Signac: Compute QC metrics on chromap quantifications
Last updated: 2022-09-14
Checks: 7 0
Knit directory: chromap_vs_cellranger_scATAC_exploration_10x/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20220912) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 10fdcb0. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
working directory clean
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/ChromapSignacQC.Rmd) and HTML (docs/ChromapSignacQC.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 10fdcb0 | jeremymsimon | 2022-09-14 | Initial commit |
Load packages and previously saved workspace
library(tidyverse)── Attaching packages ─────────────────────────────────────── tidyverse 1.3.1 ──✔ ggplot2 3.3.6 ✔ purrr 0.3.4
✔ tibble 3.1.8 ✔ dplyr 1.0.9
✔ tidyr 1.2.0 ✔ stringr 1.4.0
✔ readr 2.1.2 ✔ forcats 0.5.1── Conflicts ────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()library(GenomicRanges)Loading required package: stats4Loading required package: BiocGenerics
Attaching package: 'BiocGenerics'The following objects are masked from 'package:dplyr':
combine, intersect, setdiff, unionThe following objects are masked from 'package:stats':
IQR, mad, sd, var, xtabsThe following objects are masked from 'package:base':
anyDuplicated, append, as.data.frame, basename, cbind, colnames,
dirname, do.call, duplicated, eval, evalq, Filter, Find, get, grep,
grepl, intersect, is.unsorted, lapply, Map, mapply, match, mget,
order, paste, pmax, pmax.int, pmin, pmin.int, Position, rank,
rbind, Reduce, rownames, sapply, setdiff, sort, table, tapply,
union, unique, unsplit, which.max, which.minLoading required package: S4Vectors
Attaching package: 'S4Vectors'The following objects are masked from 'package:dplyr':
first, renameThe following object is masked from 'package:tidyr':
expandThe following objects are masked from 'package:base':
expand.grid, I, unnameLoading required package: IRanges
Attaching package: 'IRanges'The following objects are masked from 'package:dplyr':
collapse, desc, sliceThe following object is masked from 'package:purrr':
reduceLoading required package: GenomeInfoDblibrary(Seurat)Attaching SeuratObjectlibrary(Signac)
library(EnsDb.Hsapiens.v86)Loading required package: ensembldbLoading required package: GenomicFeaturesLoading required package: AnnotationDbiLoading required package: BiobaseWelcome to Bioconductor
Vignettes contain introductory material; view with
'browseVignettes()'. To cite Bioconductor, see
'citation("Biobase")', and for packages 'citation("pkgname")'.
Attaching package: 'AnnotationDbi'The following object is masked from 'package:dplyr':
selectLoading required package: AnnotationFilter
Attaching package: 'ensembldb'The following object is masked from 'package:dplyr':
filterThe following object is masked from 'package:stats':
filterload("cCRE_hg38_10x_HGMM_PBMC_chromap_fragments_MACS_q01_unionPeaks_merge100_seurat_090222.RData")HGMM
Compute QC metrics
HGMM_10x_fragmentInfo <- CountFragments(HGMM_10x_path)
rownames(HGMM_10x_fragmentInfo) <- paste0(HGMM_10x_seurat$Sample,"_",HGMM_10x_fragmentInfo$CB)
# Attach cell metadata to seurat object
HGMM_10x_seurat$fragments <- HGMM_10x_fragmentInfo[colnames(HGMM_10x_seurat), "frequency_count"]
HGMM_10x_seurat$mononucleosomal <- HGMM_10x_fragmentInfo[colnames(HGMM_10x_seurat), "mononucleosomal"]
HGMM_10x_seurat$nucleosome_free <- HGMM_10x_fragmentInfo[colnames(HGMM_10x_seurat), "nucleosome_free"]
HGMM_10x_seurat$reads_count <- HGMM_10x_fragmentInfo[colnames(HGMM_10x_seurat), "reads_count"]
# Calculate FRiP
HGMM_10x_seurat <- FRiP(
object = HGMM_10x_seurat,
assay = 'peaks',
total.fragments = "fragments"
)Calculating fraction of reads in peaks per cell# Calculate signal over excluded regions
HGMM_10x_seurat$blacklist_fraction <- FractionCountsInRegion(
object = HGMM_10x_seurat,
assay = 'peaks',
regions = blacklist_hg38
)
# Compute nucleosome signal score per cell
HGMM_10x_seurat <- NucleosomeSignal(HGMM_10x_seurat)
# Compute TSS enrichment
Annotation(HGMM_10x_seurat) <- annotations
HGMM_10x_seurat <- TSSEnrichment(HGMM_10x_seurat, fast=FALSE)Extracting TSS positionsFinding + strand cut sitesFinding - strand cut sitesComputing mean insertion frequency in flanking regionsNormalizing TSS scorePlot TSS enrichment
HGMM_10x_seurat$high.tss <- ifelse(HGMM_10x_seurat$TSS.enrichment > 2, 'High', 'Low')
TSSPlot(HGMM_10x_seurat, group.by = 'high.tss') + NoLegend()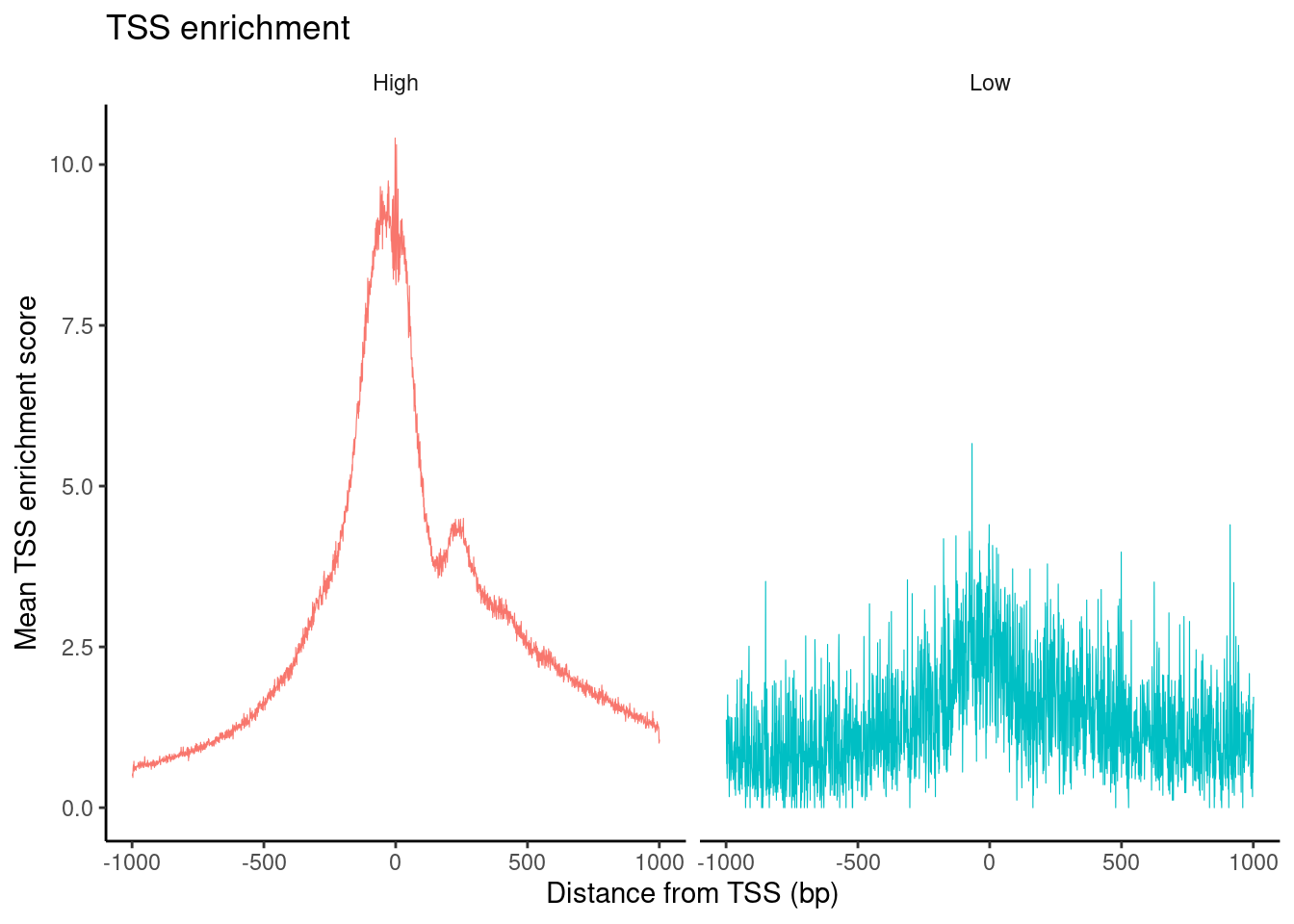
Plot nucleosome fragment length periodicity for all cells
HGMM_10x_seurat$nucleosome_group <- ifelse(HGMM_10x_seurat$nucleosome_signal > 4, 'NS > 4', 'NS < 4')
FragmentHistogram(object = HGMM_10x_seurat, group.by = 'nucleosome_group')Warning: Removed 76 rows containing non-finite values (stat_bin).Warning: Removed 4 rows containing missing values (geom_bar).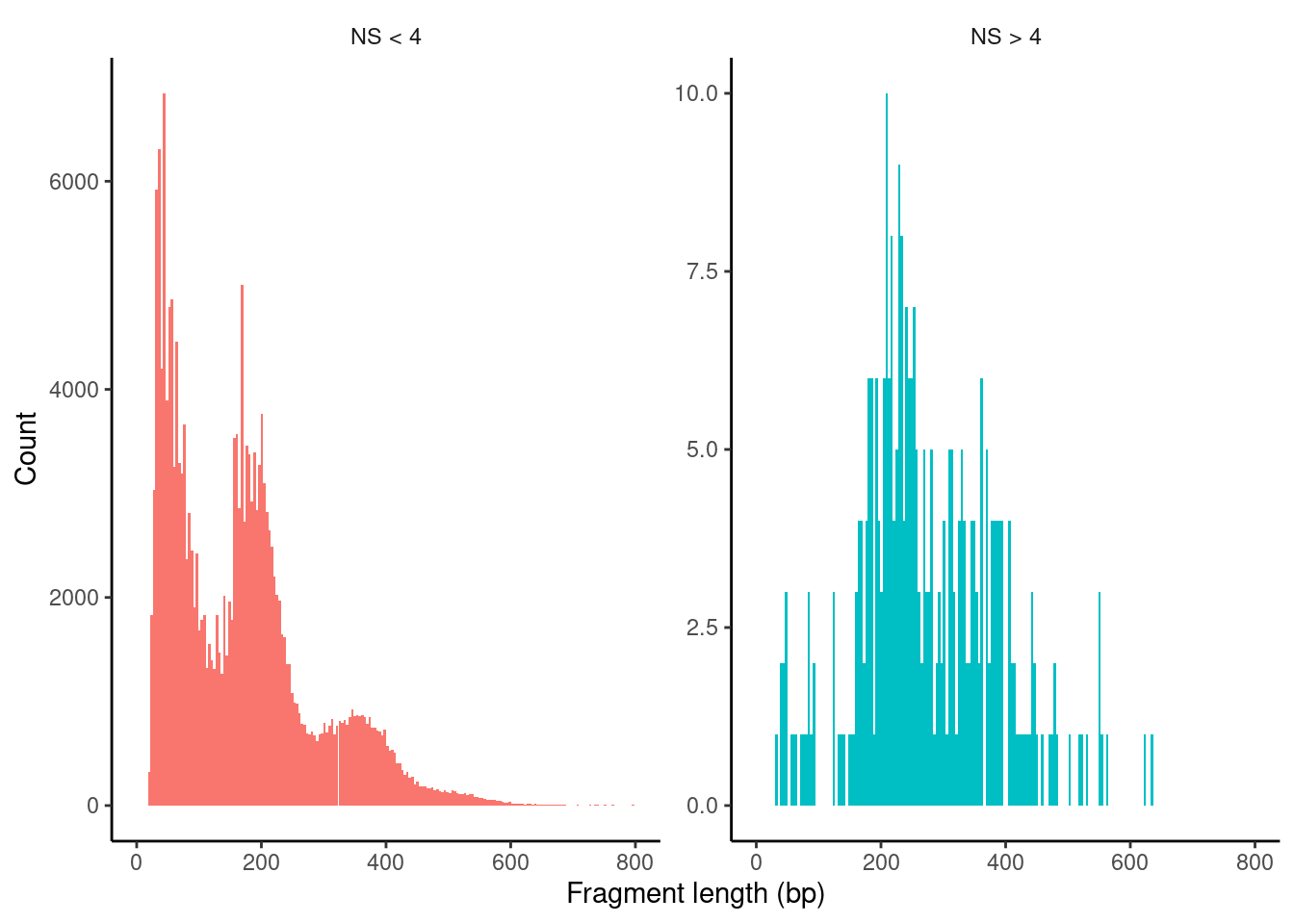
PBMC
Compute QC metrics
PBMC_10x_fragmentInfo <- CountFragments(PBMC_10x_path)
rownames(PBMC_10x_fragmentInfo) <- paste0(PBMC_10x_seurat$Sample,"_",PBMC_10x_fragmentInfo$CB)
# Attach cell metadata to seurat object
PBMC_10x_seurat$fragments <- PBMC_10x_fragmentInfo[colnames(PBMC_10x_seurat), "frequency_count"]
PBMC_10x_seurat$mononucleosomal <- PBMC_10x_fragmentInfo[colnames(PBMC_10x_seurat), "mononucleosomal"]
PBMC_10x_seurat$nucleosome_free <- PBMC_10x_fragmentInfo[colnames(PBMC_10x_seurat), "nucleosome_free"]
PBMC_10x_seurat$reads_count <- PBMC_10x_fragmentInfo[colnames(PBMC_10x_seurat), "reads_count"]
# Calculate FRiP
PBMC_10x_seurat <- FRiP(
object = PBMC_10x_seurat,
assay = 'peaks',
total.fragments = "fragments"
)Calculating fraction of reads in peaks per cell# Calculate signal over excluded regions
PBMC_10x_seurat$blacklist_fraction <- FractionCountsInRegion(
object = PBMC_10x_seurat,
assay = 'peaks',
regions = blacklist_hg38
)
# Compute nucleosome signal score per cell
PBMC_10x_seurat <- NucleosomeSignal(PBMC_10x_seurat)
# Compute TSS enrichment
Annotation(PBMC_10x_seurat) <- annotations
PBMC_10x_seurat <- TSSEnrichment(PBMC_10x_seurat, fast=FALSE)Extracting TSS positionsFinding + strand cut sitesFinding - strand cut sitesComputing mean insertion frequency in flanking regionsNormalizing TSS scorePlot TSS enrichment
PBMC_10x_seurat$high.tss <- ifelse(PBMC_10x_seurat$TSS.enrichment > 2, 'High', 'Low')
TSSPlot(PBMC_10x_seurat, group.by = 'high.tss') + NoLegend()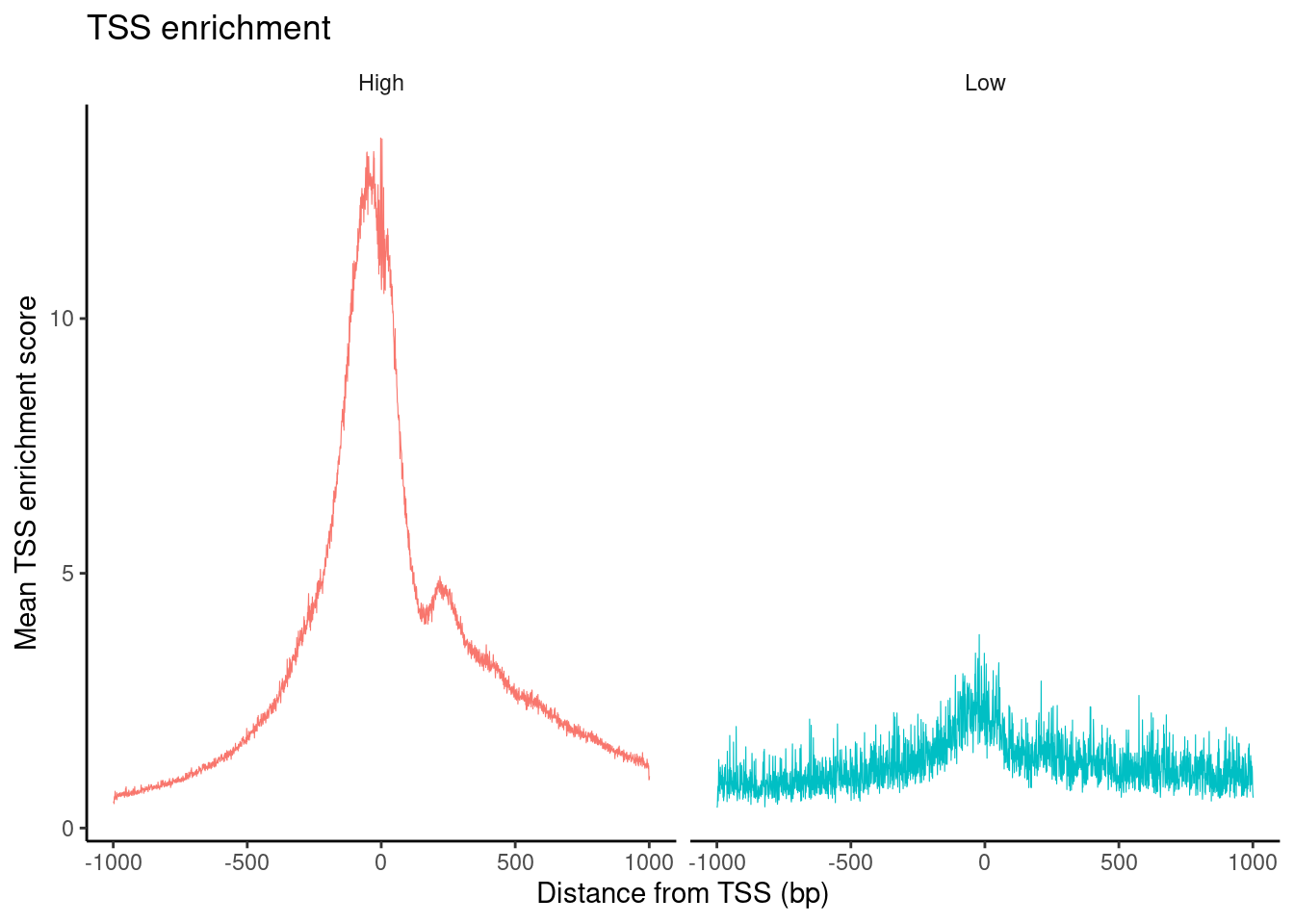
Plot nucleosome fragment length periodicity for all cells
PBMC_10x_seurat$nucleosome_group <- ifelse(PBMC_10x_seurat$nucleosome_signal > 4, 'NS > 4', 'NS < 4')
FragmentHistogram(object = PBMC_10x_seurat, group.by = 'nucleosome_group')Warning: Removed 75 rows containing non-finite values (stat_bin).Warning: Removed 4 rows containing missing values (geom_bar).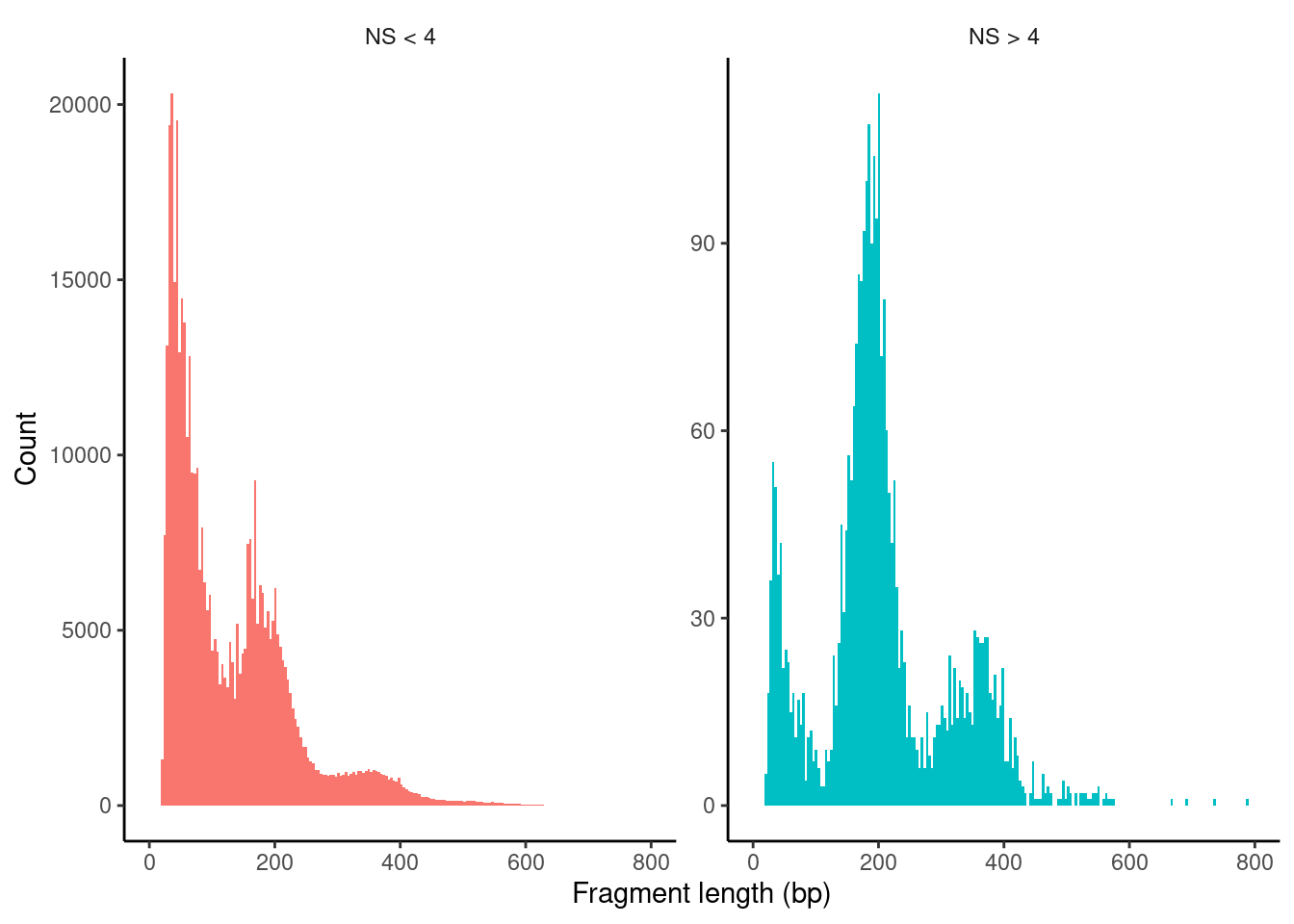
Save workspace
save.image("cCRE_hg38_10x_HGMM_PBMC_chromap_fragments_MACS_q01_unionPeaks_merge100_seurat_090222_QC.RData")
sessionInfo()R version 4.1.0 (2021-05-18)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Red Hat Enterprise Linux 8.5 (Ootpa)
Matrix products: default
BLAS/LAPACK: /nas/longleaf/rhel8/apps/r/4.1.0/lib/libopenblas_haswellp-r0.3.5.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] EnsDb.Hsapiens.v86_2.99.0 ensembldb_2.18.3
[3] AnnotationFilter_1.18.0 GenomicFeatures_1.46.5
[5] AnnotationDbi_1.56.2 Biobase_2.54.0
[7] Signac_1.7.0.9003 SeuratObject_4.0.4
[9] Seurat_4.1.0 GenomicRanges_1.46.1
[11] GenomeInfoDb_1.30.1 IRanges_2.28.0
[13] S4Vectors_0.32.4 BiocGenerics_0.40.0
[15] forcats_0.5.1 stringr_1.4.0
[17] dplyr_1.0.9 purrr_0.3.4
[19] readr_2.1.2 tidyr_1.2.0
[21] tibble_3.1.8 ggplot2_3.3.6
[23] tidyverse_1.3.1 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] utf8_1.2.2 reticulate_1.25
[3] tidyselect_1.1.2 RSQLite_2.2.10
[5] htmlwidgets_1.5.4 grid_4.1.0
[7] BiocParallel_1.28.3 Rtsne_0.15
[9] munsell_0.5.0 codetools_0.2-18
[11] ica_1.0-2 future_1.24.0
[13] miniUI_0.1.1.1 withr_2.5.0
[15] spatstat.random_2.1-0 colorspace_2.0-3
[17] filelock_1.0.2 highr_0.9
[19] knitr_1.37 rstudioapi_0.13
[21] ROCR_1.0-11 tensor_1.5
[23] listenv_0.8.0 labeling_0.4.2
[25] MatrixGenerics_1.6.0 git2r_0.30.1
[27] GenomeInfoDbData_1.2.7 polyclip_1.10-0
[29] farver_2.1.0 bit64_4.0.5
[31] rprojroot_2.0.2 parallelly_1.30.0
[33] vctrs_0.4.1 generics_0.1.2
[35] xfun_0.30 BiocFileCache_2.2.1
[37] R6_2.5.1 DelayedArray_0.20.0
[39] bitops_1.0-7 spatstat.utils_2.3-0
[41] cachem_1.0.6 assertthat_0.2.1
[43] BiocIO_1.4.0 promises_1.2.0.1
[45] scales_1.2.0 gtable_0.3.0
[47] globals_0.14.0 processx_3.5.2
[49] goftest_1.2-3 rlang_1.0.4
[51] RcppRoll_0.3.0 splines_4.1.0
[53] rtracklayer_1.54.0 lazyeval_0.2.2
[55] spatstat.geom_2.3-2 broom_1.0.0
[57] yaml_2.3.5 reshape2_1.4.4
[59] abind_1.4-5 modelr_0.1.8
[61] backports_1.4.1 httpuv_1.6.5
[63] tools_4.1.0 ellipsis_0.3.2
[65] spatstat.core_2.4-0 jquerylib_0.1.4
[67] RColorBrewer_1.1-3 ggridges_0.5.3
[69] Rcpp_1.0.8.3 plyr_1.8.7
[71] progress_1.2.2 zlibbioc_1.40.0
[73] RCurl_1.98-1.6 prettyunits_1.1.1
[75] ps_1.6.0 rpart_4.1.16
[77] deldir_1.0-6 pbapply_1.5-0
[79] cowplot_1.1.1 zoo_1.8-9
[81] SummarizedExperiment_1.24.0 haven_2.4.3
[83] ggrepel_0.9.1 cluster_2.1.2
[85] fs_1.5.2 magrittr_2.0.2
[87] data.table_1.14.2 scattermore_0.8
[89] lmtest_0.9-40 reprex_2.0.1
[91] RANN_2.6.1 whisker_0.4
[93] ProtGenerics_1.26.0 fitdistrplus_1.1-6
[95] matrixStats_0.62.0 hms_1.1.1
[97] patchwork_1.1.1 mime_0.12
[99] evaluate_0.15 xtable_1.8-4
[101] XML_3.99-0.9 readxl_1.3.1
[103] gridExtra_2.3 biomaRt_2.50.3
[105] compiler_4.1.0 KernSmooth_2.23-20
[107] crayon_1.5.1 htmltools_0.5.2
[109] mgcv_1.8-40 later_1.3.0
[111] tzdb_0.2.0 lubridate_1.8.0
[113] DBI_1.1.2 dbplyr_2.1.1
[115] rappdirs_0.3.3 MASS_7.3-55
[117] Matrix_1.4-0 cli_3.3.0
[119] parallel_4.1.0 igraph_1.3.3
[121] pkgconfig_2.0.3 GenomicAlignments_1.30.0
[123] getPass_0.2-2 plotly_4.10.0
[125] spatstat.sparse_2.1-0 xml2_1.3.3
[127] bslib_0.3.1 XVector_0.34.0
[129] rvest_1.0.2 callr_3.7.0
[131] digest_0.6.29 sctransform_0.3.3
[133] RcppAnnoy_0.0.19 spatstat.data_2.1-2
[135] Biostrings_2.62.0 rmarkdown_2.12
[137] cellranger_1.1.0 leiden_0.3.9
[139] fastmatch_1.1-3 uwot_0.1.11
[141] restfulr_0.0.13 curl_4.3.2
[143] shiny_1.7.1 Rsamtools_2.10.0
[145] rjson_0.2.21 lifecycle_1.0.1
[147] nlme_3.1-155 jsonlite_1.8.0
[149] viridisLite_0.4.0 fansi_1.0.3
[151] pillar_1.7.0 lattice_0.20-45
[153] KEGGREST_1.34.0 fastmap_1.1.0
[155] httr_1.4.2 survival_3.2-13
[157] glue_1.6.2 png_0.1-7
[159] bit_4.0.4 stringi_1.7.6
[161] sass_0.4.0 blob_1.2.2
[163] memoise_2.0.1 irlba_2.3.5
[165] future.apply_1.8.1