Merge NSCLC FRCs and TILs
Chrysa Papadopoulou
Last updated: 2024-11-05
Checks: 7 0
Knit directory: CCL19_FRCs_lung_cancer/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20240808) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 683e44a. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .DS_Store
Ignored: analysis/.DS_Store
Ignored: data/Final_submission/
Ignored: data/Human/
Ignored: data/Mouse/
Ignored: data/Public/
Ignored: output/GSEA_AdvFB_SULF1/
Ignored: output/GSEA_AdvFB_TLS/
Ignored: output/GSEA_CCR7_T/
Ignored: output/GSEA_CD8_T/
Ignored: output/GSEA_CYCL_T/
Ignored: output/GSEA_EXH_T/
Ignored: output/GSEA_SMC_PRC/
Untracked files:
Untracked: README.html
Untracked: analysis/.h5seurat
Untracked: analysis/Compare_tumors.Rmd
Untracked: analysis/NSCLC_PDAC_CAFs.Rmd
Untracked: analysis/Seurat_to_SCE.Rmd
Untracked: analysis/compression.Rmd
Untracked: analysis/index_hidden.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/merge_NSCLC_FRC_TIL.Rmd)
and HTML (docs/merge_NSCLC_FRC_TIL.html) files. If you’ve
configured a remote Git repository (see ?wflow_git_remote),
click on the hyperlinks in the table below to view the files as they
were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 683e44a | Pchryssa | 2024-11-05 | Correct figure ordering |
| html | 79d9795 | Pchryssa | 2024-09-23 | Build site. |
| Rmd | 7e218c6 | Pchryssa | 2024-09-23 | Modify figure order |
| html | 5da48e4 | Pchryssa | 2024-08-21 | Build site. |
| Rmd | c85bb48 | Pchryssa | 2024-08-21 | Merge NSCLC TIL |
| html | 532ab31 | Pchryssa | 2024-08-21 | Build site. |
| Rmd | 5a7acbb | Pchryssa | 2024-08-21 | NSCLC FRC TIL merge |
Load packages
suppressPackageStartupMessages({
library(here)
library(purrr)
library(stringr)
library(patchwork)
library(Seurat)
library(Matrix)
library(dittoSeq)
library(gridExtra)
library(CellChat)
library(NMF)
library(pheatmap)
})Infiltrating lymphocytes in NSCLC
Set directory
basedir <- here()Merge CCL19⁺ FRCs and Infiltrating lymphocytes in NSCLC
Read NSCLC TIL and FRC data
NSCLS_TIL_data <-readRDS(paste0(basedir,"/data/Human/NSCLC_TILs.rds"))
NSCLC_CCL19_FRCs <-readRDS(paste0(basedir,"/data/Human/NSCLC_CCL19_FRCs_CAFs.rds"))Merge NSCLS CCL19⁺ FRCs and NSCLS TILs
same_columns <- intersect(colnames(NSCLS_TIL_data@meta.data),colnames(NSCLC_CCL19_FRCs@meta.data))
NSCLS_TIL_data@meta.data <-NSCLS_TIL_data@meta.data[,same_columns]
NSCLC_CCL19_FRCs@meta.data <-NSCLC_CCL19_FRCs@meta.data[,same_columns]
merged_data<- merge(NSCLS_TIL_data, y = c(NSCLC_CCL19_FRCs),
add.cell.ids = c('NSCLS_TIL_data','NSCLC_CCL19_FRCs'),
project = "NSCLC_FRC_TIL")
resolution <- c(0.1, 0.25, 0.4, 0.6, 0.8, 1.,1.2,1.4,1.6,2.)
merged_data <- preprocessing(merged_data,resolution)Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.9696
Number of communities: 7
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.9427
Number of communities: 11
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.9254
Number of communities: 13
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.9050
Number of communities: 14
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8897
Number of communities: 19
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8772
Number of communities: 22
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8652
Number of communities: 23
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8546
Number of communities: 26
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8448
Number of communities: 27
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 12217
Number of edges: 420063
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8272
Number of communities: 30
Elapsed time: 1 secondsNSCLS CCL19⁺ FRCs and NSCLS TILs
#Define color palet
palet <- c("#1B9E77", "#54B0E4","#E3BE00", "#E41A1C", "#4DAF4A","#FB9A99","#377EB8","#A65628","#222F75")
names(palet) <- c( "CAF2/TRC","CAF1/PRC","AdvFB" ,"SMC/PC", paste0("CD4", "\u207A ", "T cells"), paste0("CD8", "\u207A ", "T cells"), "B cells", "Regulatory T cells",paste0("Cycling CD8", "\u207A ", "T cells"))
DimPlot(merged_data, reduction = "umap", group.by = "cell_type", cols=palet)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank(),
panel.grid.major = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2") + ggtitle(paste0("NSCLC CCL19", "\U207A ", "FRCs", " and TILs"))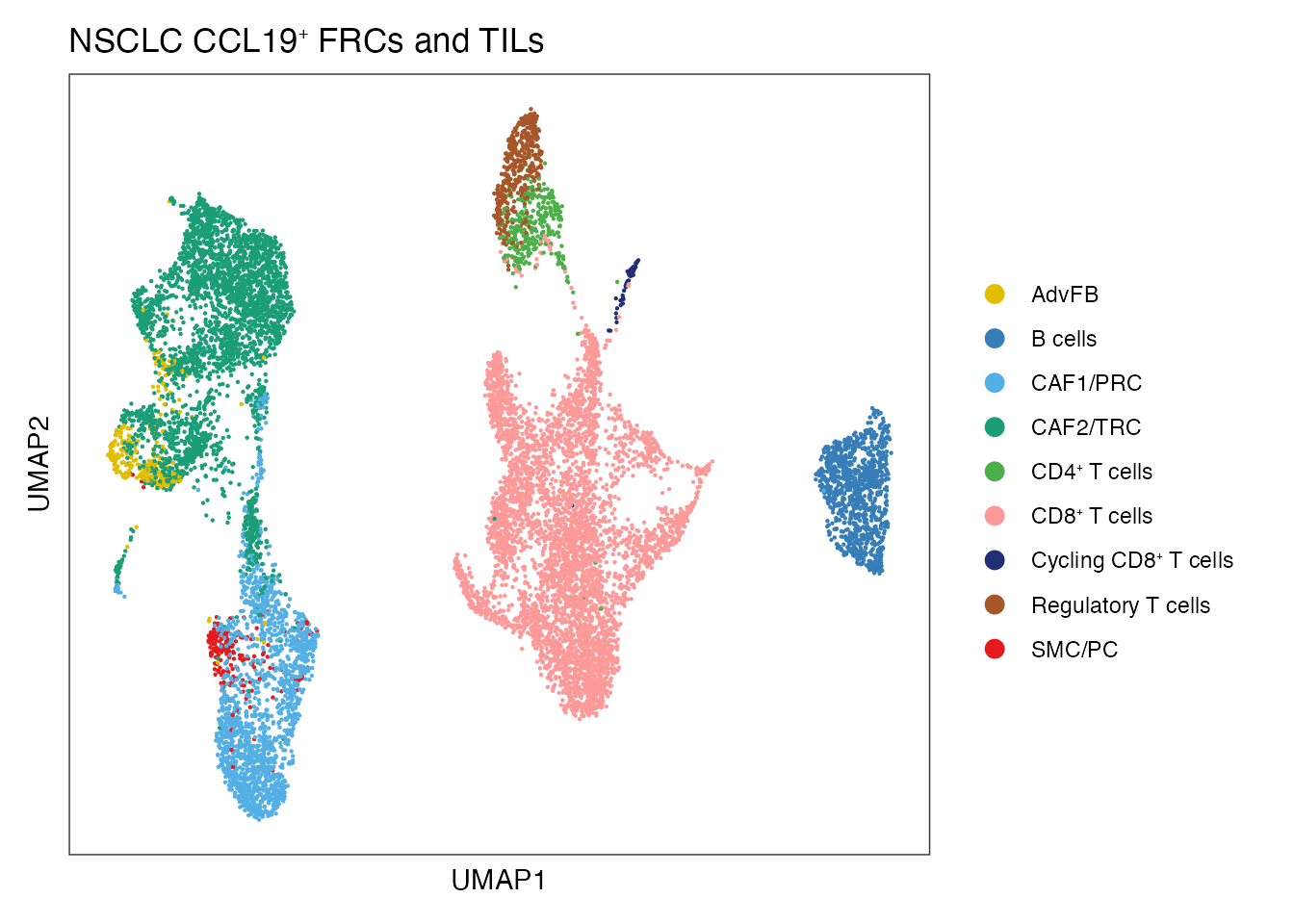
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Save merged data
#saveRDS(merged_data, paste0(basedir,"/data/Human/NSCLC_TILs_SI4.rds"))Interactome Analysis with Cellchat (Suoqin Jin et al., 2021)
Convert seurat object to cellchat object
cellchat <- Cellchat_Analysis(merged_data)[1] "Create a CellChat object from a data matrix"
Set cell identities for the new CellChat object
The cell groups used for CellChat analysis are AdvFB B cells CAF1/PRC CAF2/TRC CD4⁺ T cells CD8⁺ T cells Cycling CD8⁺ T cells Regulatory T cells SMC/PC cellchat <-CellChatDownstreamAnalysis(cellchat,"human",thresh = 0.05)triMean is used for calculating the average gene expression per cell group.
[1] ">>> Run CellChat on sc/snRNA-seq data <<< [2024-11-05 21:52:19.838019]"
[1] ">>> CellChat inference is done. Parameter values are stored in `object@options$parameter` <<< [2024-11-05 21:53:53.902528]"Save cellchat data
#saveRDS(cellchat,paste0(basedir,"/data/Human/CCL19_FRC_TIL_SIF5_cellchat.rds"))Interactome analysis (Supplementary Figure 3D)
gg <- netAnalysis_signalingRole_scatter(cellchat,color.use = palet)
gg <- gg + ggtitle("Interactome analysis (Cellchat)")
gg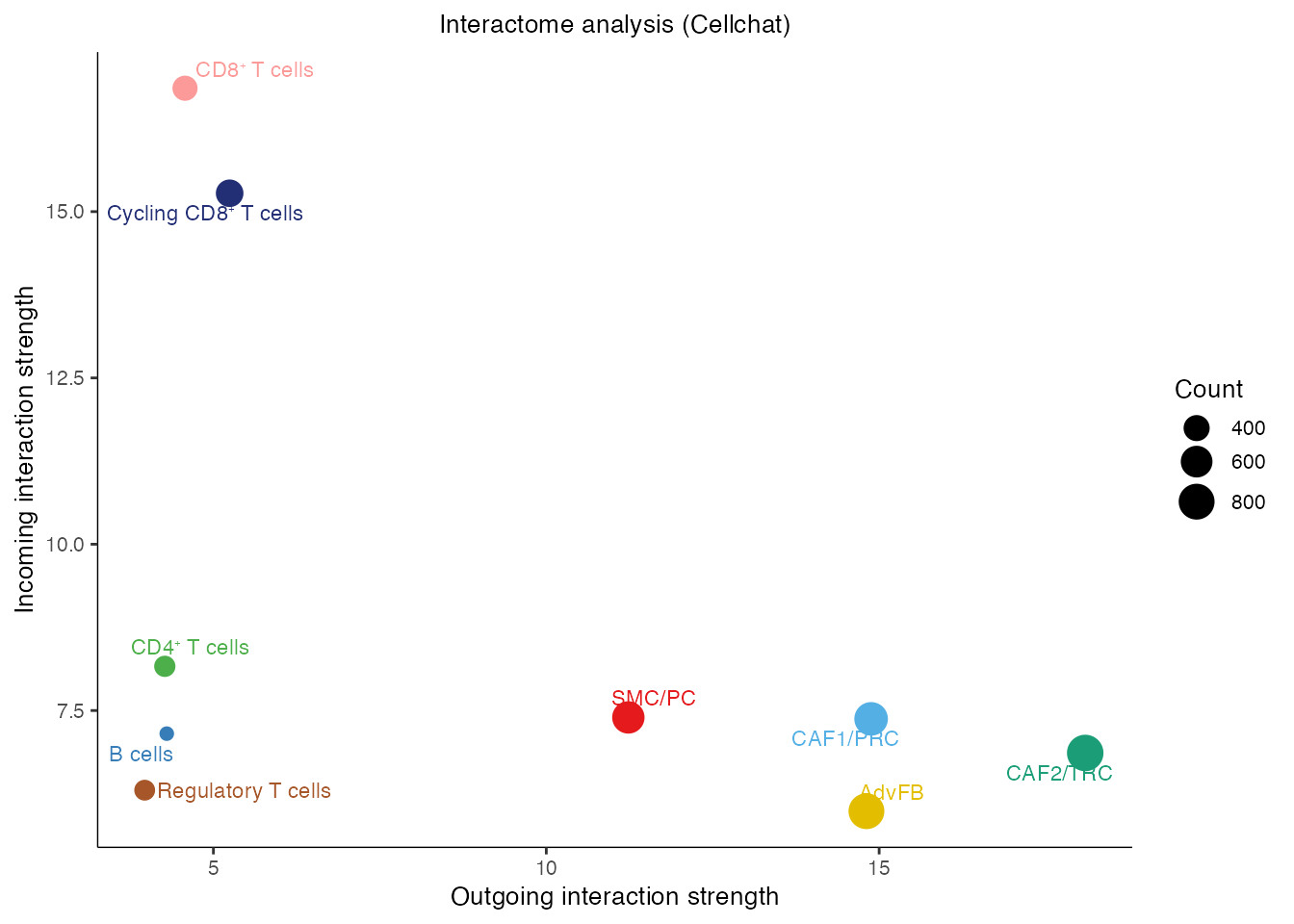
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Outgoing signaling patterns
selectK(cellchat, pattern = "outgoing")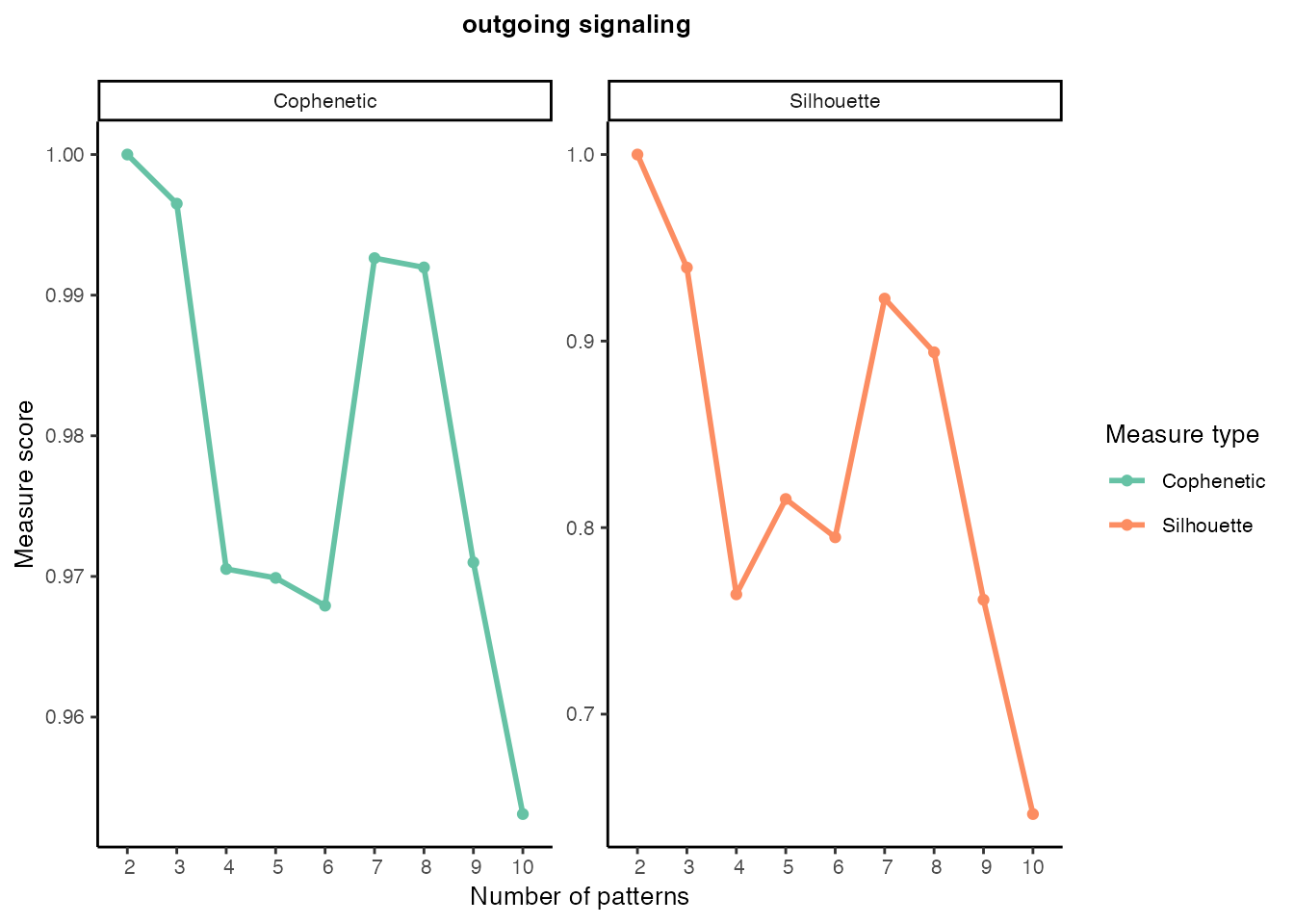
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Incoming signaling patterns
selectK(cellchat, pattern = "incoming")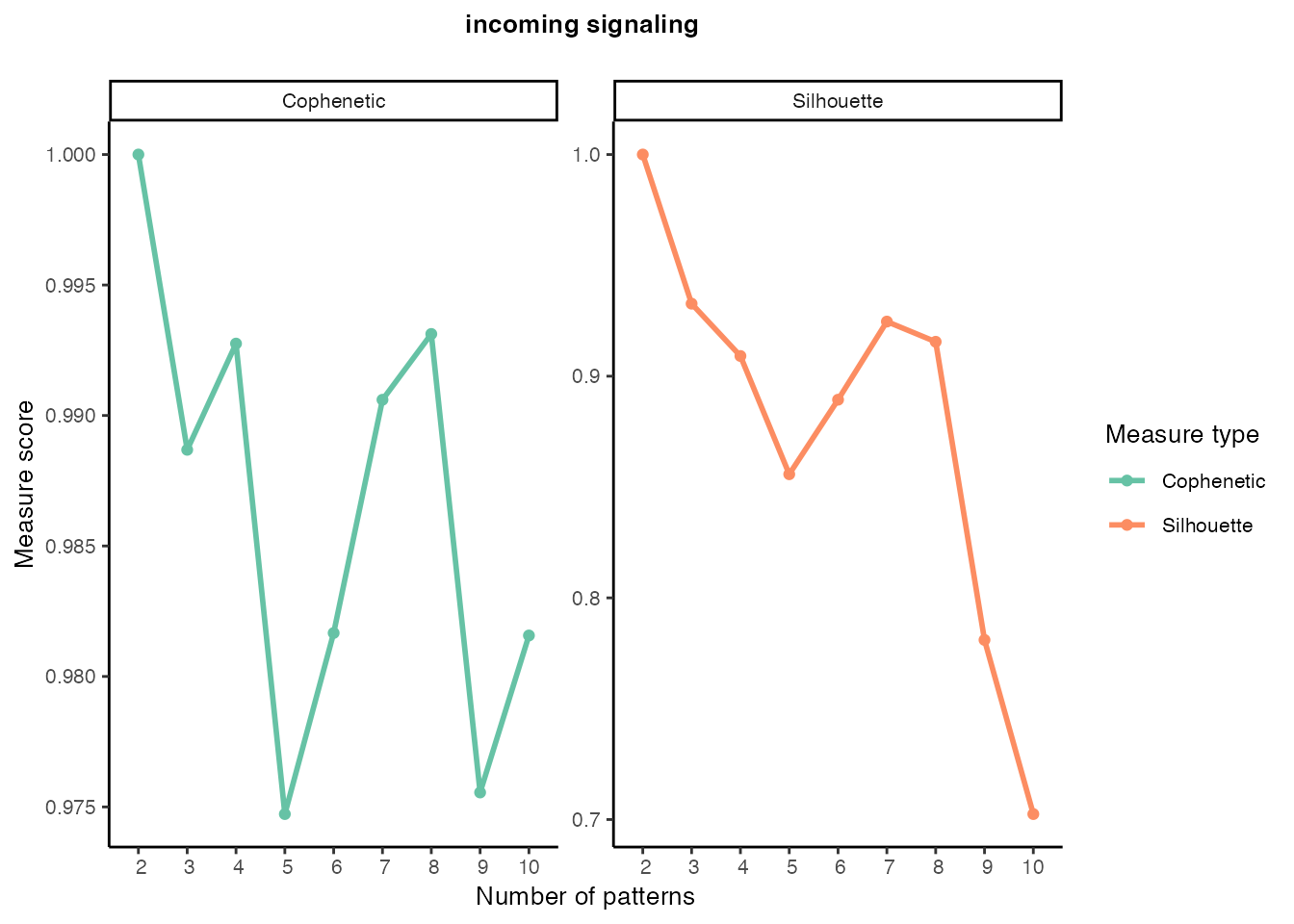
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Incoming and outgoing patterns
nPatterns <- 7
cellchat <- identifyCommunicationPatterns(cellchat, pattern = "outgoing", k = nPatterns, color.use = palet)
cellchat <- identifyCommunicationPatterns(cellchat, pattern = "incoming", k = nPatterns, color.use = palet)Joint dotplot for incoming and outcoming communication patterns (Supplementary Figure 3E)
pathways <-c("CXCL","CCL","ICAM","VCAM","MK","FGF","NECTIN","TIGIT","SELL","ITGB2","NOTCH","BAFF","SEMA3","SEMA4","LIGHT","EGF","IFN-II","CD226","IGF","FASLG")
order_list <-c("CAF2/TRC","CAF1/PRC","SMC/PC","AdvFB","Regulatory T cells", "CD4⁺ T cells","CD8⁺ T cells","Cycling CD8⁺ T cells","B cells")
netAnalysis_joint_dot(cellchat,color.use = palet,font.size = 12,pathways = pathways, order_list = order_list)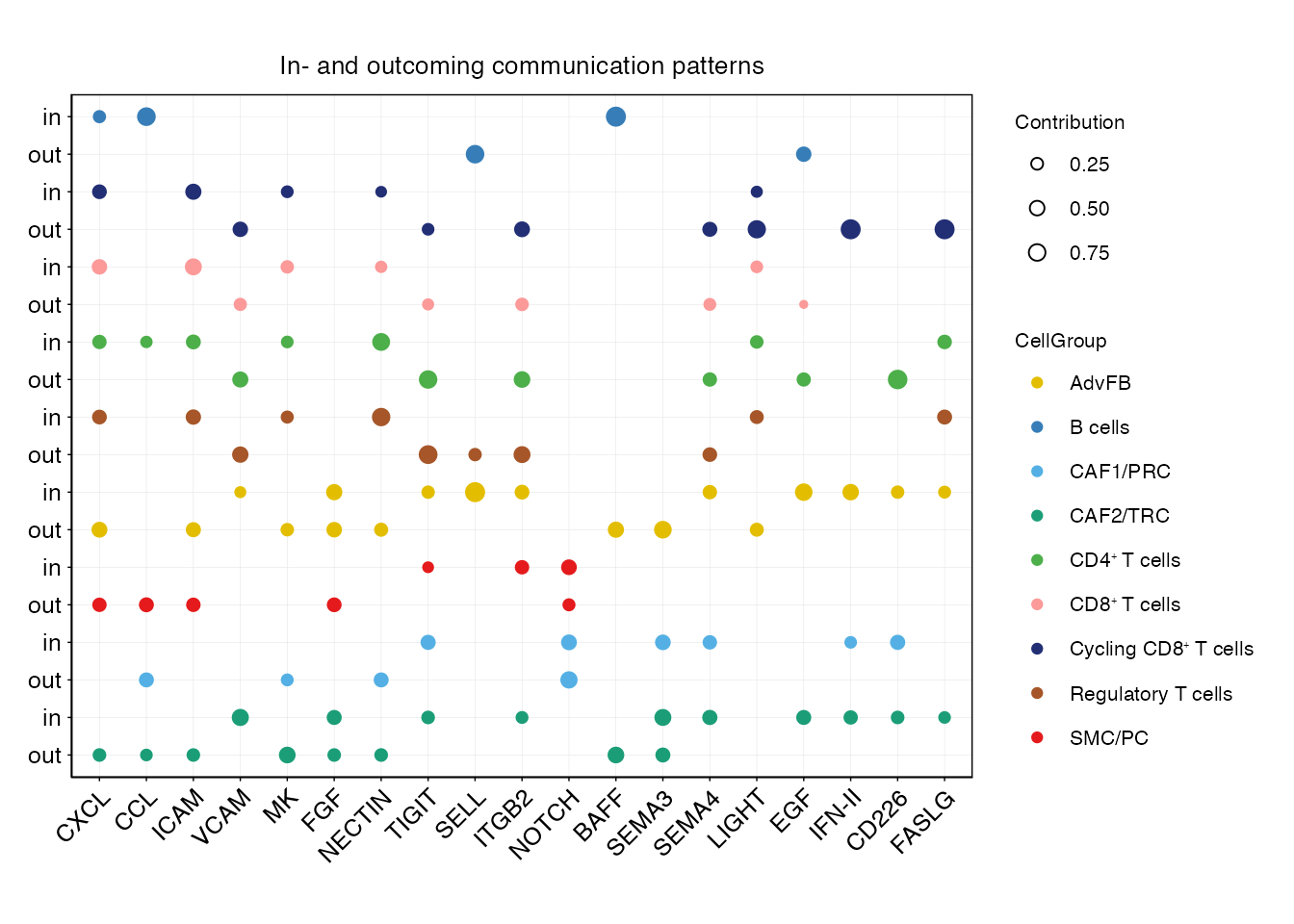
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Merge CCL19⁺ FRCs and CD8⁺ T cell subsets in NSCLC
Read CD8⁺ T cell data
NCLS_TIL_FRC_cd8 <-readRDS(paste0(basedir,"/data/Human/NSCLC_TILs_CD8_pop.rds"))Merge NSCLS CCL19⁺ FRCs and NSCLS CD8⁺ T cell subsets
same_columns <- intersect(colnames(NCLS_TIL_FRC_cd8@meta.data),colnames(NSCLC_CCL19_FRCs@meta.data))
NCLS_TIL_FRC_cd8@meta.data <-NCLS_TIL_FRC_cd8@meta.data[,same_columns]
NSCLC_CCL19_FRCs@meta.data <-NSCLC_CCL19_FRCs@meta.data[,same_columns]
merged_data<- merge(NCLS_TIL_FRC_cd8, y = c(NSCLC_CCL19_FRCs),
add.cell.ids = c('NCLS_TIL_FRC_cd8','NSCLC_CCL19_FRCs'),
project = "NSCLC_FRC_TIL_CD8")
resolution <- c(0.1, 0.25, 0.4, 0.6, 0.8, 1.,1.2,1.4,1.6,2.)
merged_data <- preprocessing(merged_data,resolution)Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.9616
Number of communities: 5
Elapsed time: 0 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.9304
Number of communities: 9
Elapsed time: 0 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.9108
Number of communities: 12
Elapsed time: 0 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8875
Number of communities: 16
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8721
Number of communities: 20
Elapsed time: 0 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8585
Number of communities: 22
Elapsed time: 1 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8473
Number of communities: 24
Elapsed time: 0 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8361
Number of communities: 23
Elapsed time: 0 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8245
Number of communities: 24
Elapsed time: 0 seconds
Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
Number of nodes: 10459
Number of edges: 356757
Running Louvain algorithm...
Maximum modularity in 10 random starts: 0.8046
Number of communities: 30
Elapsed time: 0 secondsNSCLS CCL19⁺ FRCs and NSCLS CD8⁺ T cell subsets
#Extend palet for CD8 T cell subsets
palet <- c("#1B9E77", "#54B0E4","#E3BE00", "#E41A1C","#F8766D","#00C08B","#7CAE00","#00B4F0","#F564E3")
names(palet) <- c("CAF2/TRC","CAF1/PRC","AdvFB" ,"SMC/PC",paste0("Stem-like/Naive CD8", "\u207A ", "T cells"),paste0("Exhausted CD8", "\u207A ", "T cells"), paste0("Effector-memory CD8", "\u207A ", "T cells"),paste0("Effector CD8", "\u207A ", "T cells"),paste0("Cycling CD8", "\u207A ", "T cells"))
palet <- palet[names(palet) %in% unique(merged_data$cell_type)]
DimPlot(merged_data, reduction = "umap", group.by = "cell_type", cols = palet)+
theme_bw() +
theme(axis.text = element_blank(), axis.ticks = element_blank(),
panel.grid.minor = element_blank(),
panel.grid.major = element_blank()) +
xlab("UMAP1") +
ylab("UMAP2")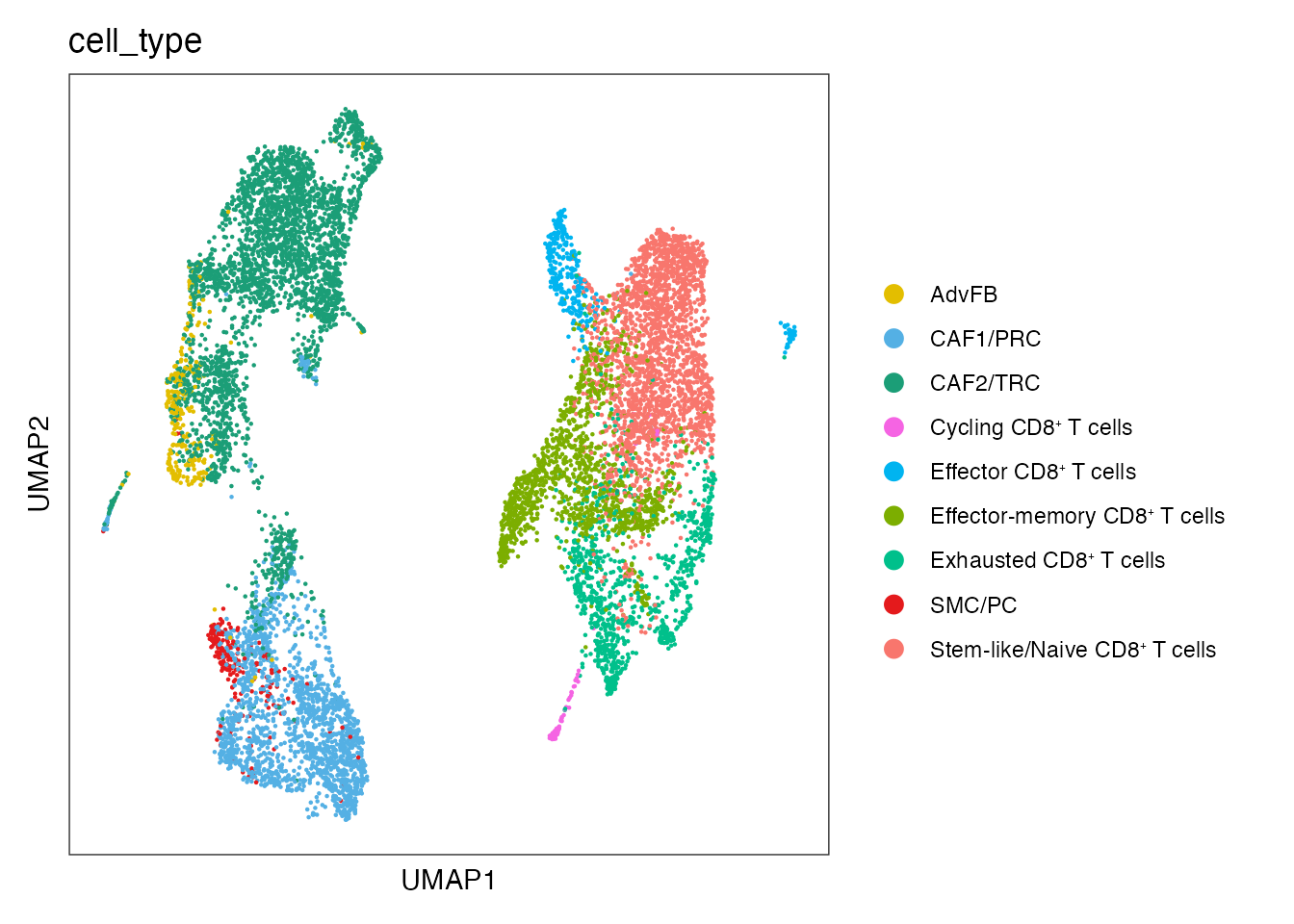
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
NSCLS CCL19⁺ FRCs and NSCLS CD8⁺ T cell data
#saveRDS(merged_data, paste0(basedir,"/data/Human/NSCLC_TILs_CD8_pop_merge.rds"))Interactome Analysis with Cellchat (Suoqin Jin et al., 2021)
Convert seurat object to cellchat object
cellchat <- Cellchat_Analysis(merged_data)[1] "Create a CellChat object from a data matrix"
Set cell identities for the new CellChat object
The cell groups used for CellChat analysis are AdvFB CAF1/PRC CAF2/TRC Cycling CD8⁺ T cells Effector CD8⁺ T cells Effector-memory CD8⁺ T cells Exhausted CD8⁺ T cells SMC/PC Stem-like/Naive CD8⁺ T cells cellchat <-CellChatDownstreamAnalysis(cellchat,"human",thresh=0.08)triMean is used for calculating the average gene expression per cell group.
[1] ">>> Run CellChat on sc/snRNA-seq data <<< [2024-11-05 22:00:33.638104]"
[1] ">>> CellChat inference is done. Parameter values are stored in `object@options$parameter` <<< [2024-11-05 22:02:02.135343]"Save cellchat data
#saveRDS(cellchat,paste0(basedir,"/data/Human/CCL19_FRC_TIL_F3E_cellchat.rds"))Interactome analysis (Figure 3E)
gg <- netAnalysis_signalingRole_scatter(cellchat,color.use = palet)
gg <- gg + ggtitle("Interactome analysis (Cellchat)")
gg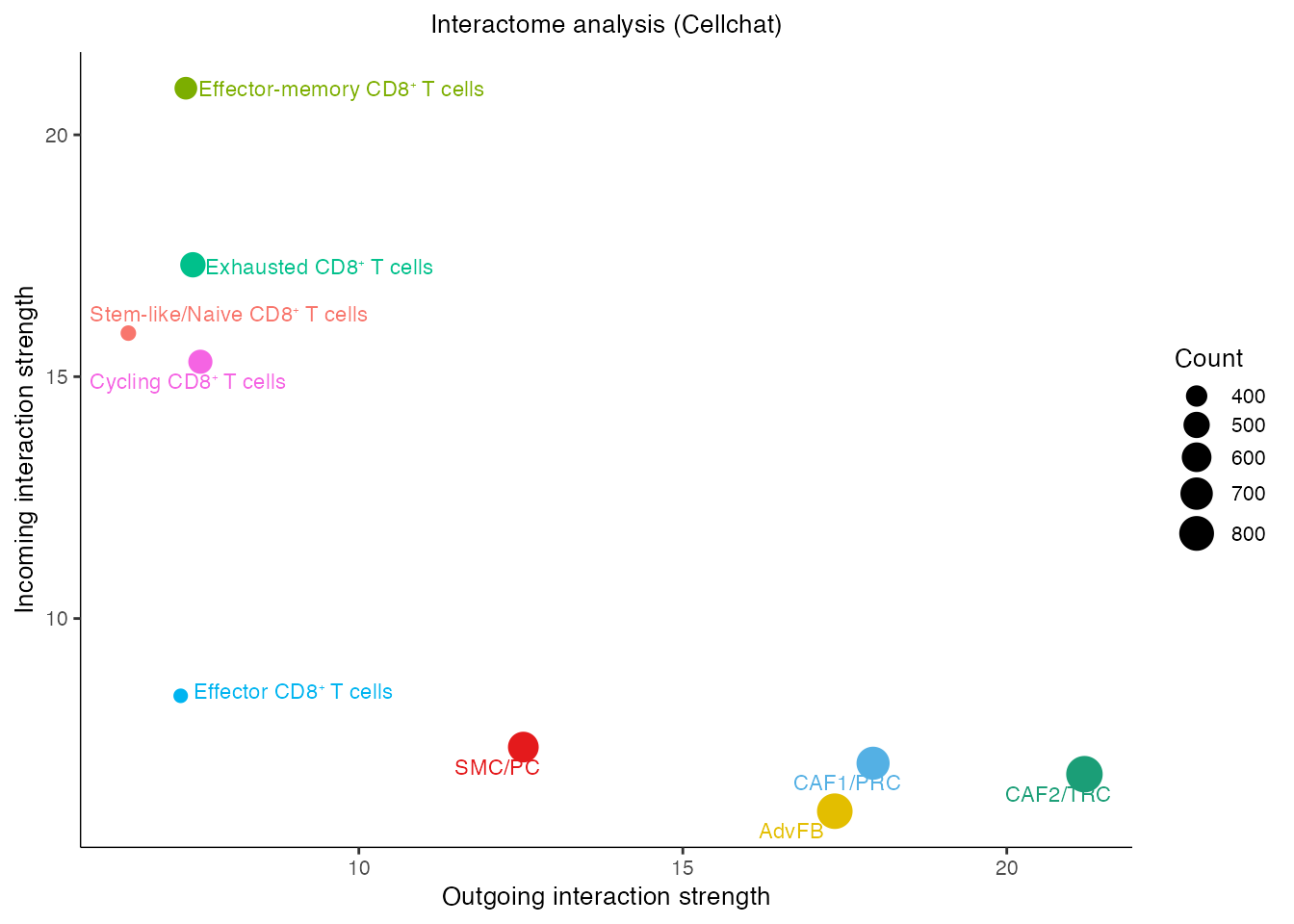
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Outgoing signaling patterns
selectK(cellchat, pattern = "outgoing")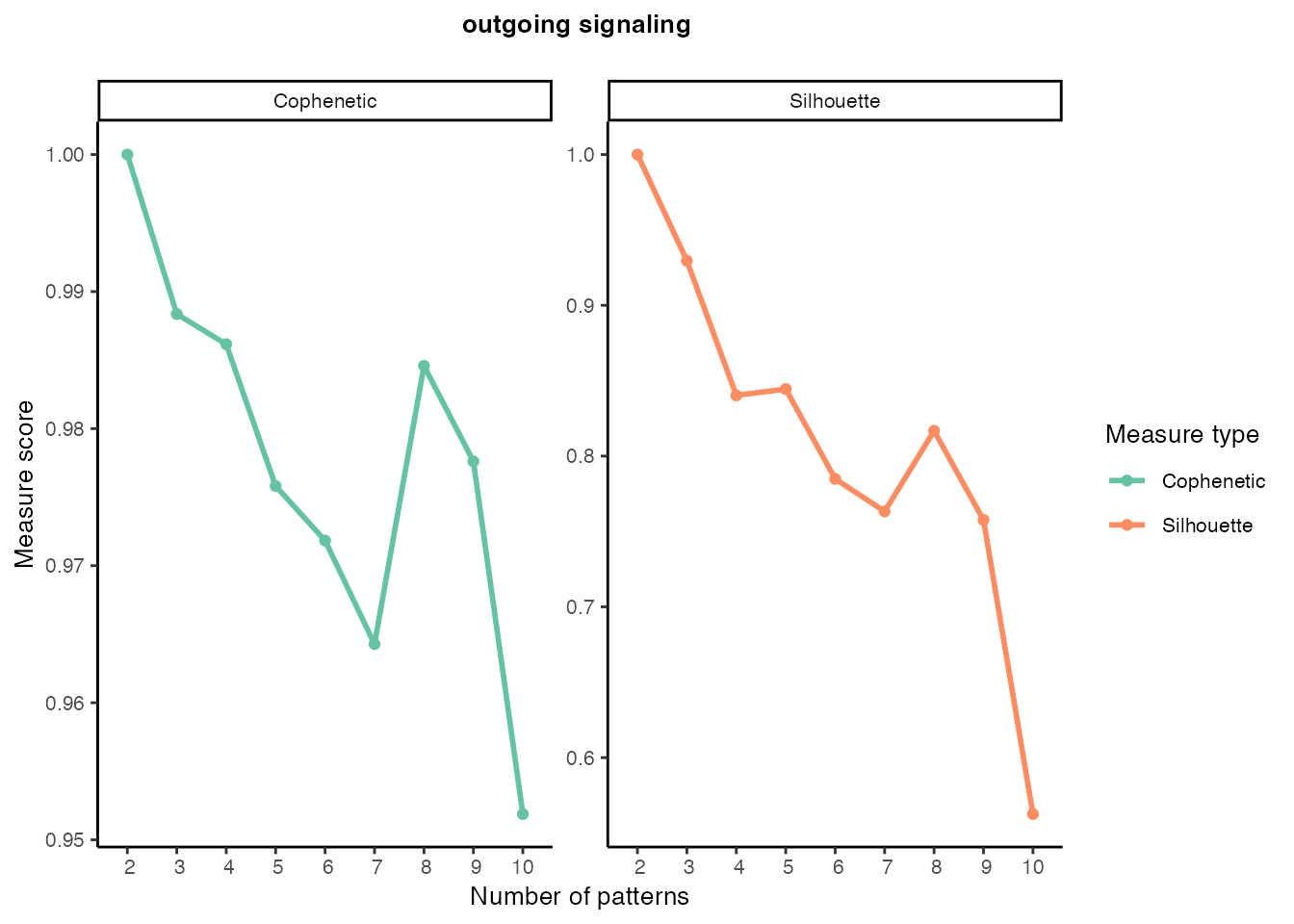
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Incoming signaling patterns
selectK(cellchat, pattern = "incoming")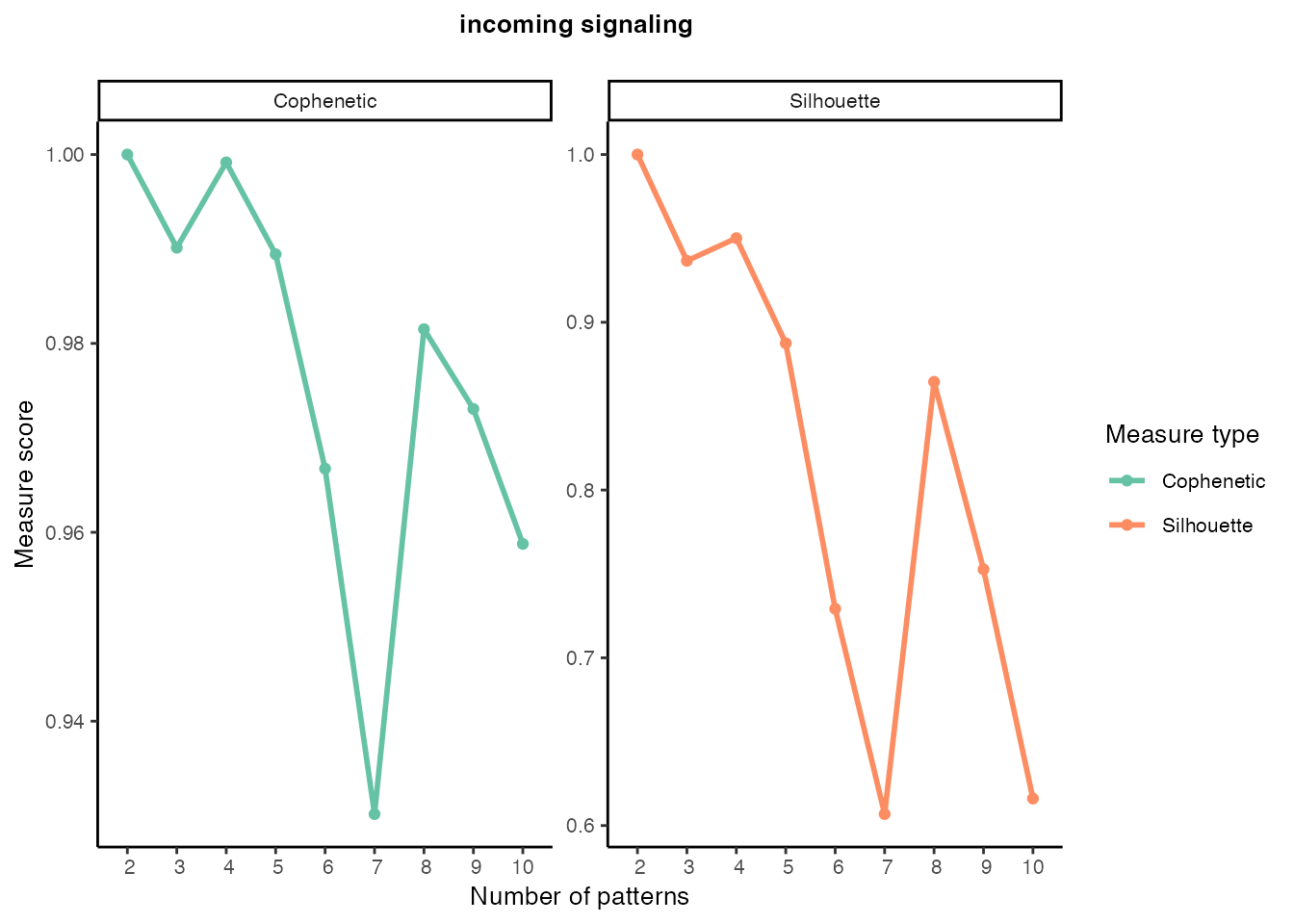
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Incoming and outgoing patterns
nPatterns <- 8
cellchat <- identifyCommunicationPatterns(cellchat, pattern = "outgoing", k = nPatterns, color.use = palet)
cellchat <- identifyCommunicationPatterns(cellchat, pattern = "incoming", k = nPatterns, color.use = palet)Joint dotplot for incoming and outcoming communication patterns (Figure 3F)
pathways <-c("MHC-II","LAMININ","FN1","CXCL","CCL","VCAM","ICAM","ITGB2","LIGHT","NECTIN","TIGIT","CD226","EGF","FASLG","SEMA4","IFN-II","IL16","GDF")
order_list <-c("CAF2/TRC","CAF1/PRC",paste0("Stem-like/Naive CD8", "\u207A ", "T cells"),
paste0("Exhausted CD8", "\u207A ", "T cells"),
paste0("Effector-memory CD8", "\u207A ", "T cells"),
paste0("Effector CD8", "\u207A ", "T cells"),
paste0("Cycling CD8", "\u207A ", "T cells"))
netAnalysis_joint_dot(cellchat,color.use = palet,font.size = 8,pathways = pathways, order_list = order_list, exclude = c("AdvFB" ,"SMC/PC"))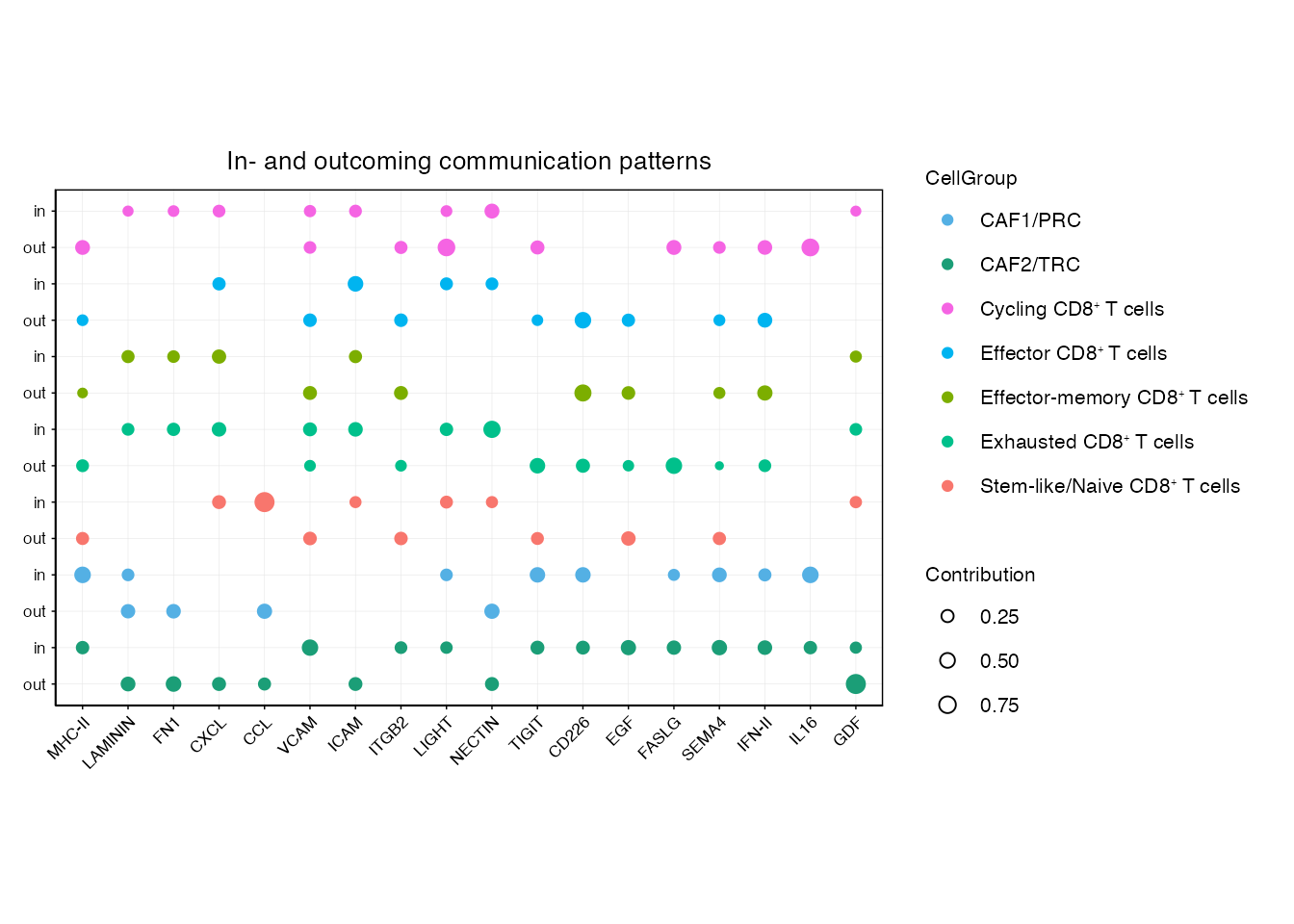
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Attraction and adhesion (Figure 3G)
Heatmap stroma
Selgenes <- c("VCAM1","CXCL16", "CD34","CXCL12")
order_list <-c("CAF1/PRC","SMC/PC", "CAF2/TRC","AdvFB")
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)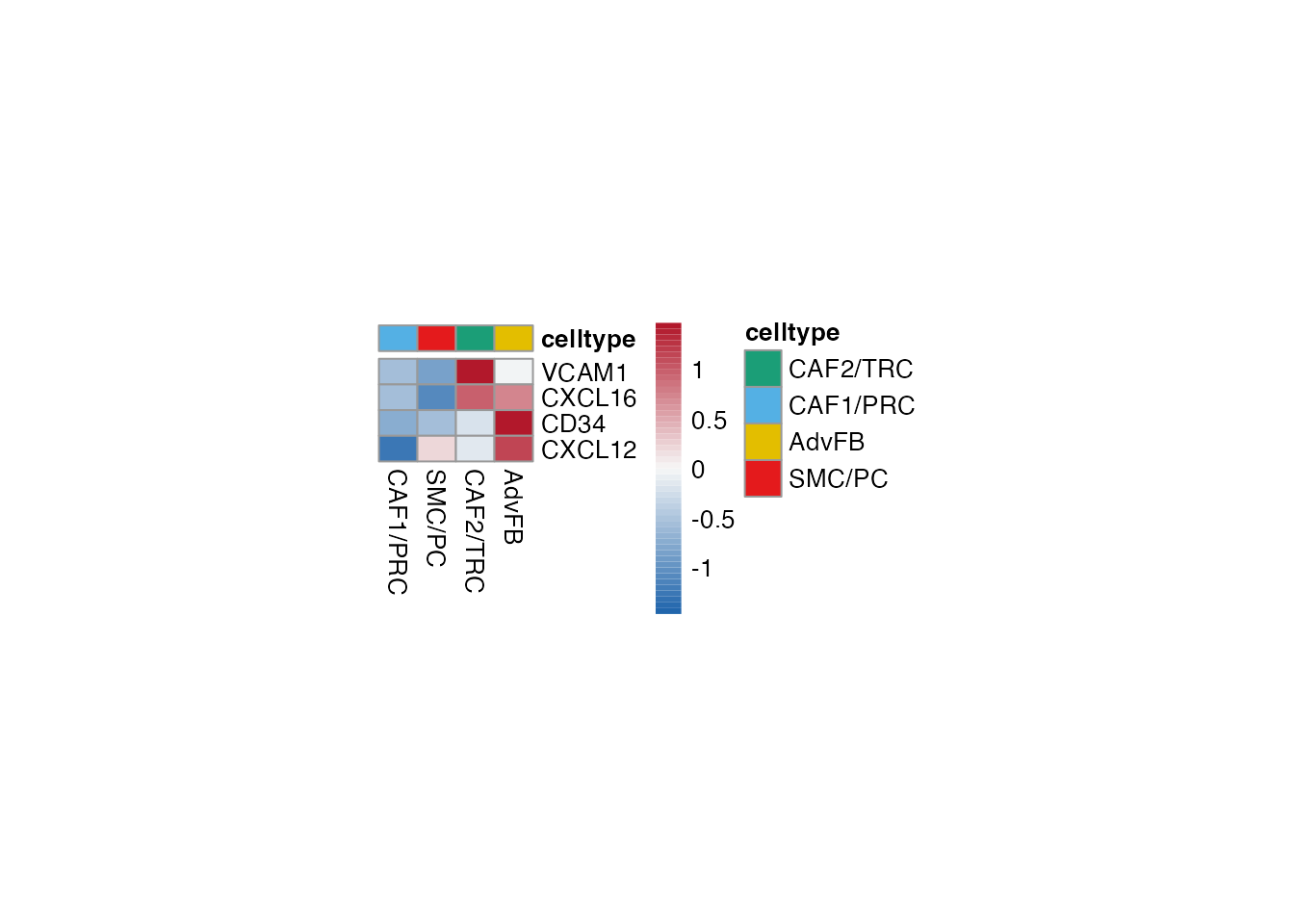
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Heatmap CD8⁺ T cell
Selgenes <- c("ITGA4","ITGB1","ITGB7","CXCR6","SELL","CXCR4")
order_list <-c(paste0("Stem-like/Naive CD8", "\u207A ", "T cells"),
paste0("Effector-memory CD8", "\u207A ", "T cells"),
paste0("Effector CD8", "\u207A ", "T cells"),
paste0("Exhausted CD8", "\u207A ", "T cells"),
paste0("Cycling CD8", "\u207A ", "T cells") )
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)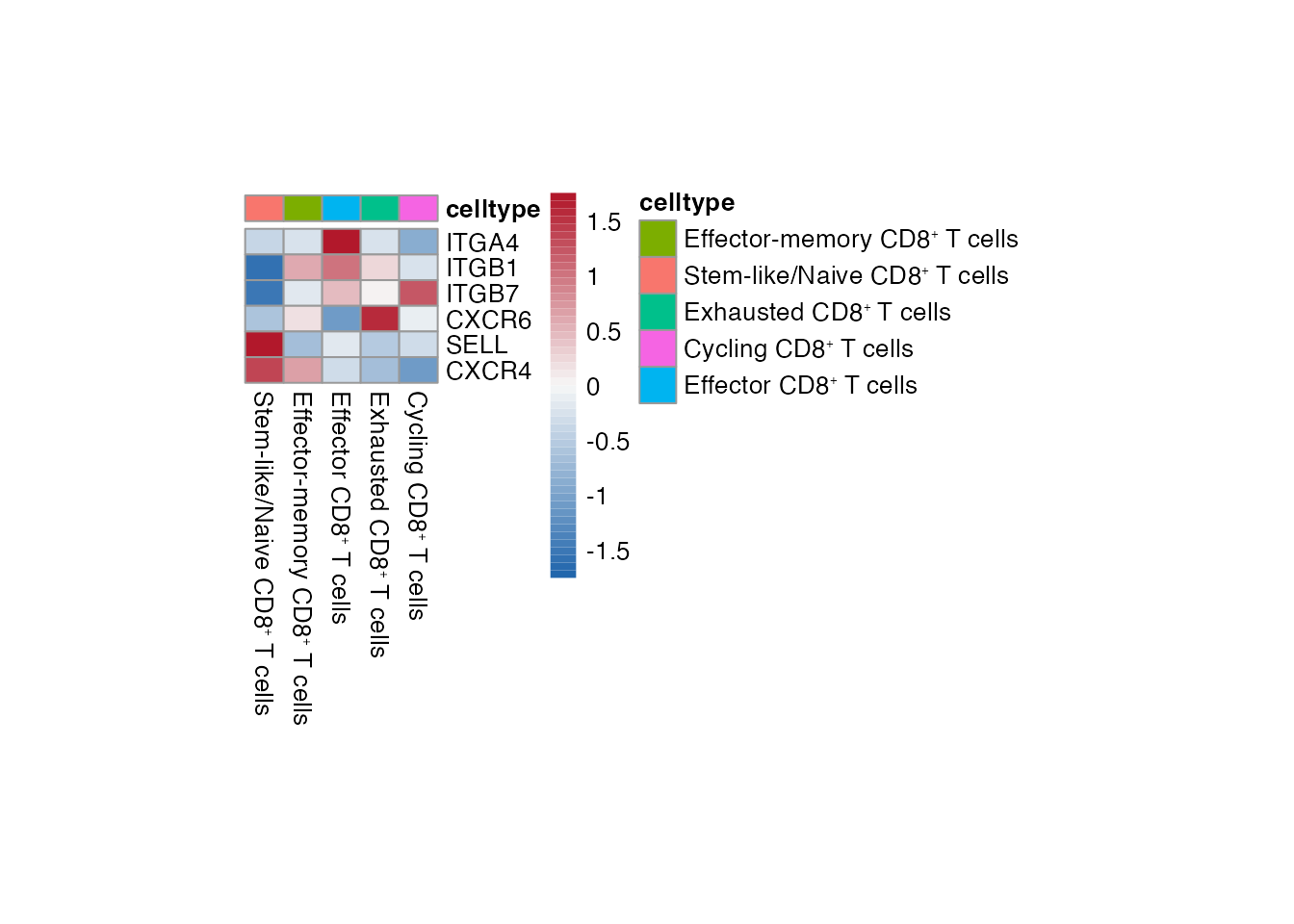
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
T cell reactivity (Figure 3H)
Heatmap stroma
Selgenes <- c("NECTIN3","NECTIN2","EGFR","FAS","IFNGR1","IFNGR2")
order_list <-c("CAF1/PRC","SMC/PC", "CAF2/TRC","AdvFB")
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)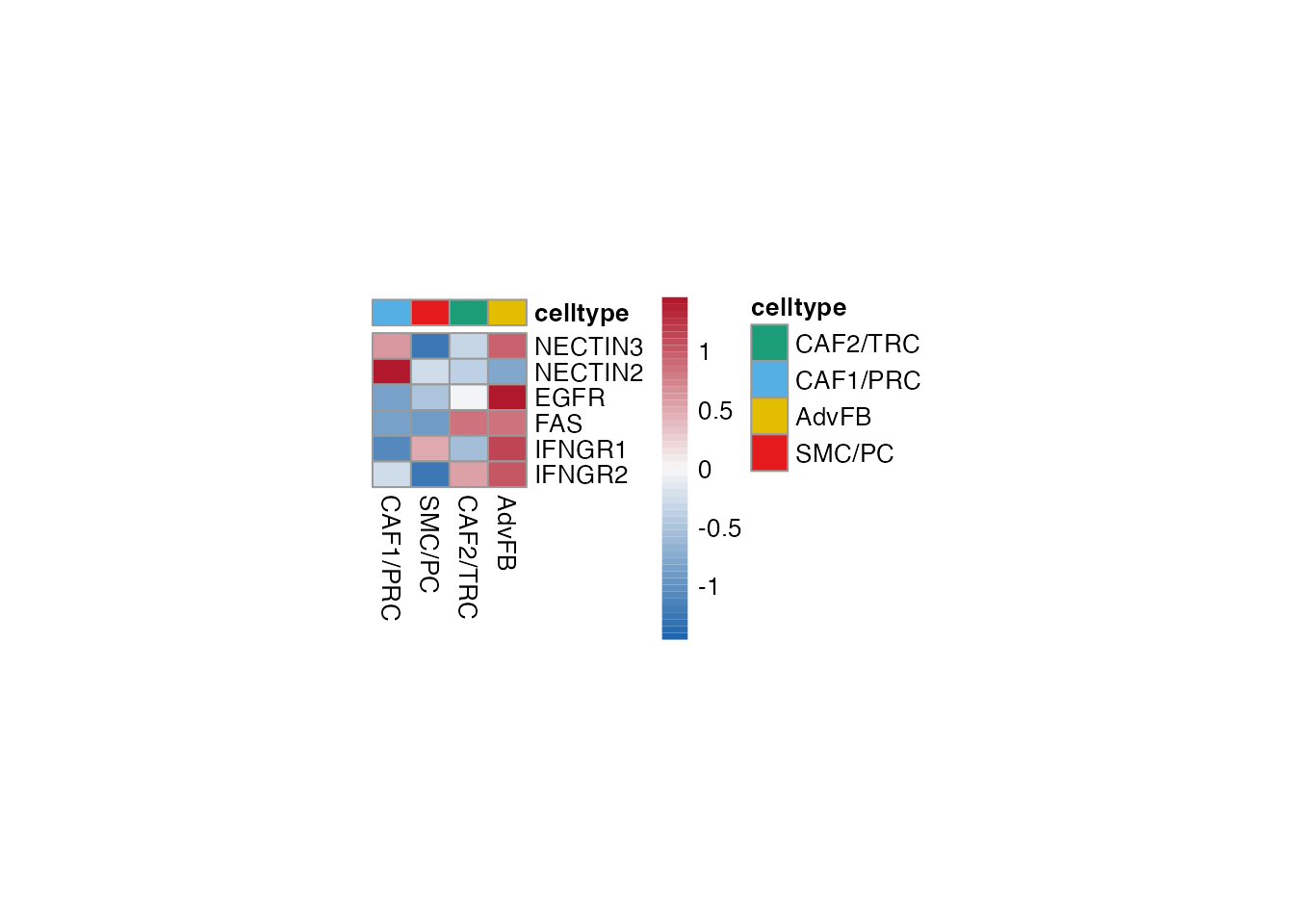
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Heatmap CD8⁺ T cell
Selgenes <- c("TIGIT","CD226","AREG","FASLG","IFNG")
order_list <-c(paste0("Stem-like/Naive CD8", "\u207A ", "T cells"),
paste0("Effector-memory CD8", "\u207A ", "T cells"),
paste0("Effector CD8", "\u207A ", "T cells"),
paste0("Exhausted CD8", "\u207A ", "T cells"),
paste0("Cycling CD8", "\u207A ", "T cells") )
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)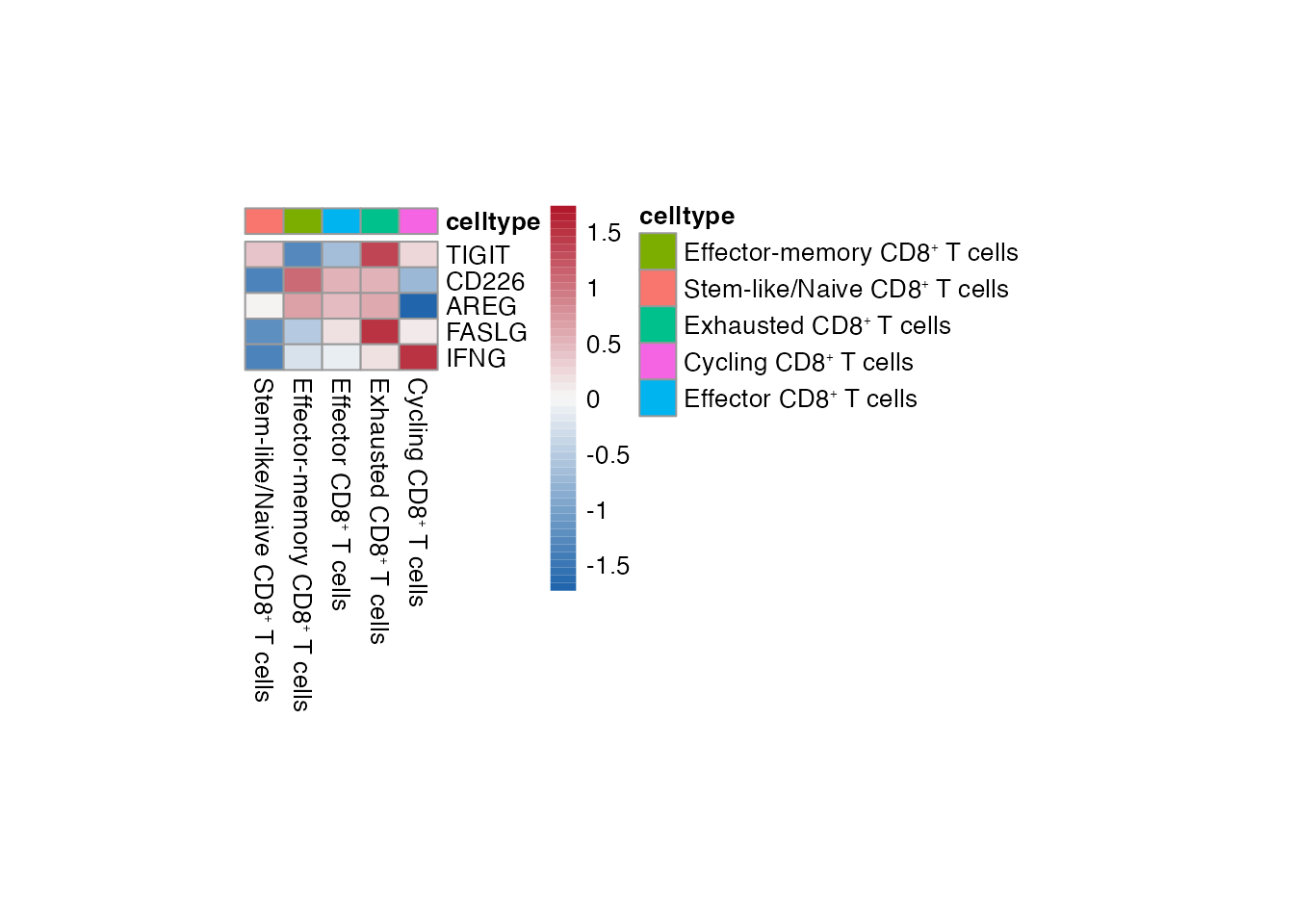
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Niche formation (Figure 3I)
Heatmap stroma
Selgenes <- c("LTBR","TNFRSF14","NOTCH3","JAG1")
order_list <-c("CAF1/PRC","SMC/PC", "CAF2/TRC","AdvFB")
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)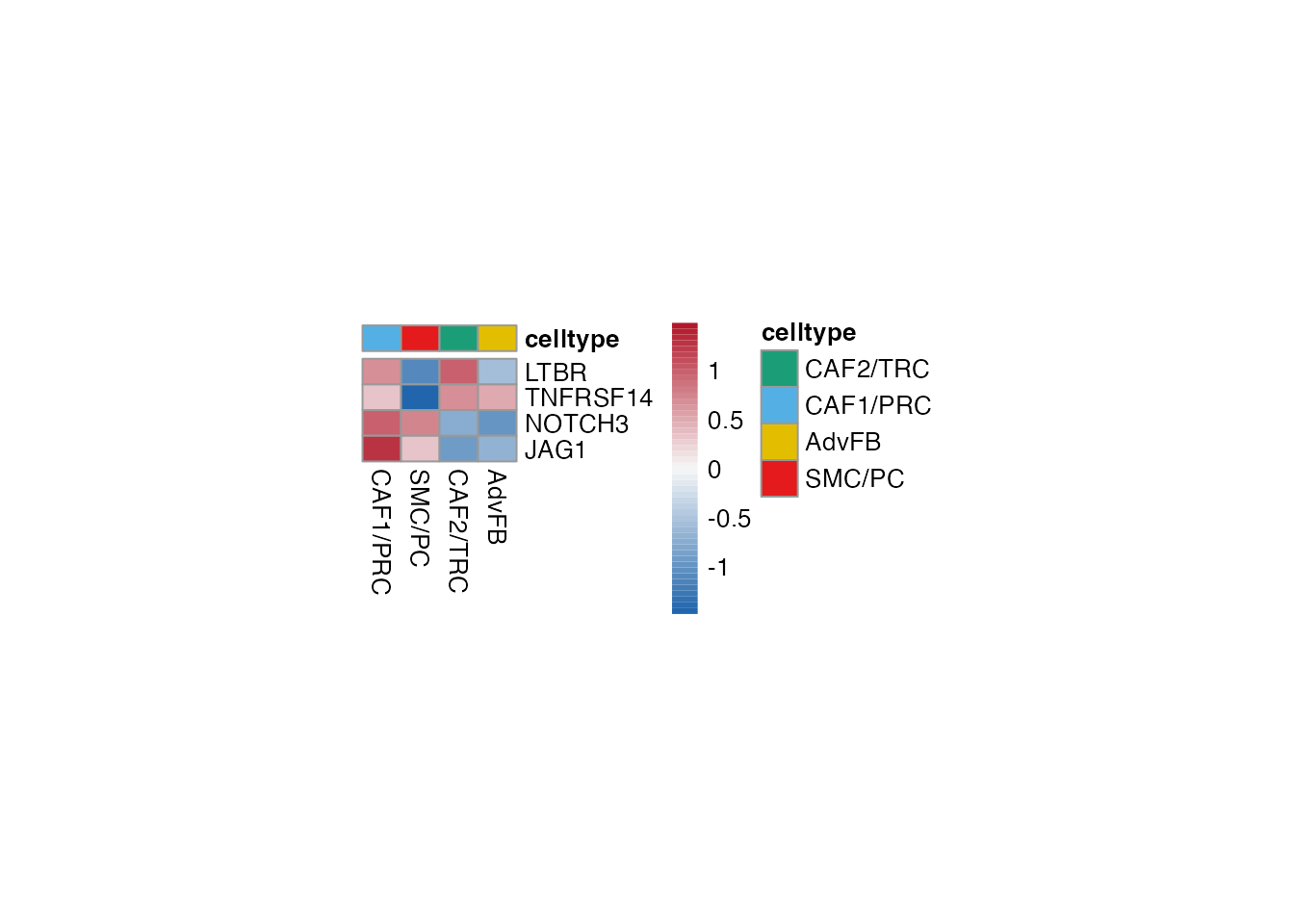
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Heatmap CD8⁺ T cell
Selgenes <- c("LTA","LTB","TNFSF14","DLL1","JAG1")
order_list <-c(paste0("Stem-like/Naive CD8", "\u207A ", "T cells"),
paste0("Effector-memory CD8", "\u207A ", "T cells"),
paste0("Effector CD8", "\u207A ", "T cells"),
paste0("Exhausted CD8", "\u207A ", "T cells"),
paste0("Cycling CD8", "\u207A ", "T cells") )
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)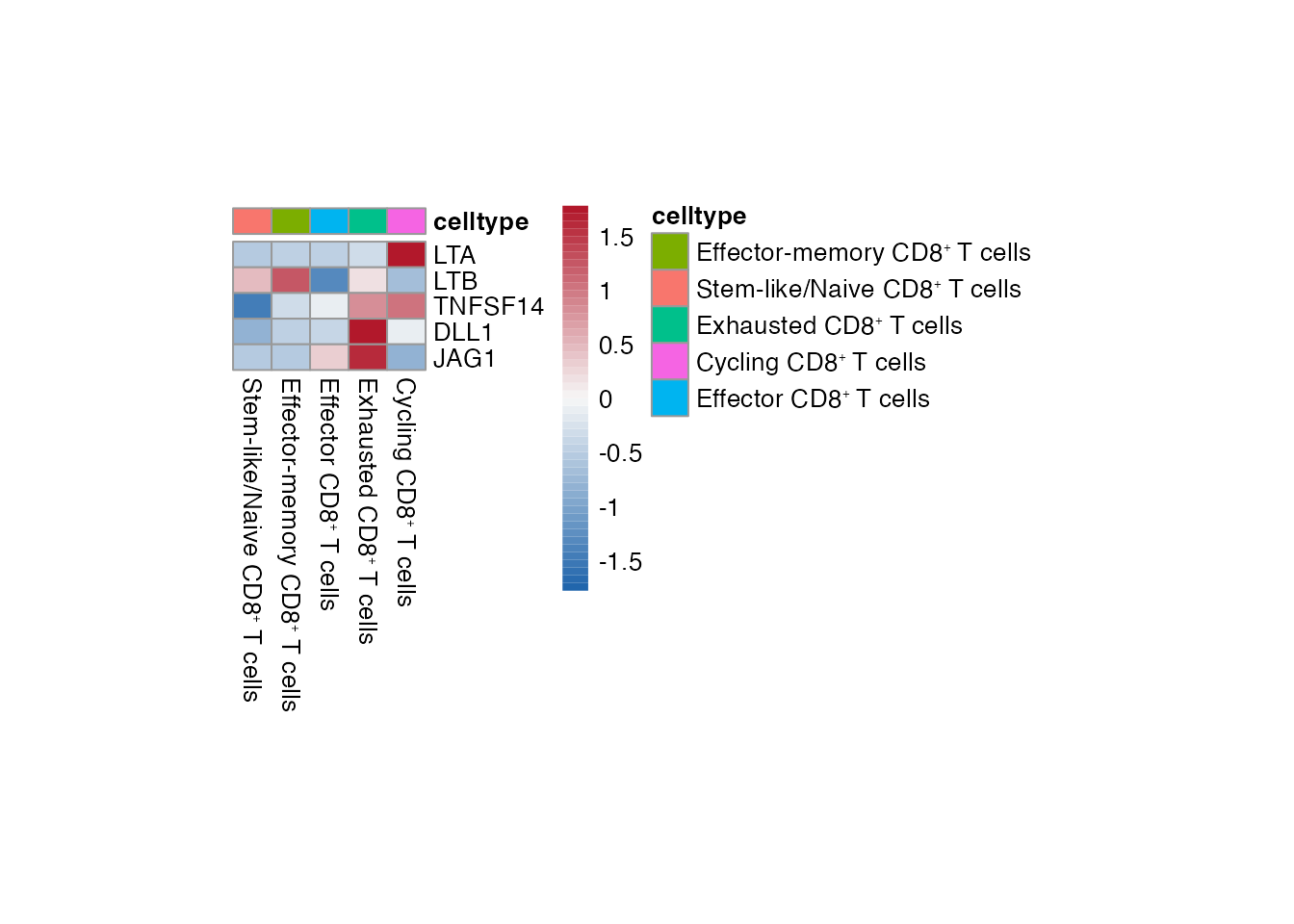
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Heatmaps (Figure 3J)
LTBR regulated
Selgenes <- c("CLU","TNFSF13B","MFGE8")
order_list <-c("CAF1/PRC","SMC/PC", "CAF2/TRC","AdvFB")
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)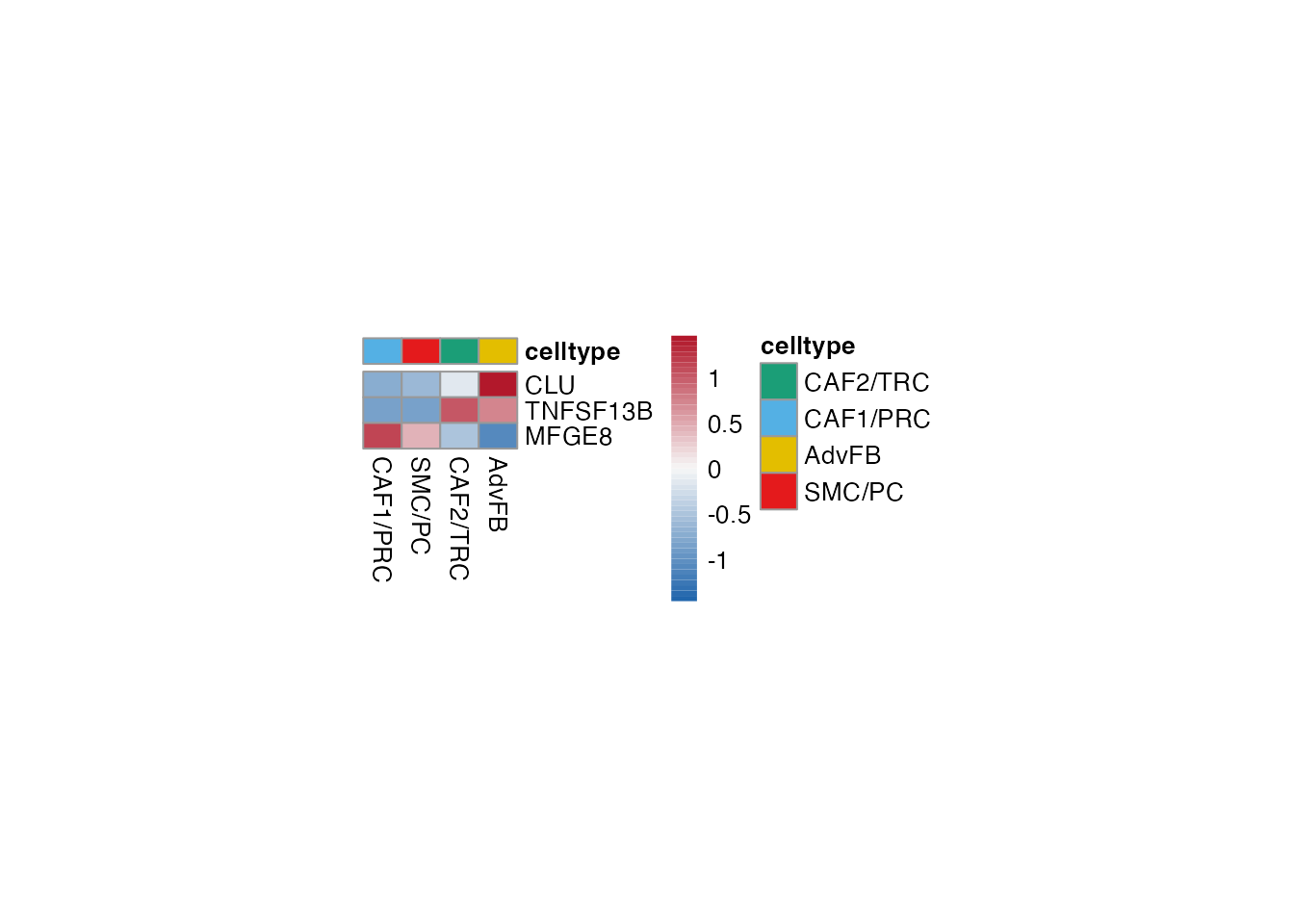
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
TNFRSF14 regulated
Selgenes <- c("DKK1", "IL32", "IL34")
order_list <-c("CAF1/PRC","SMC/PC", "CAF2/TRC","AdvFB")
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)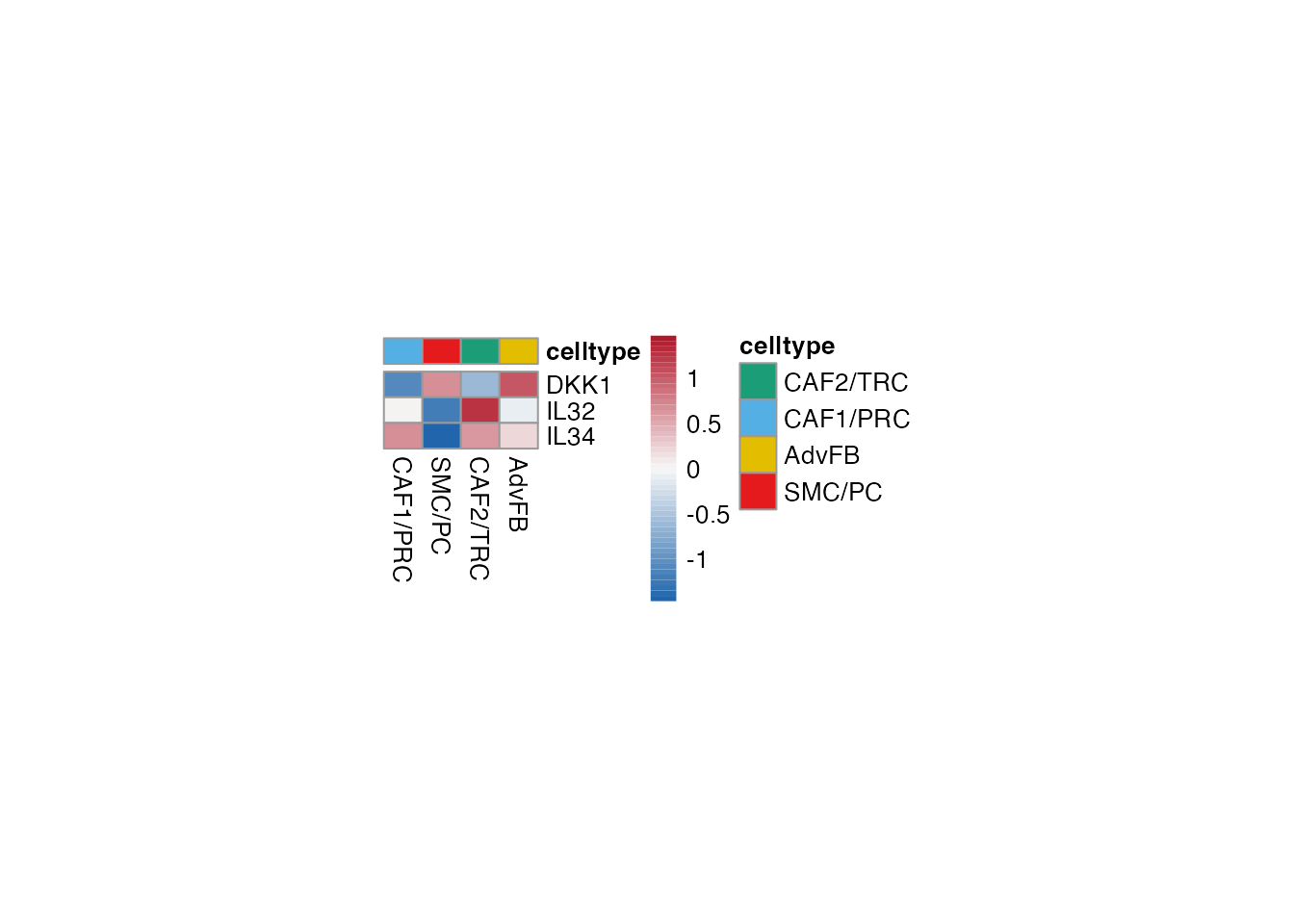
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
NOTCH3 regulated
Selgenes <- c("HEY1", "HEY2", "HEYL")
order_list <-c("CAF1/PRC","SMC/PC", "CAF2/TRC","AdvFB")
data_subset <- subset(merged_data, cell_type %in% order_list)
Heatmap(data_subset, Selgenes, order_list = order_list, palet = palet)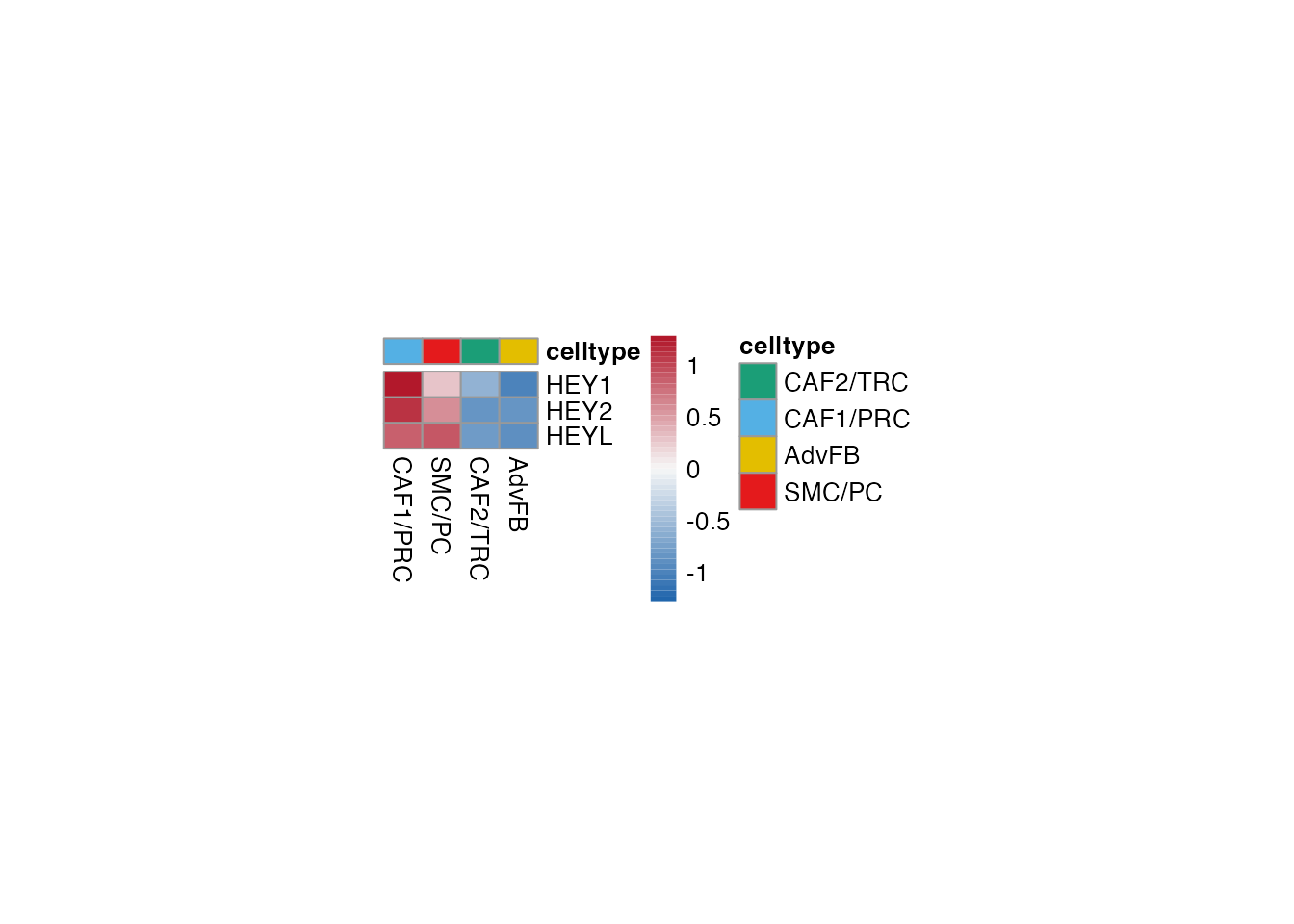
| Version | Author | Date |
|---|---|---|
| 80d46cf | Pchryssa | 2024-08-26 |
Session info
sessionInfo()R version 4.3.1 (2023-06-16)
Platform: aarch64-apple-darwin20 (64-bit)
Running under: macOS Ventura 13.6.9
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: Europe/Zurich
tzcode source: internal
attached base packages:
[1] parallel stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] doParallel_1.0.17 iterators_1.0.14 foreach_1.5.2
[4] pheatmap_1.0.12 NMF_0.26 cluster_2.1.4
[7] rngtools_1.5.2 registry_0.5-1 CellChat_1.6.1
[10] Biobase_2.60.0 BiocGenerics_0.46.0 igraph_1.5.0.1
[13] dplyr_1.1.2 gridExtra_2.3 dittoSeq_1.12.1
[16] ggplot2_3.4.2 Matrix_1.6-0 SeuratObject_4.1.3
[19] Seurat_4.3.0.1 patchwork_1.1.2 stringr_1.5.0
[22] purrr_1.0.1 here_1.0.1 magrittr_2.0.3
[25] circlize_0.4.15 tidyr_1.3.0 tibble_3.2.1
[28] workflowr_1.7.1
loaded via a namespace (and not attached):
[1] RcppAnnoy_0.0.21 splines_4.3.1
[3] later_1.3.1 bitops_1.0-7
[5] polyclip_1.10-4 ggnetwork_0.5.12
[7] lifecycle_1.0.3 rstatix_0.7.2
[9] rprojroot_2.0.3 globals_0.16.2
[11] processx_3.8.2 lattice_0.21-8
[13] MASS_7.3-60 backports_1.4.1
[15] plotly_4.10.2 sass_0.4.7
[17] rmarkdown_2.23 jquerylib_0.1.4
[19] yaml_2.3.7 httpuv_1.6.11
[21] sctransform_0.3.5 sp_2.0-0
[23] spatstat.sparse_3.0-2 reticulate_1.36.1
[25] cowplot_1.1.1 pbapply_1.7-2
[27] RColorBrewer_1.1-3 abind_1.4-5
[29] zlibbioc_1.46.0 Rtsne_0.16
[31] GenomicRanges_1.52.0 RCurl_1.98-1.12
[33] git2r_0.33.0 GenomeInfoDbData_1.2.10
[35] IRanges_2.34.1 S4Vectors_0.38.1
[37] ggrepel_0.9.3 irlba_2.3.5.1
[39] listenv_0.9.0 spatstat.utils_3.1-0
[41] RSpectra_0.16-1 goftest_1.2-3
[43] spatstat.random_3.1-5 fitdistrplus_1.1-11
[45] parallelly_1.36.0 svglite_2.1.1
[47] leiden_0.4.3 codetools_0.2-19
[49] DelayedArray_0.28.0 tidyselect_1.2.0
[51] shape_1.4.6 farver_2.1.1
[53] matrixStats_1.0.0 stats4_4.3.1
[55] spatstat.explore_3.2-1 jsonlite_1.8.7
[57] GetoptLong_1.0.5 BiocNeighbors_1.18.0
[59] ellipsis_0.3.2 progressr_0.13.0
[61] ggridges_0.5.4 ggalluvial_0.12.5
[63] survival_3.5-5 systemfonts_1.0.4
[65] tools_4.3.1 ragg_1.2.5
[67] sna_2.7-1 ica_1.0-3
[69] Rcpp_1.0.11 glue_1.6.2
[71] SparseArray_1.2.4 xfun_0.39
[73] MatrixGenerics_1.12.3 GenomeInfoDb_1.36.1
[75] withr_2.5.0 BiocManager_1.30.21.1
[77] fastmap_1.1.1 fansi_1.0.4
[79] callr_3.7.3 digest_0.6.33
[81] R6_2.5.1 mime_0.12
[83] textshaping_0.3.6 colorspace_2.1-0
[85] Cairo_1.6-1 scattermore_1.2
[87] tensor_1.5 spatstat.data_3.0-1
[89] utf8_1.2.3 generics_0.1.3
[91] data.table_1.14.8 FNN_1.1.3.2
[93] httr_1.4.6 htmlwidgets_1.6.2
[95] S4Arrays_1.2.1 whisker_0.4.1
[97] uwot_0.1.16 pkgconfig_2.0.3
[99] gtable_0.3.3 ComplexHeatmap_2.16.0
[101] lmtest_0.9-40 SingleCellExperiment_1.22.0
[103] XVector_0.40.0 htmltools_0.5.5
[105] carData_3.0-5 clue_0.3-64
[107] scales_1.2.1 png_0.1-8
[109] knitr_1.43 rstudioapi_0.15.0
[111] rjson_0.2.21 reshape2_1.4.4
[113] coda_0.19-4 statnet.common_4.9.0
[115] nlme_3.1-162 cachem_1.0.8
[117] zoo_1.8-12 GlobalOptions_0.1.2
[119] KernSmooth_2.23-22 miniUI_0.1.1.1
[121] pillar_1.9.0 grid_4.3.1
[123] vctrs_0.6.3 RANN_2.6.1
[125] ggpubr_0.6.0 promises_1.2.0.1
[127] car_3.1-2 xtable_1.8-4
[129] evaluate_0.21 cli_3.6.1
[131] compiler_4.3.1 rlang_1.1.1
[133] crayon_1.5.2 ggsignif_0.6.4
[135] future.apply_1.11.0 labeling_0.4.2
[137] ps_1.7.5 forcats_1.0.0
[139] getPass_0.2-4 plyr_1.8.8
[141] fs_1.6.3 stringi_1.7.12
[143] network_1.18.1 BiocParallel_1.34.2
[145] viridisLite_0.4.2 deldir_1.0-9
[147] gridBase_0.4-7 munsell_0.5.0
[149] lazyeval_0.2.2 spatstat.geom_3.2-4
[151] future_1.33.0 shiny_1.7.4.1
[153] highr_0.10 SummarizedExperiment_1.30.2
[155] ROCR_1.0-11 broom_1.0.5
[157] bslib_0.5.0 date()[1] "Tue Nov 5 22:05:11 2024"
sessionInfo()R version 4.3.1 (2023-06-16)
Platform: aarch64-apple-darwin20 (64-bit)
Running under: macOS Ventura 13.6.9
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: Europe/Zurich
tzcode source: internal
attached base packages:
[1] parallel stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] doParallel_1.0.17 iterators_1.0.14 foreach_1.5.2
[4] pheatmap_1.0.12 NMF_0.26 cluster_2.1.4
[7] rngtools_1.5.2 registry_0.5-1 CellChat_1.6.1
[10] Biobase_2.60.0 BiocGenerics_0.46.0 igraph_1.5.0.1
[13] dplyr_1.1.2 gridExtra_2.3 dittoSeq_1.12.1
[16] ggplot2_3.4.2 Matrix_1.6-0 SeuratObject_4.1.3
[19] Seurat_4.3.0.1 patchwork_1.1.2 stringr_1.5.0
[22] purrr_1.0.1 here_1.0.1 magrittr_2.0.3
[25] circlize_0.4.15 tidyr_1.3.0 tibble_3.2.1
[28] workflowr_1.7.1
loaded via a namespace (and not attached):
[1] RcppAnnoy_0.0.21 splines_4.3.1
[3] later_1.3.1 bitops_1.0-7
[5] polyclip_1.10-4 ggnetwork_0.5.12
[7] lifecycle_1.0.3 rstatix_0.7.2
[9] rprojroot_2.0.3 globals_0.16.2
[11] processx_3.8.2 lattice_0.21-8
[13] MASS_7.3-60 backports_1.4.1
[15] plotly_4.10.2 sass_0.4.7
[17] rmarkdown_2.23 jquerylib_0.1.4
[19] yaml_2.3.7 httpuv_1.6.11
[21] sctransform_0.3.5 sp_2.0-0
[23] spatstat.sparse_3.0-2 reticulate_1.36.1
[25] cowplot_1.1.1 pbapply_1.7-2
[27] RColorBrewer_1.1-3 abind_1.4-5
[29] zlibbioc_1.46.0 Rtsne_0.16
[31] GenomicRanges_1.52.0 RCurl_1.98-1.12
[33] git2r_0.33.0 GenomeInfoDbData_1.2.10
[35] IRanges_2.34.1 S4Vectors_0.38.1
[37] ggrepel_0.9.3 irlba_2.3.5.1
[39] listenv_0.9.0 spatstat.utils_3.1-0
[41] RSpectra_0.16-1 goftest_1.2-3
[43] spatstat.random_3.1-5 fitdistrplus_1.1-11
[45] parallelly_1.36.0 svglite_2.1.1
[47] leiden_0.4.3 codetools_0.2-19
[49] DelayedArray_0.28.0 tidyselect_1.2.0
[51] shape_1.4.6 farver_2.1.1
[53] matrixStats_1.0.0 stats4_4.3.1
[55] spatstat.explore_3.2-1 jsonlite_1.8.7
[57] GetoptLong_1.0.5 BiocNeighbors_1.18.0
[59] ellipsis_0.3.2 progressr_0.13.0
[61] ggridges_0.5.4 ggalluvial_0.12.5
[63] survival_3.5-5 systemfonts_1.0.4
[65] tools_4.3.1 ragg_1.2.5
[67] sna_2.7-1 ica_1.0-3
[69] Rcpp_1.0.11 glue_1.6.2
[71] SparseArray_1.2.4 xfun_0.39
[73] MatrixGenerics_1.12.3 GenomeInfoDb_1.36.1
[75] withr_2.5.0 BiocManager_1.30.21.1
[77] fastmap_1.1.1 fansi_1.0.4
[79] callr_3.7.3 digest_0.6.33
[81] R6_2.5.1 mime_0.12
[83] textshaping_0.3.6 colorspace_2.1-0
[85] Cairo_1.6-1 scattermore_1.2
[87] tensor_1.5 spatstat.data_3.0-1
[89] utf8_1.2.3 generics_0.1.3
[91] data.table_1.14.8 FNN_1.1.3.2
[93] httr_1.4.6 htmlwidgets_1.6.2
[95] S4Arrays_1.2.1 whisker_0.4.1
[97] uwot_0.1.16 pkgconfig_2.0.3
[99] gtable_0.3.3 ComplexHeatmap_2.16.0
[101] lmtest_0.9-40 SingleCellExperiment_1.22.0
[103] XVector_0.40.0 htmltools_0.5.5
[105] carData_3.0-5 clue_0.3-64
[107] scales_1.2.1 png_0.1-8
[109] knitr_1.43 rstudioapi_0.15.0
[111] rjson_0.2.21 reshape2_1.4.4
[113] coda_0.19-4 statnet.common_4.9.0
[115] nlme_3.1-162 cachem_1.0.8
[117] zoo_1.8-12 GlobalOptions_0.1.2
[119] KernSmooth_2.23-22 miniUI_0.1.1.1
[121] pillar_1.9.0 grid_4.3.1
[123] vctrs_0.6.3 RANN_2.6.1
[125] ggpubr_0.6.0 promises_1.2.0.1
[127] car_3.1-2 xtable_1.8-4
[129] evaluate_0.21 cli_3.6.1
[131] compiler_4.3.1 rlang_1.1.1
[133] crayon_1.5.2 ggsignif_0.6.4
[135] future.apply_1.11.0 labeling_0.4.2
[137] ps_1.7.5 forcats_1.0.0
[139] getPass_0.2-4 plyr_1.8.8
[141] fs_1.6.3 stringi_1.7.12
[143] network_1.18.1 BiocParallel_1.34.2
[145] viridisLite_0.4.2 deldir_1.0-9
[147] gridBase_0.4-7 munsell_0.5.0
[149] lazyeval_0.2.2 spatstat.geom_3.2-4
[151] future_1.33.0 shiny_1.7.4.1
[153] highr_0.10 SummarizedExperiment_1.30.2
[155] ROCR_1.0-11 broom_1.0.5
[157] bslib_0.5.0