SNPs in signal sites
Briana Mittleman
6/24/2019
Last updated: 2019-06-27
Checks: 7 0
Knit directory: apaQTL/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.4.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20190411) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: data/.DS_Store
Ignored: output/.DS_Store
Untracked files:
Untracked: .Rprofile
Untracked: ._.DS_Store
Untracked: .gitignore
Untracked: _workflowr.yml
Untracked: analysis/._PASdescriptiveplots.Rmd
Untracked: analysis/._cuttoffPercUsage.Rmd
Untracked: analysis/QTLexampleplots.Rmd
Untracked: analysis/cuttoffPercUsage.Rmd
Untracked: analysis/eQTLoverlap.Rmd
Untracked: analysis/mergeRNA.Rmd
Untracked: analysis/oldstuffNotNeeded.Rmd
Untracked: apaQTL.Rproj
Untracked: code/.NascentRNAdtPlotFirstintronicPAS.sh.swp
Untracked: code/._ApaQTL_nominalNonnorm.sh
Untracked: code/._BothFracDTPlotGeneRegions_normalized.sh
Untracked: code/._EandPqtls.sh
Untracked: code/._FC_NucintornUpandDown.sh
Untracked: code/._FC_UTR.sh
Untracked: code/._FC_intornUpandDownsteamPAS.sh
Untracked: code/._FC_nascentseq.sh
Untracked: code/._FC_newPeaks_olddata.sh
Untracked: code/._HMMpermuteTotal.py
Untracked: code/._HmmPermute.py
Untracked: code/._LC_samplegroups.py
Untracked: code/._NascentRNAdtPlot.sh
Untracked: code/._NascentRNAdtPlot3UTRPAS.sh
Untracked: code/._NascentRNAdtPlotExcludeFirstintronicPAS.sh
Untracked: code/._NascentRNAdtPlotNucPAS.sh
Untracked: code/._NascentRNAdtPlotTotPAS.sh
Untracked: code/._NascentRNAdtPlotintronicPAS.sh
Untracked: code/._NascnetRNAdtPlotPAS.sh
Untracked: code/._NetSeq_fourthintronDT.sh
Untracked: code/._NomResfromPASSNP.py
Untracked: code/._QTL2bed.py
Untracked: code/._QTL2bed_withstrand.py
Untracked: code/._RNAbam2bw.sh
Untracked: code/._SnakefilePAS
Untracked: code/._SnakefilefiltPAS
Untracked: code/._TESplots100bp.sh
Untracked: code/._TESplots150bp.sh
Untracked: code/._TESplots200bp.sh
Untracked: code/._Untitled
Untracked: code/._ZipandTabPheno.sh
Untracked: code/._aAPAqtl_nominal39ind.sh
Untracked: code/._apaQTLCorrectPvalMakeQQ.R
Untracked: code/._apaQTL_Nominal.sh
Untracked: code/._apaQTL_permuted.sh
Untracked: code/._assignNucIntonpeak2intronlocs.sh
Untracked: code/._assignTotIntronpeak2intronlocs.sh
Untracked: code/._bam2BW_5primemost.sh
Untracked: code/._bed2saf.py
Untracked: code/._bothFracDTplot1stintron.sh
Untracked: code/._bothFracDTplot4thintron.sh
Untracked: code/._bothFrac_FC.sh
Untracked: code/._callPeaksYL.py
Untracked: code/._changenomQTLres2geneName.py
Untracked: code/._chooseAnno2SAF.py
Untracked: code/._chooseSignalSite
Untracked: code/._chooseSignalSite.py
Untracked: code/._cluster.json
Untracked: code/._clusterPAS.json
Untracked: code/._clusterfiltPAS.json
Untracked: code/._codingdms2bed.py
Untracked: code/._config.yaml
Untracked: code/._config2.yaml
Untracked: code/._configOLD.yaml
Untracked: code/._convertNominal2SNPLOC.py
Untracked: code/._convertNumeric.py
Untracked: code/._correctNomeqtl.R
Untracked: code/._dag.pdf
Untracked: code/._eQTL_switch2snploc.py
Untracked: code/._eQTLgenestestedapa.py
Untracked: code/._encodeRNADTplots.sh
Untracked: code/._extractGenotypes.py
Untracked: code/._extractseqfromqtlfastq.py
Untracked: code/._fc2leafphen.py
Untracked: code/._filter5perc.R
Untracked: code/._filter5percPheno.py
Untracked: code/._filterpeaks.py
Untracked: code/._finalPASbed2SAF.py
Untracked: code/._fix4su304corr.py
Untracked: code/._fix4su604corr.py
Untracked: code/._fix4sukalisto.py
Untracked: code/._fixExandUnexeQTL
Untracked: code/._fixExandUnexeQTL.py
Untracked: code/._fixFChead.py
Untracked: code/._fixFChead_bothfrac.py
Untracked: code/._fixH3k12ac.py
Untracked: code/._fixPASregionSNPs.py
Untracked: code/._fixRNAhead4corr.py
Untracked: code/._fixRNAkalisto.py
Untracked: code/._fixgroupedtranscript.py
Untracked: code/._fixhead_netseqfc.py
Untracked: code/._getAPAfromanyeQTL.py
Untracked: code/._getApapval4eqtl.py
Untracked: code/._getApapval4eqtl_unexp.py
Untracked: code/._getDownstreamIntronNuclear.py
Untracked: code/._getIntronDownstreamPAS.py
Untracked: code/._getIntronUpstreamPAS.py
Untracked: code/._getQTLalleles.py
Untracked: code/._getQTLfastq.sh
Untracked: code/._getUpstreamIntronNuclear.py
Untracked: code/._grouptranscripts.py
Untracked: code/._intersectVCFandupPAS.sh
Untracked: code/._keep5perMAF.py
Untracked: code/._keepSNP_vcf.sh
Untracked: code/._make5percPeakbed.py
Untracked: code/._makeFileID.py
Untracked: code/._makePheno.py
Untracked: code/._makeSAFbothfrac5perc.py
Untracked: code/._makeSNP2rsidfile.py
Untracked: code/._makeeQTLempirical_unexp.py
Untracked: code/._makeeQTLempiricaldist.py
Untracked: code/._makegencondeTSSfile.py
Untracked: code/._mapSSsnps2PAS.sh
Untracked: code/._mergRNABam.sh
Untracked: code/._mergeAllBam.sh
Untracked: code/._mergeBW_norm.sh
Untracked: code/._mergeBamNascent.sh
Untracked: code/._mergeByFracBam.sh
Untracked: code/._mergePeaks.sh
Untracked: code/._mnase1stintron.sh
Untracked: code/._mnaseDT_fourthintron.sh
Untracked: code/._namePeaks.py
Untracked: code/._netseqDTplot1stIntron.sh
Untracked: code/._netseqFC.sh
Untracked: code/._peak2PAS.py
Untracked: code/._peakFC.sh
Untracked: code/._pheno2countonly.R
Untracked: code/._phenoQTLfromlist.py
Untracked: code/._processYRIgen.py
Untracked: code/._qtlRegionseq.sh
Untracked: code/._qtlsPvalOppFrac.py
Untracked: code/._quantassign2parsedpeak.py
Untracked: code/._removeXfromHmm.py
Untracked: code/._removeloc_pheno.py
Untracked: code/._runCorrectNomEqtl.sh
Untracked: code/._runHMMpermuteAPAqtls.sh
Untracked: code/._runHMMpermuteeQTLS.sh
Untracked: code/._runMakeEmpiricaleQTL_unexp.sh
Untracked: code/._runMakeeQTLempirical.sh
Untracked: code/._run_bam2bw_all3prime.sh
Untracked: code/._run_bam2bw_extra3.sh
Untracked: code/._run_getApaPval4eqtl.sh
Untracked: code/._run_getapafromeQTL.py
Untracked: code/._run_getapafromeQTL.sh
Untracked: code/._run_getapapval4eqtl_unexp.sh
Untracked: code/._run_leafcutterDiffIso.sh
Untracked: code/._run_sepUsagephen.sh
Untracked: code/._run_sepgenobychrom.sh
Untracked: code/._selectNominalPvalues.py
Untracked: code/._sepUsagePhen.py
Untracked: code/._sepgenobychrom.py
Untracked: code/._snakemakePAS.batch
Untracked: code/._snakemakefiltPAS.batch
Untracked: code/._sortindexRNAbam.sh
Untracked: code/._submit-snakemakePAS.sh
Untracked: code/._submit-snakemakefiltPAS.sh
Untracked: code/._subsetAPAnotEorPgene.py
Untracked: code/._subsetApanoteGene.py
Untracked: code/._subsetUnexplainedeQTLs.py
Untracked: code/._subsetVCF_SS.sh
Untracked: code/._subsetVCF_noSSregions.sh
Untracked: code/._subsetVCF_upstreamPAS.sh
Untracked: code/._subset_diffisopheno.py
Untracked: code/._subsetpermAPAwithGenelist.py
Untracked: code/._subsetvcf_otherreg.sh
Untracked: code/._subsetvcf_permSS.sh
Untracked: code/._subtrachfiveprimeUTR.sh
Untracked: code/._subtractExons.sh
Untracked: code/._subtractfiveprimeUTR.sh
Untracked: code/._tabixSNPS.sh
Untracked: code/._utrdms2saf.py
Untracked: code/._vcf2bed.py
Untracked: code/.snakemake/
Untracked: code/APAqtl_nominal.err
Untracked: code/APAqtl_nominal.out
Untracked: code/APAqtl_nominal_39.err
Untracked: code/APAqtl_nominal_39.out
Untracked: code/APAqtl_nominal_nonNorm.err
Untracked: code/APAqtl_nominal_nonNorm.out
Untracked: code/APAqtl_permuted.err
Untracked: code/APAqtl_permuted.out
Untracked: code/ApaQTL_nominalNonnorm.sh
Untracked: code/BothFracDTPlot1stintron.err
Untracked: code/BothFracDTPlot1stintron.out
Untracked: code/BothFracDTPlot4stintron.err
Untracked: code/BothFracDTPlot4stintron.out
Untracked: code/BothFracDTPlotGeneRegions.err
Untracked: code/BothFracDTPlotGeneRegions.out
Untracked: code/BothFracDTPlotGeneRegions_norm.err
Untracked: code/BothFracDTPlotGeneRegions_norm.out
Untracked: code/BothFracDTPlotGeneRegions_normalized.sh
Untracked: code/DistPAS2Sig.py
Untracked: code/EandPqtl.err
Untracked: code/EandPqtl.out
Untracked: code/EandPqtls.sh
Untracked: code/EncodeRNADTPlotGeneRegions.err
Untracked: code/EncodeRNADTPlotGeneRegions.out
Untracked: code/FC_NucintornUpandDown.sh
Untracked: code/FC_NucintronPASupandDown.err
Untracked: code/FC_NucintronPASupandDown.out
Untracked: code/FC_UTR.err
Untracked: code/FC_UTR.out
Untracked: code/FC_UTR.sh
Untracked: code/FC_intornUpandDownsteamPAS.sh
Untracked: code/FC_intronPASupandDown.err
Untracked: code/FC_intronPASupandDown.out
Untracked: code/FC_nascent.err
Untracked: code/FC_nascentout
Untracked: code/FC_nascentseq.sh
Untracked: code/FC_newPAS_olddata.err
Untracked: code/FC_newPAS_olddata.out
Untracked: code/FC_newPeaks_olddata.sh
Untracked: code/HMMpermuteTotal.py
Untracked: code/HmmPermute.p
Untracked: code/HmmPermute.py
Untracked: code/LC_samplegroups.py
Untracked: code/NascentDTPlotGeneRegions.err
Untracked: code/NascentDTPlotGeneRegions.out
Untracked: code/NascentDTPlotPAS.err
Untracked: code/NascentDTPlotPAS.out
Untracked: code/NascentDTPlotPAS_3utr.err
Untracked: code/NascentDTPlotPAS_3utr.out
Untracked: code/NascentDTPlotPAS_firstintron.err
Untracked: code/NascentDTPlotPAS_firstintron.out
Untracked: code/NascentDTPlotPAS_intron.err
Untracked: code/NascentDTPlotPAS_intron.out
Untracked: code/NascentDTPlotPAS_nuc.err
Untracked: code/NascentDTPlotPAS_nuc.out
Untracked: code/NascentDTPlotPAS_tot.err
Untracked: code/NascentDTPlotPAS_tot.out
Untracked: code/NascentRNAdtPlot.sh
Untracked: code/NascentRNAdtPlot3UTRPAS.sh
Untracked: code/NascentRNAdtPlotExcludeFirstintronicPAS.sh
Untracked: code/NascentRNAdtPlotFirstintronicPAS.sh
Untracked: code/NascentRNAdtPlotNucPAS.sh
Untracked: code/NascentRNAdtPlotTotPAS.sh
Untracked: code/NascentRNAdtPlotintronicPAS.sh
Untracked: code/NascnetRNAdtPlotPAS.sh
Untracked: code/NetSeq_fourthintronDT.sh
Untracked: code/NomResfromPASSNP.py
Untracked: code/Nuclear_example.err
Untracked: code/Nuclear_example.out
Untracked: code/QTL2bed.py
Untracked: code/QTL2bed_withstrand.py
Untracked: code/README.md
Untracked: code/RNABam2BW.err
Untracked: code/RNABam2BW.out
Untracked: code/RNAbam2bw.sh
Untracked: code/Rplots.pdf
Untracked: code/Script4NuclearQTLexamples.sh
Untracked: code/Script4TotalQTLexamples.sh
Untracked: code/TESplots100bp.err
Untracked: code/TESplots100bp.out
Untracked: code/TESplots100bp.sh
Untracked: code/TESplots150bp.err
Untracked: code/TESplots150bp.out
Untracked: code/TESplots150bp.sh
Untracked: code/TESplots200bp.err
Untracked: code/TESplots200bp.out
Untracked: code/TESplots200bp.sh
Untracked: code/Total_example.err
Untracked: code/Total_example.out
Untracked: code/Untitled
Untracked: code/Upstream100Bases_general.py
Untracked: code/ZipandTabPheno.sh
Untracked: code/aAPAqtl_nominal39ind.sh
Untracked: code/apaQTLCorrectPvalMakeQQ_4pc.R
Untracked: code/apaQTL_Nominal_4pc.sh
Untracked: code/apaQTL_permuted.4pc.sh
Untracked: code/apafacetboxplots.R
Untracked: code/apaqtlfacetboxplots.R
Untracked: code/assignNucIntonpeak2intronlocs.sh
Untracked: code/assignPeak2Intronicregion.err
Untracked: code/assignPeak2Intronicregion.out
Untracked: code/assignTotIntronpeak2intronlocs.sh
Untracked: code/assigntotPeak2Intronicregion.err
Untracked: code/assigntotPeak2Intronicregion.out
Untracked: code/bam2BW_5primemost.sh
Untracked: code/bam2bw.err
Untracked: code/bam2bw.out
Untracked: code/bam2bw_5primemost.err
Untracked: code/bam2bw_5primemost.out
Untracked: code/bothFracDTplot1stintron.sh
Untracked: code/bothFracDTplot4thintron.sh
Untracked: code/bothFrac_FC.err
Untracked: code/bothFrac_FC.out
Untracked: code/bothFrac_FC.sh
Untracked: code/changenomQTLres2geneName.py
Untracked: code/codingdms2bed.py
Untracked: code/convertNominal2SNPLOC.py
Untracked: code/correctNomeqtl.R
Untracked: code/dag.pdf
Untracked: code/dagPAS.pdf
Untracked: code/dagfiltPAS.pdf
Untracked: code/eQTL_switch2snploc.py
Untracked: code/eQTLgenestestedapa.py
Untracked: code/encodeRNADTplots.sh
Untracked: code/extractGenotypes.py
Untracked: code/extractseqfromqtlfastq.py
Untracked: code/fc2leafphen.py
Untracked: code/finalPASbed2SAF.py
Untracked: code/findbuginpeaks.R
Untracked: code/fix4su304corr.py
Untracked: code/fix4su604corr.py
Untracked: code/fix4sukalisto.py
Untracked: code/fixExandUnexeQTL
Untracked: code/fixExandUnexeQTL.py
Untracked: code/fixFChead_bothfrac.py
Untracked: code/fixFChead_summary.py
Untracked: code/fixH3k12ac.py
Untracked: code/fixPASregionSNPs.py
Untracked: code/fixRNAhead4corr.py
Untracked: code/fixRNAkalisto.py
Untracked: code/fixgroupedtranscript.py
Untracked: code/fixhead_netseqfc.py
Untracked: code/genotypesYRI.gen.proc.keep.vcf.log
Untracked: code/genotypesYRI.gen.proc.keep.vcf.recode.vcf
Untracked: code/get100upPAS.py
Untracked: code/getAPAfromanyeQTL.py
Untracked: code/getApapval4eqtl.py
Untracked: code/getApapval4eqtl_unexp.py
Untracked: code/getDownstreamIntronNuclear.py
Untracked: code/getIntronDownstreamPAS.py
Untracked: code/getIntronUpstreamPAS.py
Untracked: code/getQTLalleles.py
Untracked: code/getQTLfastq.sh
Untracked: code/getSeq100up.sh
Untracked: code/getUpstreamIntronNuclear.py
Untracked: code/getseq100up.err
Untracked: code/getseq100up.out
Untracked: code/grouptranscripts.err
Untracked: code/grouptranscripts.out
Untracked: code/grouptranscripts.py
Untracked: code/intersectPAS_ssSNPS.err
Untracked: code/intersectPAS_ssSNPS.out
Untracked: code/intersectVCFPAS.err
Untracked: code/intersectVCFPAS.out
Untracked: code/intersectVCFandupPAS.sh
Untracked: code/keep5perMAF.py
Untracked: code/keepSNP_vcf.sh
Untracked: code/log/
Untracked: code/makeSAFbothfrac5perc.py
Untracked: code/makeSNP2rsidfile.py
Untracked: code/makeeQTLempirical_unexp.py
Untracked: code/makeeQTLempiricaldist.py
Untracked: code/makegencondeTSSfile.py
Untracked: code/mapSSsnps2PAS.sh
Untracked: code/mergRNABam.sh
Untracked: code/mergeBW_norm.sh
Untracked: code/mergeBWnorm.err
Untracked: code/mergeBWnorm.out
Untracked: code/mergeBamNacent.err
Untracked: code/mergeBamNacent.out
Untracked: code/mergeBamNascent.sh
Untracked: code/mergeRNAbam.err
Untracked: code/mergeRNAbam.out
Untracked: code/mnase1stintron.sh
Untracked: code/mnaseDTPlot1stintron.err
Untracked: code/mnaseDTPlot1stintron.out
Untracked: code/mnaseDTPlot4thintron.err
Untracked: code/mnaseDTPlot4thintron.out
Untracked: code/mnaseDT_fourthintron.sh
Untracked: code/netDTPlot4thintron.out
Untracked: code/netseqDTplot1stIntron.sh
Untracked: code/netseqFC.err
Untracked: code/netseqFC.out
Untracked: code/netseqFC.sh
Untracked: code/neyDTPlot4thintron.err
Untracked: code/phenoQTLfromlist.py
Untracked: code/processYRIgen.py
Untracked: code/qtlFacetBoxplots.err
Untracked: code/qtlFacetBoxplots.out
Untracked: code/qtlRegionseq.sh
Untracked: code/qtlsPvalOppFrac.py
Untracked: code/removeXfromHmm.py
Untracked: code/removeloc_pheno.py
Untracked: code/runCorrectNomEqtl.sh
Untracked: code/runCorrectNomeqtl.err
Untracked: code/runCorrectNomeqtl.out
Untracked: code/runHMMpermute.err
Untracked: code/runHMMpermute.out
Untracked: code/runHMMpermuteAPAqtls.sh
Untracked: code/runHMMpermuteeQTLS.sh
Untracked: code/runHMMpermuteeQTLs.err
Untracked: code/runHMMpermuteeQTLs.out
Untracked: code/runMakeEmpiricaleQTL_unexp.sh
Untracked: code/runMakeEmpiricaleQTLs.err
Untracked: code/runMakeEmpiricaleQTLs.out
Untracked: code/runMakeEmpiricaleQTLsunex.err
Untracked: code/runMakeEmpiricaleQTLsunex.out
Untracked: code/runMakeeQTLempirical.sh
Untracked: code/run_DistPAS2Sig.err
Untracked: code/run_DistPAS2Sig.out
Untracked: code/run_bam2bw.err
Untracked: code/run_bam2bw.out
Untracked: code/run_bam2bw_all3prime.sh
Untracked: code/run_bam2bw_extra3.sh
Untracked: code/run_bam2bwexta.err
Untracked: code/run_bam2bwexta.out
Untracked: code/run_distPAS2Sig.sh
Untracked: code/run_getAPAfromanyeQTL.err
Untracked: code/run_getAPAfromanyeQTL.out
Untracked: code/run_getApaPval4eQTLs.err
Untracked: code/run_getApaPval4eQTLs.out
Untracked: code/run_getApaPval4eQTLsunexplained.err
Untracked: code/run_getApaPval4eQTLsunexplained.out
Untracked: code/run_getApaPval4eqtl.sh
Untracked: code/run_getapafromeQTL.sh
Untracked: code/run_getapapval4eqtl_unexp.sh
Untracked: code/run_leafcutterDiffIso.sh
Untracked: code/run_leafcutter_ds.err
Untracked: code/run_leafcutter_ds.out
Untracked: code/run_qtlFacetBoxplots.sh
Untracked: code/run_sepUsagephen.sh
Untracked: code/run_sepgenobychrom.err
Untracked: code/run_sepgenobychrom.out
Untracked: code/run_sepgenobychrom.sh
Untracked: code/run_sepusage.err
Untracked: code/run_sepusage.out
Untracked: code/selectNominalPvalues.py
Untracked: code/sepUsagePhen.py
Untracked: code/sepgenobychrom.py
Untracked: code/seqQTLfastq.err
Untracked: code/seqQTLfastq.out
Untracked: code/seqQTLregion.err
Untracked: code/seqQTLregion.out
Untracked: code/snakePASlog.out
Untracked: code/snakefiltPASlog.out
Untracked: code/sortindexRNABam.err
Untracked: code/sortindexRNABam.out
Untracked: code/sortindexRNAbam.sh
Untracked: code/subsetAPAnotEorPgene.py
Untracked: code/subsetApanoteGene.py
Untracked: code/subsetUnexplainedeQTLs.py
Untracked: code/subsetVCF_SS.sh
Untracked: code/subsetVCF_noSSregions.sh
Untracked: code/subsetVCF_upstreamPAS.sh
Untracked: code/subset_diffisopheno.py
Untracked: code/subsetpermAPAwithGenelist.py
Untracked: code/subsetvcf_SS.err
Untracked: code/subsetvcf_SS.out
Untracked: code/subsetvcf_noSS.err
Untracked: code/subsetvcf_noSS.out
Untracked: code/subsetvcf_otherreg.sh
Untracked: code/subsetvcf_pas.err
Untracked: code/subsetvcf_pas.out
Untracked: code/subsetvcf_perm.err
Untracked: code/subsetvcf_perm.out
Untracked: code/subsetvcf_permSS.sh
Untracked: code/subsetvcf_rand.err
Untracked: code/subsetvcf_rand.out
Untracked: code/subtract5UTR.err
Untracked: code/subtract5UTR.out
Untracked: code/subtractExons.err
Untracked: code/subtractExons.out
Untracked: code/subtractExons.sh
Untracked: code/subtractfiveprimeUTR.sh
Untracked: code/tabixSNPS.sh
Untracked: code/tabixSNPs.err
Untracked: code/tabixSNPs.out
Untracked: code/transcriptdm2bed.py
Untracked: code/utrdms2saf.py
Untracked: code/vcf2bed.py
Untracked: code/vcf_keepsnps.err
Untracked: code/vcf_keepsnps.out
Untracked: code/writeExampleQTLcode.py
Untracked: code/zipandtabPhen.err
Untracked: code/zipandtabPhen.out
Untracked: data/._.DS_Store
Untracked: data/ApaByEgene/
Untracked: data/ApaByPgene/
Untracked: data/Battle_pQTL/
Untracked: data/CompareOldandNew/
Untracked: data/DTmatrix/
Untracked: data/DiffIso/
Untracked: data/EncodeRNA/
Untracked: data/ExampleQTLPlots/
Untracked: data/GeuvadisRNA/
Untracked: data/HMMqtls/
Untracked: data/Li_eQTLs/
Untracked: data/NascentRNA/
Untracked: data/NucSpeceQTLeffect/
Untracked: data/PAS/
Untracked: data/PolyA_DB/
Untracked: data/QTLGenotypes/
Untracked: data/QTLoverlap/
Untracked: data/QTLoverlap_nonNorm/
Untracked: data/README.md
Untracked: data/RNAseq/
Untracked: data/Reads2UTR/
Untracked: data/SNPinSS/
Untracked: data/SignalSiteFiles/
Untracked: data/TF_motifdisruption/
Untracked: data/ThirtyNineIndQtl_nominal/
Untracked: data/apaQTLNominal/
Untracked: data/apaQTLNominal_4pc/
Untracked: data/apaQTLPermuted/
Untracked: data/apaQTLPermuted_4pc/
Untracked: data/apaQTLs/
Untracked: data/assignedPeaks/
Untracked: data/bam/
Untracked: data/bam_clean/
Untracked: data/bam_waspfilt/
Untracked: data/bed_10up/
Untracked: data/bed_clean/
Untracked: data/bed_clean_sort/
Untracked: data/bed_waspfilter/
Untracked: data/bedsort_waspfilter/
Untracked: data/bothFrac_FC/
Untracked: data/bw/
Untracked: data/bw_norm/
Untracked: data/eQTLs/
Untracked: data/exampleQTLs/
Untracked: data/fastq/
Untracked: data/filterPeaks/
Untracked: data/fourSU/
Untracked: data/h3k27ac/
Untracked: data/highdiffsiggenes.txt
Untracked: data/inclusivePeaks/
Untracked: data/inclusivePeaks_FC/
Untracked: data/intronRNAratio/
Untracked: data/intron_analysis/
Untracked: data/locusZoom/
Untracked: data/mergedBG/
Untracked: data/mergedBW_byfrac/
Untracked: data/mergedBW_norm/
Untracked: data/mergedBam/
Untracked: data/mergedbyFracBam/
Untracked: data/molPhenos/
Untracked: data/molQTLs/
Untracked: data/motifdistrupt/
Untracked: data/netseq/
Untracked: data/nonNorm_pheno/
Untracked: data/nuc_10up/
Untracked: data/nuc_10upclean/
Untracked: data/overlapeQTL_try2/
Untracked: data/overlapeQTLs/
Untracked: data/peakCoverage/
Untracked: data/peaks_5perc/
Untracked: data/phenotype/
Untracked: data/phenotype_5perc/
Untracked: data/sigDiffGenes.txt
Untracked: data/sort/
Untracked: data/sort_clean/
Untracked: data/sort_waspfilter/
Untracked: nohup.out
Untracked: output/._.DS_Store
Untracked: output/._meanCorrelationPhenotypes.svg
Untracked: output/dtPlots/
Untracked: output/fastqc/
Untracked: output/meanCorrelationPhenotypes.svg
Unstaged changes:
Modified: analysis/NuclearSpecAPAqtl.Rmd
Modified: analysis/Readdistagainstfeatures.Rmd
Modified: analysis/overlapapaqtlsandeqtls.Rmd
Modified: analysis/propeQTLs_explained.Rmd
Modified: analysis/signalsiteanalysis.Rmd
Modified: code/BothFracDTPlotGeneRegions.sh
Modified: code/Snakefile
Deleted: code/Upstream10Bases_general.py
Modified: code/apaQTLCorrectPvalMakeQQ.R
Modified: code/apaQTL_Nominal.sh
Modified: code/apaQTL_permuted.sh
Modified: code/apaQTLsnake.err
Modified: code/bam2bw.sh
Modified: code/bed2saf.py
Modified: code/cluster.json
Modified: code/clusterfiltPAS.json
Modified: code/config.yaml
Modified: code/environment.yaml
Modified: code/makePheno.py
Deleted: code/test.txt
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | b8e6035 | brimittleman | 2019-06-27 | add sig |
| html | 77700ac | brimittleman | 2019-06-25 | Build site. |
| Rmd | 1aa270e | brimittleman | 2019-06-25 | change order and names |
| html | 11a3069 | brimittleman | 2019-06-25 | Build site. |
| Rmd | fffb14b | brimittleman | 2019-06-25 | add pvalue and effect size |
| html | 9011f4b | brimittleman | 2019-06-25 | Build site. |
| Rmd | 1683d57 | brimittleman | 2019-06-25 | add snp write out |
| html | e767391 | brimittleman | 2019-06-25 | Build site. |
| Rmd | 45839ab | brimittleman | 2019-06-25 | results from snp in each loc |
| html | 433092b | brimittleman | 2019-06-24 | Build site. |
| Rmd | 78dd5da | brimittleman | 2019-06-24 | add snp in ss analysis |
In this analysis I want to ask if snps in a signal site are more likely to be apaQTLs than other snps close to the PAS. In order to do this i need to subset to the pas that have signal site (identified here) I will then identyify the region 50 bp upstream of the PAS and ask if there are snps in this region using the vcf files for the snps i tested.
library(workflowr)This is workflowr version 1.4.0
Run ?workflowr for help getting startedlibrary(tidyverse)── Attaching packages ───────────────────────────────────────────────────────────────────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.1.1 ✔ purrr 0.3.2
✔ tibble 2.1.1 ✔ dplyr 0.8.0.1
✔ tidyr 0.8.3 ✔ stringr 1.3.1
✔ readr 1.3.1 ✔ forcats 0.3.0 ── Conflicts ──────────────────────────────────────────────────────────────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()library(ggpubr)Loading required package: magrittr
Attaching package: 'magrittr'The following object is masked from 'package:purrr':
set_namesThe following object is masked from 'package:tidyr':
extractmkdir ../data/SNPinSSI want a bed file with 50bp upstream of these PAS.
PASwSS=read.table("../data/PAS/PASwSignalSite.txt", header = T,stringsAsFactors = F)
PAS=read.table("../data/PAS/APAPAS_GeneLocAnno.5perc_withCHR.bed", stringsAsFactors = F, header = F, col.names = c("chr", "start", "end", "PASid","score", "strand")) %>% separate(PASid, into=c("pasNum", "geneiD"), sep=":") %>% mutate(PAS=paste("peak", pasNum, sep=""),PASname=paste(PAS, geneiD, sep="_"))
PASwSSregion=PASwSS %>% inner_join(PAS, by="PAS") %>% mutate(newEnd=ifelse(strand=="+", end+50, end),newStart=ifelse(strand=="+", start, start-50)) %>% select(chr, newStart,newEnd, PASname, score, strand)
write.table(PASwSSregion,"../data/SNPinSS/FiftyupstreamPASwSS.bed", col.names = F, row.names = F, quote = F, sep="\t")sed 's/^chr//' ../data/SNPinSS/FiftyupstreamPASwSS.bed > ../data/SNPinSS/FiftyupstreamPASwSS.nochr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/FiftyupstreamPASwSS.nochr.bed > ../data/SNPinSS/FiftyupstreamPASwSS.nochr.sort.bed
sbatch subsetVCF_upstreamPAS.sh
cat ../data/SNPinSS/SNPSinFiftyupstreamPAS_chr* >../data/SNPinSS/SNPSinFiftyupstreamPAS_Allchr.recode.vcfI want to further subset to those in a signal site.
SSregions=PASwSS %>% inner_join(PAS, by="PAS") %>% mutate(absdist=abs(UpstreamDist),newEnd= ifelse(strand=="+", end-absdist, end+absdist), newStart=ifelse(strand=="+", end- (absdist+6), end + (absdist-6)), length=newEnd-newStart) %>% select(chr, newStart,newEnd, PASname, score, strand)
write.table(SSregions,"../data/SNPinSS/SignalSiteRegions.bed", col.names = F, row.names = F, quote = F, sep="\t")sed 's/^chr//' ../data/SNPinSS/SignalSiteRegions.bed > ../data/SNPinSS/SignalSiteRegions.nochr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/SignalSiteRegions.nochr.bed > ../data/SNPinSS/SignalSiteRegions.nochr.sort.bed
sbatch subsetVCF_SS.sh
cat ../data/SNPinSS/SSregions_chr* > ../data/SNPinSS/SSregions_Allchr.recode.vcfI will also need a different region to comare to. I can just shift these regions upstream by 7
SS_diffregion=SSregions %>% mutate(randStart=ifelse(strand=="+", newStart-7, newEnd), randend=ifelse(strand=="+", newStart, newEnd+7), length=randend-randStart) %>% select(chr, randStart,randend, PASname, score, strand)
write.table(SS_diffregion,"../data/SNPinSS/OtherSSRegions.bed", col.names = F, row.names = F, quote = F, sep="\t")sed 's/^chr//' ../data/SNPinSS/OtherSSRegions.bed > ../data/SNPinSS/OtherSSRegions.nochr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/OtherSSRegions.nochr.bed > ../data/SNPinSS/OtherSSRegions.nochr.sort.bed
sbatch subsetvcf_otherreg.sh
cat ../data/SNPinSS/Otherregions_chr* > ../data/SNPinSS/Otherregions_Allchr.recode.vcfAlternative option, permute the distances:
permdist=sample(PASwSS$UpstreamDist, length(PASwSS$UpstreamDist), replace = F)
SSregions_perm=as.data.frame(cbind(PASwSS, permdist))%>% inner_join(PAS, by="PAS") %>% mutate(absdist=abs(permdist),newEnd= ifelse(strand=="+", end-absdist, end+absdist), newStart=ifelse(strand=="+", end- (absdist+6), end + (absdist-6)), length=newEnd-newStart)%>% select(chr, newStart,newEnd, PASname, score, strand)
write.table(SSregions_perm,"../data/SNPinSS/SSRegions_permuted.bed", col.names = F, row.names = F, quote = F, sep="\t")sed 's/^chr//' ../data/SNPinSS/SSRegions_permuted.bed > ../data/SNPinSS/SSRegions_permuted.nochr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/SSRegions_permuted.nochr.bed > ../data/SNPinSS/SSRegions_permuted.nochr.sort.bed
sbatch subsetvcf_permSS.sh
cat ../data/SNPinSS/SSRegionsPerm_chr* > ../data/SNPinSS/SSRegionsPerm_Allchr.recode.vcf
#remove # in first linePull in QTL snps:
totQTLs=read.table("../data/apaQTLs/Total_apaQTLs4pc_5fdr.txt",header = T, stringsAsFactors = F) %>% select(sid) %>% unique()
write.table(totQTLs,"../data/apaQTLs/TotalQTLSNPsRSID.txt", col.names = F, row.names = F, quote = F)
nucQTLs=read.table("../data/apaQTLs/Nuclear_apaQTLs4pc_5fdr.txt",header = T, stringsAsFactors = F) %>% select(sid) %>% unique()
write.table(nucQTLs,"../data/apaQTLs/NuclearQTLSNPsRSID.txt", col.names = F, row.names = F, quote = F)Signal site results:
SS_snps=read.table("../data/SNPinSS/SSregions_Allchr.recode.vcf",header = T, stringsAsFactors = F) %>% select(ID) %>% mutate(totQTL=ifelse(ID %in% totQTLs$sid, "Yes", "No"), nucQTL=ifelse(ID %in% nucQTLs$sid, "Yes", "No"))
permutedSS_snps=read.table("../data/SNPinSS/SSRegionsPerm_Allchr.recode.vcf",header = T, stringsAsFactors = F) %>% select(ID) %>% mutate(totQTL=ifelse(ID %in% totQTLs$sid, "Yes", "No"), nucQTL=ifelse(ID %in% nucQTLs$sid, "Yes", "No"))
otherReg_snp=read.table("../data/SNPinSS/Otherregions_Allchr.recode.vcf",header = T, stringsAsFactors = F) %>% select(ID) %>% mutate(totQTL=ifelse(ID %in% totQTLs$sid, "Yes", "No"), nucQTL=ifelse(ID %in% nucQTLs$sid, "Yes", "No"))
fiftybp_snp=read.table("../data/SNPinSS/SNPSinFiftyupstreamPAS_Allchr.recode.vcf",header = T, stringsAsFactors = F) %>% select(ID) %>% mutate(totQTL=ifelse(ID %in% totQTLs$sid, "Yes", "No"), nucQTL=ifelse(ID %in% nucQTLs$sid, "Yes", "No"))There are only 2 qtl snps in these signal sites. This is not enough to draw anything from this.
Try with pvalues. Are the snps in pvals more likely to be significant than those not.
I need to figure out which peak is associated with each snp.
I can make a bedfile from the SS snps in python and overlap this with the Signal site regions.
python vcf2bed.py ../data/SNPinSS/SSregions_Allchr.recode.vcf ../data/SNPinSS/SSregions_Allchr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/SSregions_Allchr.bed > ../data/SNPinSS/SSregions_Allchr.sort.bed
python vcf2bed.py ../data/SNPinSS/SSRegionsPerm_Allchr.recode.vcf ../data/SNPinSS/SSRegionsPerm_Allchr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/SSRegionsPerm_Allchr.bed> ../data/SNPinSS/SSRegionsPerm_Allchr.sort.bed
python vcf2bed.py ../data/SNPinSS/SNPSinFiftyupstreamPAS_Allchr.recode.vcf ../data/SNPinSS/SNPSinFiftyupstreamPAS_Allchr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/SNPSinFiftyupstreamPAS_Allchr.bed > ../data/SNPinSS/SNPSinFiftyupstreamPAS_Allchr.sort.bed
python vcf2bed.py ../data/SNPinSS/Otherregions_Allchr.recode.vcf ../data/SNPinSS/Otherregions_Allchr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/Otherregions_Allchr.bed > ../data/SNPinSS/Otherregions_Allchr.sort.bed
sort -k1,1 -k2,2n ../data/SNPinSS/SignalSiteRegions.bed > ../data/SNPinSS/SignalSiteRegions.sort.bed
sort -k1,1 -k2,2n ../data/SNPinSS/SSRegions_permuted.bed >../data/SNPinSS/SSRegions_permuted.sort.bed
sort -k1,1 -k2,2n ../data/SNPinSS/FiftyupstreamPASwSS.bed > ../data/SNPinSS/FiftyupstreamPASwSS.sort.bed
sort -k1,1 -k2,2n ../data/SNPinSS/OtherSSRegions.bed > ../data/SNPinSS/OtherSSRegions.sort.bed
intersect with bedtools to map the snps to the regions. Then I will be able to select the snp PAS associations.
sbatch mapSSsnps2PAS.shResults to get the associations:
SSsnpswithPAS=read.table("../data/SNPinSS/SNPinSS2PAS.txt",col.names = c("chr","start", "end", "PASname", "score", "strand", "SNP")) %>% filter(SNP!=".") %>% separate(PASname, into=c("PAS", "gene", "loc"),sep="_") %>% select(PAS, SNP)
write.table(SSsnpswithPAS, "../data/SNPinSS/SS_PASandSNPs.txt", row.names = F, col.names = F, quote = F, sep="\t")
SSsnpswithPERMPAS=read.table("../data/SNPinSS/SNPinPermSS2PAS.txt",col.names = c("chr","start", "end", "PASname", "score", "strand", "SNP")) %>% filter(SNP!=".") %>% separate(PASname, into=c("PAS", "gene", "loc"),sep="_") %>% select(PAS, SNP)
write.table(SSsnpswithPERMPAS, "../data/SNPinSS/PermSS_PASandSNPs.txt", row.names = F, col.names = F, quote = F, sep="\t")
SNPregion=read.table("../data/SNPinSS/SNPSinFiftyupstream2PAS.txt",col.names = c("chr","start", "end", "PASname", "score", "strand", "SNP")) %>% filter(SNP!=".") %>% separate(PASname, into=c("PAS", "gene", "loc"),sep="_") %>% select(PAS, SNP)
write.table(SNPregion, "../data/SNPinSS/PASregion_PASandSNPs.txt", row.names = F, col.names = F, quote = F, sep="\t")
Otherregion=read.table("../data/SNPinSS/Otherregions2PAS.txt",col.names = c("chr","start", "end", "PASname", "score", "strand", "SNP")) %>% filter(SNP!=".") %>% separate(PASname, into=c("PAS", "gene", "loc"),sep="_") %>% select(PAS, SNP)
write.table(Otherregion, "../data/SNPinSS/Otherregions_PASandSNPs.txt", row.names = F, col.names = F, quote = F, sep="\t")Process the snp region with python
python fixPASregionSNPs.py
Run this with total and nuclear
python NomResfromPASSNP.py ../data/SNPinSS/SS_PASandSNPs.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Nuclear.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/SS_Nuclear_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/SS_PASandSNPs.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Total.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/SS_Total_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/PermSS_PASandSNPs.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Nuclear.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/PermSS_Nuclear_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/PermSS_PASandSNPs.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Total.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/PermSS_Total_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/PASregion_PASandSNPs.FIXED.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Nuclear.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/RegionSS_Nuclear_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/PASregion_PASandSNPs.FIXED.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Total.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/RegionSS_Total_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/Otherregions_PASandSNPs.FIXED.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Nuclear.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/OtherSS_Nuclear_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/Otherregions_PASandSNPs.FIXED.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Total.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/OtherSS_Total_nomRes.txt
Nuclear_SS=read.table('../data/apaQTLNominal_4pc/SS_Nuclear_nomRes.txt', header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Nuclear", set="SS")
Nuclear_Perm=read.table('../data/apaQTLNominal_4pc/PermSS_Nuclear_nomRes.txt',header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Nuclear", set="Permuted")
Total_SS=read.table('../data/apaQTLNominal_4pc/SS_Total_nomRes.txt', header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Total", set="SS")
Total_Perm=read.table('../data/apaQTLNominal_4pc/PermSS_Total_nomRes.txt',header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Total", set="Permuted")
Nuclear_Region=read.table('../data/apaQTLNominal_4pc/RegionSS_Nuclear_nomRes.txt',header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Nuclear", set="Region")
Total_Region=read.table('../data/apaQTLNominal_4pc/RegionSS_Total_nomRes.txt',header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Total", set="Region")
Nuclear_other=read.table('../data/apaQTLNominal_4pc/OtherSS_Nuclear_nomRes.txt',header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Nuclear", set="Upstream")
Total_other=read.table('../data/apaQTLNominal_4pc/OtherSS_Total_nomRes.txt',header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% select(pval,slope) %>% mutate(fraction="Total", set="Upstream")
all_SS_pval=bind_rows(Nuclear_SS,Nuclear_Perm,Total_SS,Total_Perm,Nuclear_Region,Total_Region,Nuclear_other,Total_other)
all_SS_pval$set <- factor(all_SS_pval$set, levels=c("SS", "Upstream", "Permuted", "Region"))ggplot(all_SS_pval, aes(x=fraction, fill=set, y=pval)) + geom_boxplot() + labs(x="Fraction", title="p-values for SNPs in Signal Sites",y="nominal P-value" ) + scale_fill_discrete(name = 'Set', labels = c('Signal Sites', 'Region upstream of Signal Site', 'Permuted Distance to Signal Site',"50 bp upstream of PAS"))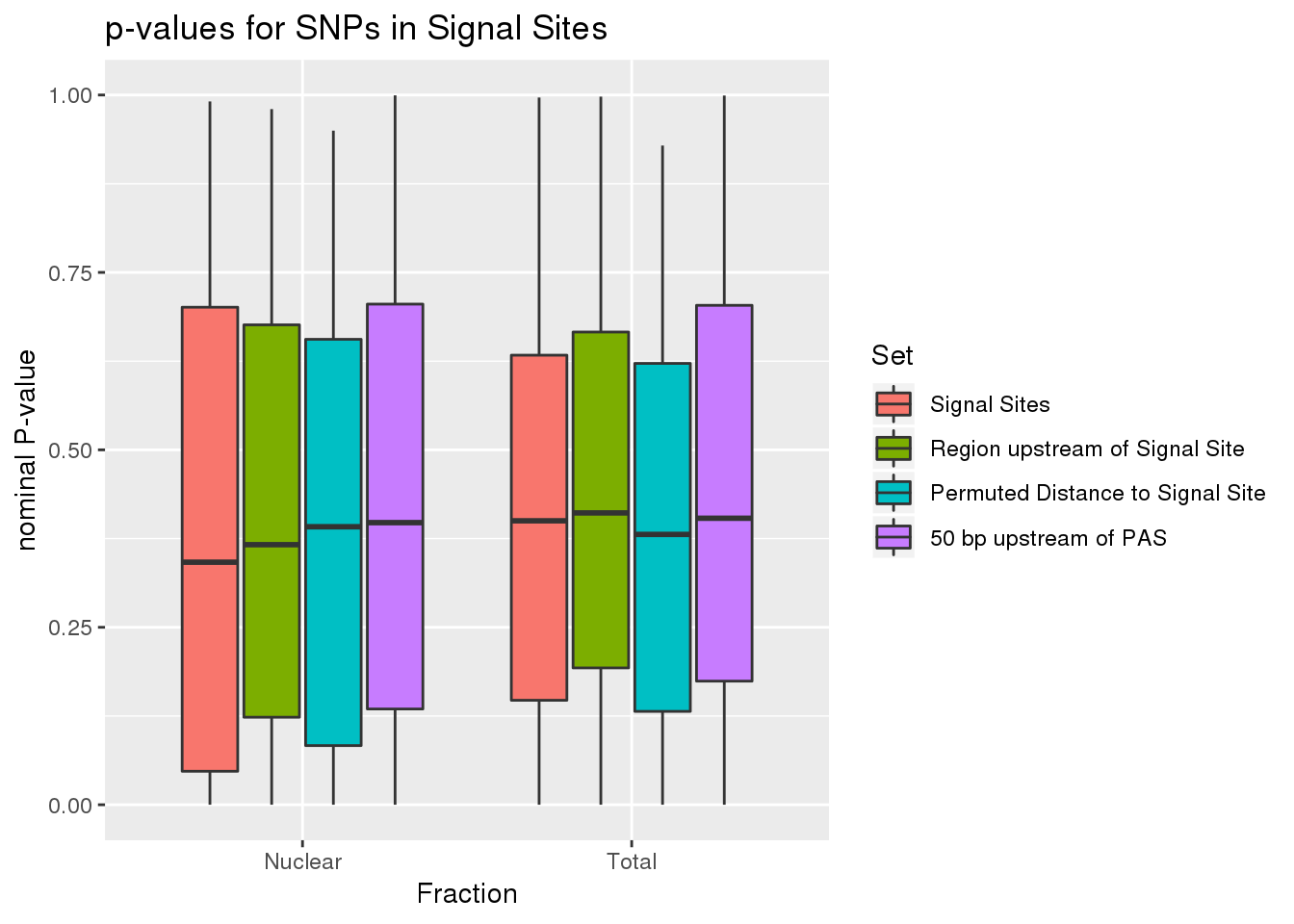
ggplot(all_SS_pval, aes(x=fraction, fill=set, y=abs(slope))) + geom_boxplot() + labs(x="Fraction", title="p-values for SNPs in Signal Sites",y="absolute value effect size" ) + scale_fill_discrete(name = 'Set', labels = c('Signal Sites', 'Region upstream of Signal Site', 'Permuted Distance to Signal Site',"50 bp upstream of PAS"))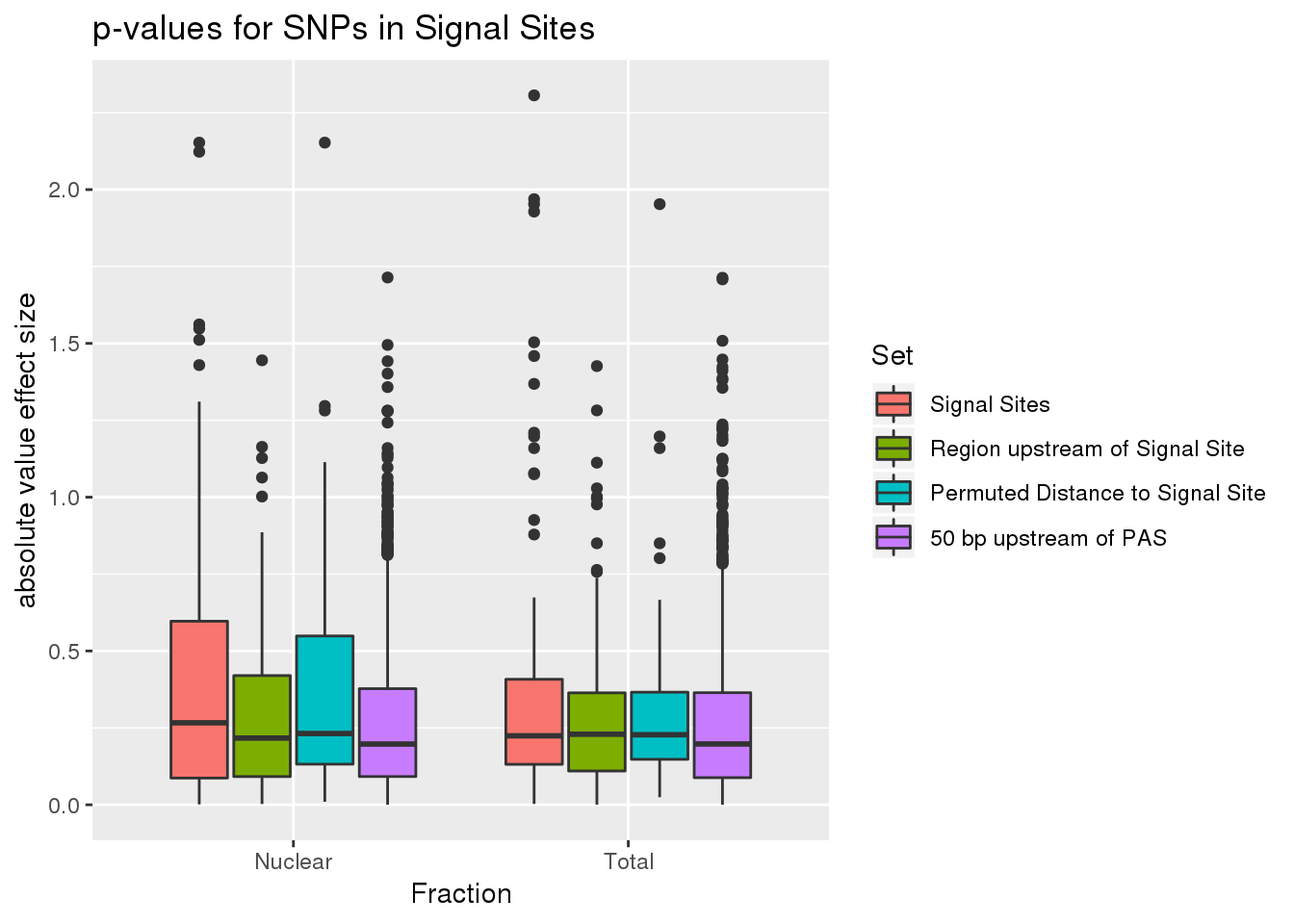
I am plotting this the wrong way. I need to make qqplots with the snps.
#plot qqplot
qqplot(-log10(runif(nrow(Nuclear_SS))), -log10(Nuclear_SS$pval))
points(sort(-log10(runif(nrow(Total_SS)))), sort(-log10(Total_SS$pval)),col= alpha("Red"))
points(sort(-log10(runif(nrow(Nuclear_Region)))), sort(-log10(Nuclear_Region$pval)),col= alpha("Orange"))
points(sort(-log10(runif(nrow(Total_Region)))), sort(-log10(Total_Region$pval)),col= alpha("Green"))
abline(0,1)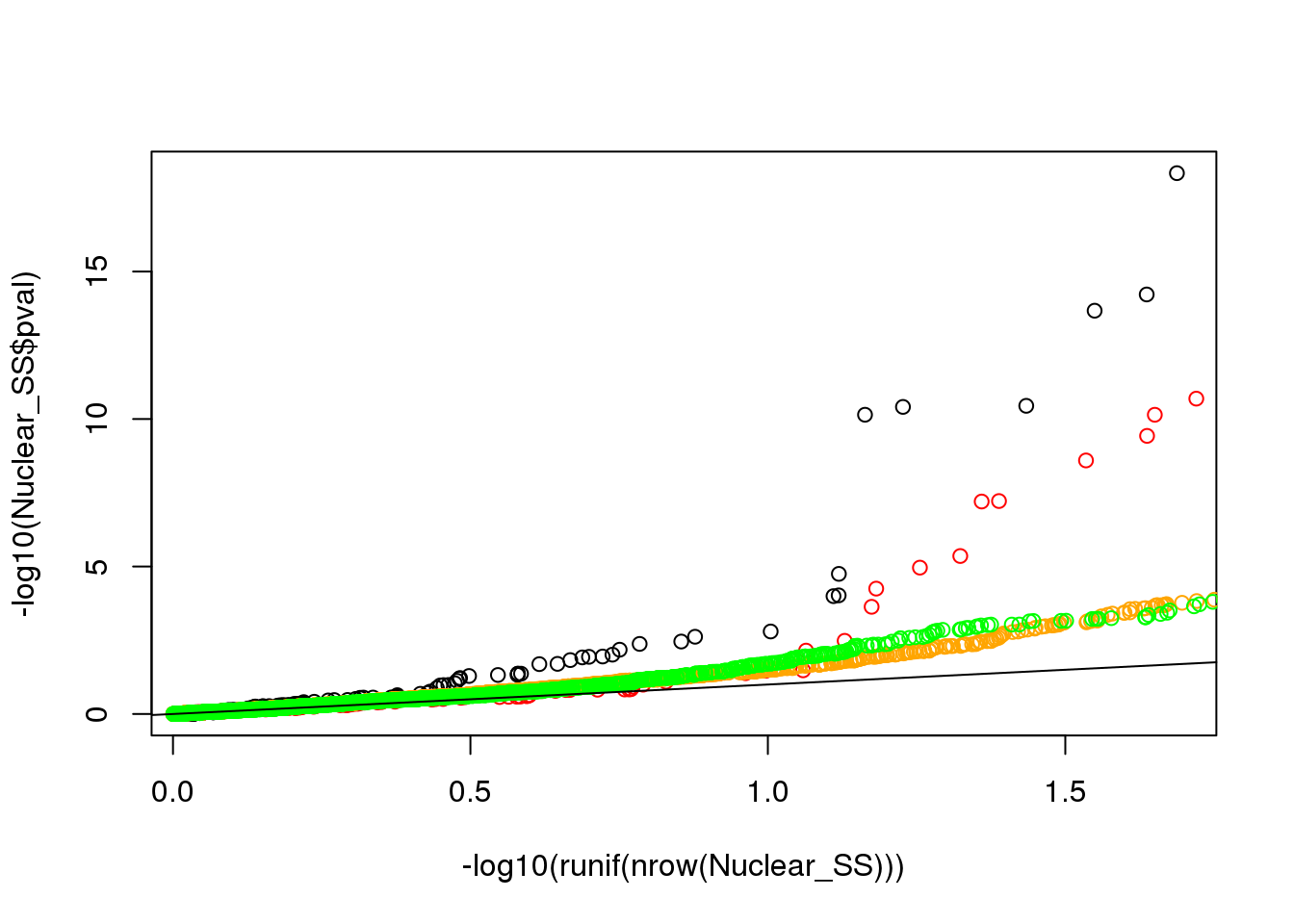
This is close but i need to make this a better comparison.I want just the UTR variants.
Nuclear_SS_all=read.table('../data/apaQTLNominal_4pc/SS_Nuclear_nomRes.txt', header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% separate(peakID, into=c("chr", "start", "end", "PASid"), sep=":")%>% separate(PASid, into=c("gene", "loc","strand", "PAS"), sep="_") %>% filter(loc=="utr3")
Total_SS_all=read.table('../data/apaQTLNominal_4pc/SS_Total_nomRes.txt', header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F)%>% separate(peakID, into=c("chr", "start", "end", "PASid"), sep=":")%>% separate(PASid, into=c("gene", "loc","strand", "PAS"), sep="_") %>% filter(loc=="utr3")The benchmark set is the UTRs without signal sites
PASwSS=read.table("../data/PAS/PASwSignalSite.txt", header = T,stringsAsFactors = F)
PAS_noSS=read.table("../data/PAS/APAPAS_GeneLocAnno.5perc_withCHR.bed", stringsAsFactors = F, header = F, col.names = c("chr", "start", "end", "PASid","score", "strand")) %>% separate(PASid, into=c("pasNum", "geneiD"), sep=":") %>% mutate(PAS=paste("peak", pasNum, sep=""),PASname=paste(PAS, geneiD, sep="_")) %>% anti_join(PASwSS, by="PAS") %>% separate(geneiD,into=c("gene", "loc"), sep="_") %>% filter(loc=="utr3") %>% mutate(newEnd=ifelse(strand=="+", end+50, end),newStart=ifelse(strand=="+", start, start-50))%>% select(chr, newStart,newEnd, PASname, score, strand)
write.table(PAS_noSS,"../data/SNPinSS/UTRregionsPASnoSS.bed", col.names = F, row.names = F, quote = F, sep="\t")sed 's/^chr//' ../data/SNPinSS/UTRregionsPASnoSS.bed > ../data/SNPinSS/UTRregionsPASnoSS.nochr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/UTRregionsPASnoSS.nochr.bed > ../data/SNPinSS/UTRregionsPASnoSS.nochr.sort.bed
sbatch subsetVCF_noSSregions.sh
cat ../data/SNPinSS/UTRnoSS_chr* >../data/SNPinSS/UTRnoSS_Allchr.recode.vcf
python vcf2bed.py ../data/SNPinSS/UTRnoSS_Allchr.recode.vcf ../data/SNPinSS/UTRnoSS_SNPsAllchr.bed
sort -k1,1 -k2,2n ../data/SNPinSS/UTRregionsPASnoSS.bed > ../data/SNPinSS/UTRregionsPASnoSS.sort.bed
sort -k1,1 -k2,2n ../data/SNPinSS/UTRnoSS_SNPsAllchr.bed > ../data/SNPinSS/UTRnoSS_SNPsAllchr.sort.bed
sbatch mapSSsnps2PAS.shNOSSsnpswithPAS=read.table("../data/SNPinSS/UTRnoSS_SNPsAllchr2PAS.txt",col.names = c("chr","start", "end", "PASname", "score", "strand", "SNP")) %>% filter(SNP!=".") %>% separate(PASname, into=c("PAS", "gene", "loc"),sep="_") %>% select(PAS, SNP)
write.table(NOSSsnpswithPAS, "../data/SNPinSS/NoSSUTR_PASandSNPs.txt", row.names = F, col.names = F, quote = F, sep="\t")Get the pval:
python NomResfromPASSNP.py ../data/SNPinSS/NoSSUTR_PASandSNPs.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Nuclear.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/NoSSUTR_Nuclear_nomRes.txt
python NomResfromPASSNP.py ../data/SNPinSS/NoSSUTR_PASandSNPs.txt ../data/apaQTLNominal_4pc/APApeak_Phenotype_GeneLocAnno.Total.5perc.fc.gz.qqnorm_AllChrom.txt ../data/apaQTLNominal_4pc/NoSSUTR_Total_nomRes.txt
Nuclear_NOSS=read.table('../data/apaQTLNominal_4pc/NoSSUTR_Nuclear_nomRes.txt', header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F) %>% separate(peakID, into=c("chr", "start", "end", "PASid"), sep=":")%>% separate(PASid, into=c("gene", "loc","strand", "PAS"), sep="_") %>% filter(loc=="utr3")
Total_NOSS=read.table('../data/apaQTLNominal_4pc/NoSSUTR_Total_nomRes.txt', header = F, col.names=c("peakID", "snp", "dist", "pval", "slope"), stringsAsFactors = F)%>% separate(peakID, into=c("chr", "start", "end", "PASid"), sep=":")%>% separate(PASid, into=c("gene", "loc","strand", "PAS"), sep="_") %>% filter(loc=="utr3")Total
#plot qqplot
qqplot(-log10(runif(nrow(Total_NOSS))), -log10(Total_NOSS$pval), xlab="-log10(Uniform)", ylab="-log10(pval)", main="Total Apa")
points(sort(-log10(runif(nrow(Total_SS)))), sort(-log10(Total_SS$pval)),col= alpha("Red"))
abline(0,1)
legend("topleft", legend=c("SNPs in UTR PAS Signal Sites", "SNPS not in Signal Sites"),col=c("red", "black"), pch=16,bty = 'n')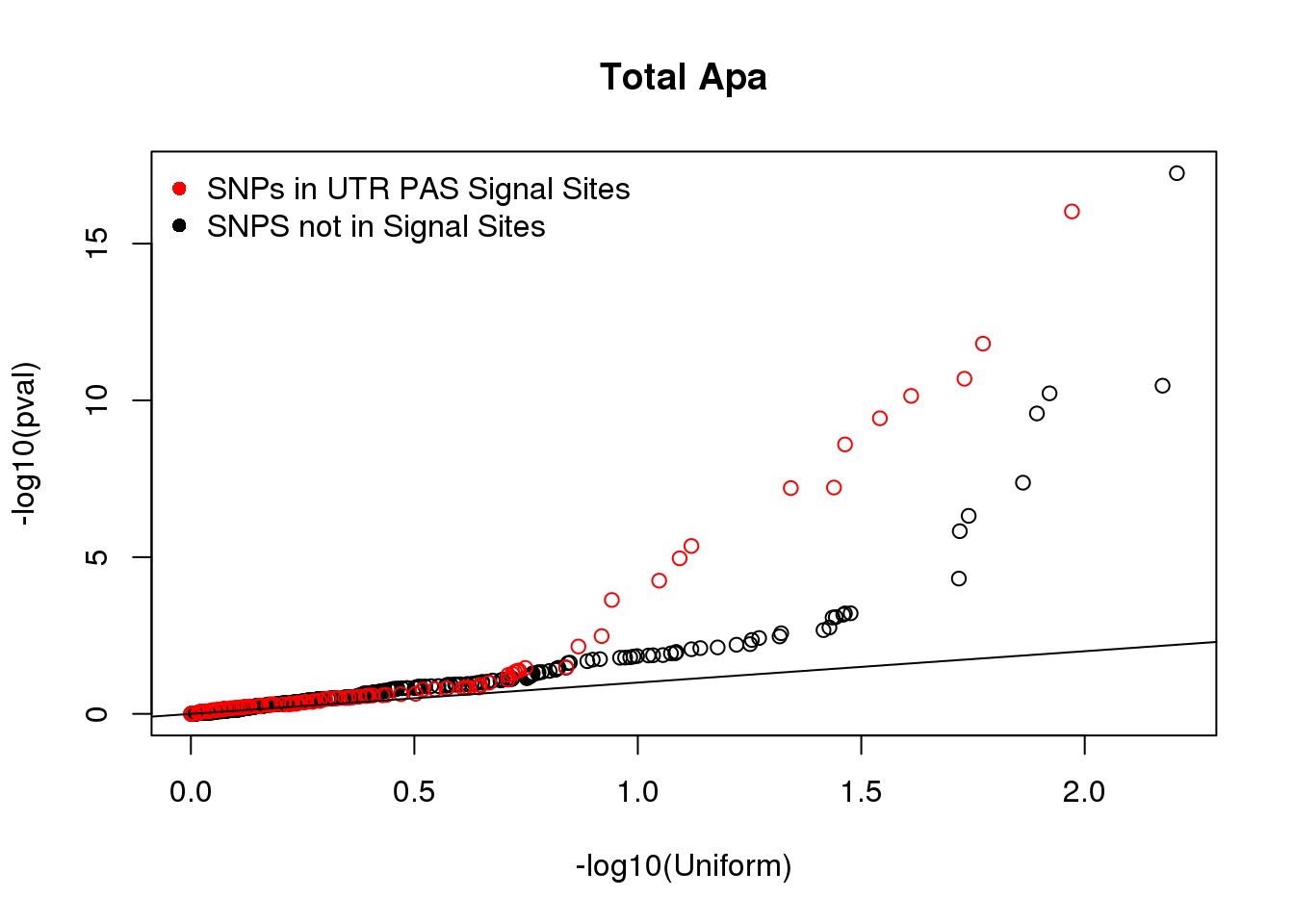 Nuclear:
Nuclear:
qqplot(-log10(runif(nrow(Nuclear_NOSS))), -log10(Nuclear_NOSS$pval),xlab="-log10(Uniform)", ylab="-log10(pval)", main="Nuclear Apa")
points(sort(-log10(runif(nrow(Nuclear_SS)))), sort(-log10(Nuclear_SS$pval)),col= alpha("Red"))
abline(0,1)
legend("topleft", legend=c("SNPs in UTR PAS Signal Sites", "SNPS not in Signal Sites"),col=c("red", "black"), pch=16,bty = 'n')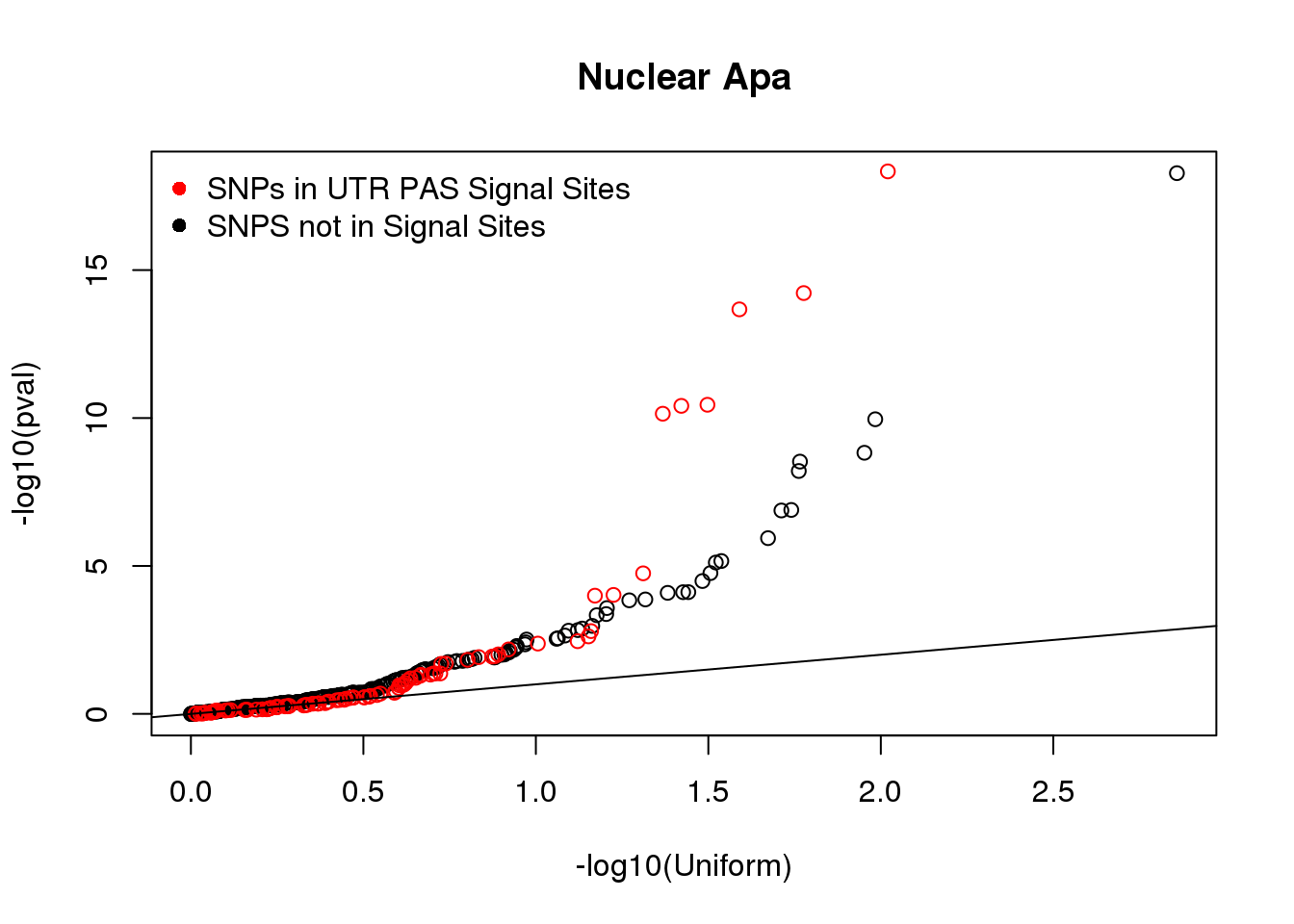
Assess significance:
wilcox.test(Nuclear_SS$pval,Nuclear_NOSS$pval,alternative = 'less')
Wilcoxon rank sum test with continuity correction
data: Nuclear_SS$pval and Nuclear_NOSS$pval
W = 14599, p-value = 0.3301
alternative hypothesis: true location shift is less than 0wilcox.test(Total_SS$pval,Total_NOSS$pval, alternative = "less")
Wilcoxon rank sum test with continuity correction
data: Total_SS$pval and Total_NOSS$pval
W = 15982, p-value = 0.4881
alternative hypothesis: true location shift is less than 0
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] ggpubr_0.2 magrittr_1.5 forcats_0.3.0 stringr_1.3.1
[5] dplyr_0.8.0.1 purrr_0.3.2 readr_1.3.1 tidyr_0.8.3
[9] tibble_2.1.1 ggplot2_3.1.1 tidyverse_1.2.1 workflowr_1.4.0
loaded via a namespace (and not attached):
[1] Rcpp_1.0.0 cellranger_1.1.0 pillar_1.3.1 compiler_3.5.1
[5] git2r_0.25.2 plyr_1.8.4 tools_3.5.1 digest_0.6.18
[9] lubridate_1.7.4 jsonlite_1.6 evaluate_0.12 nlme_3.1-137
[13] gtable_0.2.0 lattice_0.20-38 pkgconfig_2.0.2 rlang_0.3.1
[17] cli_1.0.1 rstudioapi_0.10 yaml_2.2.0 haven_1.1.2
[21] withr_2.1.2 xml2_1.2.0 httr_1.3.1 knitr_1.20
[25] hms_0.4.2 generics_0.0.2 fs_1.2.6 rprojroot_1.3-2
[29] grid_3.5.1 tidyselect_0.2.5 glue_1.3.0 R6_2.3.0
[33] readxl_1.1.0 rmarkdown_1.10 modelr_0.1.2 whisker_0.3-2
[37] backports_1.1.2 scales_1.0.0 htmltools_0.3.6 rvest_0.3.2
[41] assertthat_0.2.0 colorspace_1.3-2 labeling_0.3 stringi_1.2.4
[45] lazyeval_0.2.1 munsell_0.5.0 broom_0.5.1 crayon_1.3.4