Liftover PAS
Briana Mittleman
10/1/2019
Last updated: 2019-10-02
Checks: 7 0
Knit directory: Comparative_APA/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.4.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20190902) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: code/chimp_log/
Ignored: code/human_log/
Ignored: data/metadata_HCpanel.txt.sb-f4823d1e-qihGek/
Untracked files:
Untracked: ._.DS_Store
Untracked: Chimp/
Untracked: Human/
Untracked: code/._Config_chimp.yaml
Untracked: code/._Config_human.yaml
Untracked: code/._Snakefile
Untracked: code/._SnakefilePAS
Untracked: code/._SnakefilePASfilt
Untracked: code/._bed215upbed.py
Untracked: code/._buildStarIndex.sh
Untracked: code/._cleanbed2saf.py
Untracked: code/._cluster.json
Untracked: code/._extraSnakefiltpas
Untracked: code/._filterPASforMP.py
Untracked: code/._filterPostLift.py
Untracked: code/._fixUTRexonanno.py
Untracked: code/._formathg38Anno.py
Untracked: code/._formatpantro6Anno.py
Untracked: code/._liftPAS19to38.sh
Untracked: code/._maphg19.sh
Untracked: code/._maphg19_subjunc.sh
Untracked: code/._preparePAS4lift.py
Untracked: code/._primaryLift.sh
Untracked: code/._recLiftchim2human.sh
Untracked: code/._revLiftPAShg38to19.sh
Untracked: code/._reverseLift.sh
Untracked: code/._snakemake.batch
Untracked: code/._snakemakePAS.batch
Untracked: code/._snakemakePASchimp.batch
Untracked: code/._snakemakePAShuman.batch
Untracked: code/._snakemake_chimp.batch
Untracked: code/._snakemake_human.batch
Untracked: code/._snakemakefiltPAS.batch
Untracked: code/._snakemakefiltPAS_chimp
Untracked: code/._snakemakefiltPAS_chimp.sh
Untracked: code/._snakemakefiltPAS_human.sh
Untracked: code/._submit-snakemake-chimp.sh
Untracked: code/._submit-snakemake-human.sh
Untracked: code/._submit-snakemakePAS-chimp.sh
Untracked: code/._submit-snakemakePAS-human.sh
Untracked: code/._submit-snakemakefiltPAS-chimp.sh
Untracked: code/._submit-snakemakefiltPAS-human.sh
Untracked: code/.snakemake/
Untracked: code/Config_chimp.yaml
Untracked: code/Config_human.yaml
Untracked: code/Log.out
Untracked: code/Rev_liftoverPAShg19to38.err
Untracked: code/Rev_liftoverPAShg19to38.out
Untracked: code/SAF215upbed_gen.py
Untracked: code/Snakefile
Untracked: code/SnakefilePAS
Untracked: code/SnakefilePASfilt
Untracked: code/Upstream10Bases_general.py
Untracked: code/apaQTLsnake.err
Untracked: code/apaQTLsnake.out
Untracked: code/apaQTLsnakePAS.err
Untracked: code/apaQTLsnakePAS.out
Untracked: code/apaQTLsnakePAShuman.err
Untracked: code/bed215upbed.py
Untracked: code/bed2saf.py
Untracked: code/bg_to_cov.py
Untracked: code/buildStarIndex.sh
Untracked: code/callPeaksYL.py
Untracked: code/chooseAnno2SAF.py
Untracked: code/cleanbed2saf.py
Untracked: code/cluster.json
Untracked: code/clusterPAS.json
Untracked: code/clusterfiltPAS.json
Untracked: code/convertNumeric.py
Untracked: code/extraSnakefiltpas
Untracked: code/filter5perc.R
Untracked: code/filter5percPheno.py
Untracked: code/filterBamforMP.pysam2_gen.py
Untracked: code/filterMissprimingInNuc10_gen.py
Untracked: code/filterPASforMP.py
Untracked: code/filterPostLift.py
Untracked: code/filterSAFforMP_gen.py
Untracked: code/filterSortBedbyCleanedBed_gen.R
Untracked: code/filterpeaks.py
Untracked: code/fixFChead.py
Untracked: code/fixUTRexonanno.py
Untracked: code/formathg38Anno.py
Untracked: code/formatpantro6Anno.py
Untracked: code/generateStarIndex.err
Untracked: code/generateStarIndex.out
Untracked: code/liftPAS19to38.sh
Untracked: code/liftoverPAShg19to38.err
Untracked: code/liftoverPAShg19to38.out
Untracked: code/log/
Untracked: code/make5percPeakbed.py
Untracked: code/makeFileID.py
Untracked: code/makePheno.py
Untracked: code/maphg19.err
Untracked: code/maphg19.out
Untracked: code/maphg19.sh
Untracked: code/maphg19_sub.err
Untracked: code/maphg19_sub.out
Untracked: code/maphg19_subjunc.sh
Untracked: code/namePeaks.py
Untracked: code/peak2PAS.py
Untracked: code/pheno2countonly.R
Untracked: code/preparePAS4lift.py
Untracked: code/primaryLift.err
Untracked: code/primaryLift.out
Untracked: code/primaryLift.sh
Untracked: code/recChimpback2Human.err
Untracked: code/recChimpback2Human.out
Untracked: code/recLiftchim2human.sh
Untracked: code/revLift.err
Untracked: code/revLift.out
Untracked: code/revLiftPAShg38to19.sh
Untracked: code/reverseLift.sh
Untracked: code/slurm-62824013.out
Untracked: code/slurm-62825841.out
Untracked: code/slurm-62826116.out
Untracked: code/snakePASChimp.err
Untracked: code/snakePASChimp.out
Untracked: code/snakePAShuman.out
Untracked: code/snakemake.batch
Untracked: code/snakemakePAS.batch
Untracked: code/snakemakePASFiltChimp.err
Untracked: code/snakemakePASFiltChimp.out
Untracked: code/snakemakePASFiltHuman.err
Untracked: code/snakemakePASFiltHuman.out
Untracked: code/snakemakePASchimp.batch
Untracked: code/snakemakePAShuman.batch
Untracked: code/snakemake_chimp.batch
Untracked: code/snakemake_human.batch
Untracked: code/snakemakefiltPAS.batch
Untracked: code/snakemakefiltPAS_chimp.sh
Untracked: code/snakemakefiltPAS_human.sh
Untracked: code/submit-snakemake-chimp.sh
Untracked: code/submit-snakemake-human.sh
Untracked: code/submit-snakemakePAS-chimp.sh
Untracked: code/submit-snakemakePAS-human.sh
Untracked: code/submit-snakemakefiltPAS-chimp.sh
Untracked: code/submit-snakemakefiltPAS-human.sh
Untracked: data/._metadata_HCpanel.txt
Untracked: data/._metadata_HCpanel.txt.sb-f4823d1e-qihGek
Untracked: data/._metadata_HCpanel.xlsx
Untracked: data/chainFiles/
Untracked: data/cleanPeaks_byspecies/
Untracked: data/cleanPeaks_lifted/
Untracked: data/liftover_files/
Untracked: data/metadata_HCpanel.txt
Untracked: data/metadata_HCpanel.xlsx
Untracked: data/primaryLift/
Untracked: data/reverseLift/
Unstaged changes:
Modified: analysis/annotationInfo.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 1c6c5e2 | brimittleman | 2019-10-02 | add results for full set |
| html | 8dd5eec | brimittleman | 2019-10-02 | Build site. |
| Rmd | 558afe6 | brimittleman | 2019-10-02 | add liftover res |
| html | b5edd8e | brimittleman | 2019-10-01 | Build site. |
| Rmd | b5a2151 | brimittleman | 2019-10-01 | add analysis for liftover |
library(tidyverse)── Attaching packages ───────────────────────────────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.1.1 ✔ purrr 0.3.2
✔ tibble 2.1.1 ✔ dplyr 0.8.0.1
✔ tidyr 0.8.3 ✔ stringr 1.3.1
✔ readr 1.3.1 ✔ forcats 0.3.0 ── Conflicts ──────────────────────────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()Next step will be to prepare these for liftover. I will get the PAS as the furthest downstream base. I will liftover the regions 100bp upstream and 100bp downstream of the PAS.
As of now the PAS are still on the opposite strand.
Pos strand: pas is the end - start_new= end-100 - end_new=end + 100
neg strand: pas is the start - start_new= start-100 - end_new= start + 100
human: /project2/gilad/briana/Comparative_APA/Human/data/cleanPeaks/human_APApeaks.ALLChrom.Filtered.Named.Cleaned.bed chimp: /project2/gilad/briana/Comparative_APA/Chimp/data/cleanPeaks/chimp_APApeaks.ALLChrom.Filtered.Named.Cleaned.bed
Output:
mkdir ../data/cleanPeaks_byspecies/
python preparePAS4lift.py ../Human/data/cleanPeaks/human_APApeaks.ALLChrom.Filtered.Named.Cleaned.bed ../data/cleanPeaks_byspecies/human_APApeaks.ALLChrom.Filtered.Named.Cleaned_100bpreg.bed
python preparePAS4lift.py ../Chimp/data/cleanPeaks/chimp_APApeaks.ALLChrom.Filtered.Named.Cleaned.bed ../data/cleanPeaks_byspecies/chimp_APApeaks.ALLChrom.Filtered.Named.Cleaned_100bpreg.bed
Chain files from: http://hgdownload.soe.ucsc.edu/downloads.html#chimp dowload to ../data/chainFiles/
Liftover pipeline:
start with human- lift to chimp and back start with chimp- lift to human and back
mkdir ../data/primaryLift
mkdir ../data/reverseLift
sbatch primaryLift.shResults from primary lift:
unliftedH=read.table("../data/primaryLift/human_APApeaks_primarylift2Chimp_UNLIFTED.bed",stringsAsFactors = F) %>% nrow()
unliftedC=read.table("../data/primaryLift/chimp_APApeaks_primarylift2Human_UNLIFTED.bed",stringsAsFactors = F) %>% nrow()
liftedH=read.table("../data/primaryLift/human_APApeaks_primarylift2Chimp.bed",stringsAsFactors = F) %>% nrow()
liftedC=read.table("../data/primaryLift/chimp_APApeaks_primarylift2Human.bed",stringsAsFactors = F) %>% nrow()
primaryUnC=c("Chimp","Unlifted", unliftedC)
primaryUnH=c("Human","Unlifted", unliftedH)
primaryLH=c("Human","Lifted", liftedH)
primaryLC=c("Chimp","Lifted", liftedC)
header=c("species", "liftStat", "PAS")
primaryDF= as.data.frame(rbind(primaryLH,primaryLC, primaryUnH,primaryUnC))
colnames(primaryDF)=header
primaryDF$PAS=as.numeric(as.character(primaryDF$PAS))
primaryDF= primaryDF %>% group_by(species) %>% mutate(nPAS=sum(PAS)) %>% ungroup() %>% mutate(proportion=PAS/nPAS)ggplot(primaryDF,aes(x=species, y=PAS, fill=liftStat)) + geom_bar(stat="identity",position = "dodge") + scale_fill_brewer(palette = "Dark2") + labs(title="Primary Liftover Results")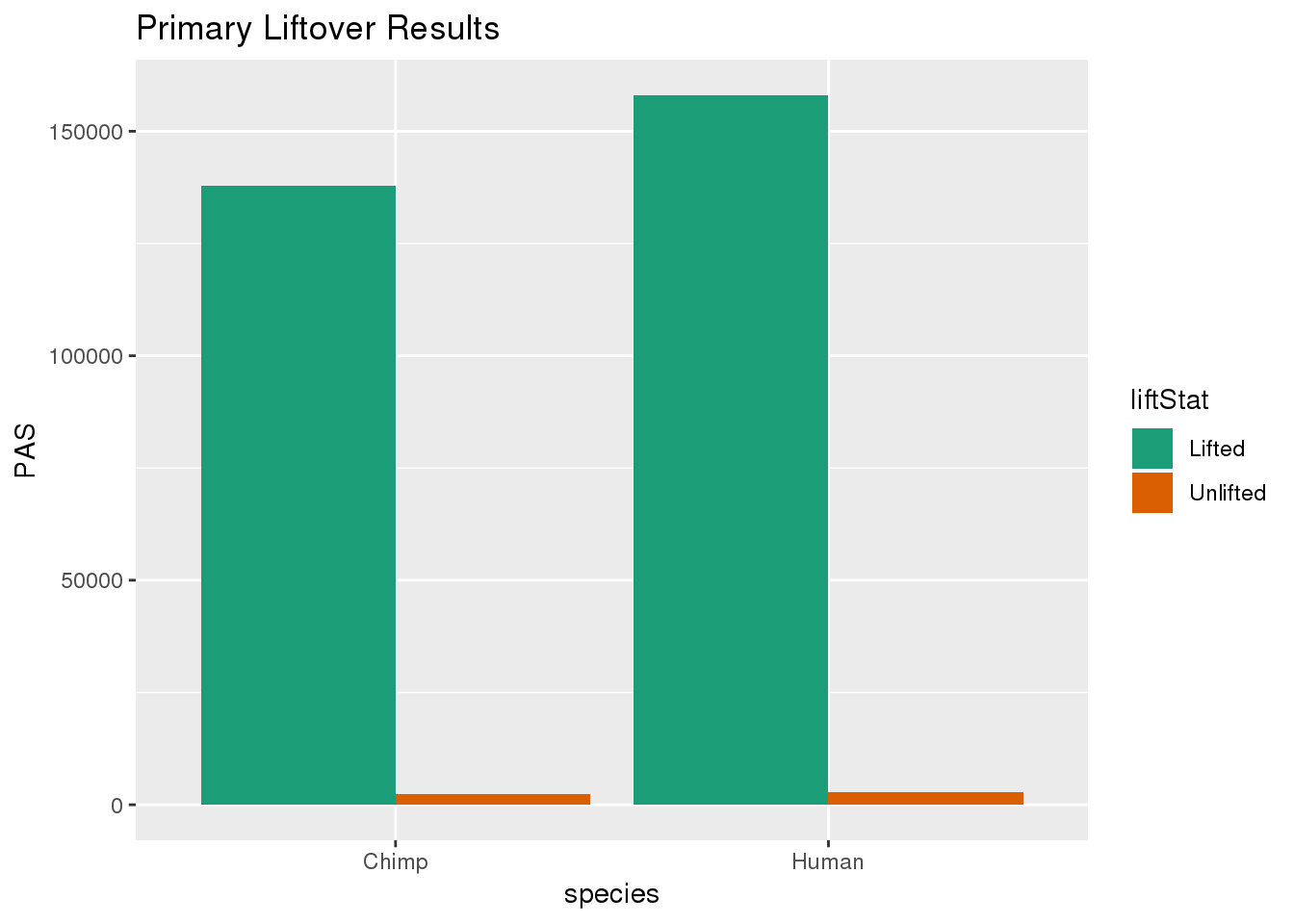
| Version | Author | Date |
|---|---|---|
| 8dd5eec | brimittleman | 2019-10-02 |
ggplot(primaryDF,aes(x=species, y=proportion, fill=liftStat)) + geom_bar(stat="identity",position = "dodge") + scale_fill_brewer(palette = "Dark2") + labs(title="Primary Liftover Results")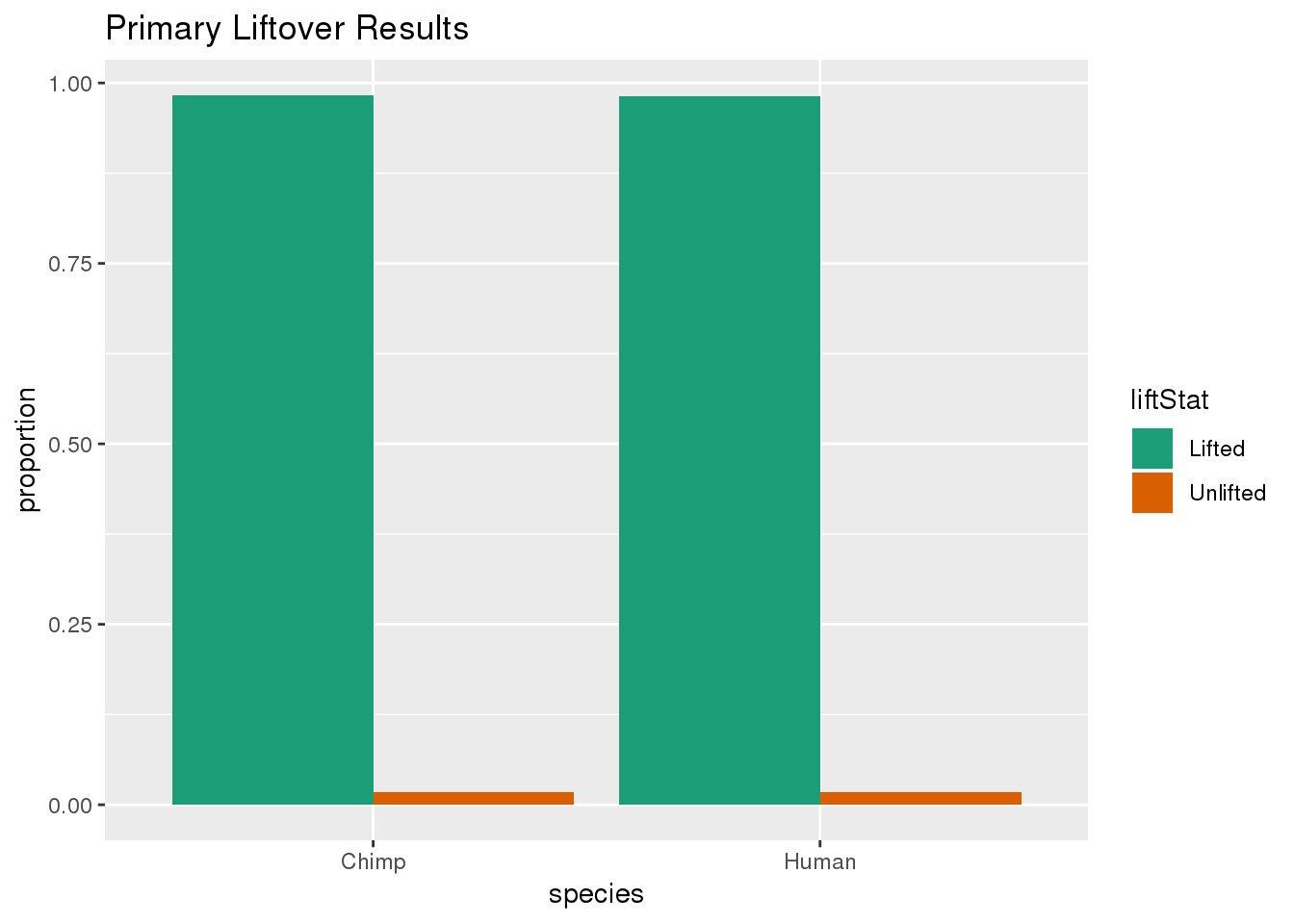
| Version | Author | Date |
|---|---|---|
| 8dd5eec | brimittleman | 2019-10-02 |
Reverse lift:
re_unliftedH=read.table("../data/reverseLift/human_APApeaks_primarylift2Human_rev2Human_UNLIFTED.bed",stringsAsFactors = F) %>% nrow()
re_unliftedC=read.table("../data/reverseLift/chimp_APApeaks_primarylift2Human_rev2Chimp_UNLIFTED.bed",stringsAsFactors = F) %>% nrow()
re_liftedH=read.table("../data/reverseLift/human_APApeaks_primarylift2Chimp_rev2Human.bed",stringsAsFactors = F) %>% nrow()
re_liftedC=read.table("../data/reverseLift/chimp_APApeaks_primarylift2Human_rev2Chimp.bed",stringsAsFactors = F) %>% nrow()
re_UnC=c("Chimp","Unlifted", re_unliftedC)
re_UnH=c("Human","Unlifted", re_unliftedH)
re_LH=c("Human","Lifted", re_liftedH)
re_LC=c("Chimp","Lifted", re_liftedC)
header=c("species", "liftStat", "PAS")
re_DF= as.data.frame(rbind(re_LH,re_LC, re_UnH,re_UnC))
colnames(re_DF)=header
re_DF$PAS=as.numeric(as.character(re_DF$PAS))
re_DF= re_DF %>% group_by(species) %>% mutate(nPAS=sum(PAS)) %>% ungroup() %>% mutate(proportion=PAS/nPAS)ggplot(re_DF,aes(x=species, y=PAS, fill=liftStat)) + geom_bar(stat="identity",position = "dodge") + scale_fill_brewer(palette = "Dark2") + labs(title="Reverse Liftover Results")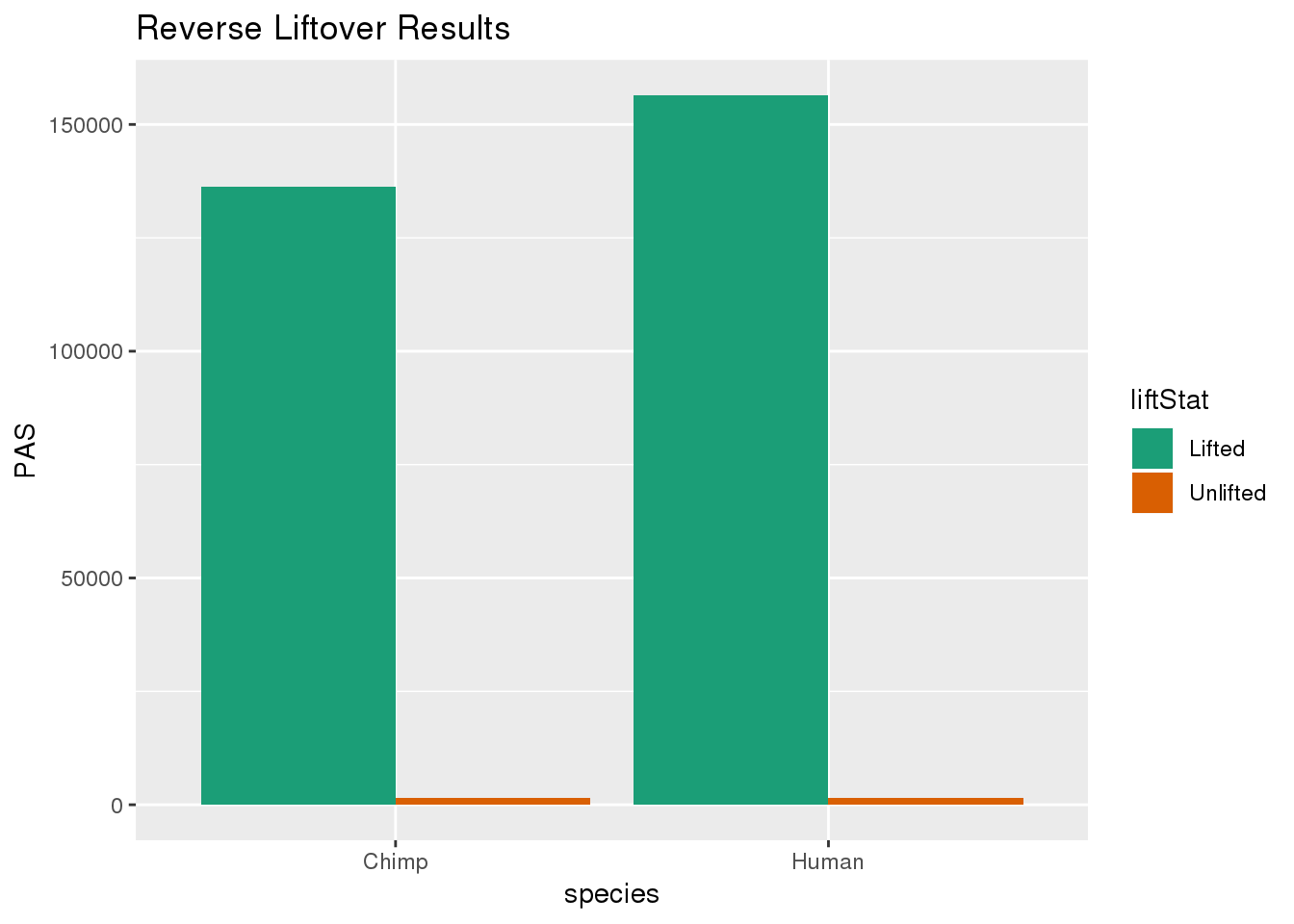
| Version | Author | Date |
|---|---|---|
| 8dd5eec | brimittleman | 2019-10-02 |
ggplot(re_DF,aes(x=species, y=proportion, fill=liftStat)) + geom_bar(stat="identity",position = "dodge") + scale_fill_brewer(palette = "Dark2")+ labs(title="Reverse Liftover Results")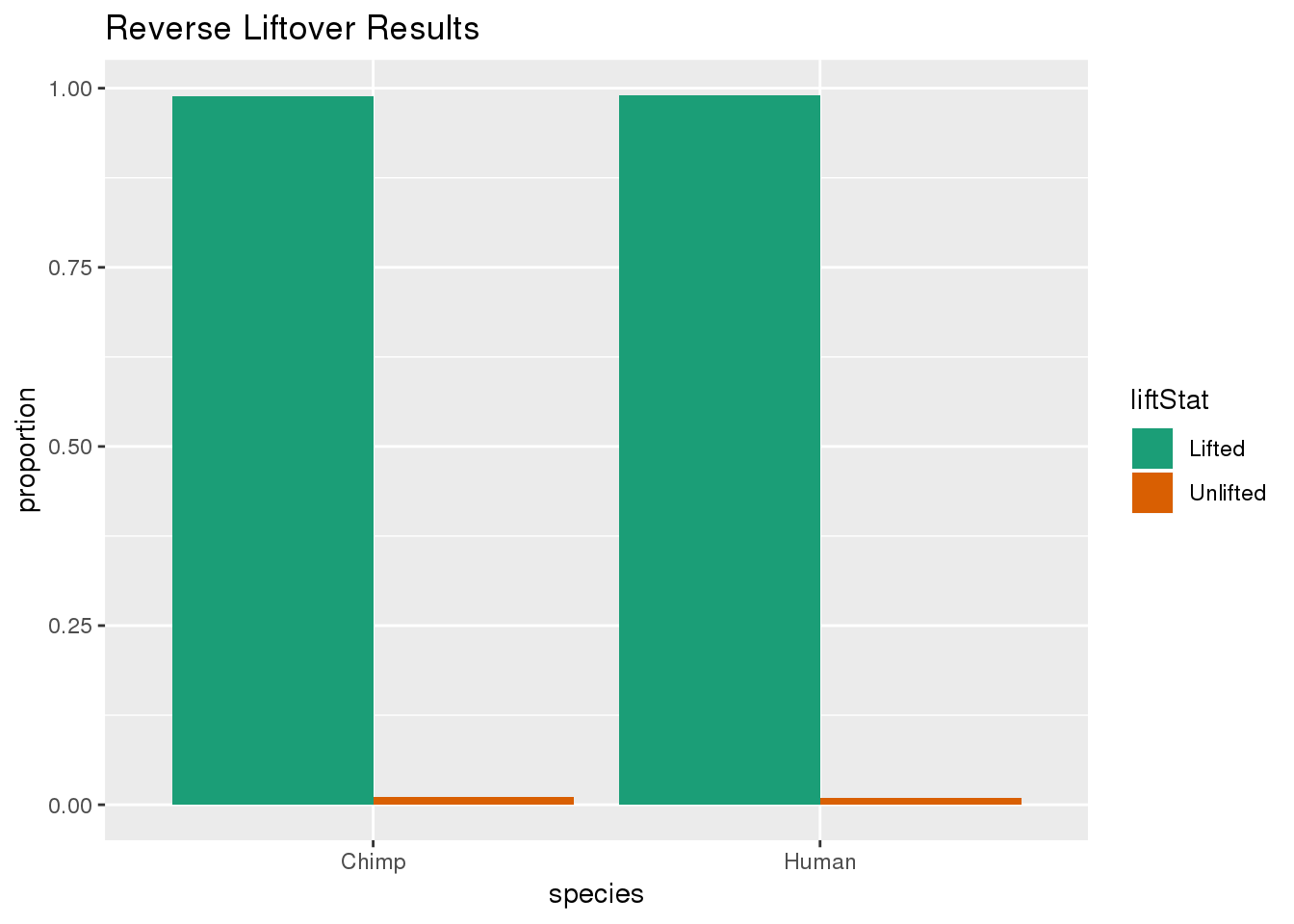
| Version | Author | Date |
|---|---|---|
| 8dd5eec | brimittleman | 2019-10-02 |
I need to now make sure they lifted to the same location. To do this I will overlap the reciprocal lifted PAS with the original files.
I subset the original files by the pas that have an exact match in the reverse map. I can do this by pas name- start:end
mkdir ../data/cleanPeaks_lifted
python filterPostLift.py ../data/cleanPeaks_byspecies/human_APApeaks.ALLChrom.Filtered.Named.Cleaned_100bpreg.bed ../data/reverseLift/human_APApeaks_primarylift2Chimp_rev2Human.bed ../data/cleanPeaks_lifted/Human_PASregions.bed
python filterPostLift.py ../data/cleanPeaks_byspecies/chimp_APApeaks.ALLChrom.Filtered.Named.Cleaned_100bpreg.bed ../data/reverseLift/chimp_APApeaks_primarylift2Human_rev2Chimp.bed ../data/cleanPeaks_lifted/Chimp_PASregions.bedResults:
Human_recLift=read.table("../data/cleanPeaks_lifted/Human_PASregions.bed",stringsAsFactors = F, col.names = c("chr", "start","end", "name", "score", "strand"))
Chimp_recLift=read.table("../data/cleanPeaks_lifted/Chimp_PASregions.bed",stringsAsFactors = F,col.names = c("chr", "start","end", "name", "score", "strand"))
originalH=unliftedH + liftedH
originalC=unliftedC + liftedC
#human
nrow(Human_recLift)/originalH[1] 0.9640866#chimp
nrow(Chimp_recLift)/originalC[1] 0.965532796% reciprocally lifted over.
lift the chimp ones back to human
sbatch recLiftchim2human.shJoin the results: If they are discovered in both say so.
Chimp_recLift_hcoord=read.table("../data/cleanPeaks_lifted/Chimp_PASregions_humanCoord.bed",stringsAsFactors = F,col.names = c("chr", "start","end", "name", "score", "strand"))
Inboth=Human_recLift %>% inner_join(Chimp_recLift_hcoord, by=c("chr", "start", "end")) %>% mutate(discover="Both", newName=paste(name.x,discover,sep=":"),meanscore=((score.x+score.y)/2)) %>% select(chr, start,end, newName, meanscore, strand.x) %>% dplyr::rename("score"=meanscore, "strand"=strand.x)
HumanOnly= Human_recLift %>% anti_join(Chimp_recLift_hcoord, by=c("chr", "start", "end")) %>% mutate(discover="Human",newName=paste(name,discover,sep=":")) %>% select(chr, start,end, newName, score, strand)
ChimpOnly=Chimp_recLift_hcoord %>% anti_join(Human_recLift, by=c("chr", "start", "end")) %>% mutate(discover="Chimp",newName=paste(name,discover,sep=":")) %>% select(chr, start,end, newName, score, strand)Graph this:
set=c("Both", "Human", "Chimp")
Number=c(nrow(Inboth), nrow(HumanOnly),nrow(ChimpOnly))
pasdiscnum=as.data.frame(cbind(set, Number))
pasdiscnum$Number=as.numeric(as.character(pasdiscnum$Number))
pasdiscnum= pasdiscnum %>% mutate(prop=Number/sum(Number))
ggplot(pasdiscnum, aes(x=set, y=Number, fill=set)) + geom_bar(stat="identity")+ scale_fill_brewer(palette = "Dark2")+ labs(y="Number of PAS", x="Discovery Set")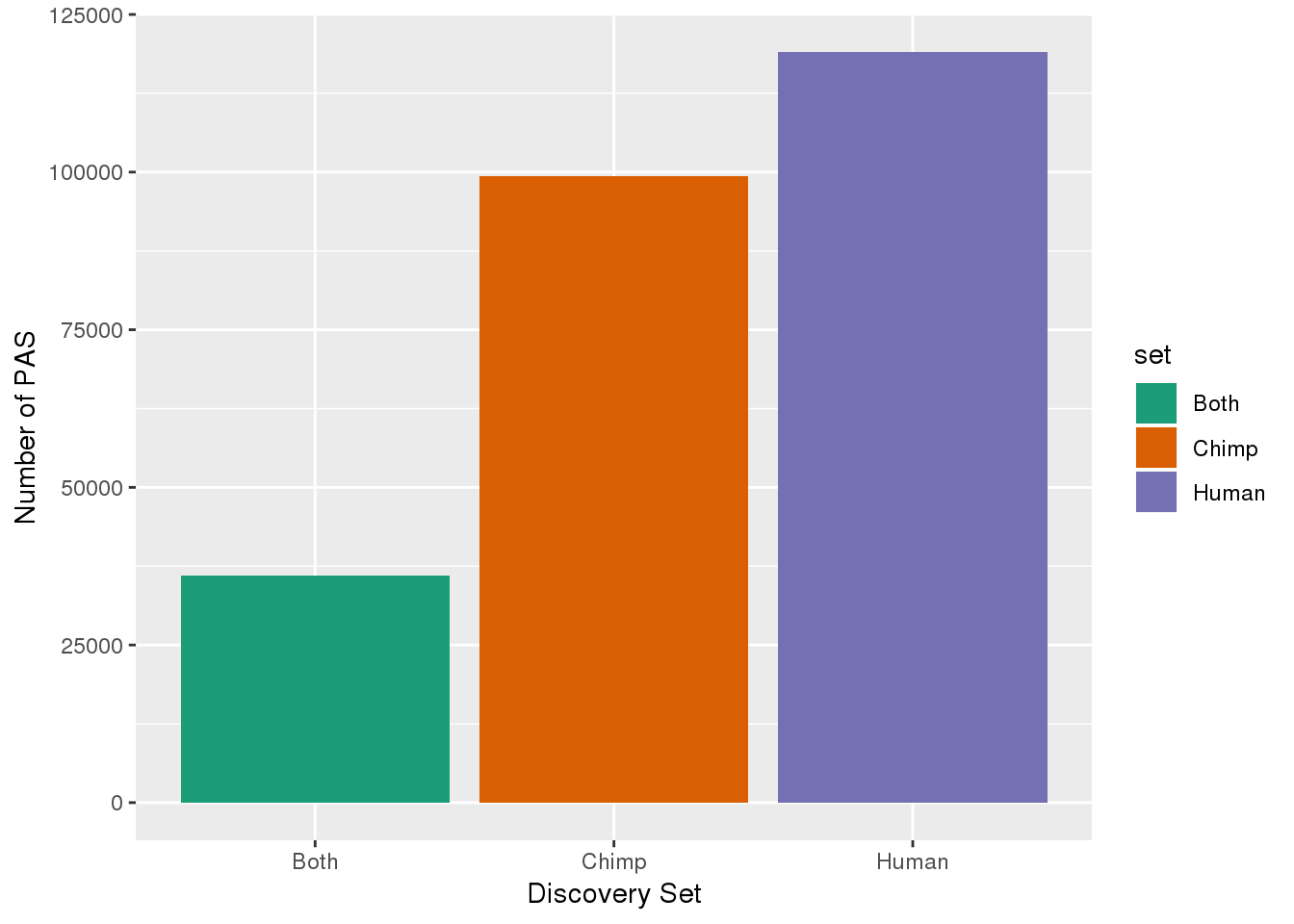
ggplot(pasdiscnum, aes(x=set, y=prop, fill=set)) + geom_bar(stat="identity")+ scale_fill_brewer(palette = "Dark2") + labs(y="Proportion of PAS", x="Discovery Set")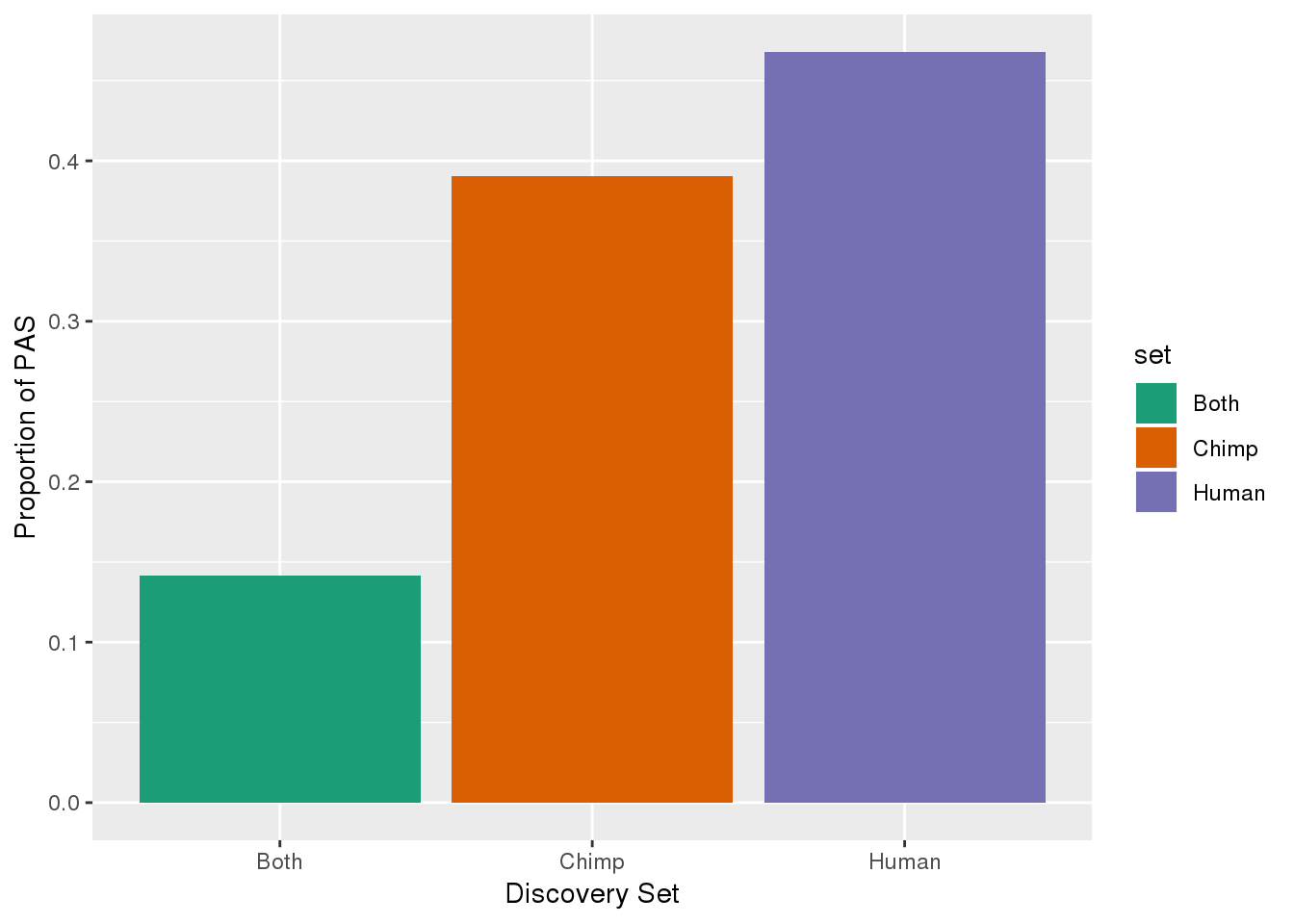
Are higher scores discovered in both?
AllPAS=rbind(Inboth,HumanOnly ,ChimpOnly) %>% separate(newName,into=c("name", "discover"))ggplot(AllPAS, aes(x=discover, y=log10(score), fill=discover)) + geom_boxplot() + scale_fill_brewer(palette = "Dark2") + labs(y="log10(Mean Read count)", title="PAS mean reads by Species of discovery")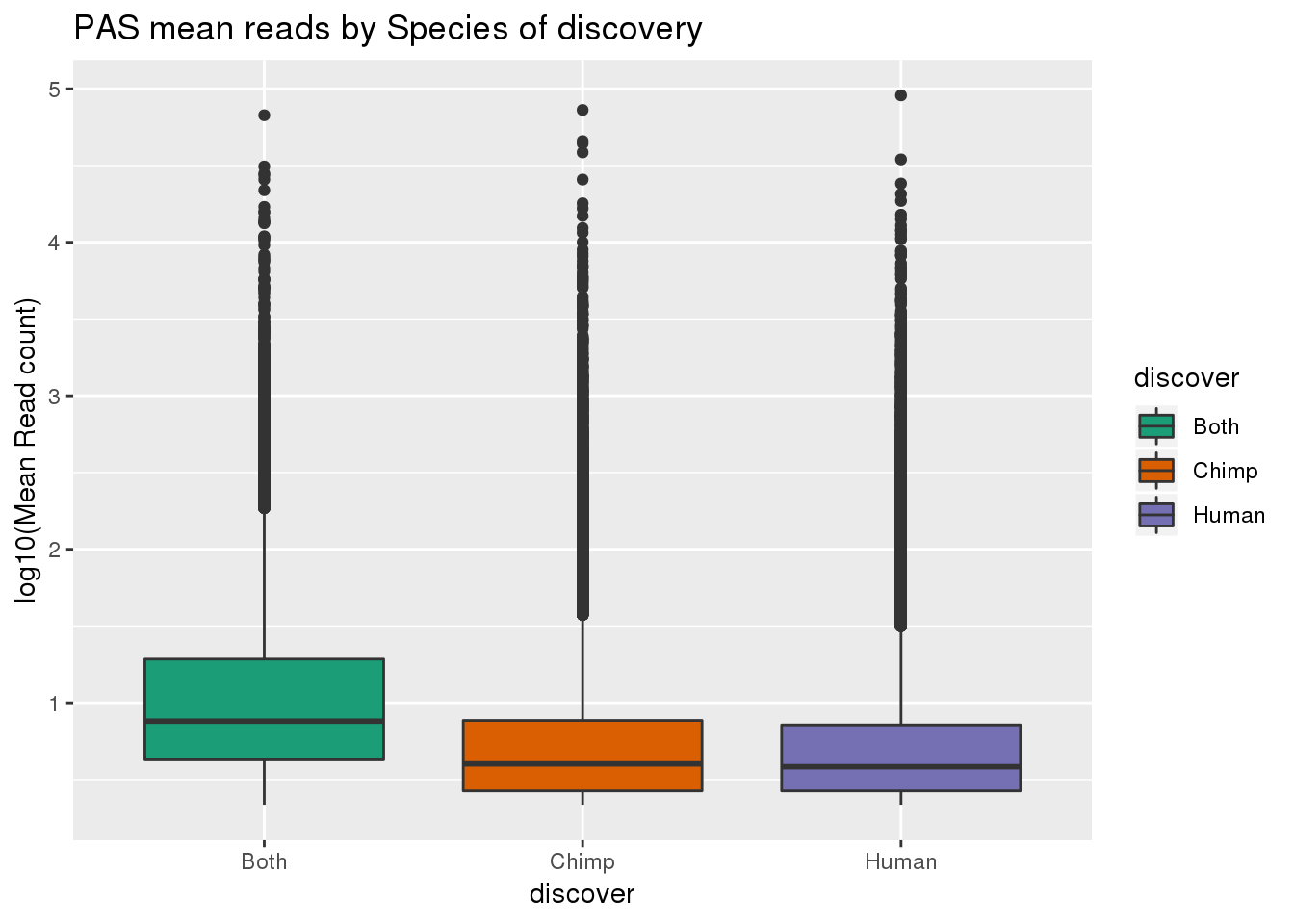
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] forcats_0.3.0 stringr_1.3.1 dplyr_0.8.0.1 purrr_0.3.2
[5] readr_1.3.1 tidyr_0.8.3 tibble_2.1.1 ggplot2_3.1.1
[9] tidyverse_1.2.1
loaded via a namespace (and not attached):
[1] Rcpp_1.0.2 RColorBrewer_1.1-2 cellranger_1.1.0
[4] pillar_1.3.1 compiler_3.5.1 git2r_0.25.2
[7] plyr_1.8.4 workflowr_1.4.0 tools_3.5.1
[10] digest_0.6.18 lubridate_1.7.4 jsonlite_1.6
[13] evaluate_0.12 nlme_3.1-137 gtable_0.2.0
[16] lattice_0.20-38 pkgconfig_2.0.2 rlang_0.4.0
[19] cli_1.1.0 rstudioapi_0.10 yaml_2.2.0
[22] haven_1.1.2 withr_2.1.2 xml2_1.2.0
[25] httr_1.3.1 knitr_1.20 hms_0.4.2
[28] generics_0.0.2 fs_1.3.1 rprojroot_1.3-2
[31] grid_3.5.1 tidyselect_0.2.5 glue_1.3.0
[34] R6_2.3.0 readxl_1.1.0 rmarkdown_1.10
[37] modelr_0.1.2 magrittr_1.5 whisker_0.3-2
[40] backports_1.1.2 scales_1.0.0 htmltools_0.3.6
[43] rvest_0.3.2 assertthat_0.2.0 colorspace_1.3-2
[46] labeling_0.3 stringi_1.2.4 lazyeval_0.2.1
[49] munsell_0.5.0 broom_0.5.1 crayon_1.3.4