Signal Site analysis
Briana Mittleman
4/23/2019
Last updated: 2019-09-17
Checks: 6 1
Knit directory: apaQTL/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.4.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
The global environment had objects present when the code in the R Markdown file was run. These objects can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment. Use wflow_publish or wflow_build to ensure that the code is always run in an empty environment.
The following objects were defined in the global environment when these results were created:
| Name | Class | Size |
|---|---|---|
| data | environment | 56 bytes |
| env | environment | 56 bytes |
The command set.seed(20190411) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: data/.DS_Store
Ignored: docs/.DS_Store
Ignored: docs/figure/.DS_Store
Ignored: docs/figure/PAS_graphs_total.Rmd/.DS_Store
Ignored: docs/figure/choosePCs.Rmd/.DS_Store
Ignored: docs/figure/exvunexpeQTL.Rmd/.DS_Store
Ignored: docs/figure/snpinSS.Rmd/.DS_Store
Ignored: output/.DS_Store
Untracked files:
Untracked: .Rprofile
Untracked: ._.DS_Store
Untracked: .gitignore
Untracked: @
Untracked: GEO_brimittleman/
Untracked: _workflowr.yml
Untracked: analysis/._PASdescriptiveplots.Rmd
Untracked: analysis/._cuttoffPercUsage.Rmd
Untracked: analysis/APApeak_Phenotype_GeneLocAnno.Nuclear.5perc.fc.gz.qqnorm.allChrom
Untracked: analysis/APApeak_Phenotype_GeneLocAnno.Total.5perc.fc.gz.qqnorm.allChrom
Untracked: analysis/QTLexampleplots.Rmd
Untracked: analysis/cuttoffPercUsage.Rmd
Untracked: analysis/eQTLoverlap.Rmd
Untracked: analysis/interpret verify bam.Rmd
Untracked: analysis/interpret_verifybam.Rmd
Untracked: analysis/mergeRNA.Rmd
Untracked: analysis/oldstuffNotNeeded.Rmd
Untracked: analysis/remove_badlines.Rmd
Untracked: analysis/totSpecInNuclear.Rmd
Untracked: analysis/totSpecIncludenotTested.Rmd
Untracked: analysis/totalspec.Rmd
Untracked: apaQTL.Rproj
Untracked: checksumsfastq.txt.gz
Untracked: code/.NascentRNAdtPlotFirstintronicPAS.sh.swp
Untracked: code/._ApaQTL_nominalNonnorm.sh
Untracked: code/._BothFracDTPlotGeneRegions.sh
Untracked: code/._BothFracDTPlotGeneRegions_normalized.sh
Untracked: code/._DistPAS2Sig_RandomIntron.py
Untracked: code/._EandPqtl_perm.sh
Untracked: code/._EandPqtls.sh
Untracked: code/._FC_NucintornUpandDown.sh
Untracked: code/._FC_UTR.sh
Untracked: code/._FC_intornUpandDownsteamPAS.sh
Untracked: code/._FC_nascentseq.sh
Untracked: code/._FC_newPeaks_olddata.sh
Untracked: code/._HMMpermuteTotal.py
Untracked: code/._HmmPermute.py
Untracked: code/._IntronicPASDT.sh
Untracked: code/._LC_samplegroups.py
Untracked: code/._LD_qtl.sh
Untracked: code/._LD_snpsproxy.sh
Untracked: code/._NascentRNAdtPlot.sh
Untracked: code/._NascentRNAdtPlot3UTRPAS.sh
Untracked: code/._NascentRNAdtPlotExcludeFirstintronicPAS.sh
Untracked: code/._NascentRNAdtPlotNucPAS.sh
Untracked: code/._NascentRNAdtPlotTotPAS.sh
Untracked: code/._NascentRNAdtPlotintronicPAS.sh
Untracked: code/._NascnetRNAdtPlotPAS.sh
Untracked: code/._NetSeq_fourthintronDT.sh
Untracked: code/._NomResfromPASSNP.py
Untracked: code/._NuclearPAS_5per.bed.py
Untracked: code/._PTTfacetboxplots.R
Untracked: code/._PrematureQTLNominal.sh
Untracked: code/._PrematureQTLPermuted.sh
Untracked: code/._QTL2bed.py
Untracked: code/._QTL2bed_withstrand.py
Untracked: code/._RNAbam2bw.sh
Untracked: code/._RNAseqDTplot.sh
Untracked: code/._RunRes2PAS.sh
Untracked: code/._SAF215upbed.py
Untracked: code/._SnakefilePAS
Untracked: code/._SnakefilefiltPAS
Untracked: code/._TESplots100bp.sh
Untracked: code/._TESplots150bp.sh
Untracked: code/._TESplots200bp.sh
Untracked: code/._TotalPAS_5perc.bed.py
Untracked: code/._Untitled
Untracked: code/._ZipandTabPheno.sh
Untracked: code/._aAPAqtl_nominal39ind.sh
Untracked: code/._allNucSpecQTLine.py
Untracked: code/._allNucSpecfromNonNorm.py
Untracked: code/._annotatePacBioPASregion.sh
Untracked: code/._annotatedPAS2bed.py
Untracked: code/._apaInPandE.py
Untracked: code/._apaQTLCorrectPvalMakeQQ.R
Untracked: code/._apaQTLCorrectpval_6or7a.R
Untracked: code/._apaQTL_Nominal.sh
Untracked: code/._apaQTL_nominalInclusive.sh
Untracked: code/._apaQTL_nominalv67.sh
Untracked: code/._apaQTL_permuted.sh
Untracked: code/._apaQTL_permuted_test6A7A.sh
Untracked: code/._apainRibo.py
Untracked: code/._assignNucIntonpeak2intronlocs.sh
Untracked: code/._assignTotIntronpeak2intronlocs.sh
Untracked: code/._bam2BW_5primemost.sh
Untracked: code/._bed2saf.py
Untracked: code/._bothFracDTplot1stintron.sh
Untracked: code/._bothFracDTplot4thintron.sh
Untracked: code/._bothFrac_FC.sh
Untracked: code/._callPeaksYL.py
Untracked: code/._changeRibonomQTLres2genename.py
Untracked: code/._changenomQTLres2geneName.py
Untracked: code/._chooseAnno2PAS_pacbio.py
Untracked: code/._chooseAnno2SAF.py
Untracked: code/._chooseSignalSite
Untracked: code/._chooseSignalSite.py
Untracked: code/._closestannotated.sh
Untracked: code/._closestannotated_byfrac.sh
Untracked: code/._cluster.json
Untracked: code/._clusterPAS.json
Untracked: code/._clusterfiltPAS.json
Untracked: code/._codingdms2bed.py
Untracked: code/._config.yaml
Untracked: code/._config2.yaml
Untracked: code/._configOLD.yaml
Untracked: code/._convertNominal2SNPLOC.py
Untracked: code/._convertNominal2SNPloc2Versions.py
Untracked: code/._convertNumeric.py
Untracked: code/._correctNomeqtl.R
Untracked: code/._createPlinkSampfile.py
Untracked: code/._dag.pdf
Untracked: code/._eQTL_switch2snploc.py
Untracked: code/._eQTLgenestestedapa.py
Untracked: code/._encodeRNADTplots.sh
Untracked: code/._extractGenotypes.py
Untracked: code/._extractseqfromqtlfastq.py
Untracked: code/._fc2leafphen.py
Untracked: code/._fc_filteredPAS6and7As.sh
Untracked: code/._fifteenBPupstreamPAS.py
Untracked: code/._fiftyBPupstreamPAS.py
Untracked: code/._filter5perc.R
Untracked: code/._filter5percPheno.py
Untracked: code/._filterLDsnps.py
Untracked: code/._filterMPPAS.py
Untracked: code/._filterMPPAS_15.py
Untracked: code/._filterMPPAS_15_7As.py
Untracked: code/._filterMPPAS_50.py
Untracked: code/._filterSAFforMP.py
Untracked: code/._filterpeaks.py
Untracked: code/._finalPASbed2SAF.py
Untracked: code/._fix4su304corr.py
Untracked: code/._fix4su604corr.py
Untracked: code/._fix4sukalisto.py
Untracked: code/._fixExandUnexeQTL
Untracked: code/._fixExandUnexeQTL.py
Untracked: code/._fixFChead.py
Untracked: code/._fixFChead_bothfrac.py
Untracked: code/._fixFChead_short.py
Untracked: code/._fixH3k12ac.py
Untracked: code/._fixPASregionSNPs.py
Untracked: code/._fixRNAhead4corr.py
Untracked: code/._fixRNAkalisto.py
Untracked: code/._fix_randomIntron.py
Untracked: code/._fixgroupedtranscript.py
Untracked: code/._fixhead_netseqfc.py
Untracked: code/._getAPAfromanyeQTL.py
Untracked: code/._getApapval4eqtl.py
Untracked: code/._getApapval4eqtl_unexp.py
Untracked: code/._getApapval4eqtl_version67.py
Untracked: code/._getDownstreamIntronNuclear.py
Untracked: code/._getIntronDownstreamPAS.py
Untracked: code/._getIntronUpstreamPAS.py
Untracked: code/._getQTLalleles.py
Untracked: code/._getQTLfastq.sh
Untracked: code/._getUpstreamIntronNuclear.py
Untracked: code/._grouptranscripts.py
Untracked: code/._intersectVCFandupPAS.sh
Untracked: code/._keep5perMAF.py
Untracked: code/._keepSNP_vcf.sh
Untracked: code/._make5percPeakbed.py
Untracked: code/._makeFileID.py
Untracked: code/._makePheno.py
Untracked: code/._makeSAFbothfrac5perc.py
Untracked: code/._makeSNP2rsidfile.py
Untracked: code/._makeeQTLempirical_unexp.py
Untracked: code/._makeeQTLempiricaldist.py
Untracked: code/._makegencondeTSSfile.py
Untracked: code/._mapSSsnps2PAS.sh
Untracked: code/._mergRNABam.sh
Untracked: code/._mergeAllBam.sh
Untracked: code/._mergeAnnotations.sh
Untracked: code/._mergeBW_norm.sh
Untracked: code/._mergeBamNascent.sh
Untracked: code/._mergeByFracBam.sh
Untracked: code/._mergePeaks.sh
Untracked: code/._mnase1stintron.sh
Untracked: code/._mnaseDT_fourthintron.sh
Untracked: code/._namePeaks.py
Untracked: code/._netseqDTplot1stIntron.sh
Untracked: code/._netseqFC.sh
Untracked: code/._nucQTLGWAS.py
Untracked: code/._nucSpecQTLineData.py
Untracked: code/._nucSpeceffectsize.py
Untracked: code/._nucspecnucPASine.py
Untracked: code/._pQTLsotherdata.py
Untracked: code/._pacbioDT.sh
Untracked: code/._pacbioIntronicDT.sh
Untracked: code/._parseBestbamid.py
Untracked: code/._peak2PAS.py
Untracked: code/._peakFC.sh
Untracked: code/._pheno2countonly.R
Untracked: code/._phenoQTLfromlist.py
Untracked: code/._processYRIgen.py
Untracked: code/._pttQTLsinapaQTL.py
Untracked: code/._qtlRegionseq.sh
Untracked: code/._qtlsPvalOppFrac.py
Untracked: code/._quantassign2parsedpeak.py
Untracked: code/._removeXfromHmm.py
Untracked: code/._removeloc_pheno.py
Untracked: code/._riboQTL.sh
Untracked: code/._runCorrectNomEqtl.sh
Untracked: code/._runHMMpermuteAPAqtls.sh
Untracked: code/._runHMMpermuteeQTLS.sh
Untracked: code/._runMakeEmpiricaleQTL_unexp.sh
Untracked: code/._runMakeeQTLempirical.sh
Untracked: code/._run_bam2bw_all3prime.sh
Untracked: code/._run_bam2bw_extra3.sh
Untracked: code/._run_bestbamid.sj
Untracked: code/._run_dist2sig_randomintron.sh
Untracked: code/._run_filtersnpLD.sh
Untracked: code/._run_getAPAfromeQTL_version6.7.sh
Untracked: code/._run_getApaPval4eqtl.sh
Untracked: code/._run_getapafromeQTL.py
Untracked: code/._run_getapafromeQTL.sh
Untracked: code/._run_getapapval4eqtl_unexp.sh
Untracked: code/._run_leafcutterDiffIso.sh
Untracked: code/._run_prxySNP.sh
Untracked: code/._run_pttfacetboxplot.sh
Untracked: code/._run_sepUsagephen.sh
Untracked: code/._run_sepgenobychrom.sh
Untracked: code/._run_verifybam.sh
Untracked: code/._selectNominalPvalues.py
Untracked: code/._sepUsagePhen.py
Untracked: code/._sepgenobychrom.py
Untracked: code/._snakemakePAS.batch
Untracked: code/._snakemakefiltPAS.batch
Untracked: code/._sortindexRNAbam.sh
Untracked: code/._specAPAinE.py
Untracked: code/._submit-snakemakePAS.sh
Untracked: code/._submit-snakemakefiltPAS.sh
Untracked: code/._subsetAPAnotEorPgene.py
Untracked: code/._subsetAPAnotEorPgene_2versions.py
Untracked: code/._subsetApanoteGene.py
Untracked: code/._subsetApanoteGene_2versions.py
Untracked: code/._subsetUnexplainedeQTLs.py
Untracked: code/._subsetVCF_SS.sh
Untracked: code/._subsetVCF_noSSregions.sh
Untracked: code/._subsetVCF_upstreamPAS.sh
Untracked: code/._subset_diffisopheno.py
Untracked: code/._subsetpermAPAwithGenelist.py
Untracked: code/._subsetpermAPAwithGenelist_2versions.py
Untracked: code/._subsetvcf_otherreg.sh
Untracked: code/._subsetvcf_permSS.sh
Untracked: code/._subtrachfiveprimeUTR.sh
Untracked: code/._subtractExons.sh
Untracked: code/._subtractfiveprimeUTR.sh
Untracked: code/._tabixSNPS.sh
Untracked: code/._tenBPupstreamPAS.py
Untracked: code/._testVerifyBam.sh
Untracked: code/._totSeceffectsize.py
Untracked: code/._twentyBPupstreamPAS.py
Untracked: code/._utrdms2saf.py
Untracked: code/._vcf2bed.py
Untracked: code/._verifyBam18517N.sh
Untracked: code/._verifyBam18517T.sh
Untracked: code/._verifyBam19128N.sh
Untracked: code/._verifyBam19128T.sh
Untracked: code/._wrap_verifybam.sh
Untracked: code/._writePTTexamplecode.py
Untracked: code/._writePTTexamplecode.sh
Untracked: code/.pversion
Untracked: code/.snakemake/
Untracked: code/1
Untracked: code/APAqtl_nominal.err
Untracked: code/APAqtl_nominal.out
Untracked: code/APAqtl_nominal_39.err
Untracked: code/APAqtl_nominal_39.out
Untracked: code/APAqtl_nominal_inclusive.err
Untracked: code/APAqtl_nominal_inclusive.out
Untracked: code/APAqtl_nominal_nonNorm.err
Untracked: code/APAqtl_nominal_nonNorm.out
Untracked: code/APAqtl_nominal_versions67.err
Untracked: code/APAqtl_nominal_versions67.out
Untracked: code/APAqtl_permuted.err
Untracked: code/APAqtl_permuted.out
Untracked: code/APAqtl_permuted_versions67.err
Untracked: code/APAqtl_permuted_versions67.out
Untracked: code/BothFracDTPlot1stintron.err
Untracked: code/BothFracDTPlot1stintron.out
Untracked: code/BothFracDTPlot4stintron.err
Untracked: code/BothFracDTPlot4stintron.out
Untracked: code/BothFracDTPlotGeneRegions.err
Untracked: code/BothFracDTPlotGeneRegions.out
Untracked: code/BothFracDTPlotGeneRegions_norm.err
Untracked: code/BothFracDTPlotGeneRegions_norm.out
Untracked: code/DistPAS2Sig_RandomIntron.py
Untracked: code/EandPqtl.err
Untracked: code/EandPqtl.out
Untracked: code/EncodeRNADTPlotGeneRegions.err
Untracked: code/EncodeRNADTPlotGeneRegions.out
Untracked: code/FC_NucintronPASupandDown.err
Untracked: code/FC_NucintronPASupandDown.out
Untracked: code/FC_UTR.err
Untracked: code/FC_UTR.out
Untracked: code/FC_intronPASupandDown.err
Untracked: code/FC_intronPASupandDown.out
Untracked: code/FC_nascent.err
Untracked: code/FC_nascentout
Untracked: code/FC_newPAS_olddata.err
Untracked: code/FC_newPAS_olddata.out
Untracked: code/HmmPermute.p
Untracked: code/IntronicPASDT.err
Untracked: code/IntronicPASDT.out
Untracked: code/LD_vcftools.hap.out
Untracked: code/NascentDTPlotGeneRegions.err
Untracked: code/NascentDTPlotGeneRegions.out
Untracked: code/NascentDTPlotPAS.err
Untracked: code/NascentDTPlotPAS.out
Untracked: code/NascentDTPlotPAS_3utr.err
Untracked: code/NascentDTPlotPAS_3utr.out
Untracked: code/NascentDTPlotPAS_firstintron.err
Untracked: code/NascentDTPlotPAS_firstintron.out
Untracked: code/NascentDTPlotPAS_intron.err
Untracked: code/NascentDTPlotPAS_intron.out
Untracked: code/NascentDTPlotPAS_nuc.err
Untracked: code/NascentDTPlotPAS_nuc.out
Untracked: code/NascentDTPlotPAS_tot.err
Untracked: code/NascentDTPlotPAS_tot.out
Untracked: code/Nuclear_example.err
Untracked: code/Nuclear_example.out
Untracked: code/PACbioDT.err
Untracked: code/PACbioDT.out
Untracked: code/PACbioDTitronic.err
Untracked: code/PACbioDTitronic.out
Untracked: code/Prematureqtl_nominal.err
Untracked: code/Prematureqtl_nominal.out
Untracked: code/Prematureqtl_permuted.err
Untracked: code/Prematureqtl_permuted.out
Untracked: code/README.md
Untracked: code/RNABam2BW.err
Untracked: code/RNABam2BW.out
Untracked: code/RNAseqDTPlotGeneRegions.err
Untracked: code/RNAseqDTPlotGeneRegions.out
Untracked: code/Rplots.pdf
Untracked: code/TESplots100bp.err
Untracked: code/TESplots100bp.out
Untracked: code/TESplots150bp.err
Untracked: code/TESplots150bp.out
Untracked: code/TESplots200bp.err
Untracked: code/TESplots200bp.out
Untracked: code/Total_example.err
Untracked: code/Total_example.out
Untracked: code/Untitled
Untracked: code/YRI_LCL.vcf.gz
Untracked: code/YRI_LCL_chr1.vcf.gz.log
Untracked: code/YRI_LCL_chr1.vcf.gz.recode.vcf
Untracked: code/annotatedPASregion.err
Untracked: code/annotatedPASregion.out
Untracked: code/apaQTL_nominalInclusive.sh
Untracked: code/assignPeak2Intronicregion.err
Untracked: code/assignPeak2Intronicregion.out
Untracked: code/assigntotPeak2Intronicregion.err
Untracked: code/assigntotPeak2Intronicregion.out
Untracked: code/bam2bw.err
Untracked: code/bam2bw.out
Untracked: code/bam2bw_5primemost.err
Untracked: code/bam2bw_5primemost.out
Untracked: code/binary_fileset.log
Untracked: code/bothFrac_FC.err
Untracked: code/bothFrac_FC.out
Untracked: code/callSHscripts.txt
Untracked: code/closestannotated.err
Untracked: code/closestannotated.out
Untracked: code/closestannotatedbyfrac.err
Untracked: code/closestannotatedbyfrac.out
Untracked: code/dag.pdf
Untracked: code/dagPAS.pdf
Untracked: code/dagfiltPAS.pdf
Untracked: code/fixExandUnexeQTL
Untracked: code/fix_randomIntron.py
Untracked: code/genotypesYRI.gen.proc.keep.vcf.log
Untracked: code/genotypesYRI.gen.proc.keep.vcf.recode.vcf
Untracked: code/getseq100up.err
Untracked: code/getseq100up.out
Untracked: code/grouptranscripts.err
Untracked: code/grouptranscripts.out
Untracked: code/intersectPAS_ssSNPS.err
Untracked: code/intersectPAS_ssSNPS.out
Untracked: code/intersectVCFPAS.err
Untracked: code/intersectVCFPAS.out
Untracked: code/log/
Untracked: code/mergeAnno.err
Untracked: code/mergeAnno.out
Untracked: code/mergeBWnorm.err
Untracked: code/mergeBWnorm.out
Untracked: code/mergeBamNacent.err
Untracked: code/mergeBamNacent.out
Untracked: code/mergeRNAbam.err
Untracked: code/mergeRNAbam.out
Untracked: code/mnaseDTPlot1stintron.err
Untracked: code/mnaseDTPlot1stintron.out
Untracked: code/mnaseDTPlot4thintron.err
Untracked: code/mnaseDTPlot4thintron.out
Untracked: code/netDTPlot4thintron.out
Untracked: code/netseqFC.err
Untracked: code/netseqFC.out
Untracked: code/neyDTPlot4thintron.err
Untracked: code/nucspecinE.py
Untracked: code/plink.log
Untracked: code/prxySNP.err
Untracked: code/prxySNP.out
Untracked: code/pttFacetBoxplots.err
Untracked: code/pttFacetBoxplots.out
Untracked: code/qtlFacetBoxplots.err
Untracked: code/qtlFacetBoxplots.out
Untracked: code/rLD_vcftools.hap.err
Untracked: code/riboqtl.err
Untracked: code/riboqtl.out
Untracked: code/runBestBamID.err
Untracked: code/runCorrectNomeqtl.err
Untracked: code/runCorrectNomeqtl.out
Untracked: code/runFilterLD.err
Untracked: code/runFilterLD.out
Untracked: code/runHMMpermute.err
Untracked: code/runHMMpermute.out
Untracked: code/runHMMpermuteeQTLs.err
Untracked: code/runHMMpermuteeQTLs.out
Untracked: code/runMakeEmpiricaleQTLs.err
Untracked: code/runMakeEmpiricaleQTLs.out
Untracked: code/runMakeEmpiricaleQTLsunex.err
Untracked: code/runMakeEmpiricaleQTLsunex.out
Untracked: code/run_DistPAS2Sig.err
Untracked: code/run_DistPAS2Sig.out
Untracked: code/run_DistPAS2Sig_intron.err
Untracked: code/run_DistPAS2Sig_intron.out
Untracked: code/run_bam2bw.err
Untracked: code/run_bam2bw.out
Untracked: code/run_bam2bwexta.err
Untracked: code/run_bam2bwexta.out
Untracked: code/run_dist2sig_randomintron.sh
Untracked: code/run_getAPAfromanyeQTL.err
Untracked: code/run_getAPAfromanyeQTL.out
Untracked: code/run_getApaPval4eQTLs.err
Untracked: code/run_getApaPval4eQTLs.out
Untracked: code/run_getApaPval4eQTLsunexplained.err
Untracked: code/run_getApaPval4eQTLsunexplained.out
Untracked: code/run_leafcutter_ds.err
Untracked: code/run_leafcutter_ds.out
Untracked: code/run_sepgenobychrom.err
Untracked: code/run_sepgenobychrom.out
Untracked: code/run_sepusage.err
Untracked: code/run_sepusage.out
Untracked: code/run_verifybam.err
Untracked: code/run_verifybam.out
Untracked: code/run_verifybam128N.err
Untracked: code/run_verifybam128N.out
Untracked: code/run_verifybam128T.err
Untracked: code/run_verifybam128T.out
Untracked: code/run_verifybam517N.err
Untracked: code/run_verifybam517N.out
Untracked: code/run_verifybam517T.err
Untracked: code/run_verifybam517T.out
Untracked: code/runprxySNP.err
Untracked: code/runprxySNP.out
Untracked: code/runres2pas.err
Untracked: code/runres2pas.out
Untracked: code/seqQTLfastq.err
Untracked: code/seqQTLfastq.out
Untracked: code/seqQTLregion.err
Untracked: code/seqQTLregion.out
Untracked: code/snakePASlog.out
Untracked: code/snakefiltPASlog.out
Untracked: code/sortindexRNABam.err
Untracked: code/sortindexRNABam.out
Untracked: code/specAPAinE.py
Untracked: code/subsetvcf_SS.err
Untracked: code/subsetvcf_SS.out
Untracked: code/subsetvcf_noSS.err
Untracked: code/subsetvcf_noSS.out
Untracked: code/subsetvcf_pas.err
Untracked: code/subsetvcf_pas.out
Untracked: code/subsetvcf_perm.err
Untracked: code/subsetvcf_perm.out
Untracked: code/subsetvcf_rand.err
Untracked: code/subsetvcf_rand.out
Untracked: code/subtract5UTR.err
Untracked: code/subtract5UTR.out
Untracked: code/subtractExons.err
Untracked: code/subtractExons.out
Untracked: code/tabixSNPs.err
Untracked: code/tabixSNPs.out
Untracked: code/test_verifybam.err
Untracked: code/test_verifybam.out
Untracked: code/vcf_keepsnps.err
Untracked: code/vcf_keepsnps.out
Untracked: code/wrap_verifybam.err
Untracked: code/wrap_verifybam.out
Untracked: code/zipandtabPhen.err
Untracked: code/zipandtabPhen.out
Untracked: data/._.DS_Store
Untracked: data/._MetaDataSequencing.txt
Untracked: data/AnnotatedPAS/
Untracked: data/ApaByEgene/
Untracked: data/ApaByPgene/
Untracked: data/BadLines/
Untracked: data/Battle_pQTL/
Untracked: data/CheckSums/
Untracked: data/CompareOldandNew/
Untracked: data/DTmatrix/
Untracked: data/DiffIso/
Untracked: data/EncodeRNA/
Untracked: data/ExampleQTLPlots/
Untracked: data/ExpressionIndependentapaQTLs.txt
Untracked: data/FlaggedPAS/
Untracked: data/GWAS_overlap/
Untracked: data/GeuvadisRNA/
Untracked: data/HMMqtls/
Untracked: data/Li_eQTLs/
Untracked: data/NascentRNA/
Untracked: data/NucSpeceQTLeffect/
Untracked: data/PAS/
Untracked: data/PAS_postFlag/
Untracked: data/PolyA_DB/
Untracked: data/PreTerm_pheno/
Untracked: data/PrematureQTLNominal/
Untracked: data/PrematureQTLPermuted/
Untracked: data/QTLGenotypes/
Untracked: data/QTLoverlap/
Untracked: data/QTLoverlap_inclusive/
Untracked: data/QTLoverlap_nonNorm/
Untracked: data/README.md
Untracked: data/RNAseq/
Untracked: data/Reads2UTR/
Untracked: data/SNPinSS/
Untracked: data/SignalSiteFiles/
Untracked: data/TF_motifdisruption/
Untracked: data/ThirtyNineIndQtl_nominal/
Untracked: data/Version15bp6As/
Untracked: data/Version15bp7As/
Untracked: data/apaQTLNominal/
Untracked: data/apaQTLNominal_4pc/
Untracked: data/apaQTLNominal_inclusive/
Untracked: data/apaQTLPermuted/
Untracked: data/apaQTLPermuted_4pc/
Untracked: data/apaQTLs/
Untracked: data/assignedPeaks/
Untracked: data/assignedPeaks_15Up/
Untracked: data/bam/
Untracked: data/bam_clean/
Untracked: data/bam_waspfilt/
Untracked: data/bed_10up/
Untracked: data/bed_clean/
Untracked: data/bed_clean_sort/
Untracked: data/bed_waspfilter/
Untracked: data/bedsort_waspfilter/
Untracked: data/bothFrac_FC/
Untracked: data/bw/
Untracked: data/bw_norm/
Untracked: data/eQTLs/
Untracked: data/exampleQTLs/
Untracked: data/fastq/
Untracked: data/filterPeaks/
Untracked: data/fourSU/
Untracked: data/h3k27ac/
Untracked: data/highdiffsiggenes.txt
Untracked: data/inclusivePeaks/
Untracked: data/inclusivePeaks_FC/
Untracked: data/intronRNAratio/
Untracked: data/intron_analysis/
Untracked: data/locusZoom/
Untracked: data/mergedBG/
Untracked: data/mergedBW_byfrac/
Untracked: data/mergedBW_norm/
Untracked: data/mergedBam/
Untracked: data/mergedbyFracBam/
Untracked: data/molPhenos/
Untracked: data/molQTLs/
Untracked: data/motifdistrupt/
Untracked: data/netseq/
Untracked: data/nonNorm_pheno/
Untracked: data/nuc_10up/
Untracked: data/nuc_10upclean/
Untracked: data/oldPASfiles/
Untracked: data/overlapeQTL_try2/
Untracked: data/overlapeQTLs/
Untracked: data/pQTLoverlap/
Untracked: data/pacbio/
Untracked: data/peakCoverage/
Untracked: data/peaks_5perc/
Untracked: data/phenotype/
Untracked: data/phenotype_5perc/
Untracked: data/phenotype_inclusivePAS/
Untracked: data/pttQTL/
Untracked: data/pttQTLplots/
Untracked: data/sigDiffGenes.txt
Untracked: data/sort/
Untracked: data/sort_clean/
Untracked: data/sort_waspfilter/
Untracked: data/twoMech/
Untracked: data/verifyBAM/
Untracked: data/verifyBAM_full/
Untracked: docs/._.DS_Store
Untracked: docs/figure/._.DS_Store
Untracked: docs/figure/PAS_graphs_total.Rmd/._.DS_Store
Untracked: docs/figure/choosePCs.Rmd/._.DS_Store
Untracked: docs/figure/exvunexpeQTL.Rmd/._.DS_Store
Untracked: docs/figure/snpinSS.Rmd/._.DS_Store
Untracked: nohup.out
Untracked: output/._.DS_Store
Untracked: output/._meanCorrelationPhenotypes.svg
Untracked: output/dtPlots/
Untracked: output/fastqc/
Untracked: output/meanCorrelationPhenotypes.svg
Untracked: run_verifybam517N.err
Untracked: run_verifybam517N.out
Unstaged changes:
Modified: analysis/NuclearSpecIncludeNotTested.Rmd
Modified: analysis/PASdescriptiveplots.Rmd
Modified: analysis/Readdistagainstfeatures.Rmd
Modified: analysis/nucSpecinEQTLs.Rmd
Modified: analysis/overlapapaqtlsandeqtls.Rmd
Modified: analysis/pQTLexampleplot.Rmd
Modified: analysis/propeQTLs_explained.Rmd
Modified: analysis/version15bpfilter.Rmd
Modified: code/DistPAS2Sig.py
Modified: code/apaQTLsnake.err
Deleted: code/test.txt
Deleted: docs/figure/signalsiteanalysis.Rmd/figure1bMain-1.pdf
Deleted: reads_graphs.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 197e7c6 | brimittleman | 2019-09-17 | fix misspell on figures |
| html | 3af0253 | brimittleman | 2019-09-17 | Build site. |
| Rmd | f8cb7b8 | brimittleman | 2019-09-17 | move inclusive, get numbers for paper |
| html | 4933ed7 | brimittleman | 2019-09-11 | Build site. |
| Rmd | 6362c16 | brimittleman | 2019-09-11 | upate y axis label |
| html | 74d7b8d | brimittleman | 2019-09-04 | Build site. |
| html | 86dbd94 | brimittleman | 2019-07-31 | Build site. |
| html | 9c38a22 | brimittleman | 2019-07-31 | Build site. |
| Rmd | 589abea | brimittleman | 2019-07-31 | paper figs |
| html | 1a928ed | brimittleman | 2019-07-17 | Build site. |
| Rmd | 07e766f | brimittleman | 2019-07-17 | 3 color signal stie plot |
| html | 16e4212 | brimittleman | 2019-07-17 | Build site. |
| Rmd | 64bcc48 | brimittleman | 2019-07-17 | fix plots meeting 7.15 |
| html | 96d85de | brimittleman | 2019-07-07 | Build site. |
| Rmd | 4a51b04 | brimittleman | 2019-07-07 | modify figures |
| html | 6b0fbce | brimittleman | 2019-06-21 | Build site. |
| Rmd | d5ec3ba | brimittleman | 2019-06-21 | change colors |
| html | bfa47ca | brimittleman | 2019-06-18 | Build site. |
| Rmd | 01f1f1d | brimittleman | 2019-06-18 | write out sites with ss |
| html | 9c1dd66 | brimittleman | 2019-06-13 | Build site. |
| html | ef7ddfe | brimittleman | 2019-04-27 | Build site. |
| Rmd | 29464ab | brimittleman | 2019-04-27 | flip axis |
| html | 4eb21ab | brimittleman | 2019-04-26 | Build site. |
| Rmd | fb7b995 | brimittleman | 2019-04-26 | add proportion with site analysis |
| html | 799dd25 | brimittleman | 2019-04-24 | Build site. |
| html | ccebe33 | brimittleman | 2019-04-24 | Build site. |
| Rmd | 4febc15 | brimittleman | 2019-04-24 | return to original SAF |
| html | 627fc45 | brimittleman | 2019-04-24 | Build site. |
| html | dd07ef7 | brimittleman | 2019-04-24 | Build site. |
| Rmd | 6dc25d8 | brimittleman | 2019-04-24 | update after SAF bug |
| html | 26058a5 | brimittleman | 2019-04-24 | Build site. |
| Rmd | 76900e4 | brimittleman | 2019-04-24 | add plots after parsing for 1 site per |
| html | 12d1cb0 | brimittleman | 2019-04-23 | Build site. |
| Rmd | e985ecd | brimittleman | 2019-04-23 | add initial plot |
| html | 214c05c | brimittleman | 2019-04-23 | Build site. |
| Rmd | 27b11e3 | brimittleman | 2019-04-23 | start signal site analysis |
library(seqLogo)Loading required package: gridlibrary(tidyverse)── Attaching packages ────────────────────────────────────────────────────────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.1.1 ✔ purrr 0.3.2
✔ tibble 2.1.1 ✔ dplyr 0.8.0.1
✔ tidyr 0.8.3 ✔ stringr 1.3.1
✔ readr 1.3.1 ✔ forcats 0.3.0 ── Conflicts ───────────────────────────────────────────────────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()library(RColorBrewer)
library(workflowr)This is workflowr version 1.4.0
Run ?workflowr for help getting startedlibrary(reshape2)
Attaching package: 'reshape2'The following object is masked from 'package:tidyr':
smithslibrary(gplots)
Attaching package: 'gplots'The following object is masked from 'package:stats':
lowesslibrary(cowplot)
Attaching package: 'cowplot'The following object is masked from 'package:ggplot2':
ggsaveIn this analysis I will plot the distribution of signal sites upstream of the PAS I have found.
First I use a python script to make a bed file with the 100 base pairs upsream of the PAS:
module load Anaconda3
source activate three-prime-env
mkdir ../data/SignalSiteFiles
python Upstream100Bases_general.py ../data/PAS/APAPAS_GeneLocAnno.5perc.bed ../data/SignalSiteFiles/APAPAS_100up.bedNow I use bedtools nuc to get the sequence for each of these regions:
sbatch getSeq100up.sh I can now run the DistPAS2Sig.py which will give me the location for the signal site for each PAS.I am running this with the 12 most common PAS signal sites.
sbatch run_distPAS2Sig.shUpload all of the results:
Loc_AATAAA= read.table("../data/SignalSiteFiles/Loc_AATAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATAAA")
Loc_AAAAAG= read.table("../data/SignalSiteFiles/Loc_AAAAAG_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AAAAAG")
Loc_AATACA= read.table("../data/SignalSiteFiles/Loc_AATACA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATACA")
Loc_AATAGA= read.table("../data/SignalSiteFiles/Loc_AATAGA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATAGA")
Loc_AATATA= read.table("../data/SignalSiteFiles/Loc_AATATA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATATA")
Loc_ACTAAA= read.table("../data/SignalSiteFiles/Loc_ACTAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="ACTAAA")
Loc_AGTAAA= read.table("../data/SignalSiteFiles/Loc_AGTAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AGTAAA")
Loc_ATTAAA= read.table("../data/SignalSiteFiles/Loc_ATTAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="ATTAAA")
Loc_CATAAA= read.table("../data/SignalSiteFiles/Loc_CATAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="CATAAA")
Loc_GATAAA= read.table("../data/SignalSiteFiles/Loc_GATAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="GATAAA")
Loc_TATAAA= read.table("../data/SignalSiteFiles/Loc_TATAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="TATAAA")
Loc_AAAAAA= read.table("../data/SignalSiteFiles/Loc_AAAAAA_Distance2end.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AAAAAA")Join these together:
AllsiteDF=as.data.frame(rbind(Loc_AATAAA,Loc_AAAAAG,Loc_AATACA,Loc_AATAGA,Loc_AATATA,Loc_ACTAAA,Loc_AGTAAA,Loc_ATTAAA, Loc_GATAAA,Loc_TATAAA,Loc_CATAAA, Loc_AAAAAA))colourCount = length(unique(AllsiteDF$Site))
getPalette = colorRampPalette(brewer.pal(8, "Set1"))AllsiteDF_sep = AllsiteDF %>% separate(PAS, int=c("GenePeak", "Location"), sep="_")
ggplot(AllsiteDF_sep, aes(x=Distance2PAS, by=Site, col=Site)) + stat_ecdf() + facet_wrap(~Location)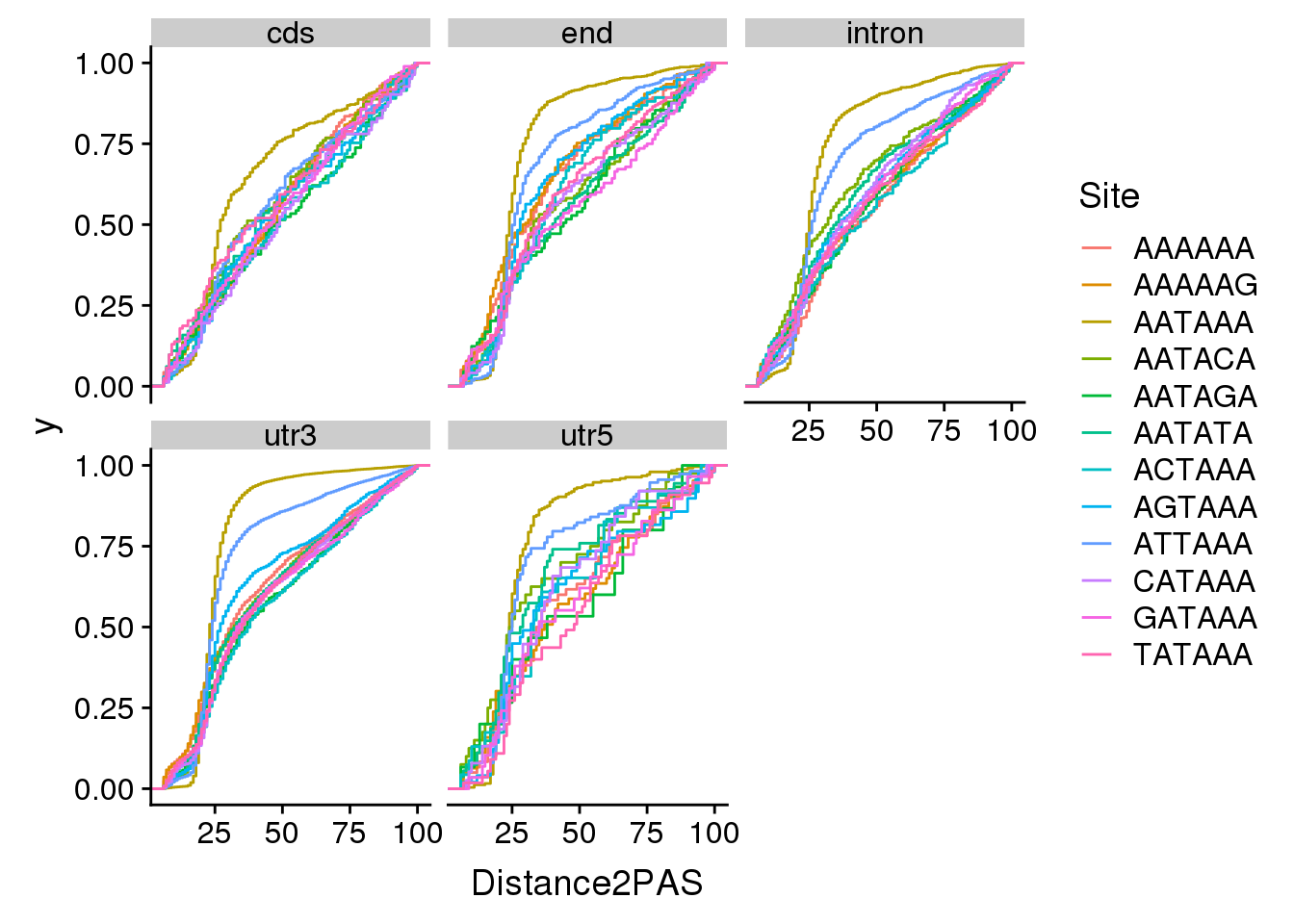
Check to see if any PAS have more than one signal site detected:
AllsiteDFmultsites=AllsiteDF %>% group_by(PAS) %>% mutate(nSites=n()) %>% filter(nSites>1)First take the perfect match within 50 bp then use the closest.
Write out the AllSite in order to use it in the chooseSignalSite.py script:
write.table(AllsiteDF, file="../data/SignalSiteFiles/AllSignalSite.txt", quote=F, col.names = F, row.names = F, sep="\t")python chooseSignalSite.py ../data/SignalSiteFiles/AllSignalSite.txt ../data/SignalSiteFiles/AllSignalSite_1perPAS.txtAllsiteDF_1per=read.table(file="../data/SignalSiteFiles/AllSignalSite_1perPAS.txt", col.names = colnames(AllsiteDF)) %>% mutate(NegCount=-1*as.integer(as.character(Distance2PAS)))Plot
dist2signalsiteplot=ggplot(AllsiteDF_1per, aes(group=Site, x=NegCount, fill=Site)) + geom_histogram(position="stack",bins=50 ) + labs(x="Distance from PAS", y="N annotated Sites", title="Location of annotated signal sites") + scale_fill_manual(values = getPalette(colourCount))
dist2signalsiteplot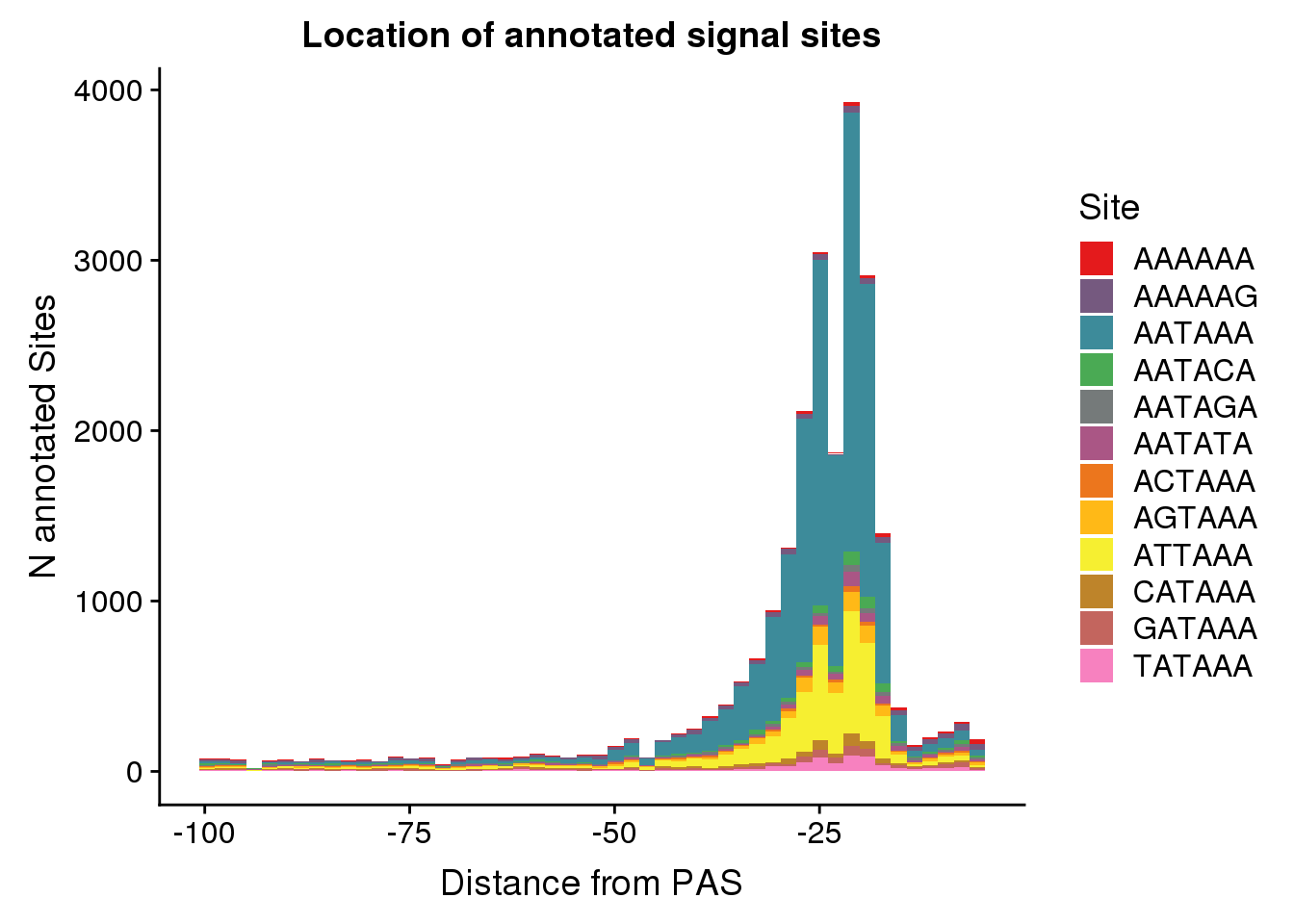
ggsave(dist2signalsiteplot, file="../output/SignalSitePlot.png")Saving 7 x 5 in imagePlot with proportion:
allPAS=read.table("../data/PAS/APAPAS_GeneLocAnno.5perc.bed", stringsAsFactors = F, col.names = c("chr","start","end","PAS","score","strand"))
AllsiteDF_1per_prop= AllsiteDF_1per %>% group_by(Site,NegCount) %>% summarise(CountperPos=n()) %>% mutate(TotCount=sum(CountperPos),prop=CountperPos/nrow(allPAS))Plot with prop:
dist2signalsiteplotprop=ggplot(AllsiteDF_1per_prop, aes(group=Site, x=NegCount,y=prop, fill=Site)) + geom_histogram(position="stack",bins=50,stat="identity" ) + labs(x="Distance from PAS", y="Proportion of annotated Sites", title="Location of annotated signal sites",fill="Site Sequence") + scale_fill_manual(values = getPalette(colourCount))+ theme(text = element_text(size=20, face="bold"),axis.text.x = element_text(size = 16),axis.text.y = element_text(size = 16), legend.position = "bottom",plot.title = element_text(size=22))+ guides(fill=guide_legend(nrow=3,byrow=TRUE))Warning: Ignoring unknown parameters: binwidth, bins, paddist2signalsiteplotprop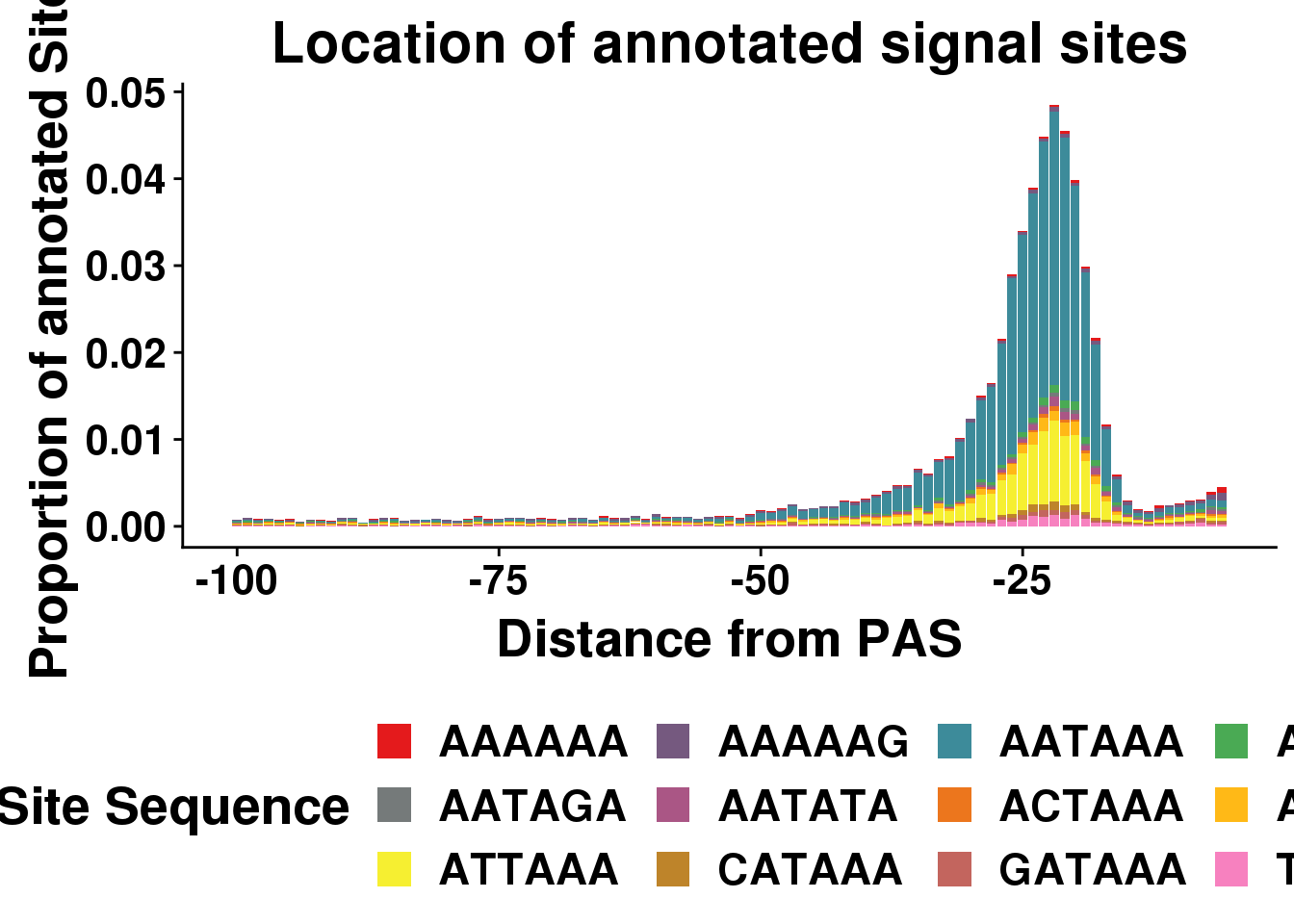
nrow(AllsiteDF_1per)/nrow(allPAS) [1] 0.5718249Seperate by location:
AllsiteDF_1per_sep= AllsiteDF_1per %>%separate(PAS, int=c("GenePeak", "Location"), sep="_")dist2signalsiteplot_byloc=ggplot(AllsiteDF_1per_sep, aes(group=Site, x=NegCount, fill=Site)) + geom_histogram(position="stack",bins=50 ) + labs(x="Distance from PAS", y="N annotated Sites", title="Location of annotated signal sites") + facet_wrap(~Location)+ scale_fill_manual(values = getPalette(colourCount))
dist2signalsiteplot_byloc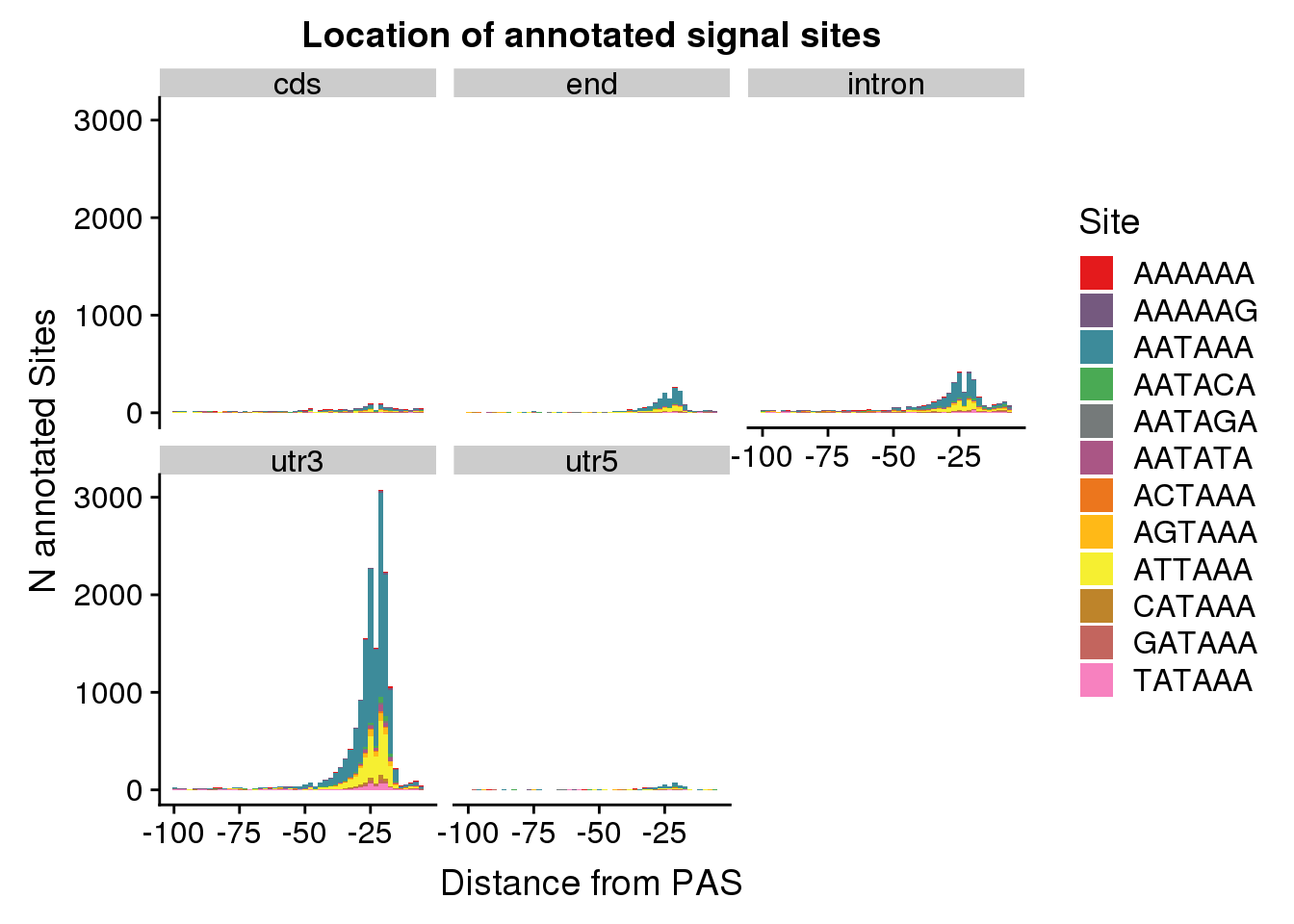
ggsave(dist2signalsiteplot_byloc, file="../output/SignalSitePlotbyLoc.png")Saving 7 x 5 in imageProportion:
allPAS_byloc=allPAS %>% separate(PAS,into=c("peakid", "loc"),sep="_") %>% group_by(loc) %>% summarise(nLoc=n())
allPAS_byloc_new=as.data.frame(allPAS_byloc$nLoc %>% t())
colnames(allPAS_byloc_new) = allPAS_byloc$loc
AllsiteDF_1per_sep_INTRON=AllsiteDF_1per_sep %>% filter(Location=="intron") %>% group_by(Site,NegCount) %>% summarise(CountperPos=n()) %>% mutate(TotCount=sum(CountperPos),prop=CountperPos/(allPAS_byloc_new$intron)) %>% mutate(Cononical=ifelse(Site=="AATAAA", "AATAAA", ifelse(Site=="ATTAAA", "AATTAA", "Other")))
ggplot(AllsiteDF_1per_sep_INTRON, aes(group=Cononical, x=NegCount,y=prop, fill=Cononical)) + geom_histogram(position="stack",bins=5, stat="identity" ) + labs(x="Distance from PAS", y="Proportion of annotated Sites", title="Location of annotated signal sites \nfor Intronic PAS", caption = "Other: AAAAAA, AAAAAG, AATACA, AATAGA,AATATA, ACTAAA, AGTAAA,CATAAA, GATAAA,TATAAA") + scale_fill_manual(values = getPalette(colourCount))+ theme(text = element_text(size=20, face="bold"),axis.text.x = element_text(size = 16), legend.position = "bottom", axis.text.y = element_text(size = 16),plot.title = element_text(size=22),plot.caption = element_text(hjust = 0,size=10))Warning: Ignoring unknown parameters: binwidth, bins, pad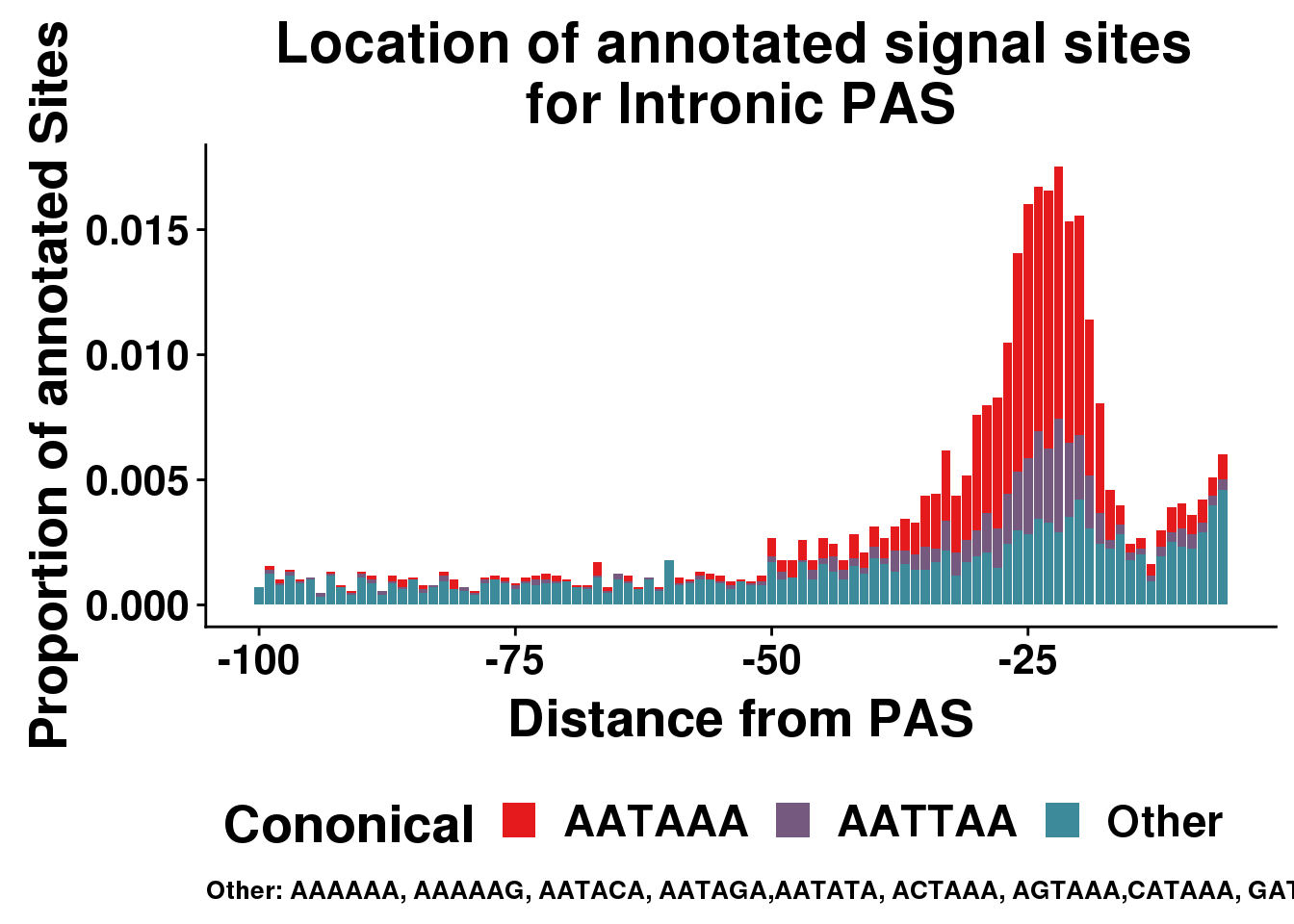
AllsiteDF_1per_sep_UTR=AllsiteDF_1per_sep %>% filter(Location=="utr3") %>% group_by(Site,NegCount) %>% summarise(CountperPos=n()) %>% mutate(TotCount=sum(CountperPos),prop=CountperPos/(allPAS_byloc_new$intron)) %>% mutate(Cononical=ifelse(Site=="AATAAA", "AATAAA", ifelse(Site=="ATTAAA", "AATTAA", "Other")))
ggplot(AllsiteDF_1per_sep_UTR, aes(group=Cononical, x=NegCount,y=prop, fill=Cononical)) + geom_histogram(position="stack", stat="identity" ) + labs(x="Distance from PAS", y="Proportion of annotated Sites", title="Location of annotated signal sites \nin UTR",caption = "Other: AAAAAA, AAAAAG, AATACA, AATAGA,AATATA, ACTAAA, AGTAAA,CATAAA, GATAAA,TATAAA") + scale_fill_manual(values = getPalette(colourCount)) + theme(text = element_text(size=20, face="bold"),axis.text.x = element_text(size = 16), legend.position = "bottom", axis.text.y = element_text(size = 16),plot.title = element_text(size=22),plot.caption = element_text(hjust = 0,size=10))+ guides(fill=guide_legend(nrow=1,byrow=TRUE))Warning: Ignoring unknown parameters: binwidth, bins, pad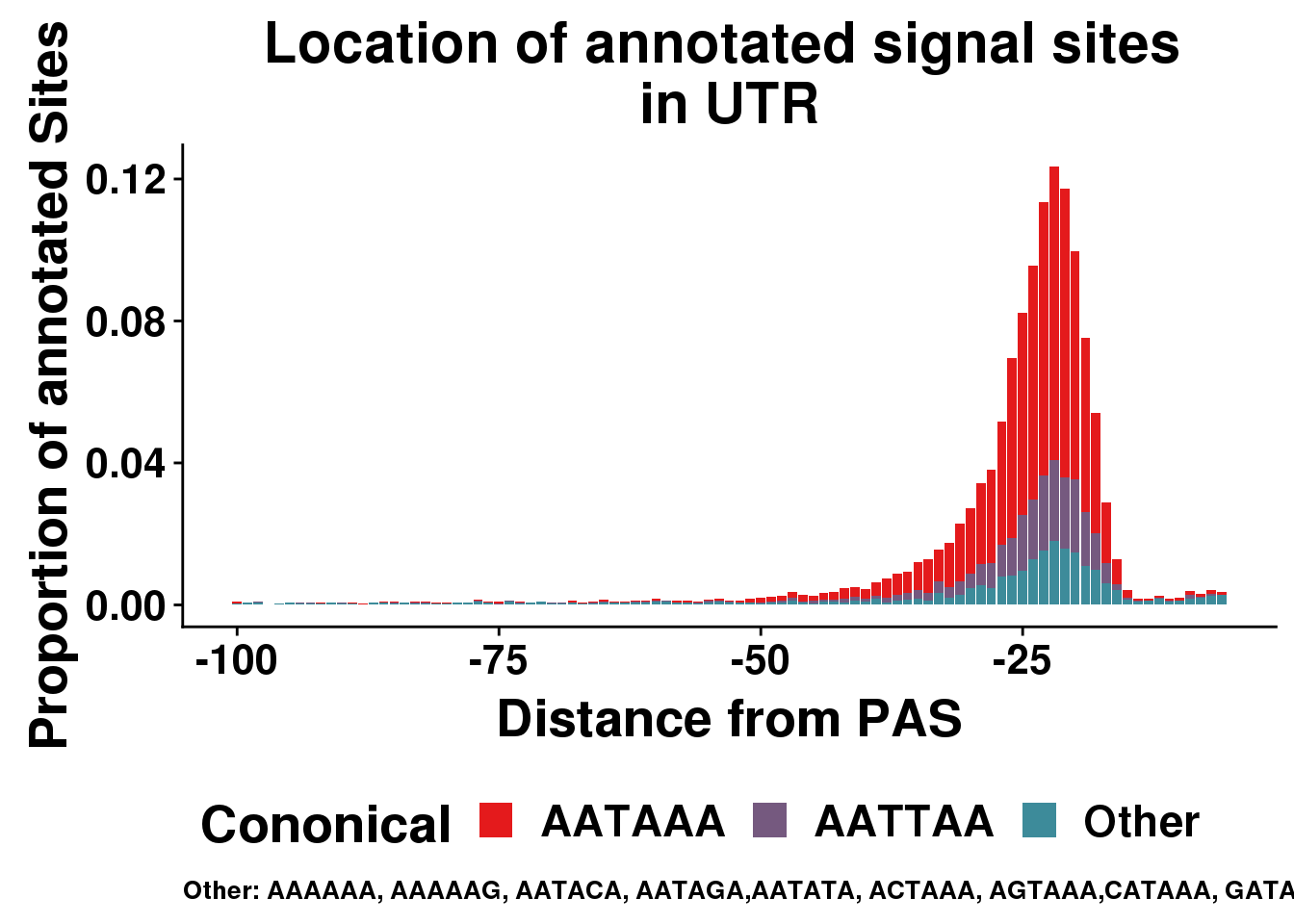
Propwith=c(nrow(AllsiteDF_1per_sep %>% filter(Location=="intron"))/allPAS_byloc_new$intron,nrow(AllsiteDF_1per_sep %>% filter(Location=="utr3"))/allPAS_byloc_new$utr3,nrow(AllsiteDF_1per_sep %>% filter(Location=="utr5"))/allPAS_byloc_new$utr5,nrow(AllsiteDF_1per_sep %>% filter(Location=="cds"))/allPAS_byloc_new$cds,nrow(AllsiteDF_1per_sep %>% filter(Location=="end"))/allPAS_byloc_new$end)
Locations=c("intron", "utr3", "utr5", "Coding", "Downstream")
propdf=as.data.frame(cbind(Location=Locations,Proportion=Propwith))
propdf$Proportion=as.numeric(as.character(propdf$Proportion))
all=ggplot(propdf,aes(x=Location, y=Proportion, fill=Location)) + geom_bar(stat="identity") + theme(axis.text.x = element_text(angle = 90, hjust = 1))
all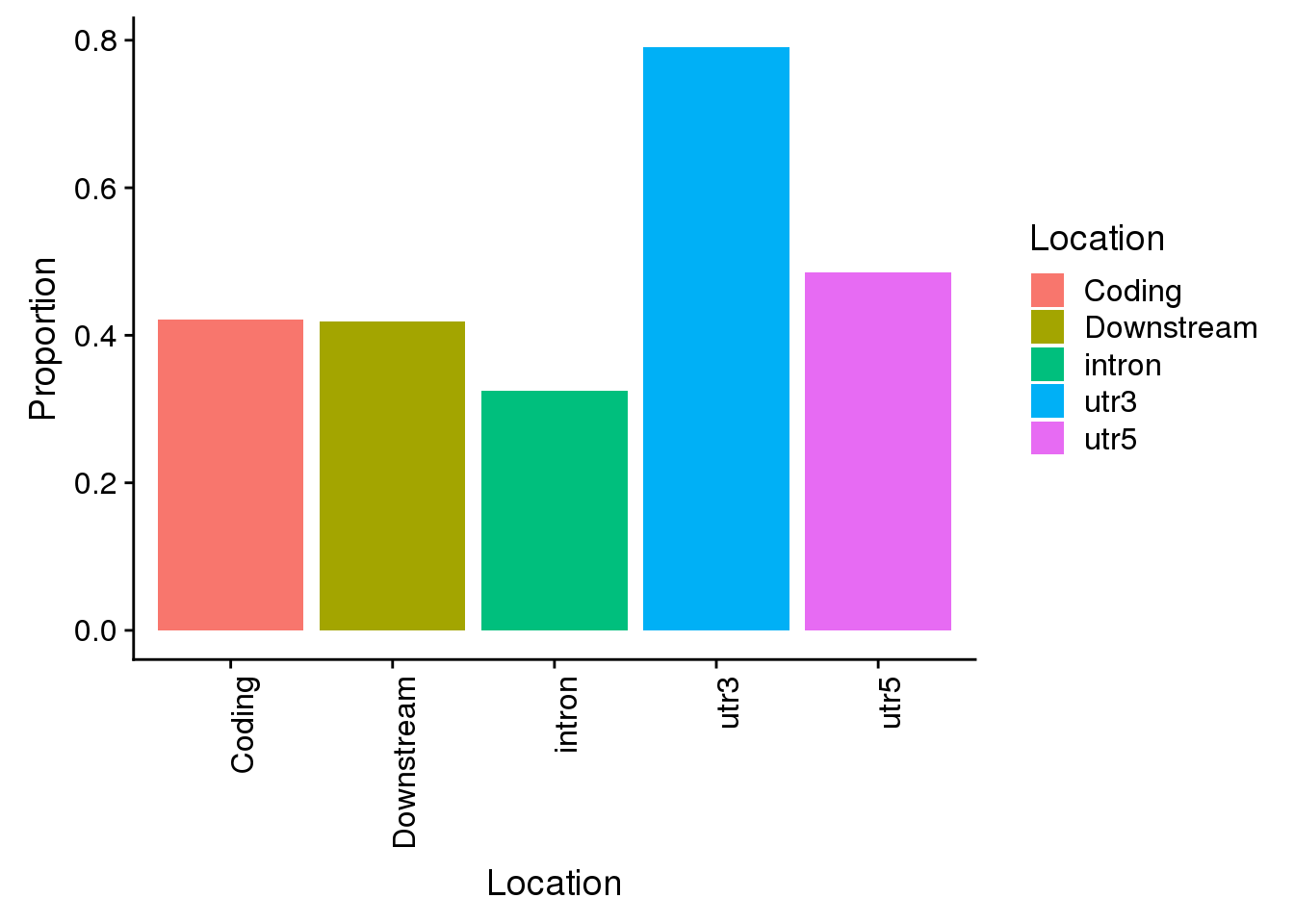
AllsiteDF_1per_sep_noncon=AllsiteDF_1per_sep %>% filter(Site != "AATAAA")
Propwithnotcon=c(nrow(AllsiteDF_1per_sep_noncon %>% filter(Location=="intron"))/allPAS_byloc_new$intron,nrow(AllsiteDF_1per_sep_noncon %>% filter(Location=="utr3"))/allPAS_byloc_new$utr3,nrow(AllsiteDF_1per_sep_noncon %>% filter(Location=="utr5"))/allPAS_byloc_new$utr5,nrow(AllsiteDF_1per_sep_noncon %>% filter(Location=="cds"))/allPAS_byloc_new$cds,nrow(AllsiteDF_1per_sep_noncon %>% filter(Location=="end"))/allPAS_byloc_new$end)
Locations=c("intron", "utr3", "utr5", "Coding", "Downstream")
propdf_noncon=as.data.frame(cbind(Location=Locations,Proportion=Propwithnotcon))
propdf_noncon$Proportion=as.numeric(as.character(propdf_noncon$Proportion))
non=ggplot(propdf_noncon,aes(x=Location, y=Proportion, fill=Location)) + geom_bar(stat="identity")+theme(axis.text.x = element_text(angle = 90, hjust = 1))
non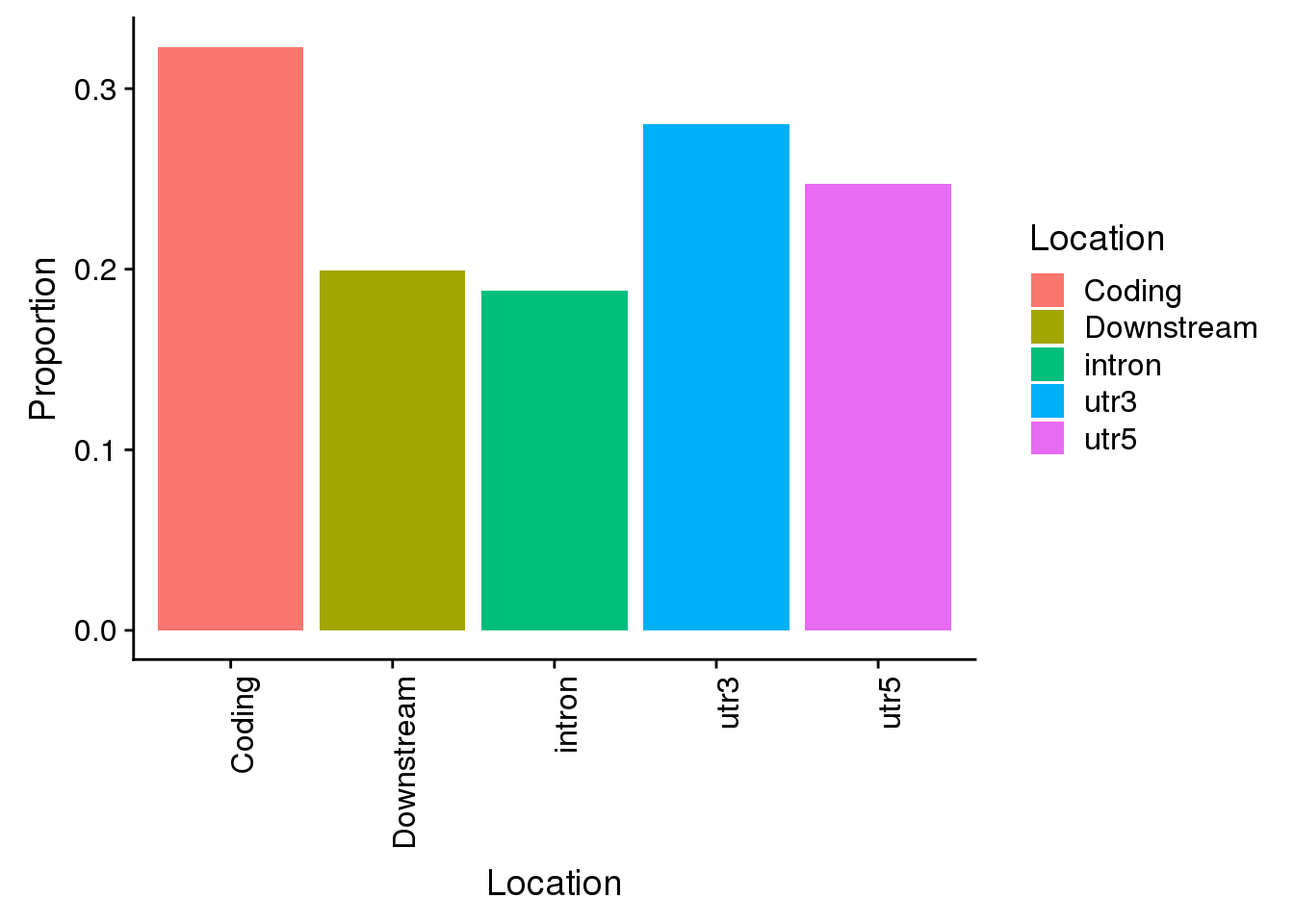
AllsiteDF_1per_sep_con=AllsiteDF_1per_sep %>% filter(Site == "AATAAA")
Propwithcon=c(nrow(AllsiteDF_1per_sep_con %>% filter(Location=="intron"))/allPAS_byloc_new$intron,nrow(AllsiteDF_1per_sep_con %>% filter(Location=="utr3"))/allPAS_byloc_new$utr3,nrow(AllsiteDF_1per_sep_con %>% filter(Location=="utr5"))/allPAS_byloc_new$utr5,nrow(AllsiteDF_1per_sep_con %>% filter(Location=="cds"))/allPAS_byloc_new$cds,nrow(AllsiteDF_1per_sep_con %>% filter(Location=="end"))/allPAS_byloc_new$end)
Locations=c("intron", "utr3", "utr5", "Coding", "Downstream")
propdf_con=as.data.frame(cbind(Location=Locations,Proportion=Propwithcon))
propdf_con$Proportion=as.numeric(as.character(propdf_con$Proportion))
con=ggplot(propdf_con,aes(x=Location, y=Proportion, fill=Location)) + geom_bar(stat="identity")+ theme(axis.text.x = element_text(angle = 90, hjust = 1))
con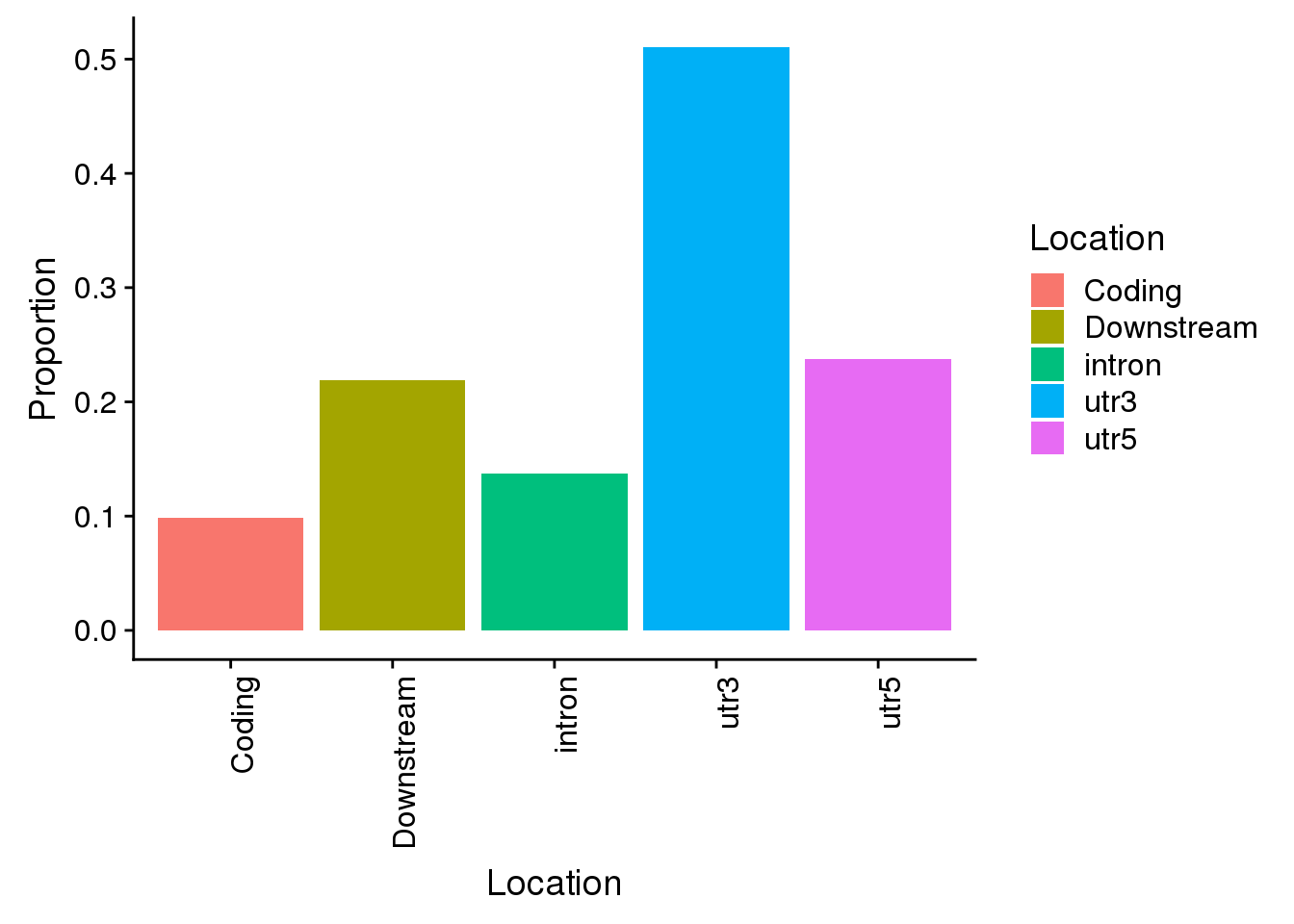
plot_grid(all, con, non, labels=c("All PAS", "Cononical PAS", "Non-conical PAS"))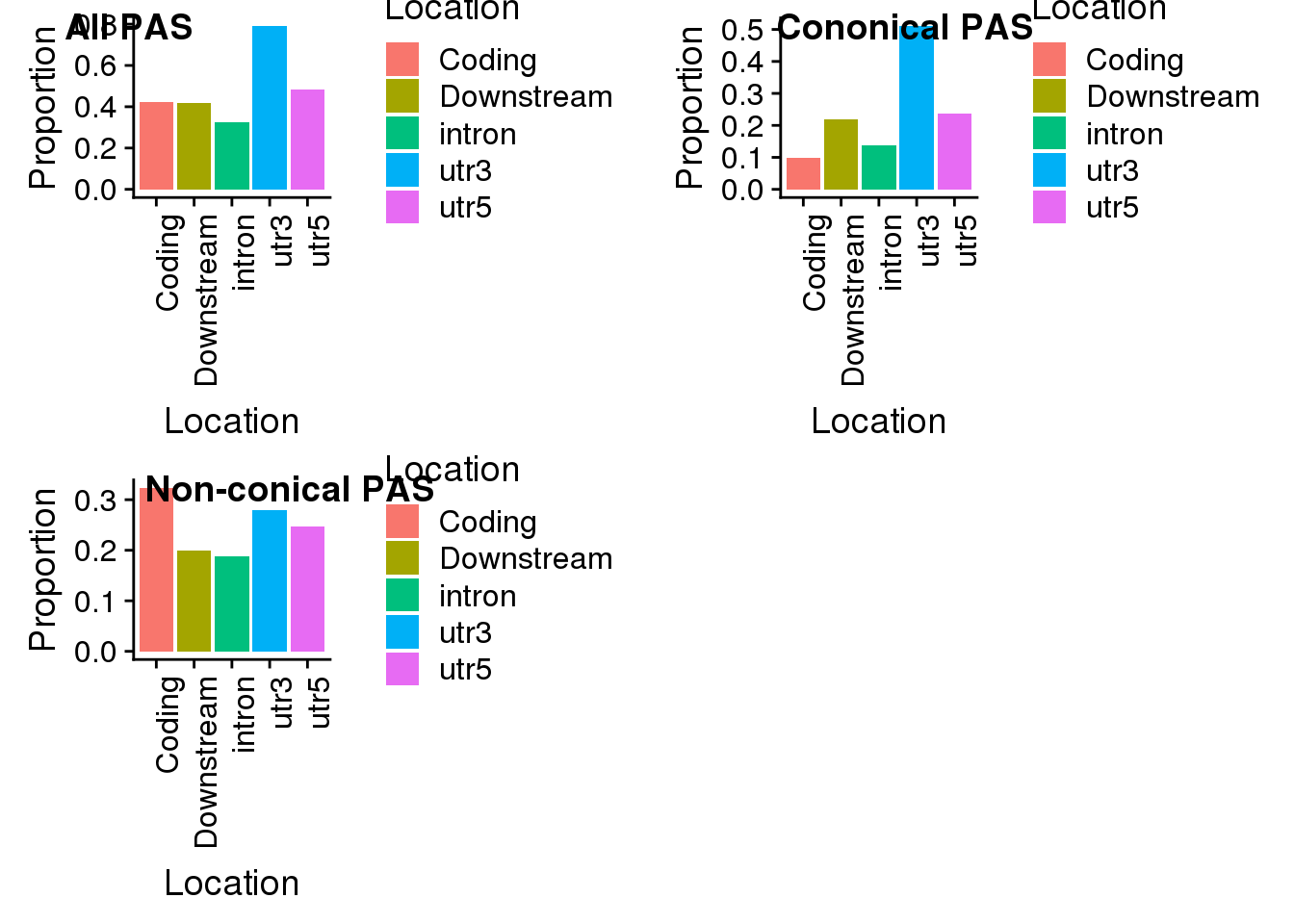
Strong evidence PAS
For future analysis I want to have a set of PAS with evidence for a signal site. I want those signal sites upstream 10-50 basepairs.
AllsiteDF_1per_use= AllsiteDF_1per %>% filter(Distance2PAS>10, Distance2PAS<50) %>% separate(PAS,into=c("peakid", "loc"),sep="_") %>% separate(peakid,into=c("Peaknum", "gene"),sep=":") %>% mutate(PAS=paste("peak", Peaknum, sep="")) %>% dplyr::rename("UpstreamDist"=NegCount) %>% select(PAS, gene, loc, Site, UpstreamDist)ggplot(AllsiteDF_1per_use, aes(x=loc)) + geom_histogram(stat="count")Warning: Ignoring unknown parameters: binwidth, bins, pad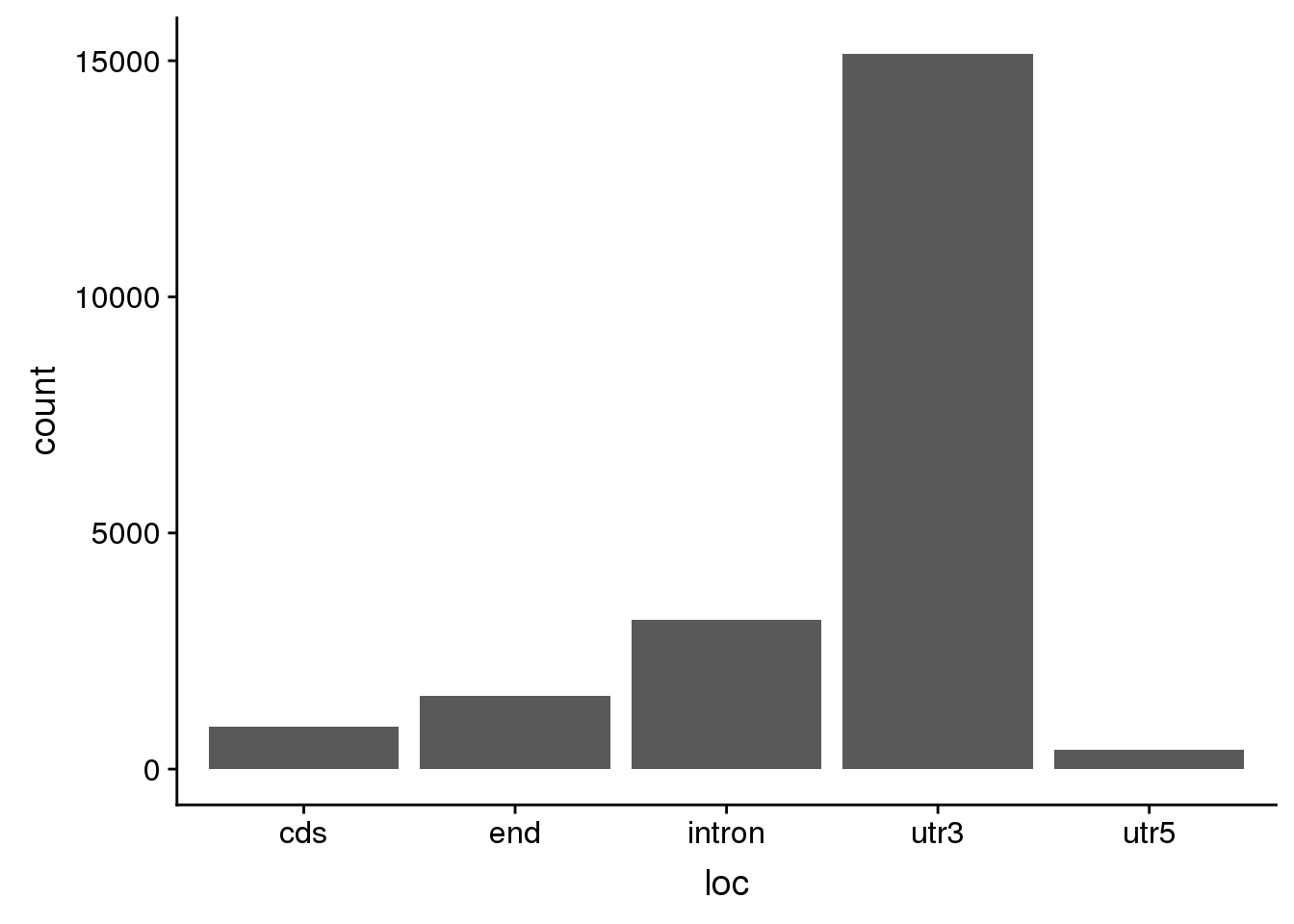
Write these out for
write.table(AllsiteDF_1per_use, file="../data/PAS/PASwSignalSite.txt", col.names = T, row.names = F, quote = F, sep="\t")Plot color cononical vs non cononical:
AllsiteDF_1per_prop_col= AllsiteDF_1per_prop %>% mutate(Cononical=ifelse(Site=="AATAAA", "AATAAA", ifelse(Site=="ATTAAA", "AATTAA", "Other")))
dist2signalsiteplotprop=ggplot(AllsiteDF_1per_prop_col, aes(group=Cononical, x=NegCount,y=prop, fill=Cononical)) + geom_histogram(position="stack",bins=50,stat="identity" ) + labs(x="Distance from PAS", y="Proportion of annotated Sites", title="Location of annotated signal sites",fill=" ", caption = "Other: AAAAAA, AAAAAG, AATACA, AATAGA,AATATA, ACTAAA, AGTAAA,CATAAA, GATAAA,TATAAA") + theme(text = element_text(size=20, face="bold"),axis.text.x = element_text(size = 16), legend.position = "bottom", axis.text.y = element_text(size = 16),plot.title = element_text(size=22),plot.caption = element_text(hjust = 0,size=10))+ guides(fill=guide_legend(nrow=1,byrow=TRUE)) + scale_fill_manual(values = getPalette(colourCount))Warning: Ignoring unknown parameters: binwidth, bins, paddist2signalsiteplotprop 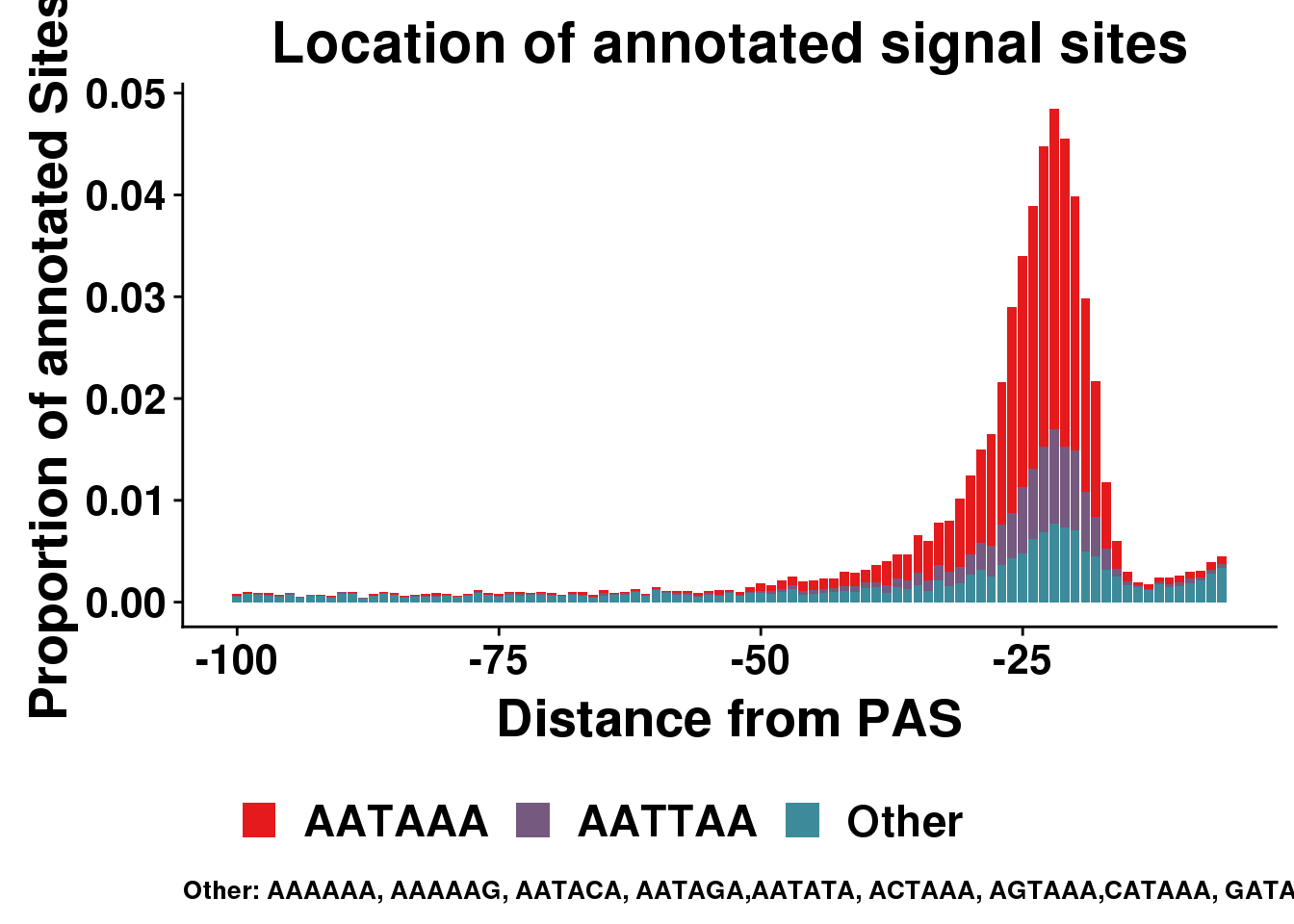
#scale_fill_discrete(name="Site", labels=c("AATAAA","ATTAAA", "AAAAAA, AAAAAG, AATACA, AATAGA, AATATA, ACTAAA, AGTAAA,CATAAA, GATAAA,TATAAA")Enrichment in intron
N intronic that have a PAS:
propwSS_intron=AllsiteDF_1per_use %>% filter(loc=="intron") %>% nrow()
#nuclear PAS
intronPAS=read.table("../data/PAS/APAPAS_GeneLocAnno.5perc.bed", col.names = c("chr","start","end", "id", "score", "strand")) %>% separate(id, into=c("pas", "loc"), sep="_") %>% filter(loc=="intron") %>% nrow()
withSS_intron=propwSS_intron/intronPAS
withSS_intron[1] 0.2477136intronannotation=read.table("/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_intron.sort.bed", col.names = c("chr", "start", "end", "loc", "gene", "score", "strand"))%>% mutate(name=paste(gene, loc, strand, sep="_")) %>% select(chr, start, end, name, score, strand)
write.table(intronannotation, "/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_intronNamed.sort.bed", col.names = F, row.names = F,quote = F, sep="\t")Compare this to a background:
I need 40 basepair regions in introns.
bedtools makewindows -i src -b /project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_intronNamed.sort.bed -w 40 > /project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_intron.sort_randomIntervals.bedMake this into a bed file (with strand):
python fix_randomIntron.pyI need to get the sequences for these with bedtools nuc.
bedtools nuc -s -seq -fi /project2/gilad/briana/genome_anotation_data/genome/Homo_sapiens.GRCh37.75.dna_sm.all.fa -bed /project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_intron.sort_randomIntervals.fixed.bed > /project2/gilad/briana/apaQTL/data/SignalSiteFiles/ncbiRefSeq_intron.sort_randomIntervalsSeq.bedsbatch run_dist2sig_randomintron.shUpload all of the results:
Loc_AATAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_AATAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATAAA")
Loc_AAAAAG_randomIntron= read.table("../data/SignalSiteFiles/Loc_AAAAAG_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AAAAAG")
Loc_AATACA_randomIntron= read.table("../data/SignalSiteFiles/Loc_AATACA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATACA")
Loc_AATAGA_randomIntron= read.table("../data/SignalSiteFiles/Loc_AATAGA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATAGA")
Loc_AATATA_randomIntron= read.table("../data/SignalSiteFiles/Loc_AATATA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AATATA")
Loc_ACTAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_ACTAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="ACTAAA")
Loc_AGTAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_AGTAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AGTAAA")
Loc_ATTAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_ATTAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="ATTAAA")
Loc_CATAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_CATAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="CATAAA")
Loc_GATAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_GATAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="GATAAA")
Loc_TATAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_TATAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="TATAAA")
Loc_AAAAAA_randomIntron= read.table("../data/SignalSiteFiles/Loc_AAAAAA_Distance2end_randomIntron.txt", header=F, col.names =c( "PAS", "Distance2PAS")) %>% mutate(Site="AAAAAA")Join these together:
AllsiteDF_randomIntron=as.data.frame(rbind(Loc_AATAAA_randomIntron,Loc_AAAAAG_randomIntron,Loc_AATACA_randomIntron,Loc_AATAGA_randomIntron,Loc_AATATA_randomIntron,Loc_ACTAAA_randomIntron,Loc_AGTAAA_randomIntron,Loc_ATTAAA_randomIntron, Loc_GATAAA_randomIntron,Loc_TATAAA_randomIntron,Loc_CATAAA_randomIntron, Loc_AAAAAA_randomIntron))Number of tested sites:
withSS_random=nrow(AllsiteDF_randomIntron)
possiblereg=84432042
propwithRandom=withSS_random/possiblereg
propwithRandom[1] 0.002426792Difference in prop test:
prop.test(x=c(withSS_random,propwSS_intron), n=c(possiblereg,intronPAS),alternative = "less" )
2-sample test for equality of proportions with continuity
correction
data: c(withSS_random, propwSS_intron) out of c(possiblereg, intronPAS)
X-squared = 313010, df = 1, p-value < 2.2e-16
alternative hypothesis: less
95 percent confidence interval:
-1.0000000 -0.2389699
sample estimates:
prop 1 prop 2
0.002426792 0.247713593
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] grid stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] cowplot_0.9.4 gplots_3.0.1 reshape2_1.4.3
[4] workflowr_1.4.0 RColorBrewer_1.1-2 forcats_0.3.0
[7] stringr_1.3.1 dplyr_0.8.0.1 purrr_0.3.2
[10] readr_1.3.1 tidyr_0.8.3 tibble_2.1.1
[13] ggplot2_3.1.1 tidyverse_1.2.1 seqLogo_1.48.0
loaded via a namespace (and not attached):
[1] gtools_3.8.1 tidyselect_0.2.5 haven_1.1.2
[4] lattice_0.20-38 colorspace_1.3-2 generics_0.0.2
[7] htmltools_0.3.6 stats4_3.5.1 yaml_2.2.0
[10] rlang_0.4.0 pillar_1.3.1 glue_1.3.0
[13] withr_2.1.2 modelr_0.1.2 readxl_1.1.0
[16] plyr_1.8.4 munsell_0.5.0 gtable_0.2.0
[19] cellranger_1.1.0 rvest_0.3.2 caTools_1.17.1.1
[22] evaluate_0.12 labeling_0.3 knitr_1.20
[25] highr_0.7 broom_0.5.1 Rcpp_1.0.2
[28] KernSmooth_2.23-15 backports_1.1.2 scales_1.0.0
[31] gdata_2.18.0 jsonlite_1.6 fs_1.3.1
[34] hms_0.4.2 digest_0.6.18 stringi_1.2.4
[37] rprojroot_1.3-2 bitops_1.0-6 cli_1.1.0
[40] tools_3.5.1 magrittr_1.5 lazyeval_0.2.1
[43] crayon_1.3.4 whisker_0.3-2 pkgconfig_2.0.2
[46] xml2_1.2.0 lubridate_1.7.4 assertthat_0.2.0
[49] rmarkdown_1.10 httr_1.3.1 rstudioapi_0.10
[52] R6_2.3.0 nlme_3.1-137 git2r_0.25.2
[55] compiler_3.5.1