Location of Signal Site
Briana Mittleman
3/6/2019
Last updated: 2019-03-07
Checks: 6 0
Knit directory: threeprimeseq/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.2.0). The Report tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(12345) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: data/.DS_Store
Ignored: data/perm_QTL_trans_noMP_5percov/
Ignored: output/.DS_Store
Untracked files:
Untracked: KalistoAbundance18486.txt
Untracked: analysis/4suDataIGV.Rmd
Untracked: analysis/DirectionapaQTL.Rmd
Untracked: analysis/EmpDistforOverlaps.Rmd
Untracked: analysis/EvaleQTLs.Rmd
Untracked: analysis/YL_QTL_test.Rmd
Untracked: analysis/groSeqAnalysis.Rmd
Untracked: analysis/ncbiRefSeq_sm.sort.mRNA.bed
Untracked: analysis/snake.config.notes.Rmd
Untracked: analysis/verifyBAM.Rmd
Untracked: analysis/verifybam_dubs.Rmd
Untracked: code/PeaksToCoverPerReads.py
Untracked: code/strober_pc_pve_heatmap_func.R
Untracked: data/18486.genecov.txt
Untracked: data/APApeaksYL.total.inbrain.bed
Untracked: data/AllPeak_counts/
Untracked: data/ApaQTLs/
Untracked: data/ApaQTLs_otherPhen/
Untracked: data/ChromHmmOverlap/
Untracked: data/DistTXN2Peak_genelocAnno/
Untracked: data/FeatureoverlapPeaks/
Untracked: data/GM12878.chromHMM.bed
Untracked: data/GM12878.chromHMM.txt
Untracked: data/LianoglouLCL/
Untracked: data/LocusZoom/
Untracked: data/LocusZoom_Unexp/
Untracked: data/LocusZoom_proc/
Untracked: data/MatchedSnps/
Untracked: data/NuclearApaQTLs.txt
Untracked: data/PeakCounts/
Untracked: data/PeakCounts_noMP_5perc/
Untracked: data/PeakCounts_noMP_genelocanno/
Untracked: data/PeakUsage/
Untracked: data/PeakUsage_noMP/
Untracked: data/PeakUsage_noMP_GeneLocAnno/
Untracked: data/PeaksUsed/
Untracked: data/PeaksUsed_noMP_5percCov/
Untracked: data/PolyA_DB/
Untracked: data/QTL_overlap/
Untracked: data/RNAkalisto/
Untracked: data/RefSeq_annotations/
Untracked: data/Replicates_usage/
Untracked: data/Signal_Loc/
Untracked: data/TotalApaQTLs.txt
Untracked: data/Totalpeaks_filtered_clean.bed
Untracked: data/UnderstandPeaksQC/
Untracked: data/WASP_STAT/
Untracked: data/YL-SP-18486-T-combined-genecov.txt
Untracked: data/YL-SP-18486-T_S9_R1_001-genecov.txt
Untracked: data/YL_QTL_test/
Untracked: data/apaExamp/
Untracked: data/apaExamp_proc/
Untracked: data/apaQTL_examp_noMP/
Untracked: data/bedgraph_peaks/
Untracked: data/bin200.5.T.nuccov.bed
Untracked: data/bin200.Anuccov.bed
Untracked: data/bin200.nuccov.bed
Untracked: data/clean_peaks/
Untracked: data/comb_map_stats.csv
Untracked: data/comb_map_stats.xlsx
Untracked: data/comb_map_stats_39ind.csv
Untracked: data/combined_reads_mapped_three_prime_seq.csv
Untracked: data/diff_iso_GeneLocAnno/
Untracked: data/diff_iso_proc/
Untracked: data/diff_iso_trans/
Untracked: data/eQTLs_Lietal/
Untracked: data/ensemble_to_genename.txt
Untracked: data/example_gene_peakQuant/
Untracked: data/explainProtVar/
Untracked: data/filtPeakOppstrand_cov_noMP_GeneLocAnno_5perc/
Untracked: data/filtered_APApeaks_merged_allchrom_refseqTrans.closest2End.bed
Untracked: data/filtered_APApeaks_merged_allchrom_refseqTrans.closest2End.noties.bed
Untracked: data/first50lines_closest.txt
Untracked: data/gencov.test.csv
Untracked: data/gencov.test.txt
Untracked: data/gencov_zero.test.csv
Untracked: data/gencov_zero.test.txt
Untracked: data/gene_cov/
Untracked: data/joined
Untracked: data/leafcutter/
Untracked: data/merged_combined_YL-SP-threeprimeseq.bg
Untracked: data/molPheno_noMP/
Untracked: data/mol_overlap/
Untracked: data/mol_pheno/
Untracked: data/nom_QTL/
Untracked: data/nom_QTL_opp/
Untracked: data/nom_QTL_trans/
Untracked: data/nuc6up/
Untracked: data/nuc_10up/
Untracked: data/other_qtls/
Untracked: data/pQTL_otherphen/
Untracked: data/pacbio_cov/
Untracked: data/peakPerRefSeqGene/
Untracked: data/peaks4DT/
Untracked: data/perm_QTL/
Untracked: data/perm_QTL_GeneLocAnno_noMP_5percov/
Untracked: data/perm_QTL_GeneLocAnno_noMP_5percov_3UTR/
Untracked: data/perm_QTL_diffWindow/
Untracked: data/perm_QTL_opp/
Untracked: data/perm_QTL_trans/
Untracked: data/perm_QTL_trans_filt/
Untracked: data/protAndAPAAndExplmRes.Rda
Untracked: data/protAndAPAlmRes.Rda
Untracked: data/protAndExpressionlmRes.Rda
Untracked: data/reads_mapped_three_prime_seq.csv
Untracked: data/smash.cov.results.bed
Untracked: data/smash.cov.results.csv
Untracked: data/smash.cov.results.txt
Untracked: data/smash_testregion/
Untracked: data/ssFC200.cov.bed
Untracked: data/temp.file1
Untracked: data/temp.file2
Untracked: data/temp.gencov.test.txt
Untracked: data/temp.gencov_zero.test.txt
Untracked: data/threePrimeSeqMetaData.csv
Untracked: data/threePrimeSeqMetaData55Ind.txt
Untracked: data/threePrimeSeqMetaData55Ind.xlsx
Untracked: data/threePrimeSeqMetaData55Ind_noDup.txt
Untracked: data/threePrimeSeqMetaData55Ind_noDup.xlsx
Untracked: data/threePrimeSeqMetaData55Ind_noDup_WASPMAP.txt
Untracked: data/threePrimeSeqMetaData55Ind_noDup_WASPMAP.xlsx
Untracked: output/LZ/
Untracked: output/deeptools_plots/
Untracked: output/picard/
Untracked: output/plots/
Untracked: output/qual.fig2.pdf
Unstaged changes:
Modified: analysis/28ind.peak.explore.Rmd
Modified: analysis/CompareLianoglouData.Rmd
Modified: analysis/NewPeakPostMP.Rmd
Modified: analysis/apaQTLoverlapGWAS.Rmd
Modified: analysis/cleanupdtseq.internalpriming.Rmd
Modified: analysis/coloc_apaQTLs_protQTLs.Rmd
Modified: analysis/dif.iso.usage.leafcutter.Rmd
Modified: analysis/diff_iso_pipeline.Rmd
Modified: analysis/explainpQTLs.Rmd
Modified: analysis/explore.filters.Rmd
Modified: analysis/fixBWChromNames.Rmd
Modified: analysis/flash2mash.Rmd
Modified: analysis/mispriming_approach.Rmd
Modified: analysis/overlapMolQTL.Rmd
Modified: analysis/overlapMolQTL.opposite.Rmd
Modified: analysis/overlap_qtls.Rmd
Modified: analysis/peakOverlap_oppstrand.Rmd
Modified: analysis/peakQCPPlots.Rmd
Modified: analysis/pheno.leaf.comb.Rmd
Modified: analysis/pipeline_55Ind.Rmd
Modified: analysis/swarmPlots_QTLs.Rmd
Modified: analysis/test.max2.Rmd
Modified: analysis/test.smash.Rmd
Modified: analysis/understandPeaks.Rmd
Modified: analysis/unexplainedeQTL_analysis.Rmd
Modified: code/Snakefile
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 72c34ce | Briana Mittleman | 2019-03-07 | signal site loc based on front side |
| html | 4bf5d09 | Briana Mittleman | 2019-03-06 | Build site. |
| Rmd | 8717550 | Briana Mittleman | 2019-03-06 | res for AATAAA |
| html | 4023fe0 | Briana Mittleman | 2019-03-06 | Build site. |
| Rmd | d561190 | Briana Mittleman | 2019-03-06 | analysis up to getting seqs |
| html | ba63ea2 | Briana Mittleman | 2019-03-06 | Build site. |
| Rmd | c200503 | Briana Mittleman | 2019-03-06 | add signal site loc analysis |
In the Signal Site enrichment analysis I looked at the peaks to see if signal sites are enriched upstream of my peaks. I found this is true but now I want to see where the signal sites are in comparison to my peaks. I am going to use the biostrings package tool matchPWM for this analysis.
library(workflowr)This is workflowr version 1.2.0
Run ?workflowr for help getting startedlibrary(tidyverse)── Attaching packages ───────────────────────────────────────────────────────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.1.0 ✔ purrr 0.3.1
✔ tibble 2.0.1 ✔ dplyr 0.8.0.1
✔ tidyr 0.8.3 ✔ stringr 1.4.0
✔ readr 1.3.1 ✔ forcats 0.4.0 Warning: package 'tibble' was built under R version 3.5.2Warning: package 'tidyr' was built under R version 3.5.2Warning: package 'purrr' was built under R version 3.5.2Warning: package 'dplyr' was built under R version 3.5.2Warning: package 'stringr' was built under R version 3.5.2Warning: package 'forcats' was built under R version 3.5.2── Conflicts ──────────────────────────────────────────────────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()library(Biostrings)Warning: package 'Biostrings' was built under R version 3.5.2Loading required package: BiocGenericsLoading required package: parallel
Attaching package: 'BiocGenerics'The following objects are masked from 'package:parallel':
clusterApply, clusterApplyLB, clusterCall, clusterEvalQ,
clusterExport, clusterMap, parApply, parCapply, parLapply,
parLapplyLB, parRapply, parSapply, parSapplyLBThe following objects are masked from 'package:dplyr':
combine, intersect, setdiff, unionThe following objects are masked from 'package:stats':
IQR, mad, sd, var, xtabsThe following objects are masked from 'package:base':
anyDuplicated, append, as.data.frame, basename, cbind,
colMeans, colnames, colSums, dirname, do.call, duplicated,
eval, evalq, Filter, Find, get, grep, grepl, intersect,
is.unsorted, lapply, lengths, Map, mapply, match, mget, order,
paste, pmax, pmax.int, pmin, pmin.int, Position, rank, rbind,
Reduce, rowMeans, rownames, rowSums, sapply, setdiff, sort,
table, tapply, union, unique, unsplit, which, which.max,
which.minLoading required package: S4VectorsLoading required package: stats4
Attaching package: 'S4Vectors'The following objects are masked from 'package:dplyr':
first, renameThe following object is masked from 'package:tidyr':
expandThe following object is masked from 'package:base':
expand.gridLoading required package: IRanges
Attaching package: 'IRanges'The following objects are masked from 'package:dplyr':
collapse, desc, sliceThe following object is masked from 'package:purrr':
reduceLoading required package: XVector
Attaching package: 'XVector'The following object is masked from 'package:purrr':
compact
Attaching package: 'Biostrings'The following object is masked from 'package:base':
strsplitlibrary(BSgenome)Loading required package: GenomeInfoDbWarning: package 'GenomeInfoDb' was built under R version 3.5.2Loading required package: GenomicRangesLoading required package: rtracklayerWarning: package 'rtracklayer' was built under R version 3.5.2library(genomation)Loading required package: gridMake location file
I need to get the coordinates for the regions I care about. I want to look at the peak and 150bp upstream. This is probably larger than I will need to look at but it will be good to have an inclusive look first.
I want to use the peak file and make a file that is the peak and upstream 150:
Upstream150Bases.py
#python
def main(Fin, Fout):
outBed=open(Fout, "w")
chrom_lengths=open("/project2/gilad/briana/genome_anotation_data/chrom_lengths2.sort.bed","r")
#make a dictionary with chrom lengths
length_dic={}
for i in chrom_lengths:
chrom, start, end = i.split()
length_dic[str(chrom)]=int(end)
#write file
for ln in open(Fin):
chrom, start, end, name, score, strand = ln.split()
chrom=str(chrom)
if strand=="+":
start_new=int(start)-150
if start_new <= 1:
start_new = 0
end_new= int(end)
if end_new == 0:
end_new=1
outBed.write("%s\t%d\t%d\t%s\t%s\t%s\n"%(chrom, start_new, end_new, name, score, strand))
if strand == "-":
start_new=int(start)
end_new=int(end) + 150
outBed.write("%s\t%d\t%d\t%s\t%s\t%s\n"%(chrom, start_new, end_new, name, score, strand))
outBed.close()
if __name__ == "__main__":
import sys
inFile = sys.argv[1]
outFile=sys.argv[2]
main(inFile, outFile)run_get150up.sh
#!/bin/bash
#SBATCH --job-name=run_get150up
#SBATCH --account=pi-yangili1
#SBATCH --time=36:00:00
#SBATCH --output=run_get150upt.out
#SBATCH --error=run_get150up.err
#SBATCH --partition=broadwl
#SBATCH --mem=16G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
python Upstream150Bases.py /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand.bed /project2/gilad/briana/threeprimeseq/data/Signal_Loc/APAPeaks_5percCov_fixedStrand_peakand150up.bed Get subject (reads)
Input the regions:
Fix chromosomes:
PeakRegions=read.table("../data/Signal_Loc/APAPeaks_5percCov_fixedStrand_peakand150up.bed", header=F,col.names = c("chr","start", "end", "peak", "score", "strand")) %>% mutate(Chrom=paste("chr", chr, sep="")) %>% select(Chrom, start,end,peak,score,strand)
write.table(PeakRegions, file="../data/Signal_Loc/APAPeaks_5percCov_fixedStrand_peakand150up_fixedChr.bed", quote=F, col.names = F, row.names = F, sep="\t")#convert to reads
reads.GR= readGeneric(file="../data/Signal_Loc/APAPeaks_5percCov_fixedStrand_peakand150up_fixedChr.bed",chr =1, start = 2, end =3, meta.cols =4, header=F, zero.based=TRUE,strand=6)I need to overlap these positions with the genome
Make motifs
AATAAA= PWM("AATAAA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
ATTAAA= PWM("ATTAAA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
AGTAAA= PWM("AGTAAA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
TATAAA= PWM("TATAAA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
CATAAA= PWM("CATAAA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
GATAAA= PWM("GATAAA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
AATATA= PWM("AATATA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
AATACA= PWM("AATACA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
AATAGA= PWM("AATAGA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
AAAAAG= PWM("AAAAAG", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))
ACTAAA= PWM("ACTAAA", type = c("log2probratio", "prob"), prior.params = c(A=0.25, C=0.25, G=0.25, T=0.25))find the mathes
genome.hg19 <- getBSgenome("BSgenome.Hsapiens.UCSC.hg19")
#matches <- matchPWM(pwm=AATAAA, subject = genome.hg19) %>% keepStandardChromosomes(., species= "Homo sapiens")
DNAstringSetPeaks=data.frame(seq=getSeq(genome.hg19, reads.GR))
x=DNAString(DNAstringSetPeaks[1,1])
hits <- matchPWM(AATAAA,x,with.score=T)
start(hits)[1] 215 219 255Look over and make hits file for all
list_AATAAA_res=c()
for (i in 1:nrow(DNAstringSetPeaks)){
x=DNAString(DNAstringSetPeaks[i,1])
list_AATAAA_res=c(list_AATAAA_res,matchPWM(AATAAA,x,with.score=T))
}Get out the start positions:
starts_AATAAA=c()
nsig=c()
last_oc_AATAAA=c()
for (i in list_AATAAA_res){
nsig=c(nsig, length(start(i)))
starts_AATAAA=c(starts_AATAAA, start(i))
#print(length(start(i)))
if (length(start(i)) != 0 ){
last_oc_AATAAA=c(last_oc_AATAAA, max(start(i),na.rm =T))
}
}Histogram of results:
summary(starts_AATAAA) Min. 1st Qu. Median Mean 3rd Qu. Max.
1.0 79.0 175.0 156.7 216.0 1612.0 hist(starts_AATAAA,breaks=10000)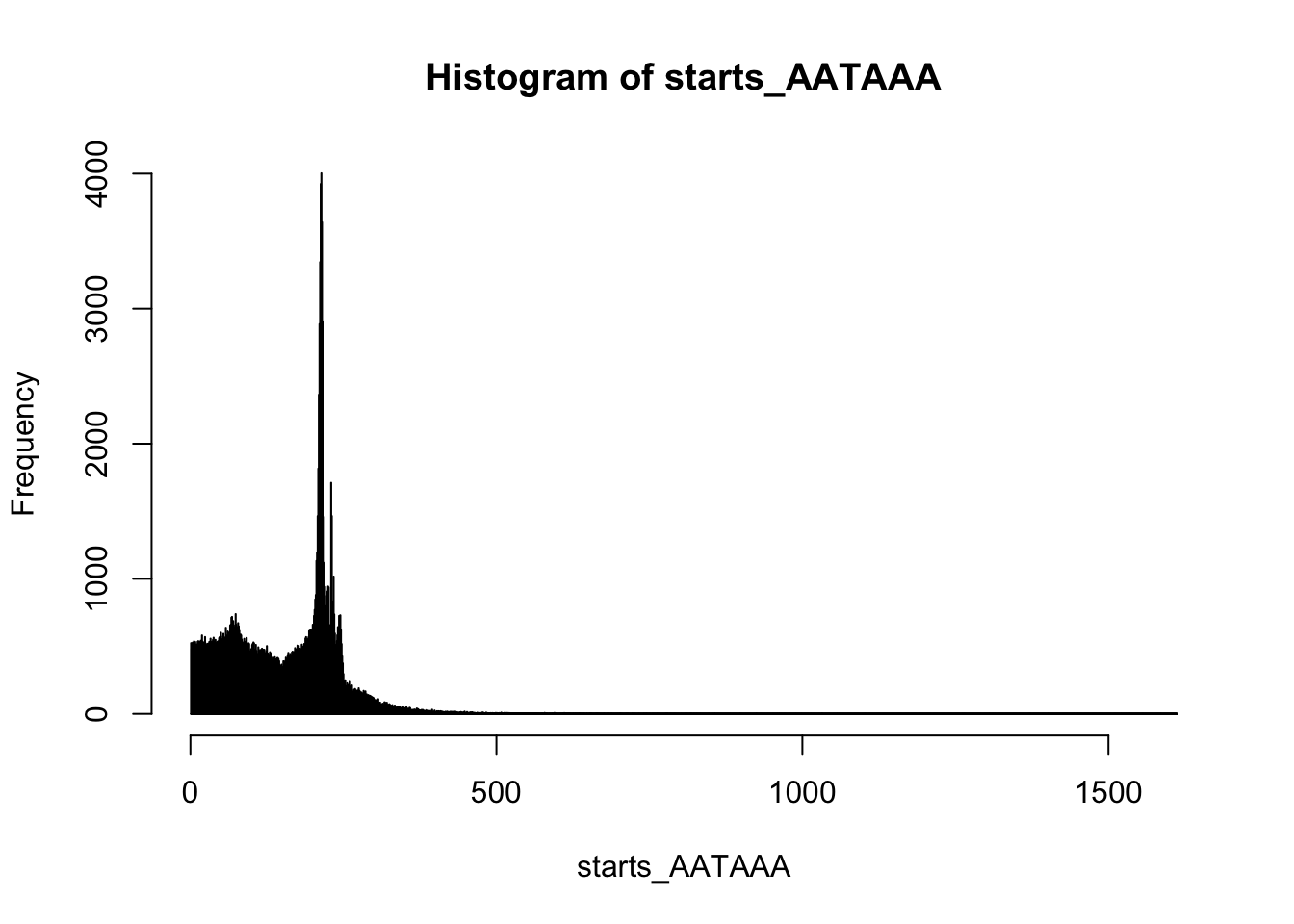
| Version | Author | Date |
|---|---|---|
| 4bf5d09 | Briana Mittleman | 2019-03-06 |
summary(nsig) Min. 1st Qu. Median Mean 3rd Qu. Max.
0.000 1.000 3.000 3.857 5.000 74.000 sum(nsig==0)[1] 3584sum(nsig==1)[1] 8358hist(nsig,breaks=100)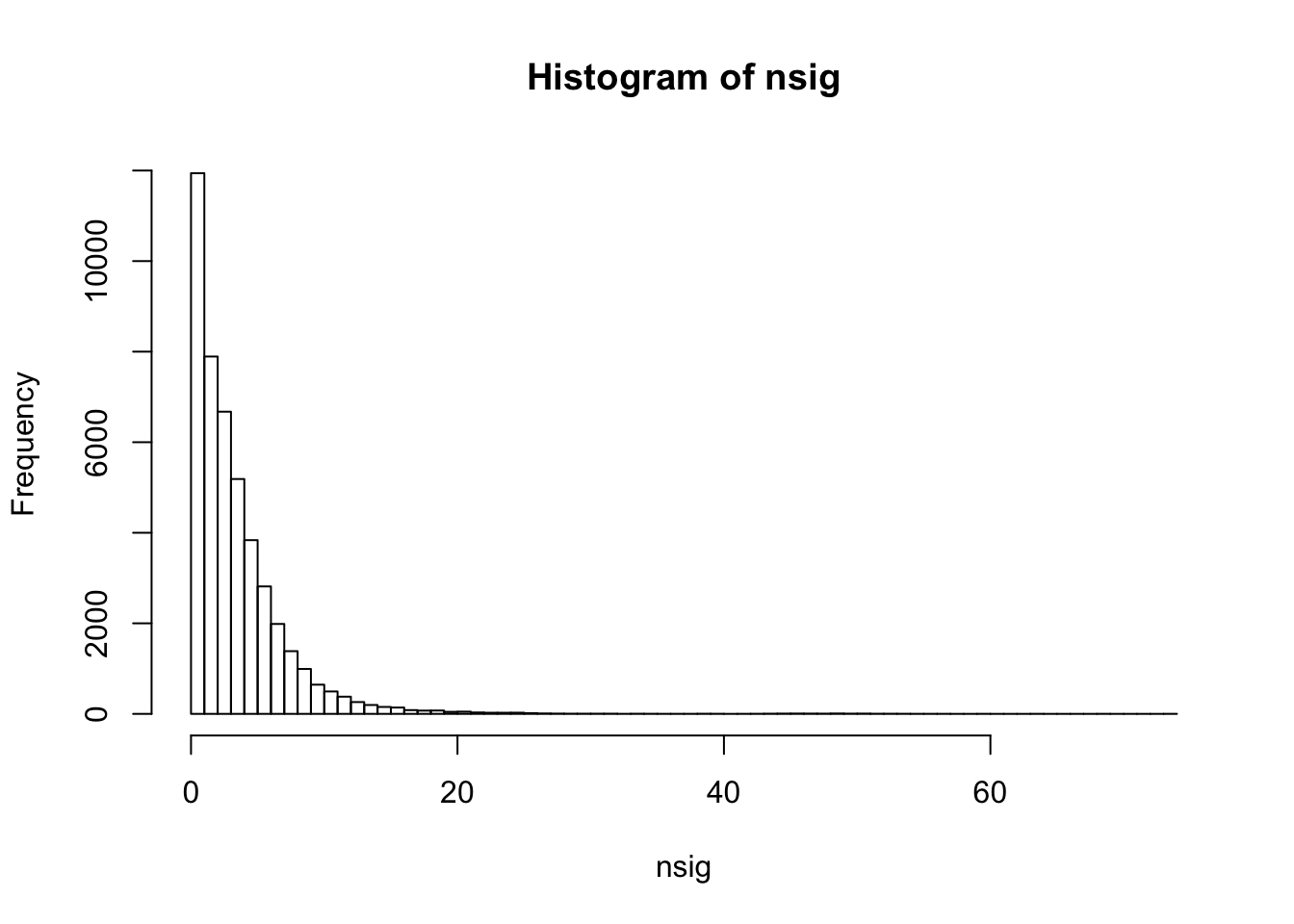
| Version | Author | Date |
|---|---|---|
| 4bf5d09 | Briana Mittleman | 2019-03-06 |
Look at the first occurence:
summary(last_oc_AATAAA) Min. 1st Qu. Median Mean 3rd Qu. Max.
1.0 209.0 215.0 213.1 231.0 1612.0 hist(last_oc_AATAAA,breaks=1000)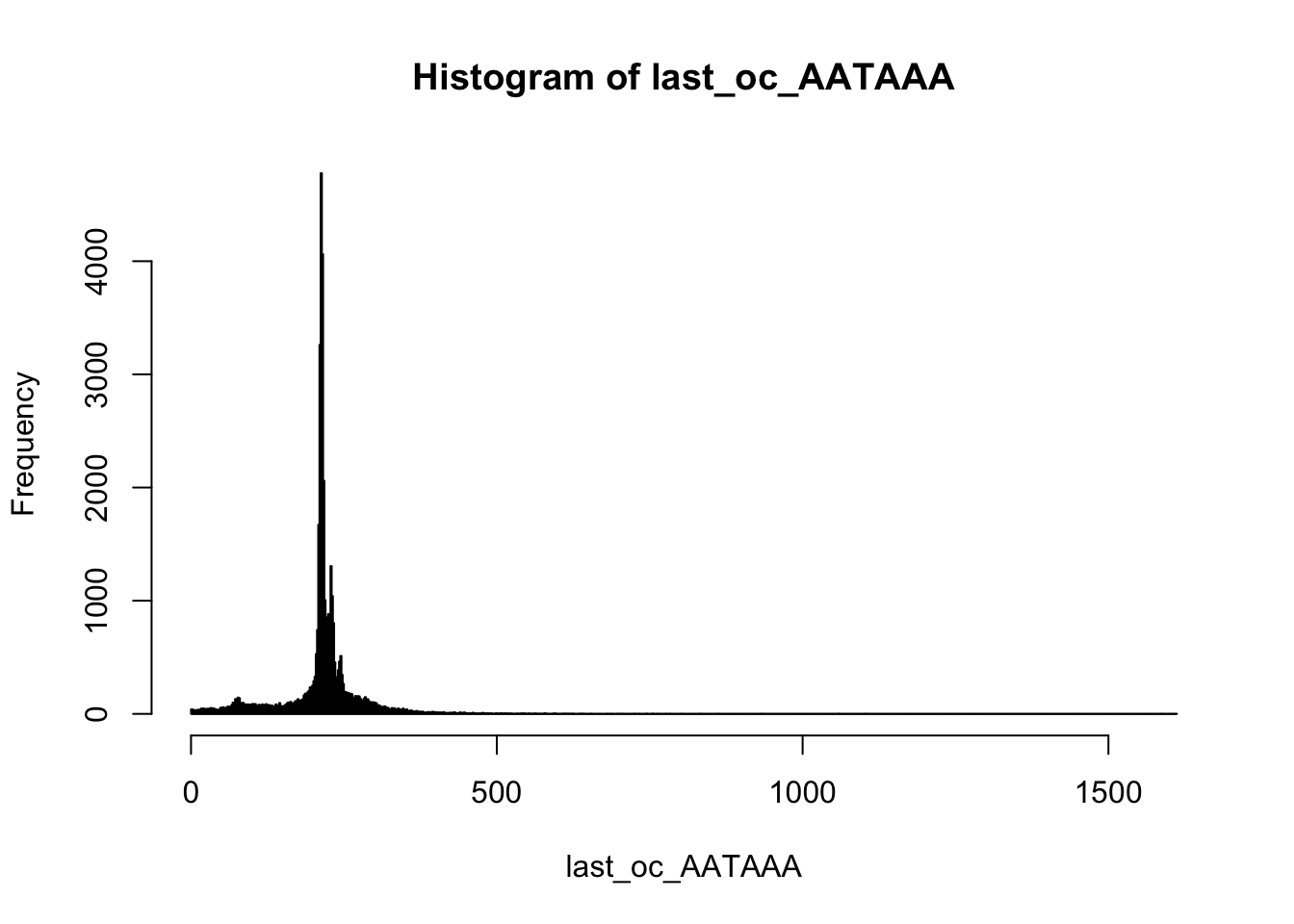
| Version | Author | Date |
|---|---|---|
| 4bf5d09 | Briana Mittleman | 2019-03-06 |
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS 10.14.1
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] grid stats4 parallel stats graphics grDevices utils
[8] datasets methods base
other attached packages:
[1] BSgenome.Hsapiens.UCSC.hg19_1.4.0 genomation_1.14.0
[3] BSgenome_1.50.0 rtracklayer_1.42.2
[5] GenomicRanges_1.34.0 GenomeInfoDb_1.18.2
[7] Biostrings_2.50.2 XVector_0.22.0
[9] IRanges_2.16.0 S4Vectors_0.20.1
[11] BiocGenerics_0.28.0 forcats_0.4.0
[13] stringr_1.4.0 dplyr_0.8.0.1
[15] purrr_0.3.1 readr_1.3.1
[17] tidyr_0.8.3 tibble_2.0.1
[19] ggplot2_3.1.0 tidyverse_1.2.1
[21] workflowr_1.2.0
loaded via a namespace (and not attached):
[1] nlme_3.1-137 bitops_1.0-6
[3] matrixStats_0.54.0 fs_1.2.6
[5] lubridate_1.7.4 httr_1.4.0
[7] rprojroot_1.3-2 tools_3.5.1
[9] backports_1.1.3 R6_2.4.0
[11] KernSmooth_2.23-15 lazyeval_0.2.1
[13] colorspace_1.4-0 seqPattern_1.14.0
[15] withr_2.1.2 tidyselect_0.2.5
[17] compiler_3.5.1 git2r_0.24.0
[19] cli_1.0.1 rvest_0.3.2
[21] Biobase_2.42.0 xml2_1.2.0
[23] DelayedArray_0.8.0 scales_1.0.0
[25] digest_0.6.18 Rsamtools_1.34.1
[27] rmarkdown_1.11 pkgconfig_2.0.2
[29] htmltools_0.3.6 plotrix_3.7-4
[31] rlang_0.3.1 readxl_1.3.0
[33] rstudioapi_0.9.0 impute_1.56.0
[35] generics_0.0.2 jsonlite_1.6
[37] BiocParallel_1.16.6 RCurl_1.95-4.12
[39] magrittr_1.5 GenomeInfoDbData_1.2.0
[41] Matrix_1.2-15 Rcpp_1.0.0
[43] munsell_0.5.0 reticulate_1.11.1
[45] stringi_1.3.1 whisker_0.3-2
[47] yaml_2.2.0 SummarizedExperiment_1.12.0
[49] zlibbioc_1.28.0 plyr_1.8.4
[51] crayon_1.3.4 lattice_0.20-38
[53] haven_2.1.0 hms_0.4.2
[55] knitr_1.21 pillar_1.3.1
[57] reshape2_1.4.3 XML_3.98-1.19
[59] glue_1.3.0 evaluate_0.13
[61] data.table_1.12.0 modelr_0.1.4
[63] cellranger_1.1.0 gtable_0.2.0
[65] assertthat_0.2.0 xfun_0.5
[67] gridBase_0.4-7 broom_0.5.1
[69] GenomicAlignments_1.18.1