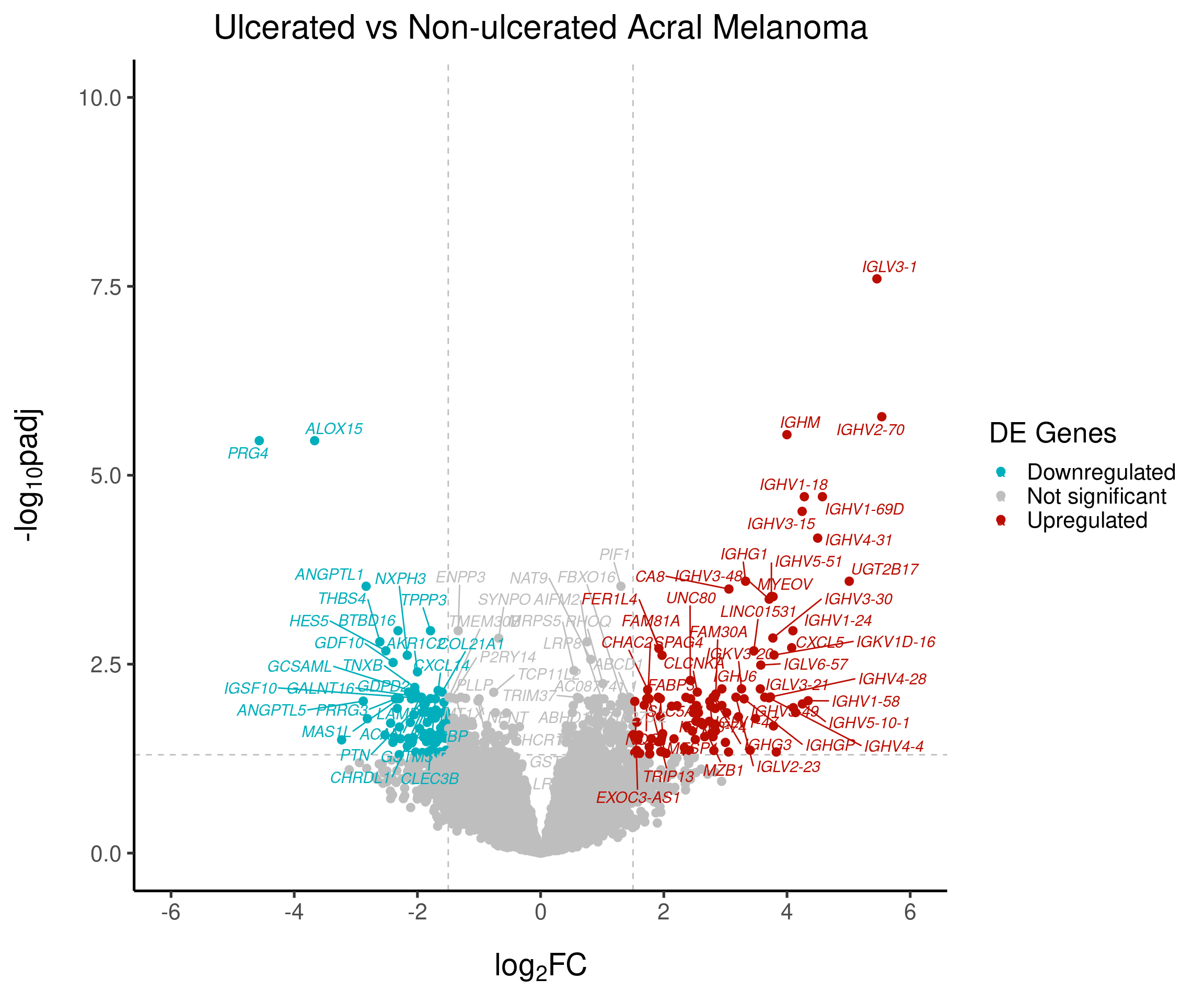
Interactive Volcano
dat <- tibble(genename = (res_final$external_gene_name),
x = res_final$log2FoldChange,
y = -log10(res_final$padj),
col = ifelse(res_final$padj < 0.05 & res_final$log2FoldChange > 1.5, "Upregulated",
ifelse(res_final$padj < 0.05 & res_final$log2FoldChange < -1.5, "Downregulated", "Non-significant")))
fig <- plot_ly(dat, x = ~x, y = ~y,
color = ~col,
colors = c("Downregulated" = "#00AFBB", "Non-significant" = "grey", "Upregulated" = "#bb0c00"),
text = ~genename,
hoverinfo = "text",
type = "scatter",
mode = "markers")
fig <- fig %>%
layout(plot_bgcolor = 'white',
paper_bgcolor = 'white',
xaxis = list(title = 'Log2 fold change',
range = c(-6, 6),
zeroline = FALSE),
yaxis = list(title = '-Log10 Padj',
range = c(0, 10)),
legend = list(title = list(text = '<b> Genes </b>'),
traceorder = "reversed"))
fig <- fig %>%
layout(
legend = list(
title = list(text = '<b> Genes </b>'),
traceorder = "normal"
)
)
figsessionInfo()R version 4.4.0 (2024-04-24)
Platform: x86_64-pc-linux-gnu
Running under: Ubuntu 22.04.4 LTS
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/blas/libblas.so.3.10.0
LAPACK: /usr/lib/x86_64-linux-gnu/lapack/liblapack.so.3.10.0
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=es_MX.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=es_MX.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=es_MX.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=es_MX.UTF-8 LC_IDENTIFICATION=C
time zone: America/Mexico_City
tzcode source: system (glibc)
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] here_1.0.1 plotly_4.10.4
[3] ggrepel_0.9.6 RColorBrewer_1.1-3
[5] factoextra_1.0.7 DESeq2_1.44.0
[7] SummarizedExperiment_1.34.0 Biobase_2.64.0
[9] MatrixGenerics_1.16.0 matrixStats_1.4.1
[11] GenomicRanges_1.56.2 GenomeInfoDb_1.40.1
[13] IRanges_2.38.1 S4Vectors_0.42.1
[15] BiocGenerics_0.50.0 lubridate_1.9.4
[17] forcats_1.0.0 stringr_1.5.1
[19] dplyr_1.1.4 purrr_1.0.2
[21] readr_2.1.5 tidyr_1.3.1
[23] tibble_3.2.1 ggplot2_3.5.1
[25] tidyverse_2.0.0 workflowr_1.7.1
loaded via a namespace (and not attached):
[1] rlang_1.1.4 magrittr_2.0.3 git2r_0.33.0
[4] compiler_4.4.0 getPass_0.2-4 callr_3.7.6
[7] vctrs_0.6.5 pkgconfig_2.0.3 crayon_1.5.3
[10] fastmap_1.2.0 XVector_0.44.0 labeling_0.4.3
[13] promises_1.3.2 rmarkdown_2.29 tzdb_0.4.0
[16] UCSC.utils_1.0.0 ps_1.8.1 xfun_0.49
[19] zlibbioc_1.50.0 cachem_1.1.0 jsonlite_1.8.9
[22] later_1.4.1 DelayedArray_0.30.1 BiocParallel_1.38.0
[25] parallel_4.4.0 R6_2.5.1 bslib_0.8.0
[28] stringi_1.8.4 jquerylib_0.1.4 numDeriv_2016.8-1.1
[31] Rcpp_1.0.13-1 knitr_1.49 httpuv_1.6.15
[34] Matrix_1.6-5 timechange_0.3.0 tidyselect_1.2.1
[37] rstudioapi_0.17.1 abind_1.4-5 yaml_2.3.10
[40] codetools_0.2-19 processx_3.8.4 lattice_0.22-5
[43] plyr_1.8.9 withr_3.0.2 coda_0.19-4.1
[46] evaluate_1.0.1 pillar_1.10.0 whisker_0.4.1
[49] generics_0.1.3 rprojroot_2.0.4 emdbook_1.3.13
[52] hms_1.1.3 munsell_0.5.1 scales_1.3.0
[55] glue_1.8.0 lazyeval_0.2.2 tools_4.4.0
[58] apeglm_1.26.1 data.table_1.16.4 locfit_1.5-9.10
[61] fs_1.6.5 mvtnorm_1.3-2 grid_4.4.0
[64] bbmle_1.0.25.1 crosstalk_1.2.1 bdsmatrix_1.3-7
[67] colorspace_2.1-1 GenomeInfoDbData_1.2.12 cli_3.6.3
[70] S4Arrays_1.4.1 viridisLite_0.4.2 gtable_0.3.6
[73] sass_0.4.9 digest_0.6.37 SparseArray_1.4.8
[76] farver_2.1.2 htmlwidgets_1.6.4 htmltools_0.5.8.1
[79] lifecycle_1.0.4 httr_1.4.7 MASS_7.3-60
sessionInfo()R version 4.4.0 (2024-04-24)
Platform: x86_64-pc-linux-gnu
Running under: Ubuntu 22.04.4 LTS
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/blas/libblas.so.3.10.0
LAPACK: /usr/lib/x86_64-linux-gnu/lapack/liblapack.so.3.10.0
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=es_MX.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=es_MX.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=es_MX.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=es_MX.UTF-8 LC_IDENTIFICATION=C
time zone: America/Mexico_City
tzcode source: system (glibc)
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] here_1.0.1 plotly_4.10.4
[3] ggrepel_0.9.6 RColorBrewer_1.1-3
[5] factoextra_1.0.7 DESeq2_1.44.0
[7] SummarizedExperiment_1.34.0 Biobase_2.64.0
[9] MatrixGenerics_1.16.0 matrixStats_1.4.1
[11] GenomicRanges_1.56.2 GenomeInfoDb_1.40.1
[13] IRanges_2.38.1 S4Vectors_0.42.1
[15] BiocGenerics_0.50.0 lubridate_1.9.4
[17] forcats_1.0.0 stringr_1.5.1
[19] dplyr_1.1.4 purrr_1.0.2
[21] readr_2.1.5 tidyr_1.3.1
[23] tibble_3.2.1 ggplot2_3.5.1
[25] tidyverse_2.0.0 workflowr_1.7.1
loaded via a namespace (and not attached):
[1] rlang_1.1.4 magrittr_2.0.3 git2r_0.33.0
[4] compiler_4.4.0 getPass_0.2-4 callr_3.7.6
[7] vctrs_0.6.5 pkgconfig_2.0.3 crayon_1.5.3
[10] fastmap_1.2.0 XVector_0.44.0 labeling_0.4.3
[13] promises_1.3.2 rmarkdown_2.29 tzdb_0.4.0
[16] UCSC.utils_1.0.0 ps_1.8.1 xfun_0.49
[19] zlibbioc_1.50.0 cachem_1.1.0 jsonlite_1.8.9
[22] later_1.4.1 DelayedArray_0.30.1 BiocParallel_1.38.0
[25] parallel_4.4.0 R6_2.5.1 bslib_0.8.0
[28] stringi_1.8.4 jquerylib_0.1.4 numDeriv_2016.8-1.1
[31] Rcpp_1.0.13-1 knitr_1.49 httpuv_1.6.15
[34] Matrix_1.6-5 timechange_0.3.0 tidyselect_1.2.1
[37] rstudioapi_0.17.1 abind_1.4-5 yaml_2.3.10
[40] codetools_0.2-19 processx_3.8.4 lattice_0.22-5
[43] plyr_1.8.9 withr_3.0.2 coda_0.19-4.1
[46] evaluate_1.0.1 pillar_1.10.0 whisker_0.4.1
[49] generics_0.1.3 rprojroot_2.0.4 emdbook_1.3.13
[52] hms_1.1.3 munsell_0.5.1 scales_1.3.0
[55] glue_1.8.0 lazyeval_0.2.2 tools_4.4.0
[58] apeglm_1.26.1 data.table_1.16.4 locfit_1.5-9.10
[61] fs_1.6.5 mvtnorm_1.3-2 grid_4.4.0
[64] bbmle_1.0.25.1 crosstalk_1.2.1 bdsmatrix_1.3-7
[67] colorspace_2.1-1 GenomeInfoDbData_1.2.12 cli_3.6.3
[70] S4Arrays_1.4.1 viridisLite_0.4.2 gtable_0.3.6
[73] sass_0.4.9 digest_0.6.37 SparseArray_1.4.8
[76] farver_2.1.2 htmlwidgets_1.6.4 htmltools_0.5.8.1
[79] lifecycle_1.0.4 httr_1.4.7 MASS_7.3-60