alt_mediation
Ittai Eres
2019-04-24
Last updated: 2019-04-24
Checks: 6 0
Knit directory: HiCiPSC/
This reproducible R Markdown analysis was created with workflowr (version 1.2.0). The Report tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20190311) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: analysis/.DS_Store
Ignored: code/.DS_Store
Ignored: data/.DS_Store
Ignored: output/.DS_Store
Untracked files:
Untracked: Rplot.jpeg
Untracked: Rplot001.jpeg
Untracked: Rplot002.jpeg
Untracked: Rplot003.jpeg
Untracked: Rplot004.jpeg
Untracked: Rplot005.jpeg
Untracked: Rplot006.jpeg
Untracked: Rplot007.jpeg
Untracked: Rplot008.jpeg
Untracked: Rplot009.jpeg
Untracked: Rplot010.jpeg
Untracked: Rplot011.jpeg
Untracked: Rplot012.jpeg
Untracked: Rplot013.jpeg
Untracked: Rplot014.jpeg
Untracked: Rplot015.jpeg
Untracked: Rplot016.jpeg
Untracked: Rplot017.jpeg
Untracked: Rplot018.jpeg
Untracked: Rplot019.jpeg
Untracked: Rplot020.jpeg
Untracked: Rplot021.jpeg
Untracked: Rplot022.jpeg
Untracked: Rplot023.jpeg
Untracked: Rplot024.jpeg
Untracked: Rplot025.jpeg
Untracked: Rplot026.jpeg
Untracked: Rplot027.jpeg
Untracked: Rplot028.jpeg
Untracked: Rplot029.jpeg
Untracked: Rplot030.jpeg
Untracked: Rplot031.jpeg
Untracked: Rplot032.jpeg
Untracked: Rplot033.jpeg
Untracked: Rplot034.jpeg
Untracked: Rplot035.jpeg
Untracked: Rplot036.jpeg
Untracked: Rplot037.jpeg
Untracked: Rplot038.jpeg
Untracked: Rplot039.jpeg
Untracked: Rplot040.jpeg
Untracked: Rplot041.jpeg
Untracked: Rplot042.jpeg
Untracked: Rplot043.jpeg
Untracked: Rplot044.jpeg
Untracked: Rplot045.jpeg
Untracked: Rplot046.jpeg
Untracked: Rplot047.jpeg
Untracked: Rplot048.jpeg
Untracked: Rplot049.jpeg
Untracked: Rplot050.jpeg
Untracked: Rplot051.jpeg
Untracked: Rplot052.jpeg
Untracked: S2A.jpeg
Untracked: S2B.jpeg
Untracked: data/Chimp_orthoexon_extended_info.txt
Untracked: data/Human_orthoexon_extended_info.txt
Untracked: data/Meta_data.txt
Untracked: data/TADs/
Untracked: data/chimp_lengths.txt
Untracked: data/counts_iPSC.txt
Untracked: data/epigenetic_enrichments/
Untracked: data/final.10kb.homer.df
Untracked: data/final.juicer.10kb.KR
Untracked: data/final.juicer.10kb.VC
Untracked: data/hic_gene_overlap/
Untracked: data/human_lengths.txt
Untracked: data/old_mediation_permutations/
Untracked: output/DC_regions.txt
Untracked: output/IEE.RPKM.RDS
Untracked: output/IEE_voom_object.RDS
Untracked: output/data.4.filtered.lm.QC
Untracked: output/data.4.fixed.init.LM
Untracked: output/data.4.fixed.init.QC
Untracked: output/data.4.init.LM
Untracked: output/data.4.init.QC
Untracked: output/data.4.lm.QC
Untracked: output/full.data.10.init.LM
Untracked: output/full.data.10.init.QC
Untracked: output/full.data.10.lm.QC
Untracked: output/full.data.annotations.RDS
Untracked: output/gene.hic.filt.RDS
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | f19d89e | Ittai Eres | 2019-04-24 | Add in secondary mediation analysis, update index, TAD and gene expression files. |
Introduction
An alternative mediation analysis examining the possibility of a species:frequency interaction term effect, as proposed by Reviewer 3.
# This script contains functions for fitting
# a linear model under the limma framework
# where the sample covariate value is differnet for each gene
# in this case, and includes and interaction term of condition and linear covariate
# note that the dimension of the expression matrix
# is the same as the dimension of the covariate matrix
library(limma)Warning: package 'limma' was built under R version 3.4.3library(assertthat)
library(data.table)Warning: package 'data.table' was built under R version 3.4.4lmFit_varying <- function(object, group_vector = NULL, cov_matrix = NULL,
# ndups = 1, spacing = 1, block = NULL,
weights = NULL, method = "ls", ...)
{
# match dimension of design matrix and covariate matrix
# the function will stop executing if their dimensions are not the same
assertthat::assert_that(are_equal(dim(object),dim(cov_matrix)))
fit <- lm.series_varying(M=object, cov_matrix=cov_matrix,
group_vector = group_vector,
weights = weights)
fit$genes <- rownames(object)
fit$Amean <- rowMeans(object, na.rm = TRUE)
fit$method <- method
fit$design <- design
new("MArrayLM", fit)
}
#' modified lm.series for interaction analysis
#'
#' @description main worker inside lmFit for our model of interest
#'
#' @param object matrix of log-expression
#' @param design a vector of sample labels. use model.matrix to make design matrix.
#' @param cov_matrix matrix of covariate values. should be the same dimension
#' as object.
#'
#' @author Joyce Hsiao
lm.series_varying <- function (M, cov_matrix,
group_vector = NULL, weights = NULL)
{
M <- as.matrix(M)
narrays <- ncol(M)
# make design matrix (not including covariates)
design <- model.matrix(~group_vector)
# compute the number of regression coefs. to be estimated
nbeta <- ncol(design) + 2
# make coeffcient names
coef.names <- c(colnames(design), "HiC", "interact")
if (is.null(colnames(design))){
coef.names <- c(paste("x", 1:ncol(design), sep = ""), "HiC", "interact")
}
# for every gene, affirm that the expression weights are
# finite and non-zero
if (!is.null(weights)) {
weights <- asMatrixWeights(weights, dim(M))
weights[weights <= 0] <- NA
M[!is.finite(weights)] <- NA
}
ngenes <- nrow(M)
stdev.unscaled <- beta <- matrix(NA, ngenes, nbeta, dimnames = list(rownames(M),
coef.names))
# start estimating beta coefficients here
beta <- stdev.unscaled
sigma <- rep(NA, ngenes)
df.residual <- rep(0, ngenes)
for (i in 1:ngenes) {
# print(i)
cc <- as.vector(unlist(cov_matrix[i,]))
design_gene <- model.matrix(~group_vector+cc+cc*group_vector)
colnames(design_gene) <- coef.names
y <- as.vector(M[i, ])
obs <- is.finite(y)
if (sum(obs) > 0) {
X <- design_gene[obs, , drop = FALSE]
y <- y[obs]
if (is.null(weights))
out <- lm.fit(X, y)
else {
w <- as.vector(weights[i, obs])
out <- lm.wfit(X, y, w)
}
est <- !is.na(out$coef)
beta[i, ] <- out$coef
stdev.unscaled[i, est] <- sqrt(diag(chol2inv(out$qr$qr,
size = out$rank)))
df.residual[i] <- out$df.residual
if (df.residual[i] > 0)
sigma[i] <- sqrt(mean(out$effects[-(1:out$rank)]^2))
}
}
QR <- qr(design_gene)
cov.coef <- chol2inv(QR$qr, size = QR$rank)
est <- QR$pivot[1:QR$rank]
dimnames(cov.coef) <- list(coef.names[est], coef.names[est])
list(coefficients = beta, stdev.unscaled = stdev.unscaled,
sigma = sigma, df.residual = df.residual, cov.coefficients = cov.coef,
pivot = QR$pivot, rank = QR$rank)
}
df <- fread("~/Desktop/Hi-C/joyce_mediation/HiC.data")
df_hic <- df[,c(1,9:16)]
head(df_hic) genes A_21792_HIC B_28126_HIC C_3649_HIC D_40300_HIC
1: ENSG00000160087 1.08688722 0.9308794 0.8452477 0.4076793
2: ENSG00000127054 0.79030376 0.5512300 0.6384713 0.5998139
3: ENSG00000240731 0.79030376 0.5512300 0.6384713 0.5998139
4: ENSG00000224051 0.37733343 0.4628101 0.6796571 0.9807702
5: ENSG00000107404 0.61419647 0.5646673 0.0951685 0.2700779
6: ENSG00000175756 -0.02453671 0.6407394 0.9644112 1.0124547
E_28815_HIC F_28834_HIC G_3624_HIC H_3651_HIC
1: 0.9411242 1.09245242 0.49777463 0.58755514
2: 0.5308121 0.67897911 0.65454221 0.49264758
3: 0.5308121 0.67897911 0.65454221 0.49264758
4: 0.2967155 0.20317751 1.02449016 0.62904609
5: 0.7123887 0.92062137 -0.03499986 0.09947955
6: 0.2476291 0.01584516 0.90435656 0.92436061df_counts <- readRDS("~/Desktop/Hi-C/joyce_mediation/count.data.RDS")
df_counts <- data.frame(df_counts)
df_counts$genes <- rownames(df_counts)
library(tidyverse)Warning: package 'tidyverse' was built under R version 3.4.2── Attaching packages ────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.1.0 ✔ purrr 0.3.2
✔ tibble 2.1.1 ✔ dplyr 0.8.0.1
✔ tidyr 0.8.3 ✔ stringr 1.4.0
✔ readr 1.3.1 ✔ forcats 0.4.0 Warning: package 'tibble' was built under R version 3.4.4Warning: package 'tidyr' was built under R version 3.4.4Warning: package 'readr' was built under R version 3.4.4Warning: package 'purrr' was built under R version 3.4.4Warning: package 'dplyr' was built under R version 3.4.4Warning: package 'stringr' was built under R version 3.4.4Warning: package 'forcats' was built under R version 3.4.4── Conflicts ───────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::between() masks data.table::between()
✖ dplyr::filter() masks stats::filter()
✖ dplyr::first() masks data.table::first()
✖ tibble::has_name() masks assertthat::has_name()
✖ dplyr::lag() masks stats::lag()
✖ dplyr::last() masks data.table::last()
✖ purrr::transpose() masks data.table::transpose()# merge count data with the HiC dataframe
df_combo <- left_join(df_hic, df_counts)Joining, by = "genes"# Hi-C
cov_matrix <- df_combo[,2:9]
counts <- df_combo[,10:17]
rownames(counts) <- df_combo$genes
rownames(cov_matrix) <- df_combo$genes
species <- factor(c(1,1,2,2,1,1,2,2))
design <- model.matrix(~species)
# compute weights
v <- voom(counts,design=model.matrix(~species), plot=T)
weights <- v$weights
log2cpm <- v$E
fit <- lmFit_varying(object=log2cpm, group_vector=species, cov_matrix=cov_matrix,
weights=weights)
fit <- eBayes(fit, robust=TRUE)
myintergenes <- topTable(fit, coef=4, number=Inf)
sum(myintergenes$adj.P.Val<=0.05) #With multiple testing correction, none are significant.[1] 0sum(myintergenes$P.Value<=0.05)[1] 395#sum(mygenes %in% filter(myintergenes, adj.P.Val<=0.8)$ID)
colors <- c(rep("black", 2), rep("red", 2), rep("black", 2), rep("red", 2))
plot(log2cpm[rownames(log2cpm)=="ENSG00000170561",], cov_matrix[rownames(log2cpm)=="ENSG00000170561",], col=colors)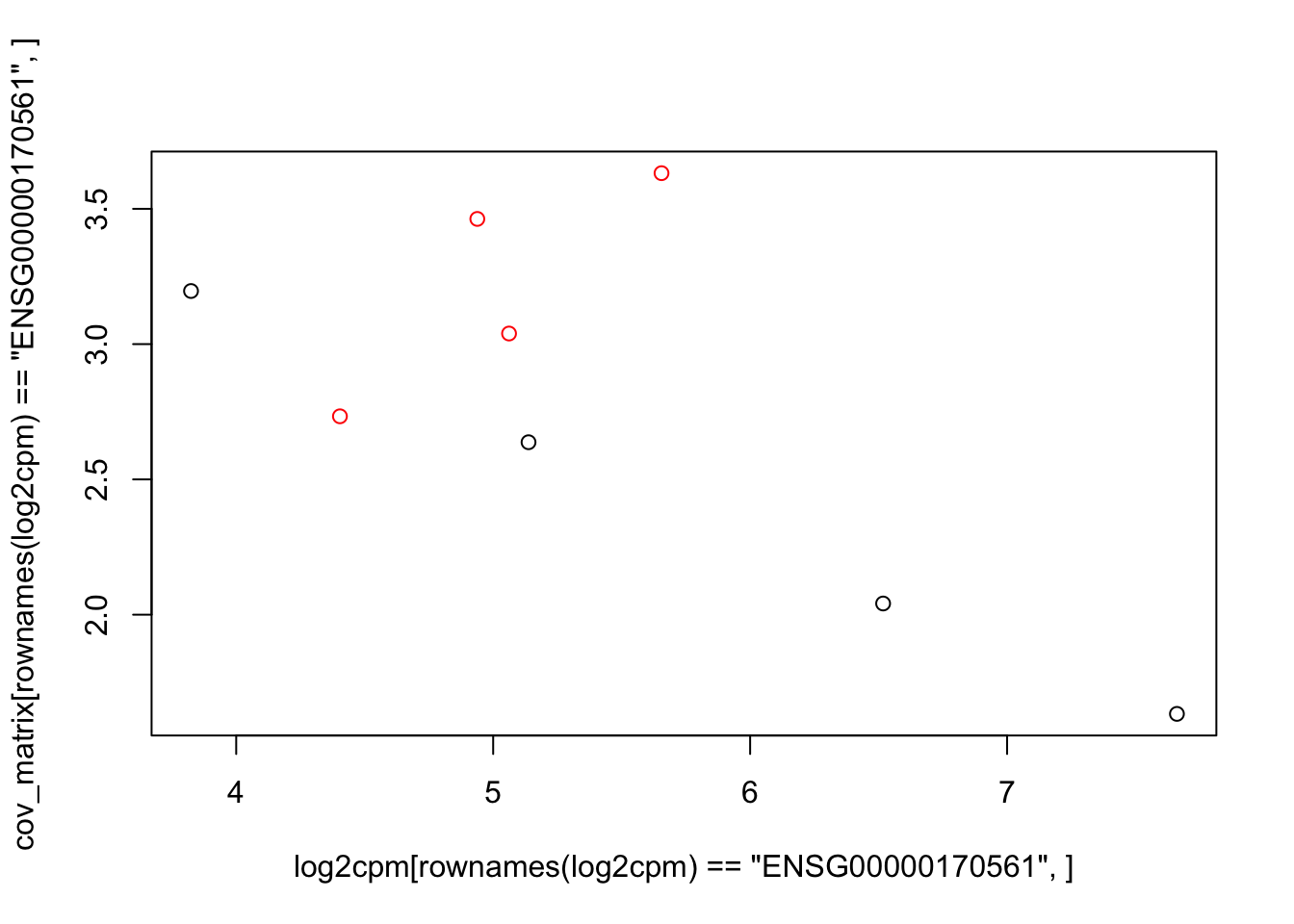
plot(log2cpm[rownames(log2cpm)=="ENSG00000280143",], cov_matrix[rownames(log2cpm)=="ENSG00000280143",], col=colors)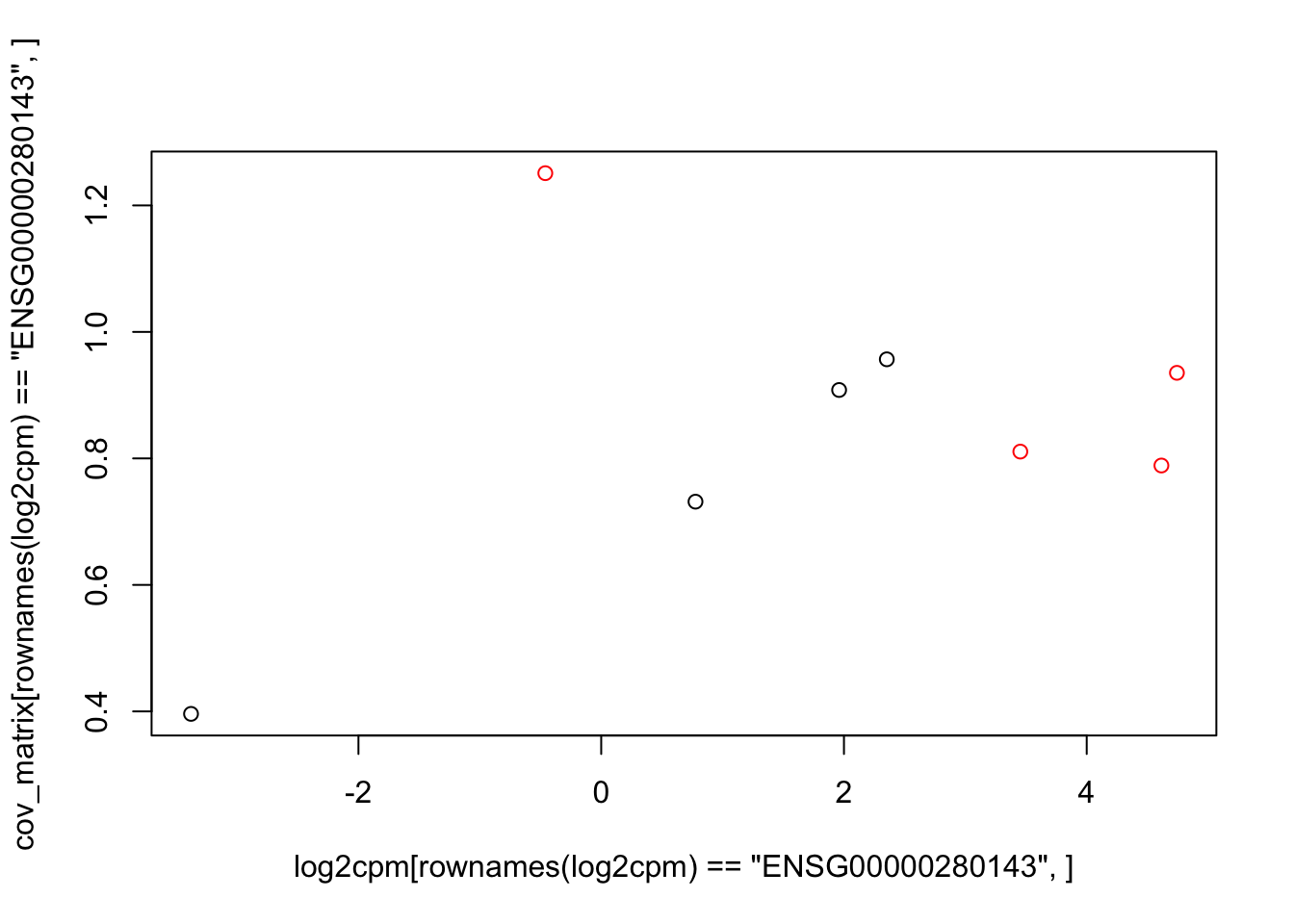
plot(log2cpm[rownames(log2cpm)=="ENSG00000124486",], cov_matrix[rownames(log2cpm)=="ENSG00000124486",], col=colors)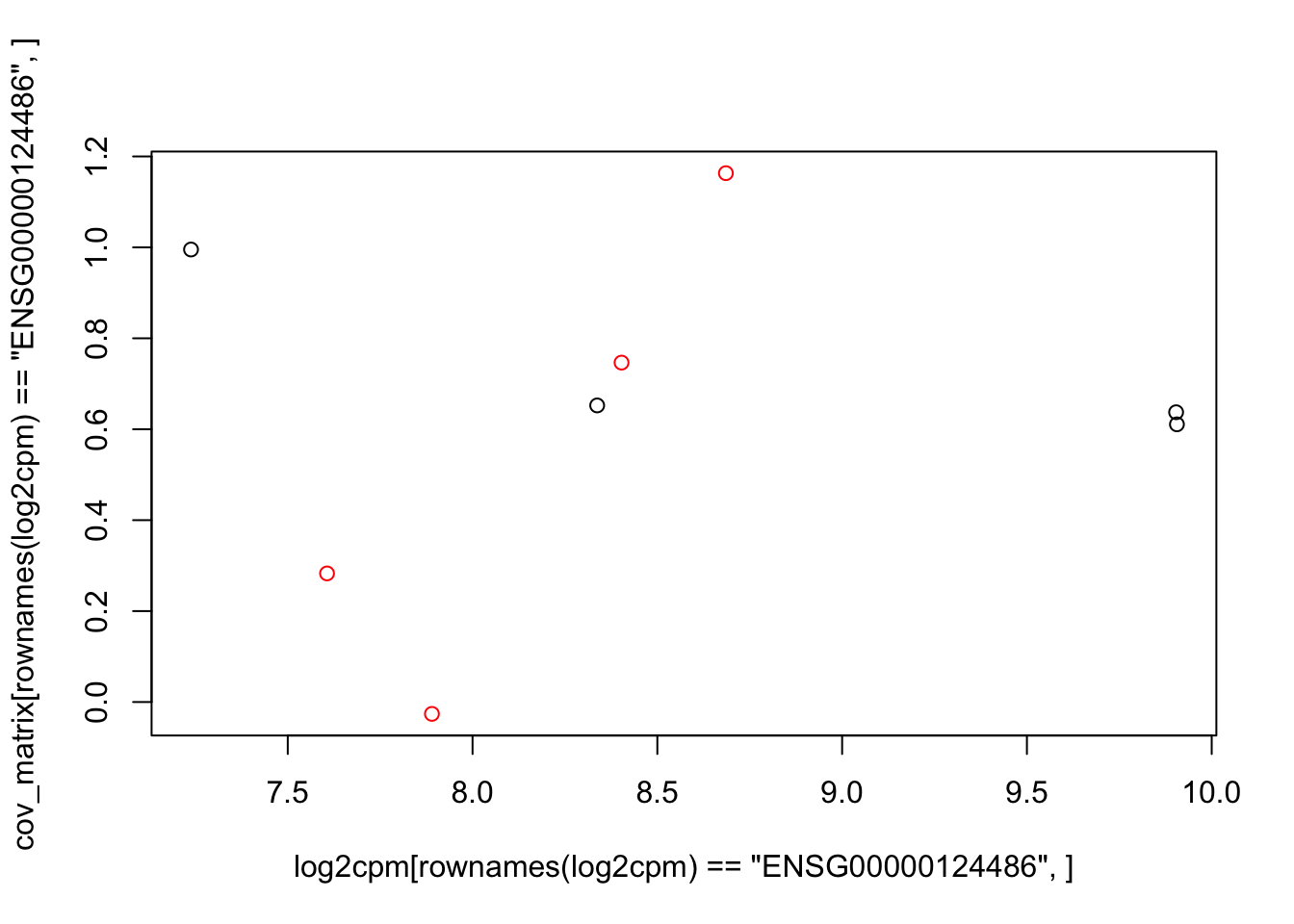
plot(log2cpm[rownames(log2cpm)=="ENSG00000100167",], cov_matrix[rownames(log2cpm)=="ENSG00000100167",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000188070",], cov_matrix[rownames(log2cpm)=="ENSG00000188070",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000169442",], cov_matrix[rownames(log2cpm)=="ENSG00000169442",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000170775",], cov_matrix[rownames(log2cpm)=="ENSG00000170775",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000267886",], cov_matrix[rownames(log2cpm)=="ENSG00000267886",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000228709",], cov_matrix[rownames(log2cpm)=="ENSG00000228709",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000178718",], cov_matrix[rownames(log2cpm)=="ENSG00000178718",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000133636",], cov_matrix[rownames(log2cpm)=="ENSG00000133636",], col=colors)
plot(log2cpm[rownames(log2cpm)=="ENSG00000204252",], cov_matrix[rownames(log2cpm)=="ENSG00000204252",], col=colors)
sessionInfo()R version 3.4.0 (2017-04-21)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: OS X El Capitan 10.11.6
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.4/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.4/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] forcats_0.4.0 stringr_1.4.0 dplyr_0.8.0.1
[4] purrr_0.3.2 readr_1.3.1 tidyr_0.8.3
[7] tibble_2.1.1 ggplot2_3.1.0 tidyverse_1.2.1
[10] data.table_1.12.0 assertthat_0.2.1 limma_3.34.9
loaded via a namespace (and not attached):
[1] statmod_1.4.30 tidyselect_0.2.5 xfun_0.5 haven_2.1.0
[5] lattice_0.20-38 colorspace_1.4-1 generics_0.0.2 htmltools_0.3.6
[9] yaml_2.2.0 rlang_0.3.3 pillar_1.3.1 glue_1.3.1
[13] withr_2.1.2 modelr_0.1.4 readxl_1.3.1 plyr_1.8.4
[17] munsell_0.5.0 gtable_0.3.0 workflowr_1.2.0 cellranger_1.1.0
[21] rvest_0.3.2 evaluate_0.13 knitr_1.22 broom_0.5.1
[25] Rcpp_1.0.1 scales_1.0.0 backports_1.1.3 jsonlite_1.6
[29] fs_1.2.7 hms_0.4.2 digest_0.6.18 stringi_1.4.3
[33] grid_3.4.0 rprojroot_1.3-2 cli_1.1.0 tools_3.4.0
[37] magrittr_1.5 lazyeval_0.2.2 crayon_1.3.4 whisker_0.3-2
[41] pkgconfig_2.0.2 xml2_1.2.0 lubridate_1.7.4 rmarkdown_1.12
[45] httr_1.4.0 rstudioapi_0.10 R6_2.4.0 nlme_3.1-137
[49] git2r_0.25.2 compiler_3.4.0