Getting started with tidymodels
2022-12-12
Last updated: 2022-12-12
Checks: 7 0
Knit directory: muse/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20200712) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version d5ddf67. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: r_packages_4.1.2/
Ignored: r_packages_4.2.0/
Untracked files:
Untracked: analysis/cell_ranger.Rmd
Untracked: data/ncrna_NONCODE[v3.0].fasta.tar.gz
Untracked: data/ncrna_noncode_v3.fa
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/tidymodels.Rmd) and HTML
(docs/tidymodels.html) files. If you’ve configured a remote
Git repository (see ?wflow_git_remote), click on the
hyperlinks in the table below to view the files as they were in that
past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | d5ddf67 | Dave Tang | 2022-12-12 | quiet |
| html | add50fe | Dave Tang | 2022-12-12 | Build site. |
| Rmd | 419613a | Dave Tang | 2022-12-12 | Tidymodels |
Build a model
https://www.tidymodels.org/start/models/
my_packages <- c('tidyverse', 'tidymodels', 'broom.mixed', 'dotwhisker', 'rstanarm')
for (my_package in my_packages){
if(!require(my_package, character.only = TRUE)){
install.packages(my_package)
}
library(my_package, character.only = TRUE)
}
theme_set(theme_bw())Data from Constable (1993) to explore how three different feeding regimes affect the size of sea urchins over time. The initial size of the sea urchins at the beginning of the experiment probably affects how big they grow as they are fed.
my_csv <- read_csv("https://tidymodels.org/start/models/urchins.csv", show_col_types = FALSE)
head(my_csv)# A tibble: 6 × 3
TREAT IV SUTW
<chr> <dbl> <dbl>
1 Initial 3.5 0.01
2 Initial 5 0.02
3 Initial 8 0.061
4 Initial 10 0.051
5 Initial 13 0.041
6 Initial 13 0.061Change the column names to be more descriptive and change
food_regime into a factor.
For each of the 72 urchins, we know their:
- experimental feeding regime group (
food_regime: eitherInitial,Low, orHigh), - size in milliliters at the start of the experiment
(
initial_volume), and - suture width at the end of the experiment (
width).
my_csv %>%
setNames(c("food_regime", "initial_volume", "width")) %>%
mutate(food_regime = factor(food_regime, levels = c("Initial", "Low", "High"))) -> urchins
dim(urchins)[1] 72 3Plot the data.
ggplot(
urchins,
aes(x = initial_volume,
y = width,
group = food_regime,
colour = food_regime)
) +
geom_point() +
geom_smooth(method = lm, se = FALSE) +
scale_color_viridis_d(option = "plasma", end = .7)`geom_smooth()` using formula = 'y ~ x'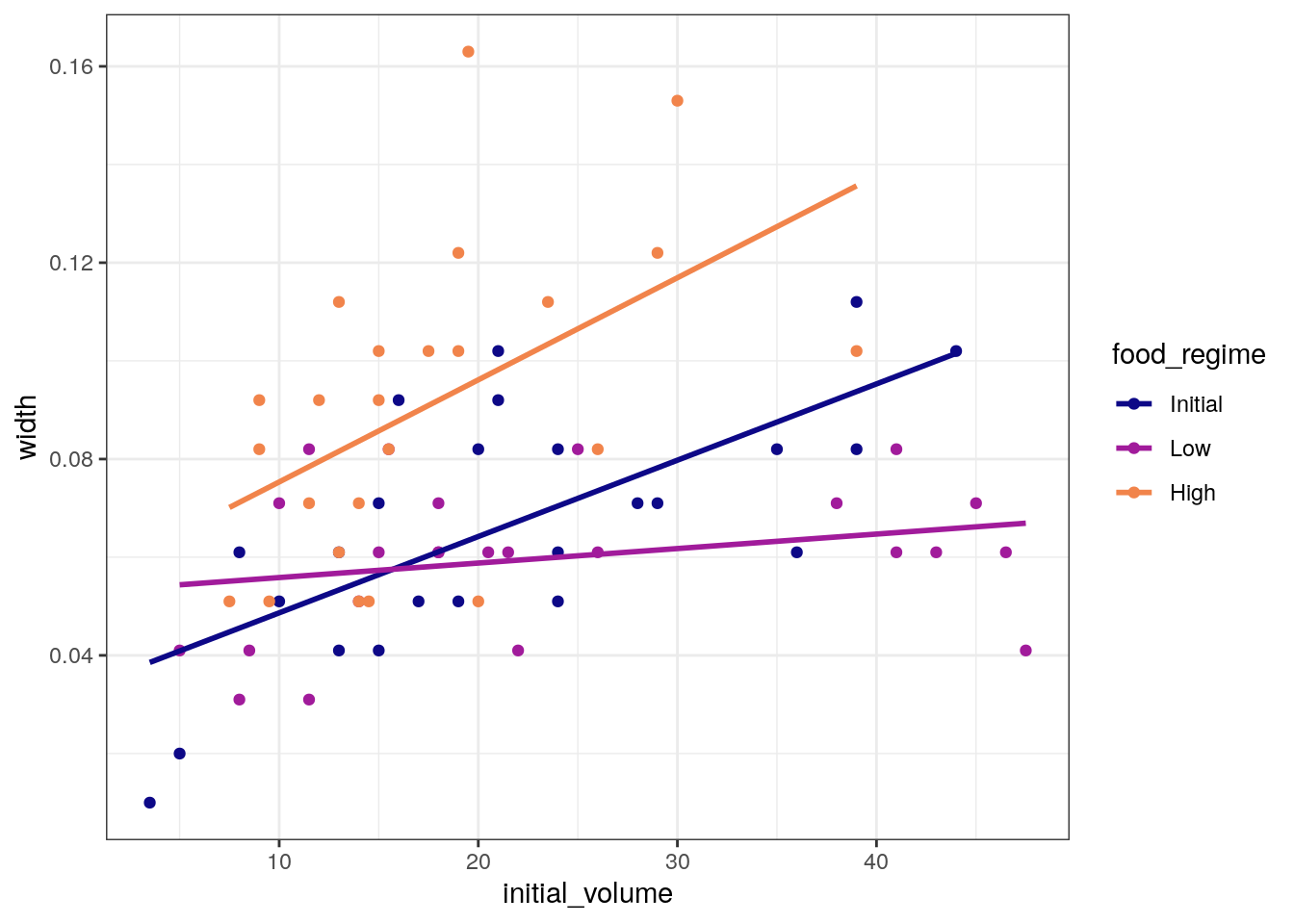
| Version | Author | Date |
|---|---|---|
| add50fe | Dave Tang | 2022-12-12 |
A standard two-way analysis of variance (ANOVA) model makes sense for
this dataset because we have both a continuous predictor
(initial_volume) and a categorical predictor
(food_regime). Since the slopes appear to be different for
at least two of the feeding regimes, let’s build a model that allows for
two-way interactions.
Specifying an R formula with our variables as defined below allows our regression model depending on initial volume to have separate slopes and intercepts for each food regime.
width ~ initial_volume * food_regimewidth ~ initial_volume * food_regimeWith tidymodels, we start by specifying the functional
form of the model that we want by using the parsnip
package. Since there is a numeric outcome and the model should be linear
with slopes and intercepts, the model type is “linear regression”.
linear_reg()Linear Regression Model Specification (regression)
Computational engine: lm Now we can think about a method for fitting or training the model,
i.e. the model engine. The engine value is often a mash-up of the
software that can be used to fit or train the model as well as the
estimation method. The default for linear_reg() is
lm for ordinary least squares. The documentation page for
linear_reg() lists all the possible engines.
We’ll save our model object as lm_mod using the default
engine.
lm_mod <- linear_reg()The model can now be estimated or trained using the
fit() function.
lm_mod %>%
fit(width ~ initial_volume * food_regime, data = urchins) -> lm_fit
lm_fitparsnip model object
Call:
stats::lm(formula = width ~ initial_volume * food_regime, data = data)
Coefficients:
(Intercept) initial_volume
0.0331216 0.0015546
food_regimeLow food_regimeHigh
0.0197824 0.0214111
initial_volume:food_regimeLow initial_volume:food_regimeHigh
-0.0012594 0.0005254 Many models have a tidy() method that provides the
summary results in a more predictable and useful format.
tidy(lm_fit)# A tibble: 6 × 5
term estimate std.error statistic p.value
<chr> <dbl> <dbl> <dbl> <dbl>
1 (Intercept) 0.0331 0.00962 3.44 0.00100
2 initial_volume 0.00155 0.000398 3.91 0.000222
3 food_regimeLow 0.0198 0.0130 1.52 0.133
4 food_regimeHigh 0.0214 0.0145 1.47 0.145
5 initial_volume:food_regimeLow -0.00126 0.000510 -2.47 0.0162
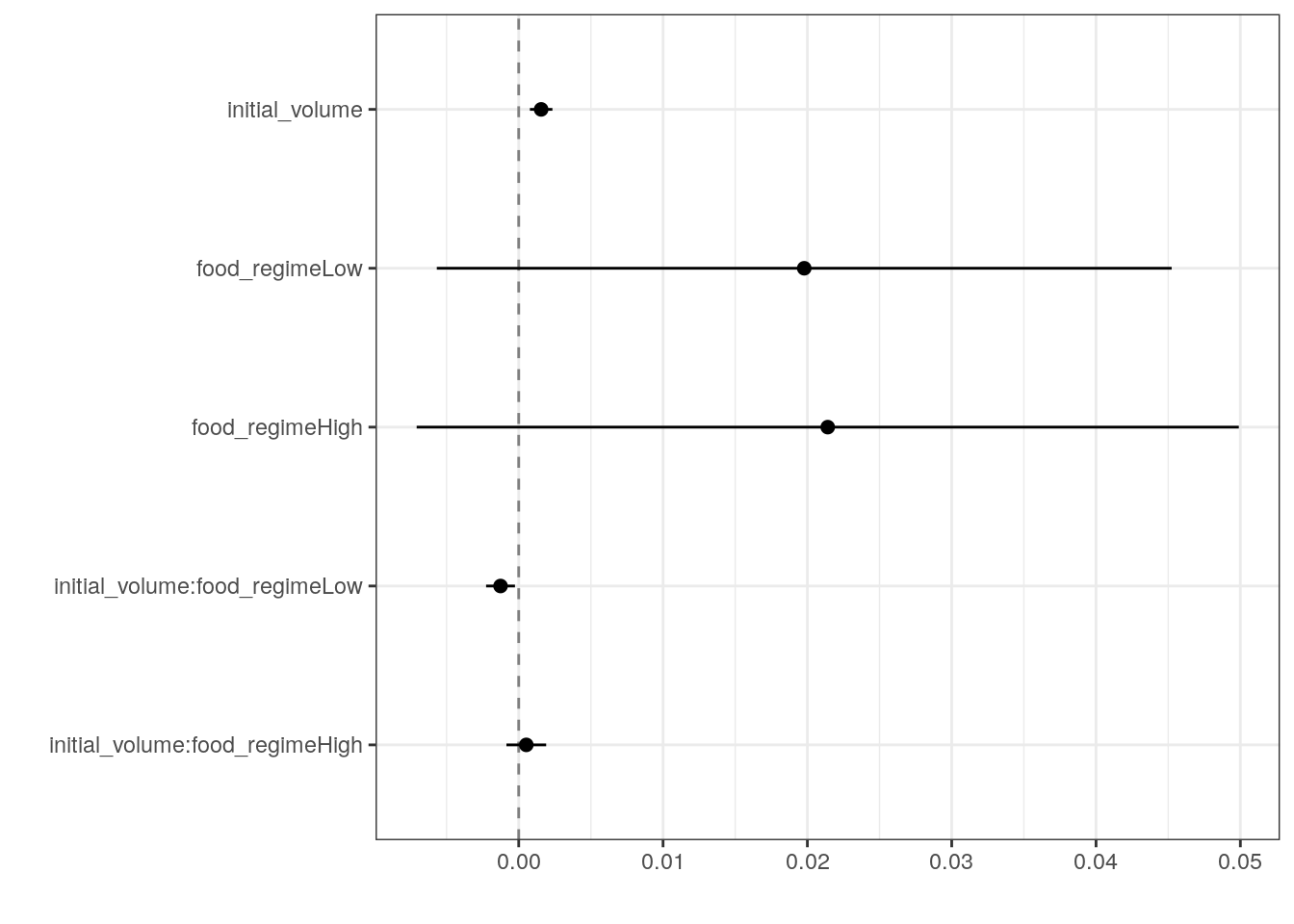
6 initial_volume:food_regimeHigh 0.000525 0.000702 0.748 0.457 Generate a dot-and-whisker plot of our regression results using the
dotwhisker package.
tidy(lm_fit) %>%
dwplot(
dot_args = list(size = 2, color = "black"),
whisker_args = list(color = "black"),
vline = geom_vline(
xintercept = 0,
colour = "grey50",
linetype = 2
)
)
| Version | Author | Date |
|---|---|---|
| add50fe | Dave Tang | 2022-12-12 |
The fitted object lm_fit has the lm model
output built-in, which you can access with lm_fit$fit, but
there are some benefits to using the fitted parsnip model
object when it comes to predicting.
Suppose that, for a publication, it would be particularly interesting to make a plot of the mean body size for urchins that started the experiment with an initial volume of 20ml. To create such a graph, we start with some new example data that we will make predictions for.
new_points <- expand.grid(
initial_volume = 20,
food_regime = c("Initial", "Low", "High")
)
new_points initial_volume food_regime
1 20 Initial
2 20 Low
3 20 HighGenerate the mean body width values.
mean_pred <- predict(lm_fit, new_data = new_points)
mean_pred# A tibble: 3 × 1
.pred
<dbl>
1 0.0642
2 0.0588
3 0.0961When making predictions, the tidymodels convention is to always produce a tibble of results with standardized column names. This makes it easy to combine the original data and the predictions in a usable format.
conf_int_pred <- predict(
lm_fit,
new_data = new_points,
type = "conf_int"
)
plot_data <-
new_points %>%
bind_cols(mean_pred) %>%
bind_cols(conf_int_pred)
ggplot(
plot_data,
aes(x = food_regime)
) +
geom_point(aes(y = .pred)) +
geom_errorbar(
aes(ymin = .pred_lower, ymax = .pred_upper),
width = .2
) +
labs(y = "urchin size")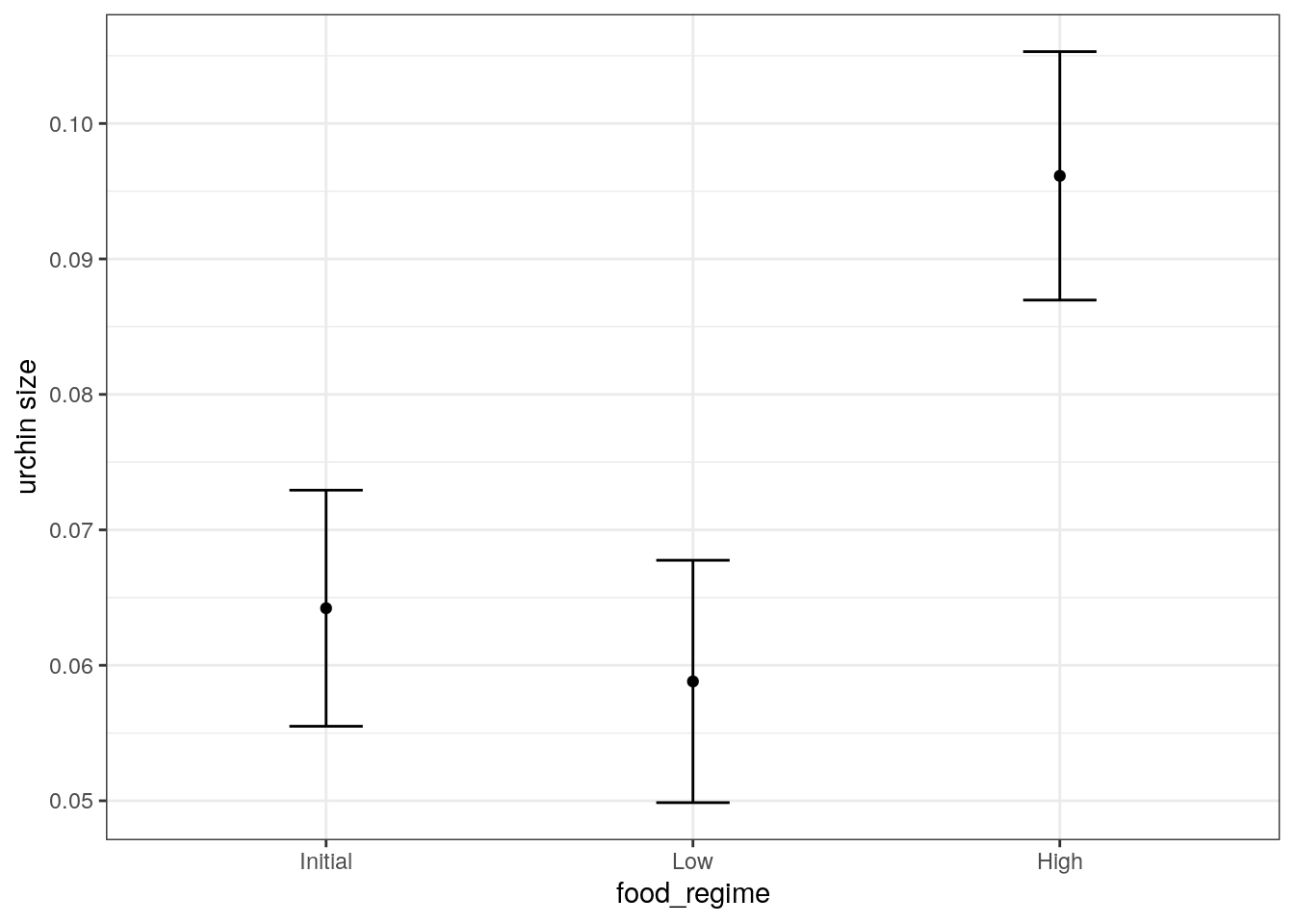
| Version | Author | Date |
|---|---|---|
| add50fe | Dave Tang | 2022-12-12 |
We are interested in knowing if the results would be different if the model were estimated using a Bayesian approach. In such an analysis, a prior distribution needs to be declared for each model parameter that represents the possible values of the parameters (before being exposed to the observed data). After some discussion, the group agrees that the priors should be bell-shaped but, since no one has any idea what the range of values should be, to take a conservative approach and make the priors wide using a Cauchy distribution (which is the same as a t-distribution with a single degree of freedom).
The documentation on the rstanarm package shows us that
the stan_glm() function can be used to estimate this model,
and that the function arguments that need to be specified are called
prior and prior_intercept.
It turns out that linear_reg() has a stan
engine. Since these prior distribution arguments are specific to the
Stan software, they are passed as arguments to
parsnip::set_engine().
# set the prior distribution
prior_dist <- rstanarm::student_t(df = 1)
set.seed(123)
# make the parsnip model
bayes_mod <-
linear_reg() %>%
set_engine("stan",
prior_intercept = prior_dist,
prior = prior_dist)
# train the model
bayes_fit <-
bayes_mod %>%
fit(width ~ initial_volume * food_regime, data = urchins)
print(bayes_fit, digits = 5)parsnip model object
stan_glm
family: gaussian [identity]
formula: width ~ initial_volume * food_regime
observations: 72
predictors: 6
------
Median MAD_SD
(Intercept) 0.03314 0.00998
initial_volume 0.00155 0.00040
food_regimeLow 0.01991 0.01332
food_regimeHigh 0.02132 0.01487
initial_volume:food_regimeLow -0.00125 0.00051
initial_volume:food_regimeHigh 0.00053 0.00072
Auxiliary parameter(s):
Median MAD_SD
sigma 0.02135 0.00185
------
* For help interpreting the printed output see ?print.stanreg
* For info on the priors used see ?prior_summary.stanregTo update the parameter table, the tidy() method is once
again used.
tidy(bayes_fit, conf.int = TRUE)# A tibble: 6 × 5
term estimate std.error conf.low conf.high
<chr> <dbl> <dbl> <dbl> <dbl>
1 (Intercept) 0.0331 0.00998 0.0167 0.0493
2 initial_volume 0.00155 0.000402 0.000891 0.00223
3 food_regimeLow 0.0199 0.0133 -0.00251 0.0418
4 food_regimeHigh 0.0213 0.0149 -0.00379 0.0462
5 initial_volume:food_regimeLow -0.00125 0.000515 -0.00212 -0.000396
6 initial_volume:food_regimeHigh 0.000533 0.000725 -0.000628 0.00170 A goal of the tidymodels packages is that the interfaces
to common tasks are standardised (as seen in the tidy()
results above). The same is true for getting predictions; we can use the
same code even though the underlying packages use very different
syntax.
bayes_plot_data <-
new_points %>%
bind_cols(predict(bayes_fit, new_data = new_points)) %>%
bind_cols(predict(bayes_fit, new_data = new_points, type = "conf_int"))
ggplot(bayes_plot_data, aes(x = food_regime)) +
geom_point(aes(y = .pred)) +
geom_errorbar(aes(ymin = .pred_lower, ymax = .pred_upper), width = .2) +
labs(y = "urchin size") +
ggtitle("Bayesian model with t(1) prior distribution")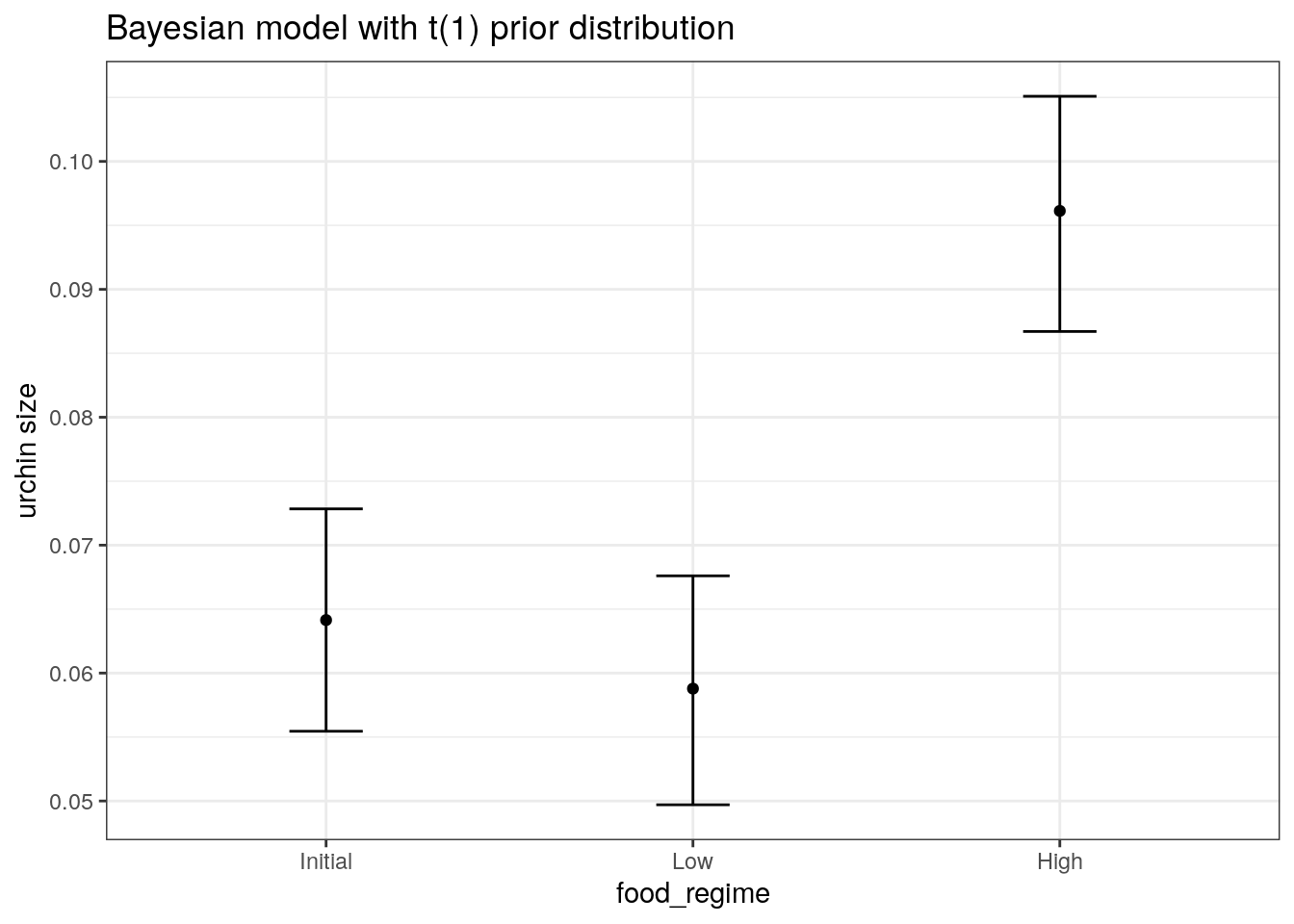
| Version | Author | Date |
|---|---|---|
| add50fe | Dave Tang | 2022-12-12 |
sessionInfo()R version 4.2.0 (2022-04-22)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Ubuntu 20.04.4 LTS
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/openblas-pthread/libblas.so.3
LAPACK: /usr/lib/x86_64-linux-gnu/openblas-pthread/liblapack.so.3
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] rstanarm_2.21.3 Rcpp_1.0.9 dotwhisker_0.7.4
[4] broom.mixed_0.2.9.4 yardstick_1.1.0 workflowsets_1.0.0
[7] workflows_1.1.2 tune_1.0.1 rsample_1.1.1
[10] recipes_1.0.3 parsnip_1.0.3 modeldata_1.0.1
[13] infer_1.0.4 dials_1.1.0 scales_1.2.1
[16] broom_1.0.1 tidymodels_1.0.0 forcats_0.5.2
[19] stringr_1.4.1 dplyr_1.0.10 purrr_0.3.5
[22] readr_2.1.3 tidyr_1.2.1 tibble_3.1.8
[25] ggplot2_3.4.0 tidyverse_1.3.2 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] utf8_1.2.2 ggstance_0.3.6 tidyselect_1.2.0
[4] lme4_1.1-31 htmlwidgets_1.5.4 grid_4.2.0
[7] munsell_0.5.0 codetools_0.2-18 DT_0.26
[10] future_1.29.0 miniUI_0.1.1.1 withr_2.5.0
[13] colorspace_2.0-3 highr_0.9 knitr_1.40
[16] rstudioapi_0.14 stats4_4.2.0 bayesplot_1.10.0
[19] listenv_0.8.0 labeling_0.4.2 git2r_0.30.1
[22] rstan_2.21.7 farver_2.1.1 bit64_4.0.5
[25] DiceDesign_1.9 datawizard_0.6.4 rprojroot_2.0.3
[28] parallelly_1.32.1 vctrs_0.5.0 generics_0.1.3
[31] ipred_0.9-13 xfun_0.34 R6_2.5.1
[34] markdown_1.3 lhs_1.1.5 cachem_1.0.6
[37] assertthat_0.2.1 promises_1.2.0.1 vroom_1.6.0
[40] nnet_7.3-18 googlesheets4_1.0.1 gtable_0.3.1
[43] globals_0.16.1 processx_3.8.0 timeDate_4021.107
[46] rlang_1.0.6 splines_4.2.0 gargle_1.2.1
[49] inline_0.3.19 yaml_2.3.6 reshape2_1.4.4
[52] modelr_0.1.10 threejs_0.3.3 crosstalk_1.2.0
[55] backports_1.4.1 httpuv_1.6.6 tools_4.2.0
[58] lava_1.7.0 ellipsis_0.3.2 jquerylib_0.1.4
[61] plyr_1.8.8 base64enc_0.1-3 ps_1.7.2
[64] prettyunits_1.1.1 rpart_4.1.19 zoo_1.8-11
[67] haven_2.5.1 fs_1.5.2 furrr_0.3.1
[70] magrittr_2.0.3 colourpicker_1.2.0 reprex_2.0.2
[73] GPfit_1.0-8 googledrive_2.0.0 whisker_0.4
[76] matrixStats_0.62.0 hms_1.1.2 shinyjs_2.1.0
[79] mime_0.12 evaluate_0.18 xtable_1.8-4
[82] shinystan_2.6.0 readxl_1.4.1 gridExtra_2.3
[85] rstantools_2.2.0 compiler_4.2.0 crayon_1.5.2
[88] minqa_1.2.5 StanHeaders_2.21.0-7 htmltools_0.5.3
[91] mgcv_1.8-41 later_1.3.0 tzdb_0.3.0
[94] RcppParallel_5.1.5 lubridate_1.8.0 DBI_1.1.3
[97] dbplyr_2.2.1 MASS_7.3-58.1 boot_1.3-28
[100] Matrix_1.5-3 cli_3.4.1 parallel_4.2.0
[103] insight_0.18.8 gower_1.0.0 igraph_1.3.5
[106] pkgconfig_2.0.3 getPass_0.2-2 xml2_1.3.3
[109] foreach_1.5.2 dygraphs_1.1.1.6 bslib_0.4.1
[112] hardhat_1.2.0 prodlim_2019.11.13 rvest_1.0.3
[115] callr_3.7.3 digest_0.6.30 parameters_0.20.0
[118] rmarkdown_2.18 cellranger_1.1.0 curl_4.3.3
[121] shiny_1.7.3 gtools_3.9.4 nloptr_2.0.3
[124] lifecycle_1.0.3 nlme_3.1-160 jsonlite_1.8.3
[127] viridisLite_0.4.1 fansi_1.0.3 pillar_1.8.1
[130] lattice_0.20-45 loo_2.5.1 fastmap_1.1.0
[133] httr_1.4.4 pkgbuild_1.4.0 survival_3.4-0
[136] glue_1.6.2 xts_0.12.2 bayestestR_0.13.0
[139] shinythemes_1.2.0 iterators_1.0.14 bit_4.0.4
[142] class_7.3-20 stringi_1.7.8 sass_0.4.2
[145] future.apply_1.10.0