Supplementary Figure 9
Florian Wuennemann
Last updated: 2024-03-21
Checks: 7 0
Knit directory: mi_spatialomics/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20230612) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 4c4da66. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/.DS_Store
Ignored: analysis/deprecated/.DS_Store
Ignored: analysis/molecular_cartography_python/.DS_Store
Ignored: analysis/seqIF_python/.DS_Store
Ignored: analysis/seqIF_python/pixie/.DS_Store
Ignored: analysis/seqIF_python/pixie/cell_clustering/
Ignored: annotations/.DS_Store
Ignored: annotations/SeqIF/.DS_Store
Ignored: annotations/molkart/.DS_Store
Ignored: annotations/molkart/Figure1_regions/.DS_Store
Ignored: annotations/molkart/Supplementary_Figure4_regions/.DS_Store
Ignored: data/.DS_Store
Ignored: data/140623.calcagno_et_al.seurat_object.rds
Ignored: data/Calcagno2022_int_logNorm_annot.h5Seurat
Ignored: data/IC_03_IF_CCR2_CD68 cell numbers.xlsx
Ignored: data/Traditional_IF_absolute_cell_counts.csv
Ignored: data/Traditional_IF_relative_cell_counts.csv
Ignored: data/pixie.cell_table_size_normalized_cell_labels.csv
Ignored: data/results_cts_100.sqm
Ignored: data/seqIF_regions_annotations/
Ignored: data/seurat/
Ignored: omnipathr-log/
Ignored: output/.DS_Store
Ignored: output/mol_cart.harmony_object.h5Seurat
Ignored: output/molkart/
Ignored: output/proteomics/
Ignored: output/results_cts.lowres.125.sqm
Ignored: output/seqIF/
Ignored: pipeline_configs/.DS_Store
Ignored: plots/
Ignored: references/.DS_Store
Ignored: renv/.DS_Store
Ignored: renv/library/
Ignored: renv/staging/
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown
(analysis/figures.supplementary_figure_9.proteomics_qc.Rmd)
and HTML
(docs/figures.supplementary_figure_9.proteomics_qc.html)
files. If you’ve configured a remote Git repository (see
?wflow_git_remote), click on the hyperlinks in the table
below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 4c4da66 | FloWuenne | 2024-03-21 | wflow_publish(c("analysis/figures.supplementary_figure_4.segmentation_metrics.Rmd", |
| html | a2cf304 | FloWuenne | 2024-03-21 | Build site. |
| Rmd | e6213a5 | FloWuenne | 2024-03-21 | Publish all updated files. |
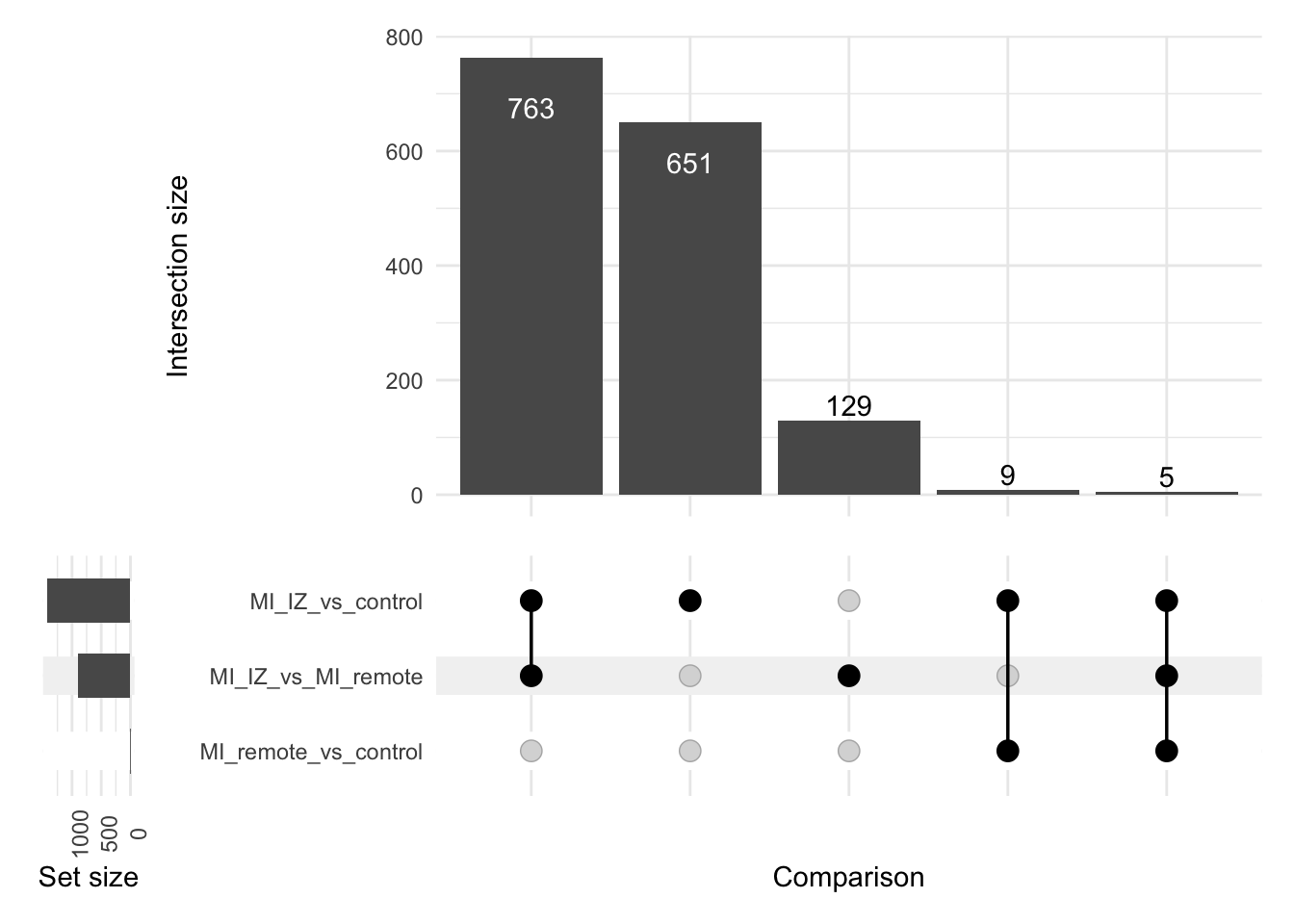
This supplementary figure contains QC plots for the proteomic data analysis.
## load data
metadata_counts <- fread("./output/proteomics/proteomics.protein_missing_stats.tsv")Subfigure A - Total proteins identified per group
## Summarize observations across group
metadata_counts_stat <- data_summary(metadata_counts,
varname = "wo_cont", groupnames = c("group"))Loading required package: plyr------------------------------------------------------------------------------You have loaded plyr after dplyr - this is likely to cause problems.
If you need functions from both plyr and dplyr, please load plyr first, then dplyr:
library(plyr); library(dplyr)------------------------------------------------------------------------------
Attaching package: 'plyr'The following objects are masked from 'package:plotly':
arrange, mutate, rename, summariseThe following object is masked from 'package:here':
hereThe following objects are masked from 'package:dplyr':
arrange, count, desc, failwith, id, mutate, rename, summarise,
summarizeThe following object is masked from 'package:purrr':
compactavg_proteins <- mean(metadata_counts$wo_cont)
avg_proteins <- sd(metadata_counts$wo_cont)
metadata_counts_stat$group <- factor(metadata_counts_stat$group,
levels = c("MI_IZ","MI_remote","control"))
## Barplot to show the number of proteins detected per group (excluding contaminants)
n_prot_plot <- ggplot(metadata_counts_stat,aes(group,wo_cont)) +
geom_bar(aes(fill = group),stat="identity", color="black",
position=position_dodge()) +
geom_point(data = metadata_counts, aes(group,wo_cont),
fill = "white",pch = 21, color= "black",size = 4) +
geom_errorbar(aes(ymin=wo_cont, ymax=wo_cont+sd), width=.2,
position=position_dodge(.9)) +
coord_flip() +
scale_fill_manual(values = proteome_palette,
labels = c("Control","MI_remote","MI_IZ")) +
labs(x = "",
y = "Proteins detected") +
theme(legend.position = "none")
n_prot_plot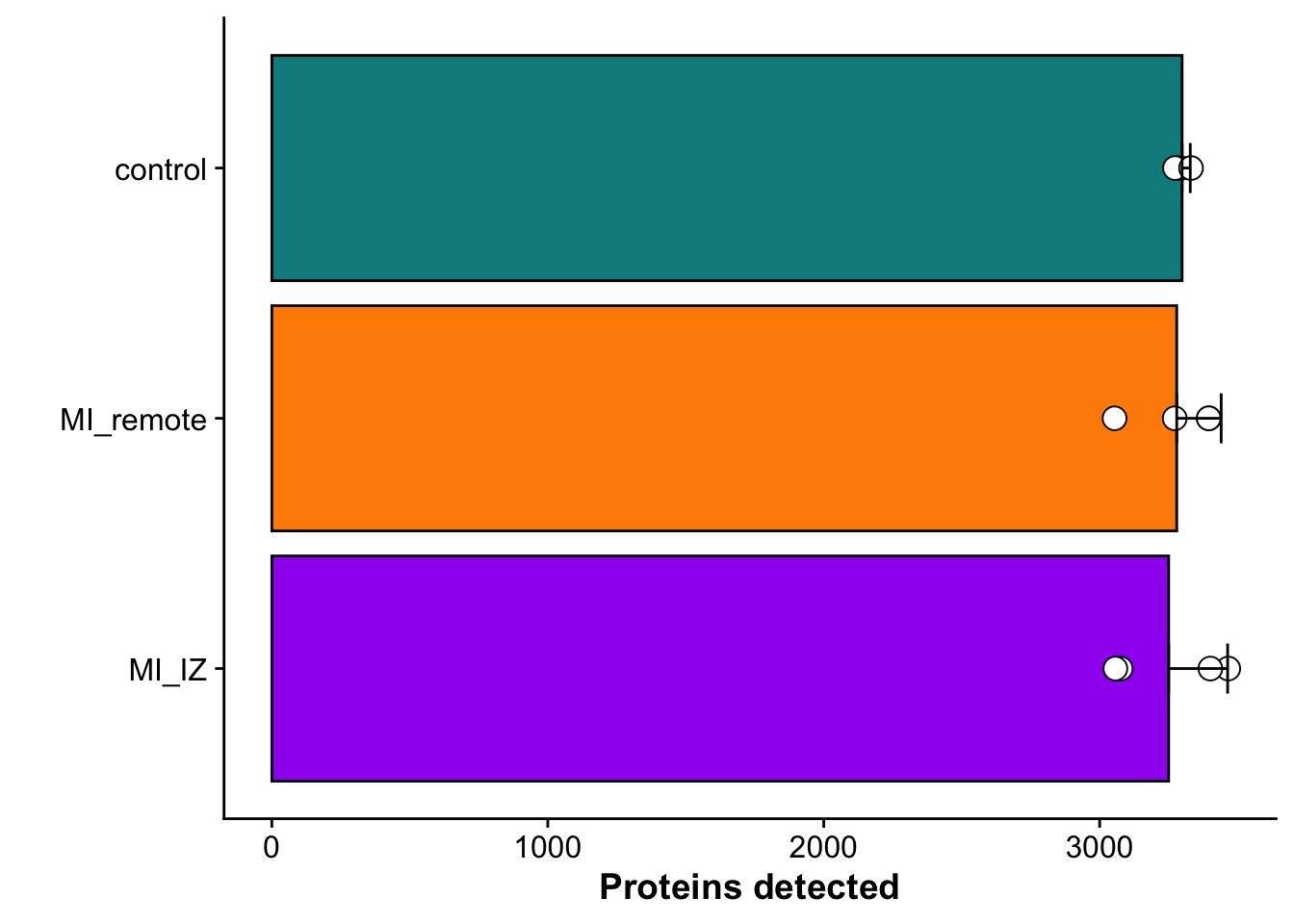
| Version | Author | Date |
|---|---|---|
| a2cf304 | FloWuenne | 2024-03-21 |
Subfigure B - % missing proteins vs number of nuclei collected
metadata_counts$group <- factor(metadata_counts$group,
levels = c("control","MI_remote","MI_IZ"))
missingness_plot <- ggplot(metadata_counts,aes(missingness,n_nuclei, label = sample)) +
geom_point(pch = 21, size = 5,aes(fill = group)) +
scale_fill_manual(values = proteome_palette,
labels = c("Control","MI_remote","MI_IZ")) +
geom_label_repel() +
labs(x = "% missing values",
y = "# nuclei in tissue collected",
fill = "Group") +
theme(legend.position = "top")
missingness_plot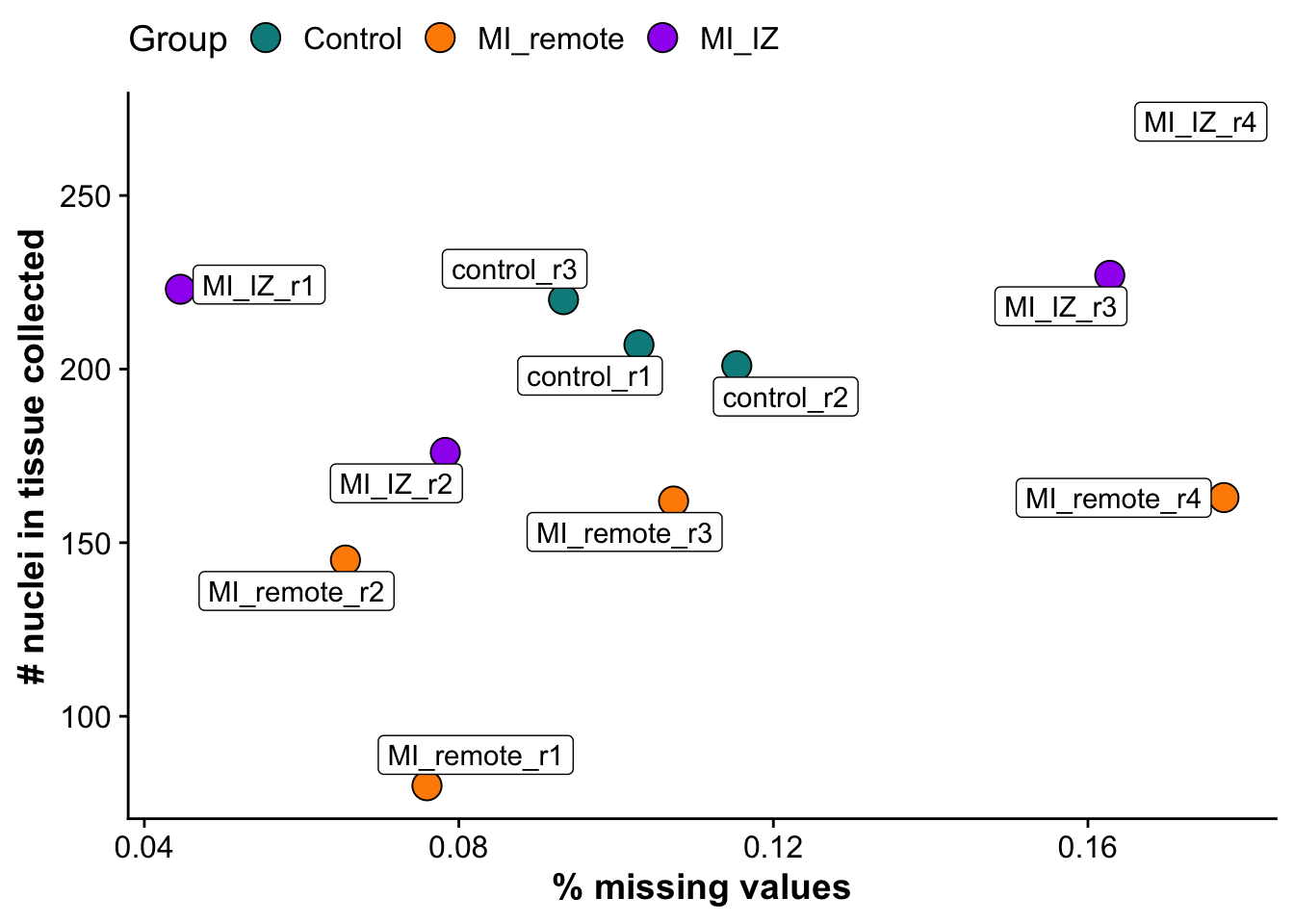
| Version | Author | Date |
|---|---|---|
| a2cf304 | FloWuenne | 2024-03-21 |
Subfigure C - % variance explained by PCs
pca_res <- readRDS("./output/proteomics/proteomics.pca_res.rds")
sdev <- pca_res$sdev[1:5]
var_explained <- data.frame(PC = paste0("PC",1:5),
var_expl = (sdev)^2/sum((sdev)^2)
)
pca_var <- ggplot(var_explained,aes(PC,var_expl)) +
geom_bar(stat = "identity", color = "black", fill = "grey30") +
geom_text(aes(label = paste(100*round(var_expl,2),sep = "")), vjust=1.4, color = "white", size =6) +
labs(x = "Principal components",
y = "% Variance explained")
pca_var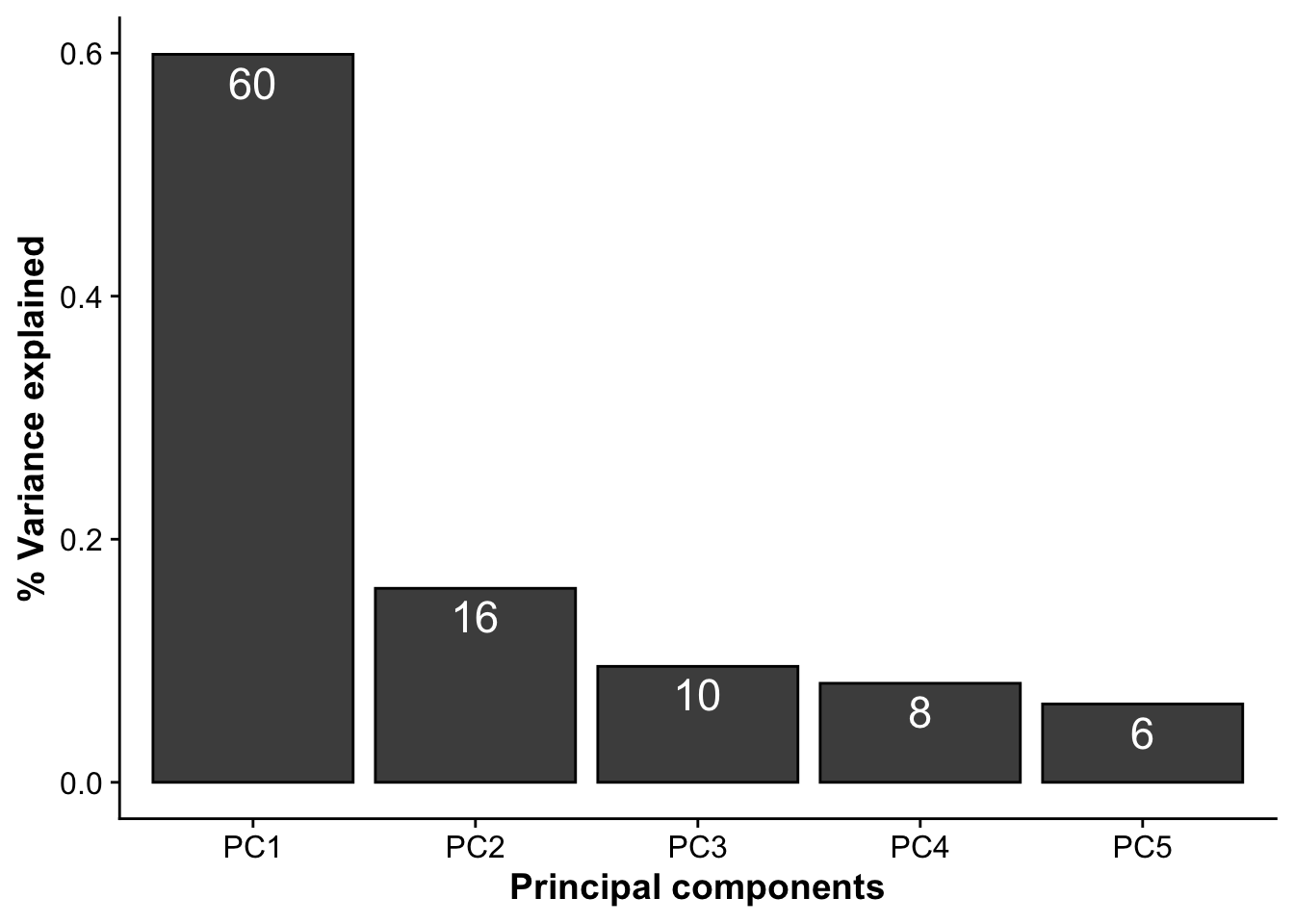
| Version | Author | Date |
|---|---|---|
| a2cf304 | FloWuenne | 2024-03-21 |
Assemble final figure
supp_figure_X <- n_prot_plot + missingness_plot + pca_var + wrap_elements(upset_plot)
supp_figure_X <- supp_figure_X +
plot_layout(nrow = 2,ncol =2 , widths = c(1,2)) +
plot_annotation(tag_levels = 'a') &
theme(plot.tag = element_text(size = 25)) &
theme(plot.background = element_rect(fill = "white"))
supp_figure_X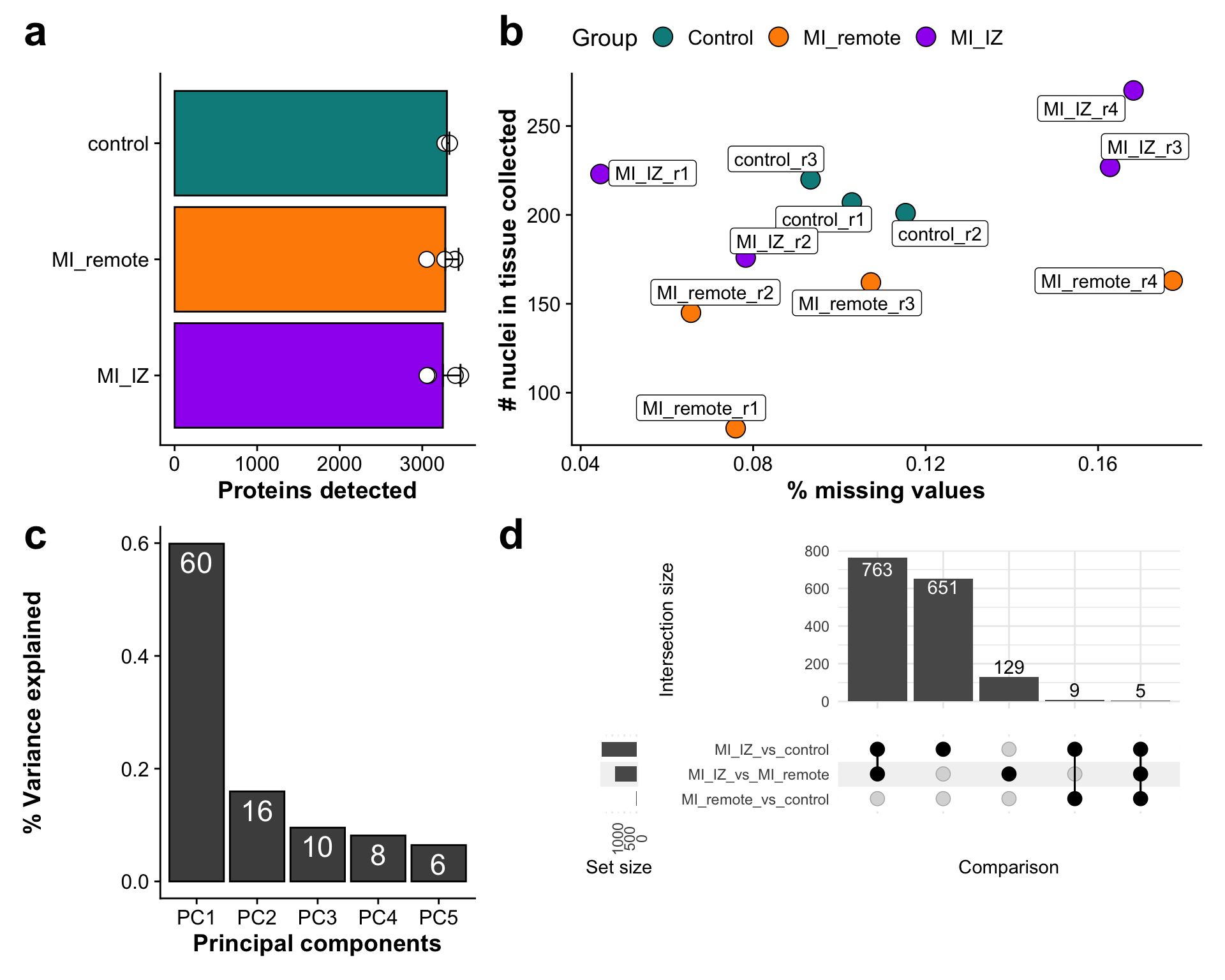
| Version | Author | Date |
|---|---|---|
| a2cf304 | FloWuenne | 2024-03-21 |
save_plot(filename = "./figures/Supplementary_figure_X.proteomics.png",
plot = supp_figure_X,
base_height = 8)
save_plot(filename = "./figures/Supplementary_figure_X.proteomics.eps",
plot = supp_figure_X,
base_height = 8)
sessionInfo()R version 4.3.1 (2023-06-16)
Platform: aarch64-apple-darwin20 (64-bit)
Running under: macOS Sonoma 14.1.2
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: Europe/Berlin
tzcode source: internal
attached base packages:
[1] stats graphics grDevices datasets utils methods base
other attached packages:
[1] ComplexUpset_1.3.3 plyr_1.8.9 RColorBrewer_1.1-3
[4] ggsci_3.0.0 cowplot_1.1.2 ggbeeswarm_0.7.2
[7] ggsignif_0.6.4 heatmaply_1.5.0 plotly_4.10.4
[10] viridis_0.6.4 viridisLite_0.4.2 ggVennDiagram_1.5.0
[13] patchwork_1.2.0 ggrepel_0.9.5 here_1.0.1
[16] data.table_1.14.10 lubridate_1.9.3 forcats_1.0.0
[19] stringr_1.5.1 dplyr_1.1.4 purrr_1.0.2
[22] readr_2.1.5 tidyr_1.3.0 tibble_3.2.1
[25] ggplot2_3.4.4 tidyverse_2.0.0 workflowr_1.7.1
loaded via a namespace (and not attached):
[1] tidyselect_1.2.0 farver_2.1.1 vipor_0.4.7
[4] fastmap_1.1.1 TSP_1.2-4 lazyeval_0.2.2
[7] promises_1.2.1 digest_0.6.34 timechange_0.2.0
[10] lifecycle_1.0.4 processx_3.8.3 magrittr_2.0.3
[13] compiler_4.3.1 rlang_1.1.3 sass_0.4.8
[16] tools_4.3.1 utf8_1.2.4 yaml_2.3.8
[19] knitr_1.45 labeling_0.4.3 htmlwidgets_1.6.4
[22] registry_0.5-1 ca_0.71.1 withr_2.5.2
[25] grid_4.3.1 fansi_1.0.6 git2r_0.33.0
[28] colorspace_2.1-0 scales_1.3.0 iterators_1.0.14
[31] cli_3.6.2 rmarkdown_2.25 ragg_1.2.7
[34] generics_0.1.3 rstudioapi_0.15.0 httr_1.4.7
[37] tzdb_0.4.0 cachem_1.0.8 assertthat_0.2.1
[40] BiocManager_1.30.22 vctrs_0.6.5 webshot_0.5.5
[43] jsonlite_1.8.8 seriation_1.5.4 callr_3.7.3
[46] hms_1.1.3 beeswarm_0.4.0 systemfonts_1.0.5
[49] dendextend_1.17.1 foreach_1.5.2 jquerylib_0.1.4
[52] glue_1.7.0 codetools_0.2-19 ps_1.7.6
[55] stringi_1.8.3 gtable_0.3.4 later_1.3.2
[58] munsell_0.5.0 pillar_1.9.0 htmltools_0.5.7
[61] R6_2.5.1 textshaping_0.3.7 rprojroot_2.0.4
[64] evaluate_0.23 highr_0.10 renv_1.0.3
[67] httpuv_1.6.14 bslib_0.6.1 Rcpp_1.0.12
[70] gridExtra_2.3 whisker_0.4.1 xfun_0.41
[73] fs_1.6.3 getPass_0.2-4 pkgconfig_2.0.3