Response_Point_09-2
Last updated: 2020-09-10
Checks: 6 1
Knit directory: Comparative_eQTL/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.5.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown is untracked by Git. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20190319) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: WorkingManuscript.zip
Ignored: WorkingManuscript/
Ignored: analysis/.DS_Store
Ignored: analysis/.Rhistory
Ignored: analysis_temp/.DS_Store
Ignored: big_data/
Ignored: code/.DS_Store
Ignored: code/snakemake_workflow/.DS_Store
Ignored: code/snakemake_workflow/.Rhistory
Ignored: data/.DS_Store
Ignored: data/PastAnalysesDataToKeep/.DS_Store
Ignored: figures/
Ignored: output/.DS_Store
Untracked files:
Untracked: analysis/20200907_Response_Point_09-2.Rmd
Unstaged changes:
Modified: analysis/20200907_Response_OriginalComments.Rmd
Deleted: output/CellType.GWAS.Manhattan.png
Deleted: output/CellType.GWAS.QQ.png
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
There are no past versions. Publish this analysis with wflow_publish() to start tracking its development.
Intro
I performed the cell type QTL analysis, using code in the snakemake. This was a GWAS with all GTEX left-venticle samples, (~300 individuals) using qq-normalized cell type phenotypes (the first PC of the CIBERSORT cell type composition estimates), with the same genotyping related PCs used by GTEX for eQTL mapping as covariates in a simple additive linear model. Here are the results…
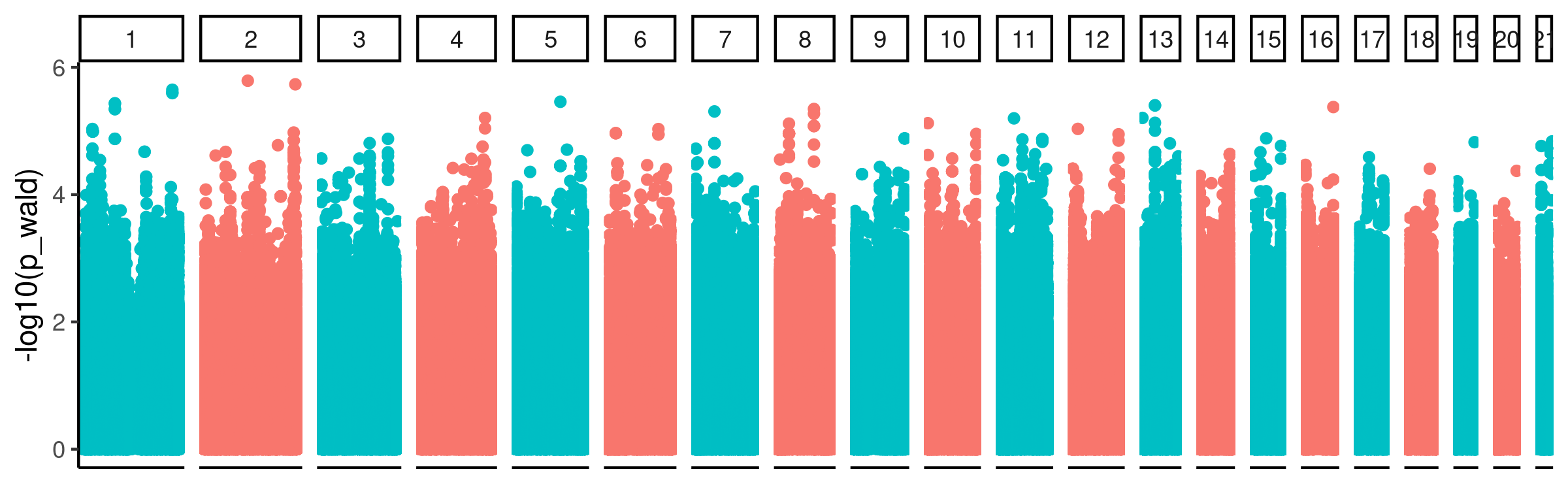
GWAS_Manhattan
Nothing seems to reach genome wide significance. Nonetheless, there may be true signal buried in here that we can answer with directed hypothesis tests. For example, it still seems reasonable to me to check if eQTL SNPs have inflated P-values for this cell type QTL analysis.
I checked if eQTL SNPs (top SNPs for GTEX heart left ventricle eGenes (FDR<0.01) ) have generally smaller P-values than a same sized random sample of non eQTL SNPs, using a QQ-plot:
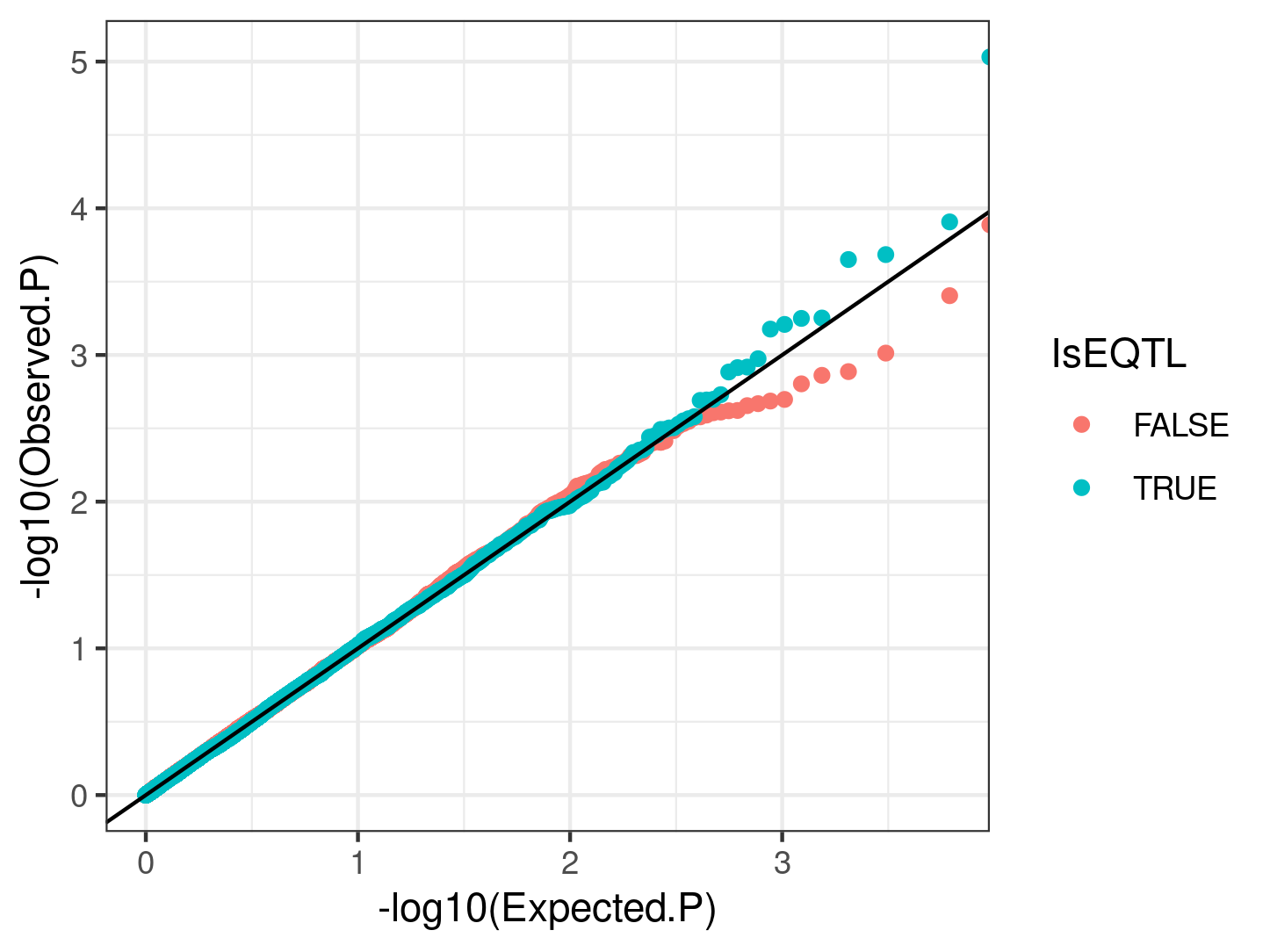 .
.
My interpretation of this is that eQTLs generally are not driven by cell type QTLs. Another thing I could ask is if the top cell type QTLs (some which may be false positives), are closer to highly dispersed genes compared to lowly dispersed genes.
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS Catalina 10.15.5
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
loaded via a namespace (and not attached):
[1] workflowr_1.5.0 Rcpp_1.0.5 rprojroot_1.3-2 digest_0.6.23
[5] later_1.0.0 R6_2.4.1 backports_1.1.5 git2r_0.26.1
[9] magrittr_1.5 evaluate_0.14 stringi_1.4.3 rlang_0.4.1
[13] fs_1.3.1 promises_1.1.0 rmarkdown_1.18 tools_3.6.1
[17] stringr_1.4.0 glue_1.3.1 httpuv_1.5.2 xfun_0.11
[21] yaml_2.2.0 compiler_3.6.1 htmltools_0.4.0 knitr_1.26