Peak Plots
Briana Mittleman
2/16/2019
Last updated: 2019-02-21
Checks: 6 0
Knit directory: threeprimeseq/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.2.0). The Report tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(12345) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/figure/
Ignored: data/.DS_Store
Ignored: data/perm_QTL_trans_noMP_5percov/
Ignored: output/.DS_Store
Untracked files:
Untracked: KalistoAbundance18486.txt
Untracked: analysis/4suDataIGV.Rmd
Untracked: analysis/DirectionapaQTL.Rmd
Untracked: analysis/EvaleQTLs.Rmd
Untracked: analysis/YL_QTL_test.Rmd
Untracked: analysis/mergeReplicates.Rmd
Untracked: analysis/ncbiRefSeq_sm.sort.mRNA.bed
Untracked: analysis/snake.config.notes.Rmd
Untracked: analysis/verifyBAM.Rmd
Untracked: analysis/verifybam_dubs.Rmd
Untracked: code/PeaksToCoverPerReads.py
Untracked: code/strober_pc_pve_heatmap_func.R
Untracked: data/18486.genecov.txt
Untracked: data/APApeaksYL.total.inbrain.bed
Untracked: data/AllPeak_counts/
Untracked: data/ApaQTLs/
Untracked: data/ApaQTLs_otherPhen/
Untracked: data/ChromHmmOverlap/
Untracked: data/DistTXN2Peak_genelocAnno/
Untracked: data/GM12878.chromHMM.bed
Untracked: data/GM12878.chromHMM.txt
Untracked: data/LianoglouLCL/
Untracked: data/LocusZoom/
Untracked: data/LocusZoom_proc/
Untracked: data/MatchedSnps/
Untracked: data/NuclearApaQTLs.txt
Untracked: data/PeakCounts/
Untracked: data/PeakCounts_noMP_5perc/
Untracked: data/PeakCounts_noMP_genelocanno/
Untracked: data/PeakUsage/
Untracked: data/PeakUsage_noMP/
Untracked: data/PeakUsage_noMP_GeneLocAnno/
Untracked: data/PeaksUsed/
Untracked: data/PeaksUsed_noMP_5percCov/
Untracked: data/QTL_overlap/
Untracked: data/RNAkalisto/
Untracked: data/RefSeq_annotations/
Untracked: data/TotalApaQTLs.txt
Untracked: data/Totalpeaks_filtered_clean.bed
Untracked: data/UnderstandPeaksQC/
Untracked: data/WASP_STAT/
Untracked: data/YL-SP-18486-T-combined-genecov.txt
Untracked: data/YL-SP-18486-T_S9_R1_001-genecov.txt
Untracked: data/YL_QTL_test/
Untracked: data/apaExamp/
Untracked: data/apaExamp_proc/
Untracked: data/apaQTL_examp_noMP/
Untracked: data/bedgraph_peaks/
Untracked: data/bin200.5.T.nuccov.bed
Untracked: data/bin200.Anuccov.bed
Untracked: data/bin200.nuccov.bed
Untracked: data/clean_peaks/
Untracked: data/comb_map_stats.csv
Untracked: data/comb_map_stats.xlsx
Untracked: data/comb_map_stats_39ind.csv
Untracked: data/combined_reads_mapped_three_prime_seq.csv
Untracked: data/diff_iso_GeneLocAnno/
Untracked: data/diff_iso_proc/
Untracked: data/diff_iso_trans/
Untracked: data/ensemble_to_genename.txt
Untracked: data/example_gene_peakQuant/
Untracked: data/explainProtVar/
Untracked: data/filtPeakOppstrand_cov_noMP_GeneLocAnno_5perc/
Untracked: data/filtered_APApeaks_merged_allchrom_refseqTrans.closest2End.bed
Untracked: data/filtered_APApeaks_merged_allchrom_refseqTrans.closest2End.noties.bed
Untracked: data/first50lines_closest.txt
Untracked: data/gencov.test.csv
Untracked: data/gencov.test.txt
Untracked: data/gencov_zero.test.csv
Untracked: data/gencov_zero.test.txt
Untracked: data/gene_cov/
Untracked: data/joined
Untracked: data/leafcutter/
Untracked: data/merged_combined_YL-SP-threeprimeseq.bg
Untracked: data/molPheno_noMP/
Untracked: data/mol_overlap/
Untracked: data/mol_pheno/
Untracked: data/nom_QTL/
Untracked: data/nom_QTL_opp/
Untracked: data/nom_QTL_trans/
Untracked: data/nuc6up/
Untracked: data/nuc_10up/
Untracked: data/other_qtls/
Untracked: data/pQTL_otherphen/
Untracked: data/peakPerRefSeqGene/
Untracked: data/perm_QTL/
Untracked: data/perm_QTL_GeneLocAnno_noMP_5percov/
Untracked: data/perm_QTL_GeneLocAnno_noMP_5percov_3UTR/
Untracked: data/perm_QTL_diffWindow/
Untracked: data/perm_QTL_opp/
Untracked: data/perm_QTL_trans/
Untracked: data/perm_QTL_trans_filt/
Untracked: data/protAndAPAAndExplmRes.Rda
Untracked: data/protAndAPAlmRes.Rda
Untracked: data/protAndExpressionlmRes.Rda
Untracked: data/reads_mapped_three_prime_seq.csv
Untracked: data/smash.cov.results.bed
Untracked: data/smash.cov.results.csv
Untracked: data/smash.cov.results.txt
Untracked: data/smash_testregion/
Untracked: data/ssFC200.cov.bed
Untracked: data/temp.file1
Untracked: data/temp.file2
Untracked: data/temp.gencov.test.txt
Untracked: data/temp.gencov_zero.test.txt
Untracked: data/threePrimeSeqMetaData.csv
Untracked: data/threePrimeSeqMetaData55Ind.txt
Untracked: data/threePrimeSeqMetaData55Ind.xlsx
Untracked: data/threePrimeSeqMetaData55Ind_noDup.txt
Untracked: data/threePrimeSeqMetaData55Ind_noDup.xlsx
Untracked: data/threePrimeSeqMetaData55Ind_noDup_WASPMAP.txt
Untracked: data/threePrimeSeqMetaData55Ind_noDup_WASPMAP.xlsx
Untracked: output/deeptools_plots/
Untracked: output/picard/
Untracked: output/plots/
Untracked: output/qual.fig2.pdf
Unstaged changes:
Modified: analysis/28ind.peak.explore.Rmd
Modified: analysis/CompareLianoglouData.Rmd
Modified: analysis/NewPeakPostMP.Rmd
Modified: analysis/apaQTLoverlapGWAS.Rmd
Modified: analysis/cleanupdtseq.internalpriming.Rmd
Modified: analysis/coloc_apaQTLs_protQTLs.Rmd
Modified: analysis/dif.iso.usage.leafcutter.Rmd
Modified: analysis/diffIsoAnalysisNewMapping.Rmd
Modified: analysis/diff_iso_pipeline.Rmd
Modified: analysis/explainpQTLs.Rmd
Modified: analysis/explore.filters.Rmd
Modified: analysis/flash2mash.Rmd
Modified: analysis/mispriming_approach.Rmd
Modified: analysis/overlapMolQTL.Rmd
Modified: analysis/overlapMolQTL.opposite.Rmd
Modified: analysis/overlap_qtls.Rmd
Modified: analysis/peakOverlap_oppstrand.Rmd
Modified: analysis/peakQCPPlots.Rmd
Modified: analysis/pheno.leaf.comb.Rmd
Modified: analysis/pipeline_55Ind.Rmd
Modified: analysis/swarmPlots_QTLs.Rmd
Modified: analysis/test.max2.Rmd
Modified: analysis/test.smash.Rmd
Modified: analysis/understandPeaks.Rmd
Modified: code/Snakefile
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | bd23058 | Briana Mittleman | 2019-02-21 | add top 200 |
| html | 382f5dc | Briana Mittleman | 2019-02-21 | Build site. |
| Rmd | 3fea87f | Briana Mittleman | 2019-02-21 | add pi plot |
| html | 031978b | Briana Mittleman | 2019-02-20 | Build site. |
| Rmd | 11f0bee | Briana Mittleman | 2019-02-20 | modify dT |
| html | a39ad03 | Briana Mittleman | 2019-02-20 | Build site. |
| Rmd | e07197b | Briana Mittleman | 2019-02-20 | fix strands |
| html | a4fd0e0 | Briana Mittleman | 2019-02-19 | Build site. |
| Rmd | d3a76f3 | Briana Mittleman | 2019-02-19 | fix link |
| html | 69dfcbc | Briana Mittleman | 2019-02-18 | Build site. |
| Rmd | af9929e | Briana Mittleman | 2019-02-18 | rna alone |
| html | d27ed26 | Briana Mittleman | 2019-02-18 | Build site. |
| Rmd | f0cf945 | Briana Mittleman | 2019-02-18 | add deeptools plot code |
| html | 879be33 | Briana Mittleman | 2019-02-16 | Build site. |
| Rmd | f8c76ea | Briana Mittleman | 2019-02-16 | move peak QC plots |
library(workflowr)This is workflowr version 1.2.0
Run ?workflowr for help getting startedlibrary(tidyverse)── Attaching packages ──────────────────────────────────────────────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.0.0 ✔ purrr 0.2.5
✔ tibble 1.4.2 ✔ dplyr 0.7.6
✔ tidyr 0.8.1 ✔ stringr 1.4.0
✔ readr 1.1.1 ✔ forcats 0.3.0Warning: package 'stringr' was built under R version 3.5.2── Conflicts ─────────────────────────────────────────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()library(reshape2)
Attaching package: 'reshape2'The following object is masked from 'package:tidyr':
smithslibrary(cowplot)
Attaching package: 'cowplot'The following object is masked from 'package:ggplot2':
ggsaveI want to remake a lot of the peak QC plots I have been making with the new mapped and proccessed data created in the accounting for mappping bias analysis
Peaks per gene
Number of genes with 1 peak, 2 peaks, more peaks
Distance between gene and TES
Peaks in each category
Peak Size
Peak per gene:
I will do this for total and nuclear 5% seperatly then for the peaks I used in the QTL analysis.
Nuclear peaks: 42127: /project2/gilad/briana/threeprimeseq/data/phenotypes_filtPeakTranscript_noMP_GeneLocAnno/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno.NoMP_sm_quant.Nuclear_fixed.pheno.5percPeaks.txt
Total peaks: 36915: /project2/gilad/briana/threeprimeseq/data/phenotypes_filtPeakTranscript_noMP_GeneLocAnno/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno.NoMP_sm_quant.Total_fixed.pheno.5percPeaks.txt
peakNames=c("chr", 'start','end','gene','strand','name', 'mean')
totalPeaks=read.table("../data/PeaksUsed_noMP_5percCov/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno.NoMP_sm_quant.Total_fixed.pheno.5percPeaks.txt", stringsAsFactors = F, col.names = peakNames)
nuclearPeaks=read.table("../data/PeaksUsed_noMP_5percCov/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno.NoMP_sm_quant.Nuclear_fixed.pheno.5percPeaks.txt", stringsAsFactors = F, col.names = peakNames)Peaks per gene:
totalPeaks_genes=totalPeaks %>% group_by(gene) %>% summarise(nPeaks=n()) %>% group_by(nPeaks) %>% summarise(GenesWithNPeaks=n())
nuclearPeaks_genes=nuclearPeaks %>% group_by(gene) %>% summarise(nPeaks=n())%>% group_by(nPeaks) %>% summarise(GenesWithNPeaks=n())
nPeaksBoth=totalPeaks_genes %>% full_join(nuclearPeaks_genes, by="nPeaks")
colnames(nPeaksBoth)= c("Npeaks", "Total", "Nuclear")
nPeaksBoth$Total= nPeaksBoth$Total %>% replace_na(0)
#melt nPeaksBoth
nPeaksBoth_melt=melt(nPeaksBoth, id.var="Npeaks")
colnames(nPeaksBoth_melt)= c("PAS", "Fraction", "Genes")
peakUsage5perc=ggplot(nPeaksBoth_melt, aes(x=PAS, y=Genes, fill=Fraction)) + geom_bar(stat="identity", position = "dodge") + labs(title="Number of Genes by PAS Number \n 5% Usage",x="Number of PAS in Gene") + theme(axis.text.y = element_text(size=12),axis.title.y=element_text(size=10,face="bold"), axis.title.x=element_text(size=12,face="bold"))+ scale_fill_manual(values=c("darkviolet","deepskyblue3")) + facet_grid(~Fraction)
peakUsage5perc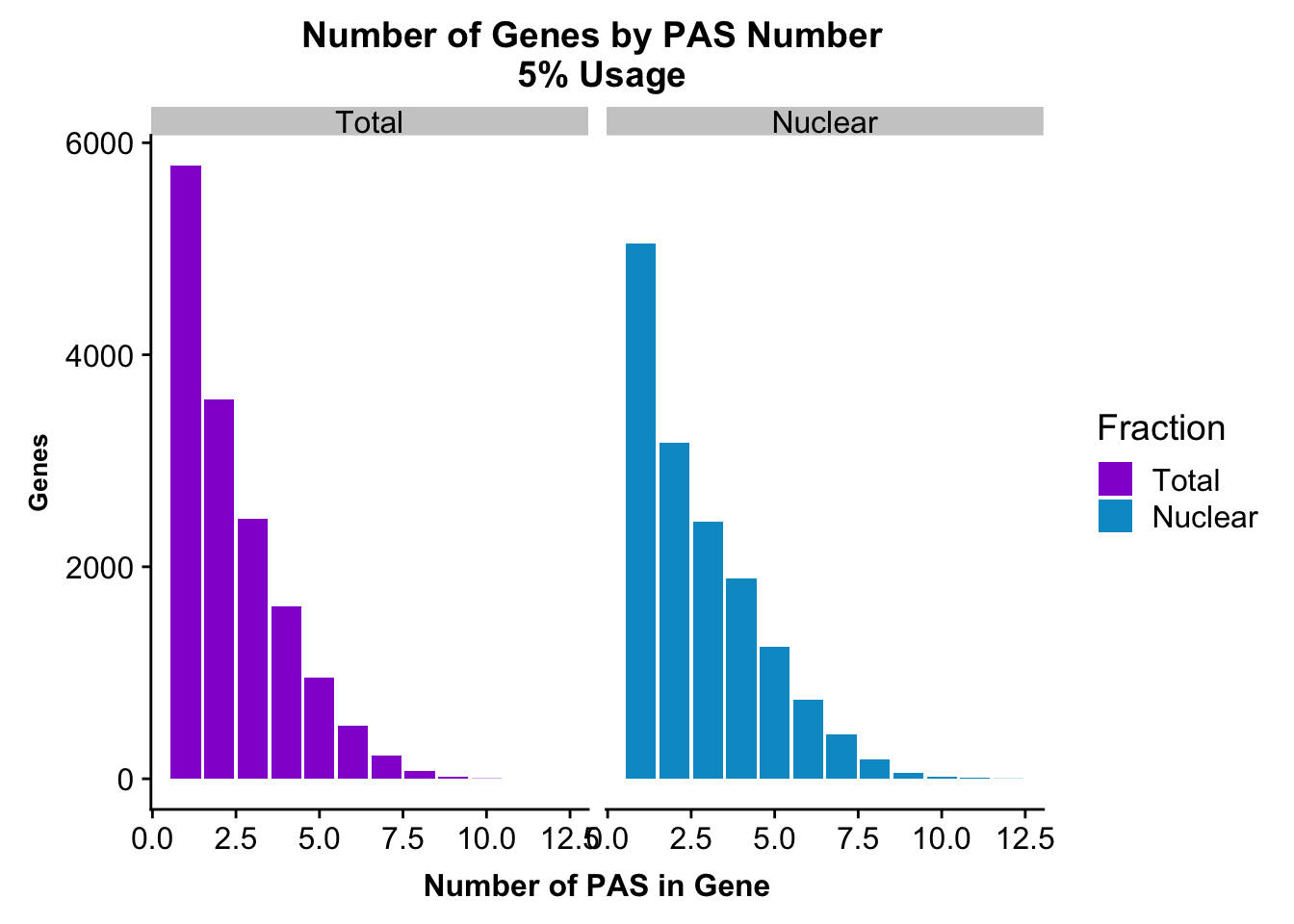
| Version | Author | Date |
|---|---|---|
| 879be33 | Briana Mittleman | 2019-02-16 |
ggsave(peakUsage5perc, file="../output/plots/PeakNumberPerGenebyFrac.png")Saving 7 x 5 in imagePlot this with the peaks used in the fraction
allPeaks=read.table("../data/PeaksUsed_noMP_5percCov/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.bed", stringsAsFactors = F, col.names = c("chr", 'start','end', 'id', 'score', 'strand')) %>% separate(id, into=c("gene", "peak"), sep=":")%>% group_by(gene) %>% summarise(nPeaks=n()) %>% group_by(nPeaks) %>% summarise(GenesWithNPeaks=n())
colnames(allPeaks)=c("PAS","Genes" )
allPeaksGenes=ggplot(allPeaks, aes(x=PAS, y=Genes)) + geom_bar(stat="identity",fill="blue") + labs(title="Number of Genes by PAS Count: \n PAS Used in QTL analysis",x="Number of PAS in Gene") + theme(axis.text.y = element_text(size=12),axis.title.y=element_text(size=10,face="bold"), axis.title.x=element_text(size=12,face="bold"))
allPeaksGenes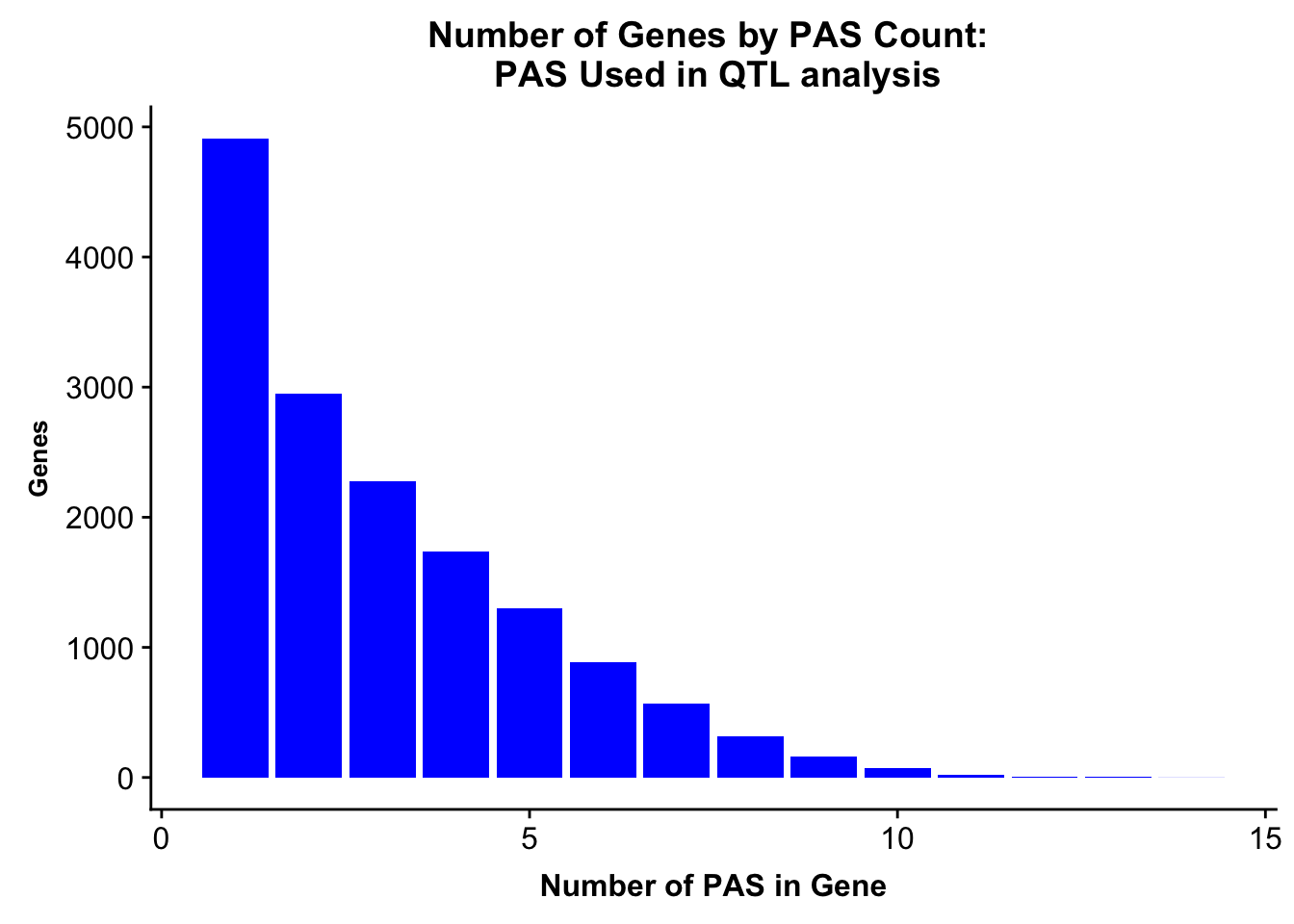
| Version | Author | Date |
|---|---|---|
| 879be33 | Briana Mittleman | 2019-02-16 |
ggsave(allPeaksGenes, file="../output/plots/PeakNumberPerGeneUsedinQTL.png")Saving 7 x 5 in imageNumber of genes with 1 peak, 2 peaks, more peaks
Make this as a boxplot
GeneAnno=read.table("../data/RefSeq_annotations/Transcript2GeneName.dms", stringsAsFactors = F, header=T) %>% select(name2) %>% unique()
colnames(GeneAnno)="gene"
genesWithpeak= read.table("../data/PeaksUsed_noMP_5percCov/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.bed", stringsAsFactors = F, col.names = c("chr", 'start','end', 'id', 'score', 'strand')) %>% separate(id, into=c("gene", "peak"), sep=":") %>% select(gene) %>% unique()
Geneswith0= GeneAnno %>% anti_join(genesWithpeak, by="gene") %>% nrow()
Geneswith0[1] 11896To get the genes with 0 peaks I need to pull in the gene annotation file
morethan2= allPeaks %>% filter(PAS > 2)
colSums(morethan2) PAS Genes
102 7361 Category=c("0 PAS", "1 PAS", "2 PAS", "More than 2 PAS")
genesPerCat=c(11896/27115, 4909/27115, 2949/27115, 7361/27115)
genesPerCat_df=as.data.frame(cbind(Category,genesPerCat))
genesPerCat_df$genesPerCat=as.numeric(as.character(genesPerCat_df$genesPerCat))
lab0=paste("Genes =", "11896", sep=" ")
lab1=paste("Genes =", "4909", sep=" ")
lab2=paste("Genes =", "2949", sep=" ")
labMore=paste("Genes =", "7361", sep=" ")
propGenesbyPAS=ggplot(genesPerCat_df, aes(x="", y=genesPerCat, fill=Category)) + geom_bar(stat="identity") + labs(x="Total Genes = 27115", y="Proportion of Genes", title="Proportion of Genes by number of PAS") + annotate("text", x="", y= .7, label=lab0) + annotate("text", x="", y= .5, label=lab1) + annotate("text", x="", y= .33, label=lab2) + annotate("text", x="", y= .2, label=labMore) + scale_fill_brewer(palette="RdYlBu")
propGenesbyPAS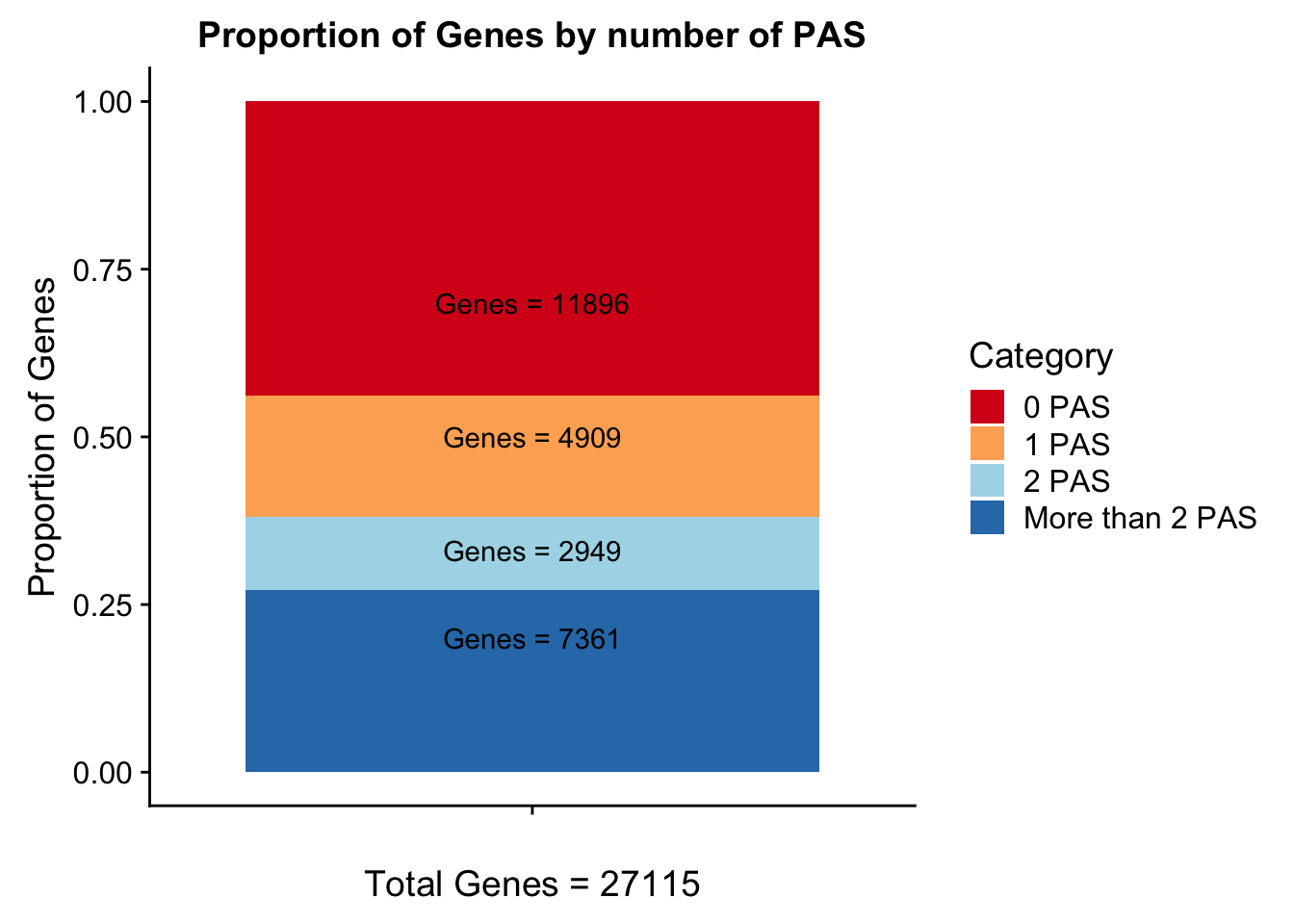
ggsave(propGenesbyPAS, file="../output/plots/PropOfGenesByPASnum.png",height=8, width=5)Box plot
propGenesbyPASpi=ggplot(genesPerCat_df, aes(x="", y=genesPerCat, fill=Category)) + geom_bar(stat="identity") + labs(x="Total Genes = 27115", y="Proportion of Genes", title="Proportion of Genes by number of PAS") +coord_polar("y") + scale_fill_brewer(palette="RdYlBu")
propGenesbyPASpi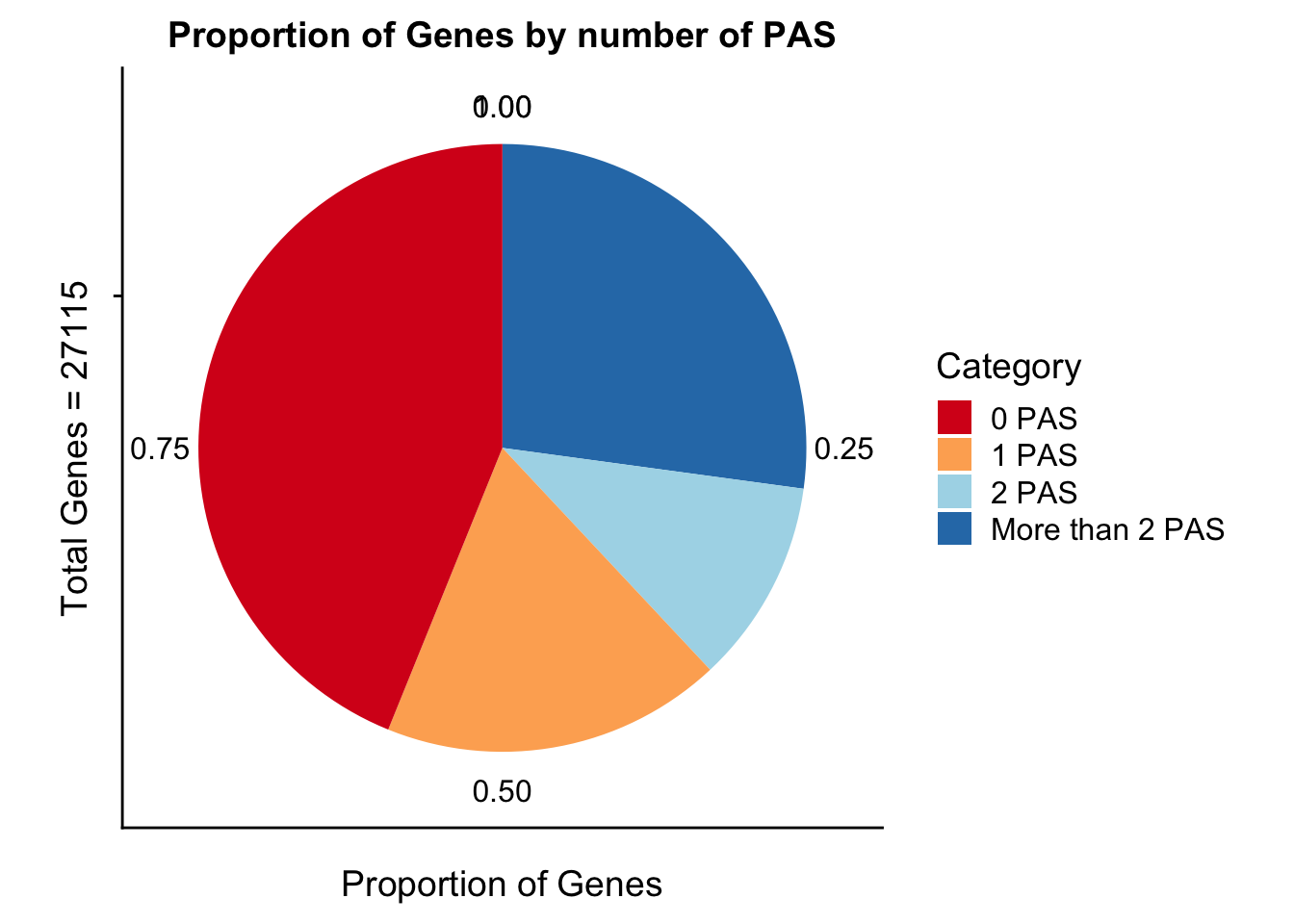
| Version | Author | Date |
|---|---|---|
| 382f5dc | Briana Mittleman | 2019-02-21 |
ggsave(propGenesbyPASpi, file="../output/plots/PropOfGenesByPASnum_piplot.png")Saving 7 x 5 in imageDistance between TES and peak
- GetDistTXNend2Peak.py
convert /project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.SAF to bed file
peaksGeneLocAnno_5percSAF2Bed.py
distTXN2Peak=read.table("../data/DistTXN2Peak_genelocAnno/distPeak2EndTXN.txt", col.names = c("Peak", "name2", "Distance", "Gene_Strand"),stringsAsFactors = F)
txnanno=read.table("../data/RefSeq_annotations/Transcript2GeneName.dms", header=T,stringsAsFactors = F) %>% mutate(length=abs(txEnd-txStart)) %>% semi_join(distTXN2Peak, by="name2")
distTXN2Peak =distTXN2Peak %>% mutate(AbsDist=abs(Distance))
mean(txnanno$length)[1] 60808.79distTXN2PeakPlot=ggplot(distTXN2Peak, aes(x=AbsDist + 1)) + geom_density() + scale_x_log10() + labs(x="Absolute Distance between end of Transcription and center of Peak", title="Distribution of transcription to peak absolute distance") + geom_vline(xintercept=mean(txnanno$length), col="red") + annotate("text", x=1000000, y=.4, label="Average transcript length \n for genes in peaks", col='red')
distTXN2PeakPlot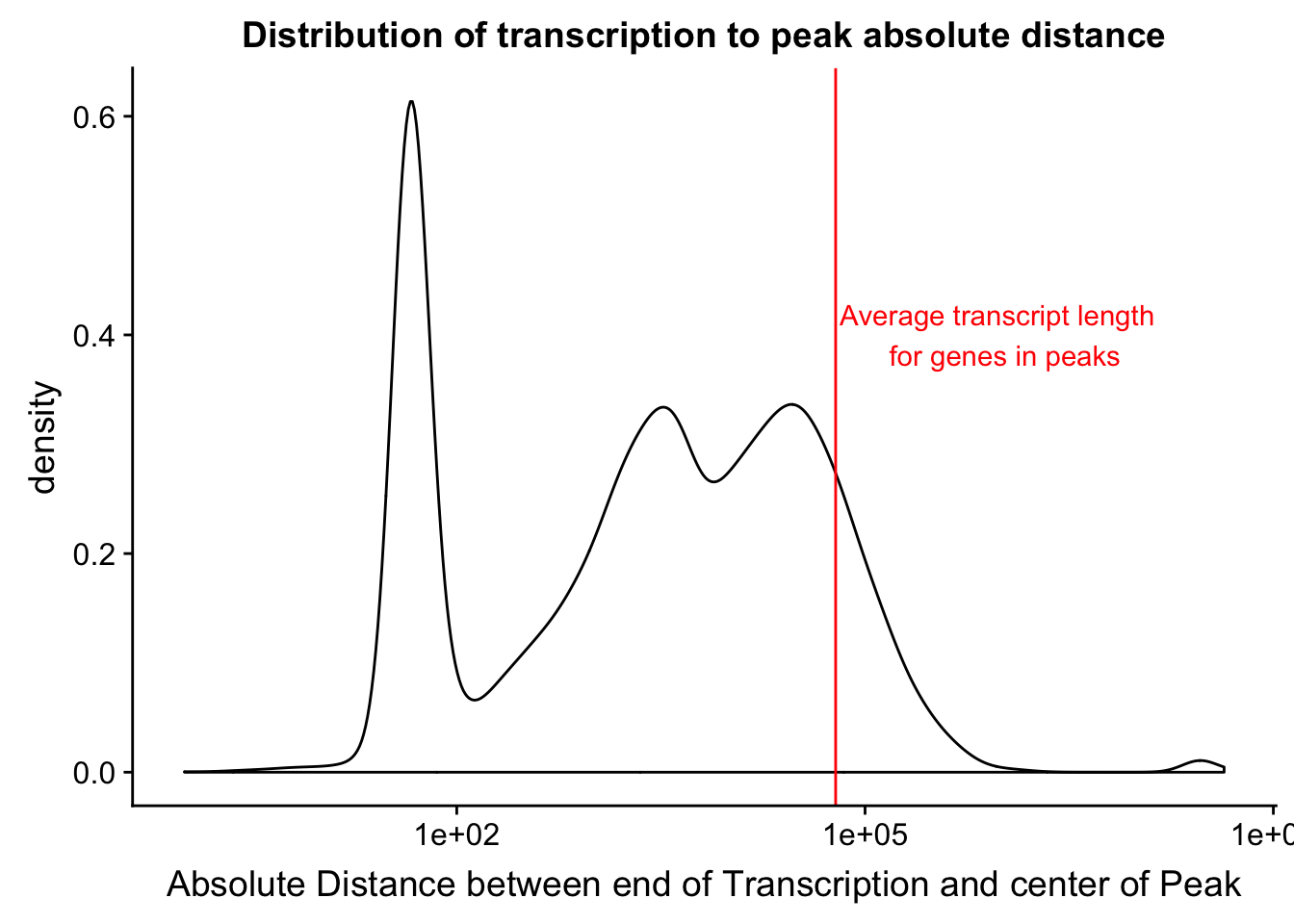
| Version | Author | Date |
|---|---|---|
| 382f5dc | Briana Mittleman | 2019-02-21 |
ggsave(distTXN2PeakPlot, file="../output/plots/DistanceBetweenPeakandTES.png")Saving 7 x 5 in imagePeaks per category
- processGenLocPeakAnno2SAF_withAnno.py
- filternamePeaks5percCov_GeneLocAnno_withAnno.py
peakswAnno=read.table("../data/PeaksUsed_noMP_5percCov/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov_withAnno.SAF", header=T) %>% separate(GeneID, into=c("Peak", "chrom", "start", "end", "strand", "gene", "loc"),sep=":") %>% select(Peak, loc) %>% group_by(loc) %>% summarise(Num=n())locationOfPeaks=ggplot(peakswAnno, aes(x=loc, y=Num)) + geom_bar(stat="identity", fill="blue") + labs(x="Gene Location", y="Number of Peaks", title="Location distribution for all PAS with 5% Usage")
locationOfPeaks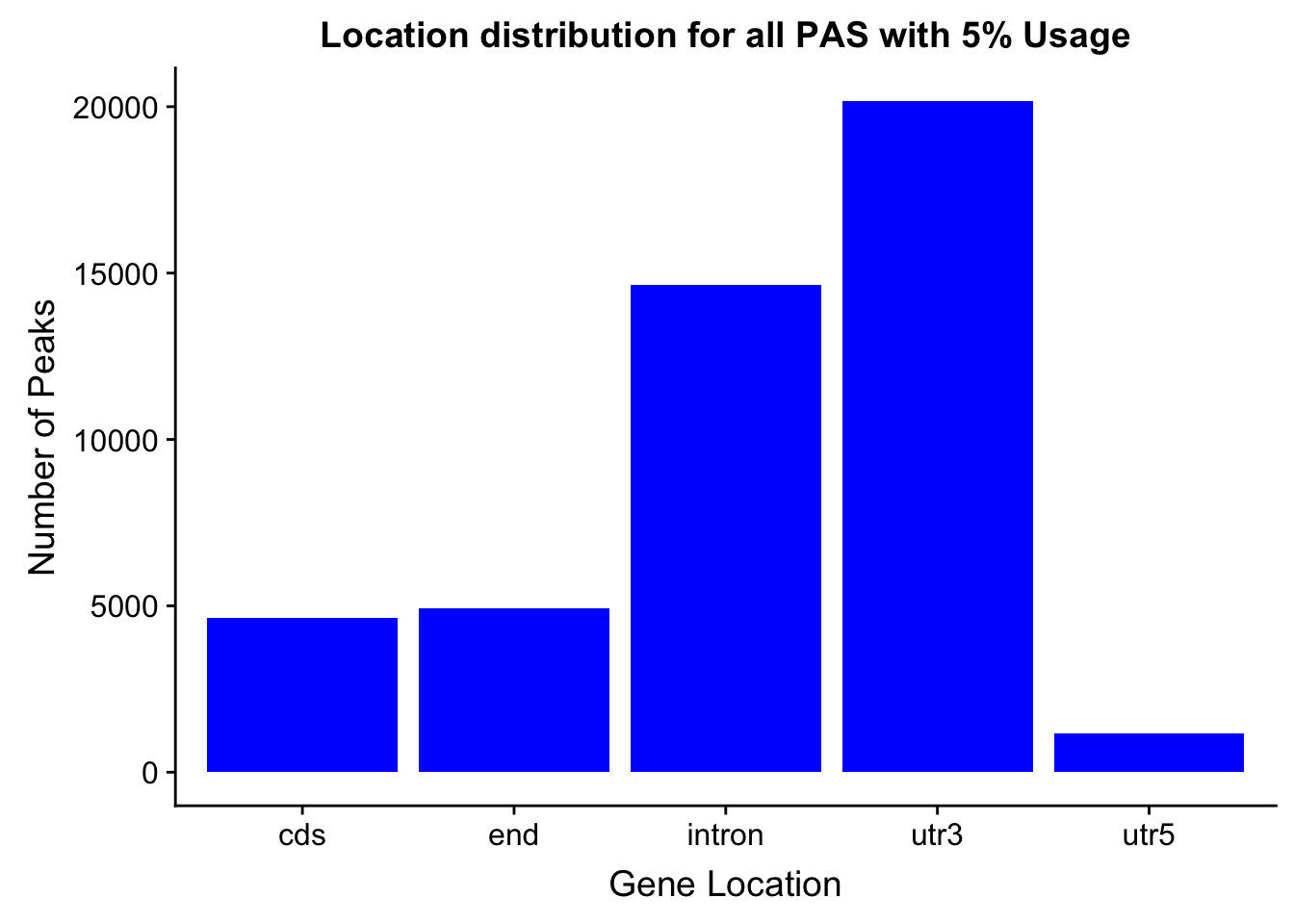
| Version | Author | Date |
|---|---|---|
| 382f5dc | Briana Mittleman | 2019-02-21 |
ggsave(locationOfPeaks, file="../output/plots/PeakLocationByAnnotation.png")Saving 7 x 5 in imagePeak Size
Peak length:
peaks=read.table("../data/PeaksUsed_noMP_5percCov/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.bed",col.names=c("chr", 'start','end', 'peak', 'score', 'strand')) %>% mutate(length=end-start)ggplot(peaks,aes(x=length)) + geom_histogram(bins=300) + labs(title="Peak Size", x="number of basepairs") + geom_vline(xintercept =mean(peaks$length),col="red")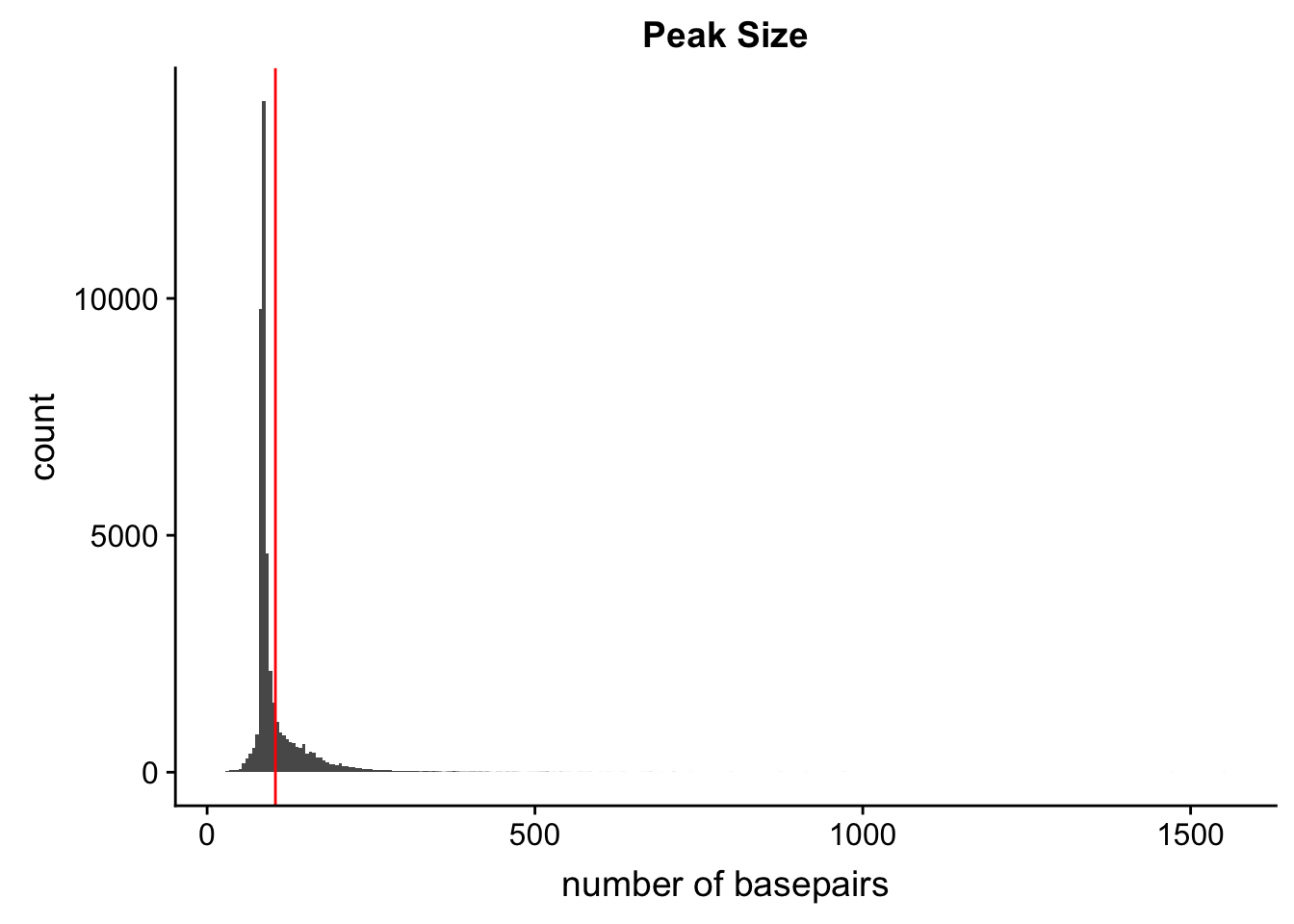
| Version | Author | Date |
|---|---|---|
| 382f5dc | Briana Mittleman | 2019-02-21 |
Deep tools:
files to remake:
Merged bam files are in /project2/gilad/briana/threeprimeseq/data/mergedBams_NoMP
Code is mergeBam2BW.sh
mergeBam2BW.sh
#!/bin/bash
#SBATCH --job-name=mergeBam2B
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=mergeBam2BW.out
#SBATCH --error=mergeBam2BW.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
#total
bamCoverage -b /project2/gilad/briana/threeprimeseq/data/mergedBams_NoMP/AllTotalSamples.MergedBamFiles.noMP.sort.bam -o /project2/gilad/briana/threeprimeseq/data/mergedBW/Total_MergedBamCoverage.bw
#nuclear
bamCoverage -b /project2/gilad/briana/threeprimeseq/data/mergedBams_NoMP/AllNuclearSamples.MergedBamFiles.noMP.sort.bam -o /project2/gilad/briana/threeprimeseq/data/mergedBW/Nuclear_MergedBamCoverage.bw I need to remake the peak files with the strand opposite (peaks are opposite strand from the reads!)
fixStrand4DTplots.py
peaksIn="/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.bed"
intronIn="/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5percCov_INTRON.bed"
PeakOut="/project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand.bed"
intronOut="/project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed"
def fix_strand(Fin,Fout):
fout=open(Fout,"w")
for ln in open(Fin, "r"):
chrom, start, end, name, score, strand = ln.split()
if strand=="+":
fout.write("%s\t%s\t%s\t%s\t%s\t-\n"%(chrom,start,end,name,score))
else:
fout.write("%s\t%s\t%s\t%s\t%s\t+\n"%(chrom,start,end,name,score))
fout.close()
fix_strand(peaksIn, PeakOut)
fix_strand(intronIn, intronOut)/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.bed
/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5percCov_INTRON.bed
BothFracDTPlotmyPeaks_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=BothFracDTPlotmyPeaks_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=BothFracDTPlotmyPeaks_noMPFilt.out
#SBATCH --error=BothFracDTPlotmyPeaks_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/mergedBW/Total_MergedBamCoverage.bw /project2/gilad/briana/threeprimeseq/data/mergedBW/Nuclear_MergedBamCoverage.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_myPeaksNompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_myPeaksNompfilt.gz --refPointLabel "Called PAS" --plotTitle "Combined Reads at All Called PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_myPeaksNompfilt.pngRNADTPlotmyPeaks_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=RNADTPlotmyPeaks_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=RNADTPlotmyPeaks_noMPFilt.out
#SBATCH --error=RNADTPlotmyPeaks_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksNompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksNompfilt.gz --refPointLabel "Called PAS" --plotTitle "Combined Reads at All Called PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksNompfilt.pngI want to make one of these that look at total, nuclear, and RNA at peaks assigned to an intron. This means I need to subset the peak file to only include these. I can do this similar to how I did the UTR subset in this analysis
I want to make a bedfile with these peaks. I need to also make sure they are in the final clean peaks
makeIntronPeakBed.py
inFile="/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLoc.bed"
outFile=open("/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5percCov_INTRON.bed" , "w")
okPeaks=open("/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.bed", "r")
okPeak_dic={}
for ln in okPeaks:
peak=ln.split()[3].split(":")[1]
peak_num=peak[4:]
okPeak_dic[peak_num]=""
for ln in open(inFile, "r"):
chrom, start, end, peak, cov, strand, score, anno = ln.split()
if anno==".":
continue
anno_lst=anno.split(",")
if len(anno_lst)==1:
gene=anno_lst[0].split(":")[1]
if anno_lst[0].split(":")[0]=="intron":
if peak in okPeak_dic.keys():
peak_i=int(peak)
start_i=int(start)
end_i=int(end)
type="intron"
outFile.write("%s\t%d\t%d\t%s\t%s\t%s\n"%(chrom, start_i, end_i, type,score, strand))
else:
type_dic={}
for each in anno_lst:
type_dic[each.split(":")[0]]=each.split(":")[1]
if "utr3" in type_dic.keys():
continue
if "intron" in type_dic.keys():
if peak in okPeak_dic.keys():
peak_i=int(peak)
start_i=int(start)
end_i=int(end)
type="intron"
outFile.write("%s\t%d\t%d\t%s\t%s\t%s\n"%(chrom, start_i, end_i,type ,score, strand))
outFile.close()BothFracDTPlotmyIntronPeaks_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=BothFracDTPlotmyIntronPeaks_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=BothFracRNADTPlotmyIntronPeaks_noMPFilt.out
#SBATCH --error=BothFracRNADTPlotmyIntronPeaks_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/mergedBW/Total_MergedBamCoverage.bw /project2/gilad/briana/threeprimeseq/data/mergedBW/Nuclear_MergedBamCoverage.bw /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_myPeaksIntron_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_myPeaksIntron_Nompfilt.gz --refPointLabel "Called Intronic PAS" --plotTitle "Combined Reads at Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_myPeaksIntronNompfilt.pngRNA seq only:
RNADTPlotmyIntronPeaks_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=RNADTPlotmyIntronPeaks_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=RNADTPlotmyIntronPeaks_noMPFilt.out
#SBATCH --error=RNADTPlotmyIntronPeaks_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksIntron_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksIntron_Nompfilt.gz --refPointLabel "Called Intronic PAS" --plotTitle "Combined Reads at Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksIntronNompfilt.pngI should try this with the nuclear RNA samples
I need to merge them and make a BW.
mergeNucRNAseq.sh
#!/bin/bash
#SBATCH --job-name=mergeNucRNAseq
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=mergeNucRNAseq.out
#SBATCH --error=mergeNucRNAseq.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
samtools merge /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.bam /project2/gilad/briana/Total_Nuc_RNA_seq_data/170428_K00242_0214_AHK2GMBBXX-YG-SP20/data/sort/YG-SP20-Nuc-2_S5_L005_R1_001-sort.bam /project2/gilad/briana/Total_Nuc_RNA_seq_data/170428_K00242_0214_AHK2GMBBXX-YG-SP20/data/sort/YG-SP20-Nuc-1_S2_L005_R1_001-sort.bam
samtools sort /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.bam > /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bam
samtools index /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bamNucBam2BW.sh
#!/bin/bash
#SBATCH --job-name=NucBam2BW
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=NucBam2BW.out
#SBATCH --error=NucBam2BW.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
#total
bamCoverage -b /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bam -o /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bw
NucRNADTPlotmyIntronPeaks_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=NucRNADTPlotmyIntronPeaks_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=NucRNADTPlotmyIntronPeaks_noMPFilt.out
#SBATCH --error=NucRNADTPlotmyIntronPeaks_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Nompfilt.gz --refPointLabel "Called Intronic PAS" --plotTitle "Combined Reads at Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntronNompfilt.pngNucRNADTPlotmyIntronPeaks_noMPFilt_150.sh
#!/bin/bash
#SBATCH --job-name=NucRNADTPlotmyIntronPeaks_noMPFilt_150
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=NucRNADTPlotmyIntronPeaks_noMPFilt150.out
#SBATCH --error=NucRNADTPlotmyIntronPeaks_noMPFilt150.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed -b 150 -a 150 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Nompfilt150.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Nompfilt150.gz --refPointLabel "Called Intronic PAS" --plotTitle "Combined Reads at Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntronNompfilt150.pngNucRNADTPlotmyIntronPeaks_noMPFilt_300.sh
#!/bin/bash
#SBATCH --job-name=NucRNADTPlotmyIntronPeaks_noMPFilt_300
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=NucRNADTPlotmyIntronPeaks_noMPFilt300.out
#SBATCH --error=NucRNADTPlotmyIntronPeaks_noMPFilt300.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed -b 300 -a 300 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Nompfilt300.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Nompfilt300.gz --refPointLabel "Called Intronic PAS" --plotTitle "Combined Reads at Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntronNompfilt300.pngTES and TSS (not looking right- coding end doesnt work)
fixStrand4DTplots_TESTSS.py Fix strand for these:
TSSIn="/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_TSSAllGenes.bed"
TESIn="/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_TESAllGenes.bed"
TSSOut="/project2/gilad/briana/threeprimeseq/data/peaks4DT/ncbiRefSeq_TSSAllGenes_fixedStrand.bed"
TESOut="/project2/gilad/briana/threeprimeseq/data/peaks4DT/ncbiRefSeq_TESAllGenes_fixedStrand.bed"
def fix_strand(Fin,Fout):
fout=open(Fout,"w")
for ln in open(Fin, "r"):
chrom, start, end, name, score, strand = ln.split()
if strand=="+":
fout.write("%s\t%s\t%s\t%s\t%s\t-\n"%(chrom,start,end,name,score))
else:
fout.write("%s\t%s\t%s\t%s\t%s\t+\n"%(chrom,start,end,name,score))
fout.close()
fix_strand(TSSIn, TSSOut)
fix_strand(TESIn, TESOut)files to make: new TSS file from the annotation in the new gene loc annocation pipeline
getTss.py
TXN2Gene_file=open("/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/Transcript2GeneName.dms","r")
outFile=open("/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_TSSAllGenes.bed", "w")
for i, ln in enumerate(TXN2Gene_file):
if i >0 :
chrom=ln.split()[2]
chromf=chrom[3:]
start=int(ln.split()[6])-1
end=int(ln.split()[6])
txn=ln.split()[1]
genename=ln.split()[12]
id=txn + ":" + genename
strand=ln.split()[3]
score="."
outFile.write("%s\t%s\t%s\t%s\t%s\t%s\n"%(chromf, start, end, id, score, strand))
outFile.close()RNADTPlotTSS_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=RNADTPlotTSS_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=RNADTPlotTSS_noMPFilt.out
#SBATCH --error=RNADTPlotTSS_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/genome_anotation_data/ncbiRefSeq.mRNA.transcriptTSS.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_TSS_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_TSS_Nompfilt.gz --refPointLabel "Called TSS" --plotTitle "Combined Reads at TSS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_TSS_Nompfilt.pngBothFracDTPlotTSS_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=BothFracDTPlotTSS_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=BothFracDTPlotTSS_noMPFilt.out
#SBATCH --error=BothFracDTPlotTSS_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/mergedBW/Total_MergedBamCoverage.bw /project2/gilad/briana/threeprimeseq/data/mergedBW/Nuclear_MergedBamCoverage.bw -R /project2/gilad/briana/genome_anotation_data/ncbiRefSeq.mRNA.transcriptTSS.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_TSS_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_TSS_Nompfilt.gz --refPointLabel "Called TSS" --plotTitle "Combined Reads at TSS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFrac_TSS_Nompfilt.pnggetTES.py
TXN2Gene_file=open("/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/Transcript2GeneName.dms","r")
outFile=open("/project2/gilad/briana/genome_anotation_data/RefSeq_annotations/ncbiRefSeq_TESAllGenes.bed", "w")
for i, ln in enumerate(TXN2Gene_file):
if i >0 :
chrom=ln.split()[2]
chromf=chrom[3:]
start=int(ln.split()[7])-1
end=int(ln.split()[7])
txn=ln.split()[1]
genename=ln.split()[12]
id=txn + ":" + genename
strand=ln.split()[3]
score="."
outFile.write("%s\t%s\t%s\t%s\t%s\t%s\n"%(chromf, start, end, id, score, strand))
outFile.close()BothFracRNADTPlotTES_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=BothFracRNADTPlotTES_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=BothFracRNADTPlotTES_noMPFilt.out
#SBATCH --error=BothFracRNADTPlotTESnoMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/mergedBW/Total_MergedBamCoverage.bw /project2/gilad/briana/threeprimeseq/data/mergedBW/Nuclear_MergedBamCoverage.bw /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/ncbiRefSeq_TESAllGenes_fixedStrand.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFracRNA_TES_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFracRNA_TES_Nompfilt.gz --refPointLabel "Called TES" --plotTitle "Combined Reads at TES" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/BothFracRNA_TES_Nompfilt.pngRNADTPlotTES_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=RNADTPlotTES_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=RNADTPlotTES_noMPFilt.out
#SBATCH --error=RNADTPlotTESnoMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/ncbiRefSeq_TESAllGenes_fixedStrand.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_TES_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_TES_Nompfilt.gz --refPointLabel "Called TES" --plotTitle "Combined Reads at TES" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_TES_Nompfilt.png/project2/gilad/briana/genome_anotation_data/ncbiRefSeq.mRNA.transcriptTSS.bed
ATAC seq
ATAC seq bam files are in /project2/yangili1/LCL/ATAC/ I will merge the bam files.
mergeBamFiles_ATAC.sh
#!/bin/bash
#SBATCH --job-name=mergeBamFiles_ATAC
#SBATCH --account=pi-yangili1
#SBATCH --time=8:00:00
#SBATCH --output=mergeBamFiles_ATAC.out
#SBATCH --error=mergeBamFiles_ATAC.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
samtools merge /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBam/ATAC_merged.bam /project2/yangili1/LCL/ATAC/*.sort.bam
samtools sort /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBam/ATAC_merged.bam > /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBam/ATAC_merged.sort.bam
samtools index /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBam/ATAC_merged.sort.bambam2BW_ATAC.sh
#!/bin/bash
#SBATCH --job-name=bam2BW_ATAC
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=bam2BW_ATAC.out
#SBATCH --error=bam2BW_ATAC.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
#total
bamCoverage -b /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBam/ATAC_merged.sort.bam -o /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBW/ATAC_merged.sort.bw
ATACDTPlotmyIntronPeaks_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=ATACDTPlotmyIntronPeaks_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=ATACDTPlotmyIntronPeaks_noMPFilt.out
#SBATCH --error=ATACDTPlotmyIntronPeaks_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBW/ATAC_merged.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed -b 1000 -a 1000 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/ATAC_myPeaksIntron_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/ATAC_myPeaksIntron_Nompfilt.gz --refPointLabel "Called Intronic PAS" --plotTitle "ATAC-seq at Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/ATAC_myPeaksIntronNompfilt.png3’ UTR peaks
Do it with the 3’ UTR peaks
Find the scripts for the UTR only peaks
processGenLocPeakAnno2SAF_3UTRonly.py
I will subset Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_3UTR.SAF for those in the 5percent coverage.
The peak needs to be in /project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.SAF
filter_UTRpeak_5perc.py
fiveperc=open("/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed.5percCov.SAF","r")
utr=open("/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc_3UTR/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_3UTR.SAF","r")
outBed=open("/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc_3UTR/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5perc_3UTR.bed","w")
#make ok dic
okPeak={}
for i, ln in enumerate(fiveperc):
if i >0:
peak=ln.split()[0]
okPeak[peak]=""
#filter and write bed
for i,ln in enumerate(utr):
if i > 0:
peak = ln.split()[0]
if peak in okPeak.keys():
num=peak.split(":")[0]
gene=peak.split(":")[-1]
chrom=ln.split()[1]
start=ln.split()[2]
end=ln.split()[3]
strand=ln.split()[4]
id=num + ":" + gene
score="."
outBed.write("%s\t%s\t%s\t%s\t%s\t%s\n"%(chrom, start, end,id,score,strand ))
outBed.close()This is 20 thousand peaks.
Fix strand:
fix3UTRPeakStrand.py
In="/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc_3UTR/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5perc_3UTR.bed"
Out="/project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc_3UTR/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5perc_FixedStrand_3UTR.bed"
def fix_strand(Fin,Fout):
fout=open(Fout,"w")
for ln in open(Fin, "r"):
chrom, start, end, name, score, strand = ln.split()
if strand=="+":
fout.write("%s\t%s\t%s\t%s\t%s\t-\n"%(chrom,start,end,name,score))
else:
fout.write("%s\t%s\t%s\t%s\t%s\t+\n"%(chrom,start,end,name,score))
fout.close()
fix_strand(In, Out)
Make the deep tools plots:
- Nuclear RNA
- ATAC
ATACDTPlotUTRPeaks_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=ATACDTPlotUTRPeaks_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=ATACDTPlotUTRPeaks_noMPFilt.out
#SBATCH --error=ATACDTPlotUTRPeaks_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/ATAC_mergeBW/ATAC_merged.sort.bw -R /project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc_3UTR/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5perc_FixedStrand_3UTR.bed -b 300 -a 300 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/ATAC_myPeaksUTR_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/ATAC_myPeaksUTR_Nompfilt.gz --refPointLabel "Called 3' UTR PAS" --plotTitle "ATAC-seq at 3' UTR PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/ATAC_myPeaksUTR_Nompfilt.png NucRNADTPlotmyUTR_noMPFilt.sh
#!/bin/bash
#SBATCH --job-name=NucRNADTPlotmyUTR_noMPFilt
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=NucRNADTPlotmyUTR_noMPFilt.out
#SBATCH --error=NucRNADTPlotmyUTR_noMPFilt.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/threeprimeseq/data/mergedPeaks_noMP_GeneLoc_3UTR/Filtered_APApeaks_merged_allchrom_noMP.sort.named.noCHR_geneLocParsed_5perc_FixedStrand_3UTR.bed -b 300 -a 300 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksUTR_Nompfilt.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksUTR_Nompfilt.gz --refPointLabel "Called 3' UTR PAS" --plotTitle "Combined Reads at 3' UTR PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksUTRNompfilt.pngFilter internal to higher usage:
I need to find the usage for each of the peaks and take only the top 200 intronic by average usage. I can do this in R.
I need the file with the average usage.
I can do this based on the nuclear peaks
filterTop200UsageNuclearIntronPeaks.R
library(tidyverse)
#get nuclear mean usage
nuclearPeakUs=read.table("/project2/gilad/briana/threeprimeseq/data/phenotypes_filtPeakTranscript_noMP_GeneLocAnno/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno_NoMP_sm_quant.Nuclear.fixed.pheno.fc", header = T, stringsAsFactors = F) %>% separate(chrom, sep = ":", into = c("chr", "start", "end", "id")) %>% separate(id, sep="_", into=c("gene", "strand", "peak"))
ind=colnames(nuclearPeakUs)[7:dim(nuclearPeakUs)[2]]
nuclearPeakUs_CountNum=read.table("/project2/gilad/briana/threeprimeseq/data/phenotypes_filtPeakTranscript_noMP_GeneLocAnno/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno_NoMP_sm_quant.Nuclear.fixed.pheno.CountsOnlyNumeric.txt", col.names = ind)
nuclearPeak=as.data.frame(cbind(nuclearPeakUs[,1:6], nuclearPeakUs_CountNum))
nuclearPeakUs_CountNum_mean=rowMeans(nuclearPeakUs_CountNum)
NuclearPeakUSMean=as.data.frame(cbind(nuclearPeakUs[,1:6],nuclearPeakUs_CountNum_mean))
#load in intron peaks
intron=read.table("/project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed", stringsAsFactors = F, col.names = c("ch", "start", "end", "loc", "score", "strand")) %>% mutate(chr=paste("chr", ch, sep=""))
NuclearPeakUSMean$start=as.integer(NuclearPeakUSMean$start)
NuclearPeakUSMean$end=as.integer(NuclearPeakUSMean$end)
#join
intron_usage= intron %>% inner_join(NuclearPeakUSMean, by=c("chr","start", "end")) %>% select(ch, start, end, loc, score, strand.x, nuclearPeakUs_CountNum_mean) %>% arrange(desc(nuclearPeakUs_CountNum_mean)) %>% select(-nuclearPeakUs_CountNum_mean) %>%slice(1:200)
write.table(intron_usage, file="/project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage.bed", quote=F, row.names = F, col.names = F)DO this for total as well
filterTop200UsageNuclearIntronPeaks_total.R
library(tidyverse)
#get nuclear mean usage
totalPeakUs=read.table("/project2/gilad/briana/threeprimeseq/data/phenotypes_filtPeakTranscript_noMP_GeneLocAnno/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno_NoMP_sm_quant.Total.fixed.pheno.fc", header = T, stringsAsFactors = F) %>% separate(chrom, sep = ":", into = c("chr", "start", "end", "id")) %>% separate(id, sep="_", into=c("gene", "strand", "peak"))
ind=colnames(totalPeakUs)[7:dim(totalPeakUs)[2]]
totalPeakUs_CountNum=read.table("/project2/gilad/briana/threeprimeseq/data/phenotypes_filtPeakTranscript_noMP_GeneLocAnno/filtered_APApeaks_merged_allchrom_refseqGenes.GeneLocAnno_NoMP_sm_quant.Total.fixed.pheno.CountsOnlyNumeric.txt", col.names = ind)
totalPeak=as.data.frame(cbind(totalPeakUs[,1:6], totalPeakUs_CountNum))
totalPeakUs_CountNum_mean=rowMeans(totalPeakUs_CountNum)
TotalPeakUSMean=as.data.frame(cbind(totalPeakUs[,1:6],totalPeakUs_CountNum_mean))
#load in intron peaks
intron=read.table("/project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON.bed", stringsAsFactors = F, col.names = c("ch", "start", "end", "loc", "score", "strand")) %>% mutate(chr=paste("chr", ch, sep=""))
TotalPeakUSMean$start=as.integer(TotalPeakUSMean$start)
TotalPeakUSMean$end=as.integer(TotalPeakUSMean$end)
#join
intron_usage= intron %>% inner_join(TotalPeakUSMean, by=c("chr","start", "end")) %>% select(ch, start, end, loc, score, strand.x, totalPeakUs_CountNum_mean) %>% arrange(desc(totalPeakUs_CountNum_mean)) %>% select(-totalPeakUs_CountNum_mean)%>% slice(1:200)
write.table(intron_usage, file="/project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage_Total.bed", quote=F, row.names = F, col.names = F, sep="\t")Sort these files to use in DT:
sort -k1,1 -k2,2n /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage_Total.bed > /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage_Total.sort.bed
sort -k1,1 -k2,2n /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage.bed > /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage.Nuclear.sort.bedNucRNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150.sh
#!/bin/bash
#SBATCH --job-name=NucRNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=NucRNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150.out
#SBATCH --error=NucRNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage.Nuclear.sort.bed -b 300 -a 300 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Top200Nuc_Nompfilt150.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Top200Nuc_Nompfilt150.gz --refPointLabel "Called Intronic PAS" --plotTitle "Nuclear RNAseq at Nuclear Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Top200Nuc_Nompfilt150.pngNucRNADTPlotmyIntronPeaks_Top200Tot_noMPFilt_150.sh
#!/bin/bash
#SBATCH --job-name=NucRNADTPlotmyIntronPeaks_noMPFilt_150
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=NucRNADTPlotmyIntronPeaks_noMPFilt150.out
#SBATCH --error=NucRNADTPlotmyIntronPeaks_noMPFilt150.err
#SBATCH --partition=bigmem2
#SBATCH --mem=100G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/NuclearRNA/NuclearRNA_merged.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage.Nuclear.sort.bed -b 300 -a 300 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Top200Tot_Nompfilt150.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Top200Tot_Nompfilt150.gz --refPointLabel "Called Intronic PAS" --plotTitle "Nuclear RNAseq at Total Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/NucRNA_myPeaksIntron_Top200Tot_Nompfilt150.pngLook at these in the top nuclear in RNA seq:
RNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150.sh
#!/bin/bash
#SBATCH --job-name=RNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150
#SBATCH --account=pi-yangili1
#SBATCH --time=24:00:00
#SBATCH --output=RNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150.out
#SBATCH --error=RNADTPlotmyIntronPeaks_Top200Nuc_noMPFilt_150.err
#SBATCH --partition=broadwl
#SBATCH --mem=50G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
computeMatrix reference-point -S /project2/gilad/briana/threeprimeseq/data/rnaseq_bw/RNAseqGeuvadis_STAR_6samp_MergedBams.sort.bw -R /project2/gilad/briana/threeprimeseq/data/peaks4DT/APAPeaks_5percCov_fixedStrand_INTRON_top200Usage.Nuclear.sort.bed -b 300 -a 300 -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksIntron_Top200Nuc_Nompfilt150.gz
plotHeatmap --sortRegions descend -m /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksIntron_Top200Nuc_Nompfilt150.gz --refPointLabel "Called Intronic PAS" --plotTitle "RNAseq at Nuclear Intronic PAS" --heatmapHeight 7 --colorMap YlGnBu -out /project2/gilad/briana/threeprimeseq/data/LianoglouDeepTools/RNA_myPeaksIntron_Top200Nuc_Nompfilt150.png
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS 10.14.1
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] bindrcpp_0.2.2 cowplot_0.9.3 reshape2_1.4.3 forcats_0.3.0
[5] stringr_1.4.0 dplyr_0.7.6 purrr_0.2.5 readr_1.1.1
[9] tidyr_0.8.1 tibble_1.4.2 ggplot2_3.0.0 tidyverse_1.2.1
[13] workflowr_1.2.0
loaded via a namespace (and not attached):
[1] tidyselect_0.2.4 haven_1.1.2 lattice_0.20-35
[4] colorspace_1.3-2 htmltools_0.3.6 yaml_2.2.0
[7] rlang_0.2.2 pillar_1.3.0 glue_1.3.0
[10] withr_2.1.2 RColorBrewer_1.1-2 modelr_0.1.2
[13] readxl_1.1.0 bindr_0.1.1 plyr_1.8.4
[16] munsell_0.5.0 gtable_0.2.0 cellranger_1.1.0
[19] rvest_0.3.2 evaluate_0.13 labeling_0.3
[22] knitr_1.20 broom_0.5.0 Rcpp_0.12.19
[25] scales_1.0.0 backports_1.1.2 jsonlite_1.6
[28] fs_1.2.6 hms_0.4.2 digest_0.6.17
[31] stringi_1.2.4 grid_3.5.1 rprojroot_1.3-2
[34] cli_1.0.1 tools_3.5.1 magrittr_1.5
[37] lazyeval_0.2.1 crayon_1.3.4 whisker_0.3-2
[40] pkgconfig_2.0.2 xml2_1.2.0 lubridate_1.7.4
[43] assertthat_0.2.0 rmarkdown_1.11 httr_1.3.1
[46] rstudioapi_0.9.0 R6_2.3.0 nlme_3.1-137
[49] git2r_0.24.0 compiler_3.5.1