Visualising metagenome differences
Philipp Bayer
20th Nov 2023
Last updated: 2023-11-21
Checks: 6 1
Knit directory:
Amphibolis_Posidonia_Comparison/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of
the R Markdown file created these results, you’ll want to first commit
it to the Git repo. If you’re still working on the analysis, you can
ignore this warning. When you’re finished, you can run
wflow_publish to commit the R Markdown file and build the
HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20210414) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version aa839e6. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .Rproj.user/
Ignored: renv/library/
Ignored: renv/staging/
Untracked files:
Untracked: data/metagenome/MAG_counts.tsv
Unstaged changes:
Modified: analysis/metagenome.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/metagenome.Rmd) and HTML
(docs/metagenome.html) files. If you’ve configured a remote
Git repository (see ?wflow_git_remote), click on the
hyperlinks in the table below to view the files as they were in that
past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | aa839e6 | Philipp Bayer | 2023-11-21 | add missing ifles |
| html | aa839e6 | Philipp Bayer | 2023-11-21 | add missing ifles |
| Rmd | 52025b5 | Philipp Bayer | 2023-11-21 | Final figures and changes |
| html | 52025b5 | Philipp Bayer | 2023-11-21 | Final figures and changes |
| html | 1d2eef4 | Philipp Bayer | 2023-11-20 | Build site. |
| Rmd | 3ae555e | Philipp Bayer | 2023-11-20 | workflowr::wflow_publish(files = c("analysis/index.Rmd", "analysis/metagenome.Rmd")) |
| Rmd | b5757ac | Philipp Bayer | 2023-11-20 | Expand metagenome analysis |
| html | b5757ac | Philipp Bayer | 2023-11-20 | Expand metagenome analysis |
| Rmd | bf735ee | Philipp Bayer | 2023-11-20 | Add reformatted CAT for easier plotting |
| Rmd | f0e4ba7 | Philipp Bayer | 2023-11-20 | add metagenomics files. add renv. |
library(tidyverse)
library(microshades)
library(ggtext)
library(scales)
knitr::opts_knit$set(root.dir = rprojroot::find_rstudio_root_file())trunc_str <- function(latin_name) {
tmp <- str_split(latin_name, pattern = ' ')[[1]]
return(paste0(substring(tmp[1], 1, 1), '. ', tmp[2]))
}Here we compare metagenomes (taxonomy and genes) across Amphibolis vs the others.
metadata <- read_tsv('./data/metagenome/samples.tsv')Rows: 64 Columns: 4
── Column specification ────────────────────────────────────────────────────────
Delimiter: "\t"
chr (3): Sample, Species, Tissue
dbl (1): Replicate
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.metadata <- metadata |> rename(Sample_species = Species,
Sample_replicate = Replicate) |>
mutate(Tissue = str_to_title(Tissue))Taxonomies
We have two taxonomies: one based on CAT, one based on GTDB. GTDB is less complete but more accurate. Let’s see what they look like.
GTDB
tax <- read_tsv('./data/metagenome/all_gtdb.summary.tsv.gz')Rows: 520 Columns: 20
── Column specification ────────────────────────────────────────────────────────
Delimiter: "\t"
chr (17): user_genome, classification, fastani_reference, fastani_reference_...
dbl (3): msa_percent, translation_table, red_value
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.Need to put some extra work in; MAGs with no classification are not in the output file.
all_mag_counts <- read_tsv('./data/metagenome/MAG_counts.tsv') |> mutate(Sample = str_replace(Sample, '_L2', '')) |>
rename(TotalMAGs = MAGs)Rows: 64 Columns: 2
── Column specification ────────────────────────────────────────────────────────
Delimiter: "\t"
chr (1): Sample
dbl (1): MAGs
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.clean_tax <- tax |>
separate(user_genome, into = c('sample', 'MAG'), sep='\\.') |>
separate(classification, into = c('domain', 'phylum',
'class', 'order',
'family', 'genus',
'species'), sep=';') |>
mutate(sample = str_replace_all(sample, 'MEGAHIT-', ''),
sample = str_replace_all(sample, '_L2', ''))
clean_tax <- clean_tax |> left_join(metadata, by = c('sample'='Sample'))Next, we keep only Posidonia australis and Amphibolis antarctica samples
amph_pos_tax <- clean_tax |> filter(Sample_species %in% c('Amphibolis antarctica', 'Posidonia australis')) |>
left_join(all_mag_counts, by = c('sample'='Sample'))GTDB by order
colors <-c(microshades_palette("micro_blue", 4, lightest = FALSE),
microshades_palette("micro_purple", 4, lightest = FALSE),
microshades_palette("micro_green", 4, lightest = FALSE),
microshades_palette("micro_orange", 4, lightest = FALSE))plot_orders_gtdb <- function(amph_pos_tax, group_remainder = TRUE) {
temp <- amph_pos_tax |>
group_by(sample, Tissue, Sample_species,TotalMAGs) |>
mutate(Sample_species = trunc_str(Sample_species)) |>
mutate(Sample_species = paste0('*', Sample_species, '*')) |>
mutate(order = str_replace_all(order, 'o__','')) |>
count(order, .drop = FALSE)
if(group_remainder) {
temp <- temp |>
mutate(counted_orders = case_when(n == 1 ~ 'Remainder',
TRUE ~ order))
} else {
temp <- temp |>
mutate(counted_orders = order)
}
temp2 <- temp |>
select(-order) |>
group_by(sample, Tissue, Sample_species, n, counted_orders) |>
summarise(n = sum(n)) |>
filter(counted_orders != '' )
# we need to count the unclassified MAGs that are not in the output
group_presents <- temp2 |> group_by(sample) |> summarise(classifiedMAGs = sum(n))
missings <- group_presents |>
left_join(temp |>
select(sample, TotalMAGs)) |>
unique() |>
mutate(unclassifiedMAGs = TotalMAGs - classifiedMAGs) |>
select(-c(classifiedMAGs,TotalMAGs)) |>
rename(Unclassified = unclassifiedMAGs) |>
pivot_longer(-c(sample, Tissue, Sample_species)) |>
rename(counted_orders=name, n=value)
temp2 <-rbind(temp2, missings)
uniques <- c(setdiff(unique(temp2$counted_orders), c('Unclassified', 'Remainder')), 'Remainder', 'Unclassified')
temp2 |>
mutate(counted_orders = factor(counted_orders, levels=uniques)) |>
mutate(sample = str_remove(sample, "[A-Z]+")) |>
mutate(sample = str_replace(sample, '31', '3')) |>
ggplot(aes(x = factor(sample, levels=rev(unique(sample))), y = n, fill=counted_orders)) +
geom_col() +
theme_minimal() +
facet_wrap(~Tissue+Sample_species, scales = 'free_y', ncol=2) +
coord_flip() +
ylab('Number of contigs') + xlab('Replicate') +
labs(fill = 'Order') +
scale_y_continuous(breaks=pretty_breaks()) +
# move legend position to bottom
theme(legend.position = "bottom",
strip.text.x = element_markdown()) +
scale_fill_manual(values = colors)
}
plot_orders_gtdb(amph_pos_tax)`summarise()` has grouped output by 'sample', 'Tissue', 'Sample_species', 'n'.
You can override using the `.groups` argument.
Adding missing grouping variables: `Tissue`, `Sample_species`
Joining with `by = join_by(sample)`
GTDB by family
amph_pos_tax |>
group_by(sample, Tissue) |>
mutate(family = str_replace_all(family, 'f__','')) |>
count(family) |>
filter ( n > 1) |>
filter(family != '' ) |>
ggplot(aes(x = sample, y = n, fill=family)) +
geom_col() +
theme_minimal() +
#theme(axis.text.x = element_text(angle = 90, hjust = 1)) +
facet_wrap(~Tissue) + coord_flip() +
ylab('Number of contigs') + xlab('Sample')
| Version | Author | Date |
|---|---|---|
| b5757ac | Philipp Bayer | 2023-11-20 |
amph_pos_tax |> group_by(sample, species) |> filter(species != 's__') |>
count(species)# A tibble: 0 × 3
# Groups: sample, species [0]
# ℹ 3 variables: sample <chr>, species <chr>, n <int>CAT
Let’s do the same using CAT
tax_cat <- read_tsv('./data/metagenome/all_cat.summary.reformatted.tsv.gz')Warning: One or more parsing issues, call `problems()` on your data frame for details,
e.g.:
dat <- vroom(...)
problems(dat)Rows: 1454 Columns: 12
── Column specification ────────────────────────────────────────────────────────
Delimiter: "\t"
chr (10): # bin, classification, lineage, lineage scores, Superkingdom, Clas...
dbl (2): number of ORFs in bin, number of ORFs classification is based on
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.clean_tax_cat <- tax_cat |>
separate(`# bin`, into = c('sample', 'MAG', 'fa'), sep='\\.') |>
mutate(sample = str_replace_all(sample, 'MEGAHIT-', ''),
sample = str_replace_all(sample, '_L2', '')) |>
select(-fa)
clean_tax_cat <- clean_tax_cat |> left_join(metadata, by = c('sample'='Sample')) |> left_join(all_mag_counts, by =c('sample'='Sample'))Next, we keep only Posidonia australis and Amphibolis antarctica samples
amph_pos_tax_cat <- clean_tax_cat |> filter(Sample_species %in% c('Amphibolis antarctica', 'Posidonia australis'))CAT by order
plot_orders_cat <-
function(amph_pos_tax_cat, group_remainder = TRUE) {
temp <- amph_pos_tax_cat |>
separate(Order, into = c('Order', 'Order_score')) |>
rename(order = Order) |>
group_by(sample, Tissue, Sample_species, TotalMAGs) |>
mutate(Sample_species = trunc_str(Sample_species)) |>
mutate(Sample_species = paste0('*', Sample_species, '*')) |>
count(order)
if (group_remainder) {
temp <- temp |>
mutate(counted_orders = case_when(n == 1 ~ 'Remainder',
TRUE ~ order))
} else {
temp <- temp |>
mutate(counted_orders = order)
}
temp2 <- temp |>
select(-order) |>
group_by(sample, Tissue, Sample_species, n, counted_orders,TotalMAGs) |>
summarise(n = sum(n)) |>
filter(counted_orders != '')
# we need to count the unclassified MAGs that are not in the output
group_presents <- temp2 |> group_by(sample) |> summarise(classifiedMAGs = sum(n))
missings <- group_presents |>
left_join(temp2) |>
select(classifiedMAGs, Tissue, Sample_species, sample, TotalMAGs)|>
unique() |>
mutate(unclassifiedMAGs = TotalMAGs - classifiedMAGs) |>
select(-c(classifiedMAGs,TotalMAGs)) |>
rename(Unclassified = unclassifiedMAGs) |>
pivot_longer(-c(sample, Tissue, Sample_species)) |>
rename(counted_orders=name, n=value)
temp2 <-rbind(temp2, missings)
uniques <- c(setdiff(unique(temp2$counted_orders), c('Unclassified', 'Remainder')), 'Remainder', 'Unclassified')
temp2 |>
mutate(counted_orders = factor(counted_orders, levels = uniques)) |>
mutate(sample = str_remove(sample, "[A-Z]+")) |>
mutate(sample = str_replace(sample, '31', '3')) |>
ggplot(aes(
x = factor(sample, levels = rev(unique(sample))),
y = n,
fill = counted_orders
)) +
geom_col() +
theme_minimal() +
facet_wrap( ~ Sample_species + Tissue, ncol = 2, scales = 'free_y') +
coord_flip() +
ylab('Number of contigs') + xlab('Replicate') +
labs(fill = 'Order') +
scale_y_continuous(breaks = pretty_breaks()) +
# move legend position to bottom
theme(legend.position = "bottom",
strip.text.x = element_markdown()) +
scale_fill_manual(values = colors)
}
plot_orders_cat(amph_pos_tax_cat)Warning: Expected 2 pieces. Additional pieces discarded in 345 rows [1, 2, 3, 4, 5, 7,
8, 11, 13, 14, 15, 17, 22, 25, 26, 27, 29, 30, 33, 34, ...].`summarise()` has grouped output by 'sample', 'Tissue', 'Sample_species', 'n',
'counted_orders'. You can override using the `.groups` argument.
Joining with `by = join_by(sample)`
CAT by family
amph_pos_tax_cat |>
separate(Family, into = c('Family', 'Family_score')) |>
group_by(sample, Tissue) |>
count(Family) |>
filter ( n > 1) |>
filter(Family != '' ) |>
ggplot(aes(x = sample, y = n, fill=Family)) +
geom_col() +
theme_minimal() +
#theme(axis.text.x = element_text(angle = 90, hjust = 1)) +
facet_wrap(~Tissue) + coord_flip() +
ylab('Number of contigs') + xlab('Sample')Warning: Expected 2 pieces. Additional pieces discarded in 178 rows [3, 4, 5, 26, 29,
30, 33, 47, 56, 75, 79, 81, 82, 84, 88, 92, 93, 95, 97, 101, ...].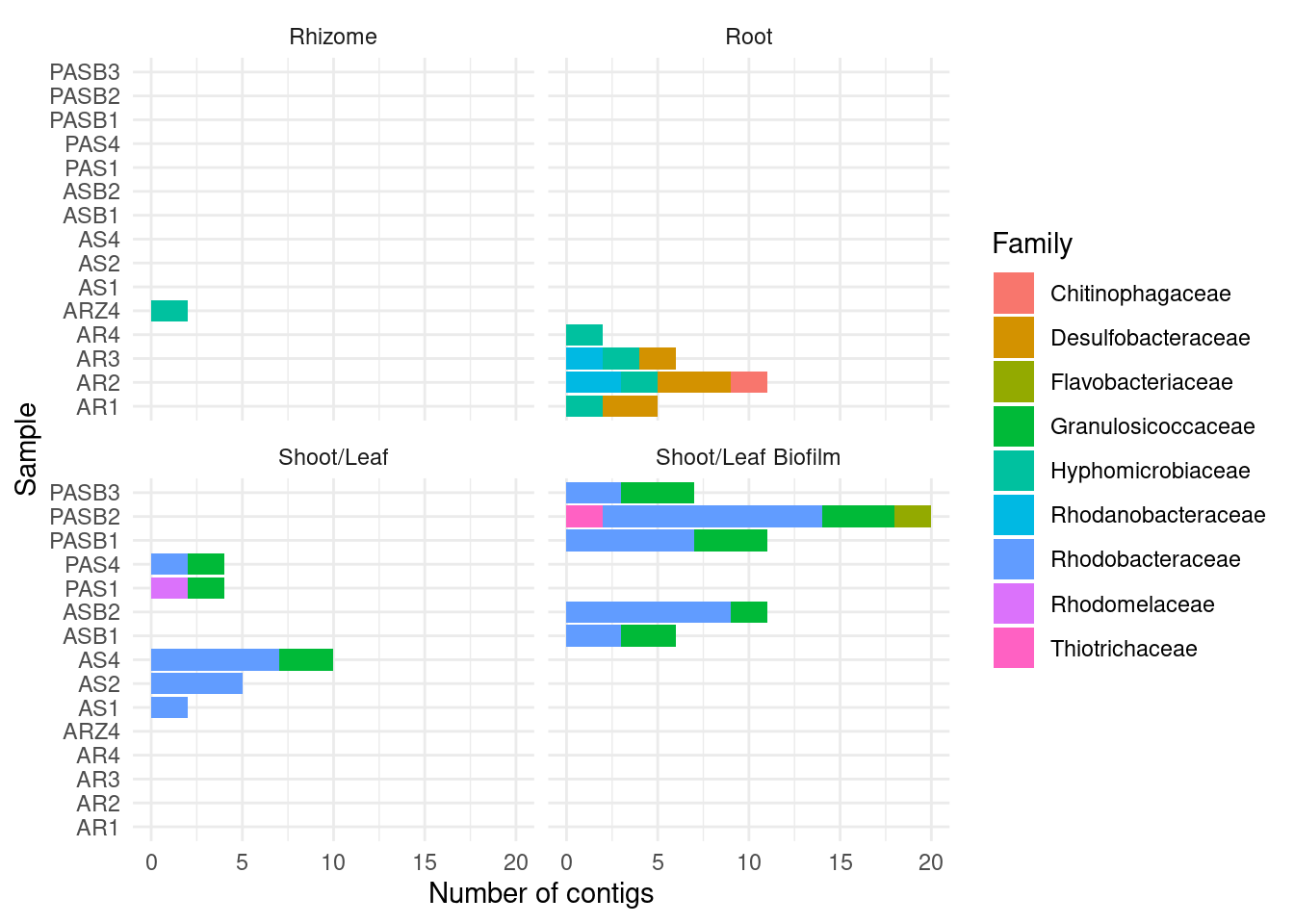
| Version | Author | Date |
|---|---|---|
| b5757ac | Philipp Bayer | 2023-11-20 |
amph_pos_tax_cat |> separate(Species, into = c('Species', 'Species_score'), sep =':') |> filter(!is.na(Species)) |> group_by(sample, Species) |>
count(Species)# A tibble: 68 × 3
# Groups: sample, Species [68]
sample Species n
<chr> <chr> <int>
1 AR1 Acidihalobacter ferrooxydans 1
2 AR1 Deltaproteobacteria bacterium 1
3 AR1 Desulfobacterales bacterium 1
4 AR1 Gammaproteobacteria bacterium 1
5 AR1 Solirubrobacterales bacterium 70-9 1
6 AR1 Spirochaetaceae bacterium 4572_59 1
7 AR2 Acidihalobacter ferrooxydans 1
8 AR2 Desulfobacterales bacterium 1
9 AR2 Flammeovirgaceae bacterium 1
10 AR2 Gammaproteobacteria bacterium 2
# ℹ 58 more rowsamph_pos_tax_cat |> separate(Species, into = c('Species', 'Species_score'), sep =':') |> filter(!is.na(Species)) |> group_by(sample, Species) |>
count(Species) |> filter(str_detect(Species, 'Acidihalobacter'))# A tibble: 4 × 3
# Groups: sample, Species [4]
sample Species n
<chr> <chr> <int>
1 AR1 Acidihalobacter ferrooxydans 1
2 AR2 Acidihalobacter ferrooxydans 1
3 AR3 Acidihalobacter ferrooxydans 1
4 AR4 Acidihalobacter ferrooxydans 1There we go :) Which MAGs are those?
amph_pos_tax_cat |> filter(str_detect(Species, 'Acidihalobacter')) |> knitr::kable()| sample | MAG | classification | number of ORFs in bin | number of ORFs classification is based on | lineage | lineage scores | Superkingdom | Class | Order | Family | Genus | Species | Sample_replicate | Sample_species | Tissue | TotalMAGs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR1 | 36 | classified | 3947 | 3706 | 1;131567;2;1224;1236;135613;72276;1765964;1765967 | 1.00;0.99;0.99;0.93;0.79;0.69;0.68;0.67;0.46 | Bacteria: 0.99 | Gammaproteobacteria: 0.79 | Chromatiales: 0.69 | Ectothiorhodospiraceae: 0.68 | Acidihalobacter: 0.67 | Acidihalobacter ferrooxydans: 0.46 | 1 | Amphibolis antarctica | Root | 76 |
| AR2 | 57 | classified | 5702 | 5052 | 1;131567;2;1224;1236;135613;72276;1765964;1765967 | 1.00;0.99;0.98;0.86;0.70;0.54;0.53;0.52;0.35 | Bacteria: 0.98 | Gammaproteobacteria: 0.70 | Chromatiales: 0.54 | Ectothiorhodospiraceae: 0.53 | Acidihalobacter: 0.52 | Acidihalobacter ferrooxydans: 0.35 | 2 | Amphibolis antarctica | Root | 79 |
| AR3 | 6 | classified | 3558 | 3381 | 1;131567;2;1224;1236;135613;72276;1765964;1765967 | 1.00;0.99;0.99;0.94;0.82;0.75;0.73;0.73;0.49 | Bacteria: 0.99 | Gammaproteobacteria: 0.82 | Chromatiales: 0.75 | Ectothiorhodospiraceae: 0.73 | Acidihalobacter: 0.73 | Acidihalobacter ferrooxydans: 0.49 | 3 | Amphibolis antarctica | Root | 87 |
| AR4 | 41 | classified | 3552 | 3379 | 1;131567;2;1224;1236;135613;72276;1765964;1765967 | 1.00;0.99;0.99;0.94;0.82;0.74;0.73;0.72;0.49 | Bacteria: 0.99 | Gammaproteobacteria: 0.82 | Chromatiales: 0.74 | Ectothiorhodospiraceae: 0.73 | Acidihalobacter: 0.72 | Acidihalobacter ferrooxydans: 0.49 | 4 | Amphibolis antarctica | Root | 62 |
amph_pos_tax_cat |> filter(str_detect(Family, 'Ectothiorhodospiraceae'))# A tibble: 4 × 17
sample MAG classification `number of ORFs in bin` number of ORFs classific…¹
<chr> <chr> <chr> <dbl> <dbl>
1 AR1 36 classified 3947 3706
2 AR2 57 classified 5702 5052
3 AR3 6 classified 3558 3381
4 AR4 41 classified 3552 3379
# ℹ abbreviated name: ¹`number of ORFs classification is based on`
# ℹ 12 more variables: lineage <chr>, `lineage scores` <chr>,
# Superkingdom <chr>, Class <chr>, Order <chr>, Family <chr>, Genus <chr>,
# Species <chr>, Sample_replicate <dbl>, Sample_species <chr>, Tissue <chr>,
# TotalMAGs <dbl>Are these in the GTDB classification?
amph_pos_tax |> filter(str_detect(family, 'Ectothiorhodospiraceae'))# A tibble: 0 × 31
# ℹ 31 variables: sample <chr>, MAG <chr>, domain <chr>, phylum <chr>,
# class <chr>, order <chr>, family <chr>, genus <chr>, species <chr>,
# fastani_reference <chr>, fastani_reference_radius <chr>,
# fastani_taxonomy <chr>, fastani_ani <chr>, fastani_af <chr>,
# closest_placement_reference <chr>, closest_placement_radius <chr>,
# closest_placement_taxonomy <chr>, closest_placement_ani <chr>,
# closest_placement_af <chr>, pplacer_taxonomy <chr>, …Nope!
Gene comparison
Let’s compare gene presence/absence by names first
genes <- read_tsv('././data/metagenome/no_hypothetical.all_genes.tsv.gz')Rows: 1362907 Columns: 8
── Column specification ────────────────────────────────────────────────────────
Delimiter: "\t"
chr (8): filename, locus_tag, ftype, length_bp, gene, EC_number, COG, product
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.genes <- genes |>
separate(filename, into = c('sample', 'MAG'), sep='\\.', extra = 'drop') |> # get rid of extra filenaming stuff
mutate(sample = str_replace(sample, 'MEGAHIT-', '')) |>
separate(MAG, into = c('MAG', 'rest'), sep ='/') |>
select(-rest)Warning: Expected 2 pieces. Missing pieces filled with `NA` in 1443 rows [2106, 4306,
7036, 7144, 7539, 10011, 12436, 12584, 13618, 17164, 17303, 19074, 21967,
22288, 28457, 30064, 32221, 32315, 34439, 34480, ...].genessplit <- genes |> separate(gene, into = c('gene', 'gene_copy_number'), sep='_')Warning: Expected 2 pieces. Missing pieces filled with `NA` in 692502 rows [2, 3, 5, 6,
7, 10, 12, 13, 14, 15, 17, 18, 25, 26, 30, 31, 33, 35, 36, 37, ...].genessplit <- genessplit |> mutate(sample = str_replace(sample, '_L2', '')) |>
left_join(metadata, by = c('sample'='Sample')) |>
filter(Sample_species %in% c('Amphibolis antarctica', 'Posidonia australis'))count_diffs <- genessplit |>
group_by(Sample_species, gene) |>
count(gene) |>
pivot_wider(names_from = Sample_species, values_from = n) |>
mutate(difference = `Amphibolis antarctica` - `Posidonia australis`) |>
arrange(desc(difference))count_diffs |> filter(`Posidonia australis` <100) |> head() |> knitr::kable()| gene | Amphibolis antarctica | Posidonia australis | difference |
|---|---|---|---|
| betI | 408 | 80 | 328 |
| korA | 396 | 76 | 320 |
| malT | 374 | 63 | 311 |
| htpG | 392 | 92 | 300 |
| mftC | 382 | 95 | 287 |
| recD2 | 362 | 78 | 284 |
We hypothesise that the ACC precursor-existing genes in Amphibolis lead to different microbiomes. ACC is broken down by ACC deaminases, which are present in Amphibolis but not Posidonia. Let’s see if we can find ACC deaminases in the metagenomes (acdS).
genessplit |> filter(gene == 'acdS') |>
group_by(Sample_species, Tissue, Sample_replicate) |>
count(gene) |>
mutate(Sample_species = trunc_str(Sample_species)) |>
mutate(Sample_species = paste0('*', Sample_species, '*')) |>
ggplot(aes(x= interaction(factor(Sample_replicate, levels=4:1), Tissue), y = n, fill = Sample_species)) +
geom_col() +
facet_wrap(~Sample_species) +
coord_flip()+ theme_minimal() +
scale_y_continuous(breaks=pretty_breaks()) +
ylab('Total number of *acdS*-containing MAGs') +
xlab('Sample species') +
theme(legend.position = 'none',
strip.text.x = element_markdown(),
axis.title.x = element_markdown())
What are those acdS-containing MAGs?
genessplit |> filter(gene == 'acdS') |> left_join(amph_pos_tax, by=c('sample', 'MAG'), multiple = 'any') |> knitr::kable()| sample | MAG | locus_tag | ftype | length_bp | gene | gene_copy_number | EC_number | COG | product | Sample_replicate.x | Sample_species.x | Tissue.x | domain | phylum | class | order | family | genus | species | fastani_reference | fastani_reference_radius | fastani_taxonomy | fastani_ani | fastani_af | closest_placement_reference | closest_placement_radius | closest_placement_taxonomy | closest_placement_ani | closest_placement_af | pplacer_taxonomy | classification_method | note | other_related_references(genome_id,species_name,radius,ANI,AF) | msa_percent | translation_table | red_value | warnings | Sample_replicate.y | Sample_species.y | Tissue.y | TotalMAGs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR1 | 20 | DKHMKEDN_06522 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AR1 | 47 | BGAOAMKE_02990 | CDS | 1017 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AR1 | 63 | NHMBFEEI_02351 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AR2 | 50 | BOJIEKPI_04502 | CDS | 1017 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 2 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AR3 | 14 | AIHODAJD_01310 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AR3 | 27 | OKIFEOJJ_02417 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AR3 | 40 | FMKMBAOD_01701 | CDS | 1206 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Amphibolis antarctica | Root | d__Bacteria | p__Desulfobacterota | c__Desulfarculia | o__Desulfarculales | f__Desulfarculaceae | g__Desulfocarbo | s__ | N/A | N/A | N/A | N/A | N/A | GCF_001184205.1 | 95.0 | d__Bacteria;p__Desulfobacterota;c__Desulfarculia;o__Desulfarculales;f__Desulfarculaceae;g__Desulfocarbo;s__Desulfocarbo indianensis | 80.56 | 0.55 | d__Bacteria;p__Desulfobacterota;c__Desulfarculia;o__Desulfarculales;f__Desulfarculaceae;g;s | taxonomic classification defined by topology and ANI | N/A | N/A | 83.32 | 11 | 0.9207634 | N/A | 3 | Amphibolis antarctica | Root | 87 |
| AR3 | 73 | KKDHNOJN_03268 | CDS | 1020 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Amphibolis antarctica | Root | d__Bacteria | p__Chloroflexota | c__Anaerolineae | o__SBR1031 | f__UBA3940 | g__ | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Chloroflexota;c__Anaerolineae;o__SBR1031;f__UBA3940;g;s | taxonomic classification fully defined by topology | N/A | N/A | 87.39 | 11 | 0.8196529 | N/A | 3 | Amphibolis antarctica | Root | 87 |
| AR3 | 81 | MCEFDNIP_01072 | CDS | 450 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AR4 | 58 | DIBNLNHP_00637 | CDS | 1017 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 4 | Amphibolis antarctica | Root | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ARZ1 | 11 | BJHMODOA_03098 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Amphibolis antarctica | Rhizome | d__Bacteria | p__Desulfobacterota | c__Desulfobacteria | o__Desulfobacterales | f__Desulfosarcinaceae | g__ | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Desulfobacterota;c__Desulfobacteria;o__Desulfobacterales;f__Desulfosarcinaceae;g;s | taxonomic classification fully defined by topology | N/A | N/A | 82.41 | 11 | 0.8240578 | Genome has more than 11.7% of markers with multiple hits | 1 | Amphibolis antarctica | Rhizome | 29 |
| ARZ2 | 17 | FMKMFIHB_02180 | CDS | 294 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 2 | Amphibolis antarctica | Rhizome | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ARZ2 | 5 | OLJLPPPD_02508 | CDS | 1014 | acdS | 1 | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 2 | Amphibolis antarctica | Rhizome | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ARZ2 | 5 | OLJLPPPD_07778 | CDS | 1017 | acdS | 2 | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 2 | Amphibolis antarctica | Rhizome | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ARZ4 | 10 | IODBOAAD_01716 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 4 | Amphibolis antarctica | Rhizome | d__Bacteria | p__Actinobacteriota | c__Acidimicrobiia | o__Acidimicrobiales | f__SZUA-35 | g__CADEDH01 | s__ | N/A | N/A | N/A | N/A | N/A | GCA_902826025.1 | 95.0 | d__Bacteria;p__Actinobacteriota;c__Acidimicrobiia;o__Acidimicrobiales;f__SZUA-35;g__CADEDH01;s__CADEDH01 sp902826025 | 78.07 | 0.37 | d__Bacteria;p__Actinobacteriota;c__Acidimicrobiia;o__Acidimicrobiales;f__SZUA-35;g;s | taxonomic classification defined by topology and ANI | N/A | N/A | 83.62 | 11 | 0.8691864 | N/A | 4 | Amphibolis antarctica | Rhizome | 23 |
| ARZ4 | 15 | MGGPOGNO_00561 | CDS | 294 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 4 | Amphibolis antarctica | Rhizome | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AS1 | 9 | LBHBPACF_04841 | CDS | 1044 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Amphibolis antarctica | Shoot/Leaf | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__Arenicellales | f__Arenicellaceae | g__ | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__Arenicellales;f__Arenicellaceae;g;s | taxonomic novelty determined using RED | N/A | N/A | 55.33 | 11 | 0.7901209 | N/A | 1 | Amphibolis antarctica | Shoot/Leaf | 21 |
| AS2 | 24 | KIMMHMKH_01726 | CDS | 759 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 2 | Amphibolis antarctica | Shoot/Leaf | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AS3 | 4 | JMLBOELL_02752 | CDS | 1038 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Amphibolis antarctica | Shoot/Leaf | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AS4 | 16 | DBHNGLOI_00802 | CDS | 786 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 4 | Amphibolis antarctica | Shoot/Leaf | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AS4 | 20 | LNPAPPIA_02154 | CDS | 1044 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 4 | Amphibolis antarctica | Shoot/Leaf | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ASB1 | 3 | OFIFNHEM_00574 | CDS | 1044 | acdS | 1 | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Amphibolis antarctica | Shoot/Leaf Biofilm | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__Granulosicoccales | f__Granulosicoccaceae | g__ | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__Granulosicoccales;f__Granulosicoccaceae;g;s | taxonomic novelty determined using RED | N/A | N/A | 50.43 | 11 | 0.8452805 | N/A | 1 | Amphibolis antarctica | Shoot/Leaf Biofilm | 20 |
| ASB1 | 3 | OFIFNHEM_03206 | CDS | 1044 | acdS | 2 | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Amphibolis antarctica | Shoot/Leaf Biofilm | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__Granulosicoccales | f__Granulosicoccaceae | g__ | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__Granulosicoccales;f__Granulosicoccaceae;g;s | taxonomic novelty determined using RED | N/A | N/A | 50.43 | 11 | 0.8452805 | N/A | 1 | Amphibolis antarctica | Shoot/Leaf Biofilm | 20 |
| ASB2 | 25 | GKJLHJPG_02215 | CDS | 1044 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 2 | Amphibolis antarctica | Shoot/Leaf Biofilm | d__Bacteria | p__Proteobacteria | c__Alphaproteobacteria | o__Rhodobacterales | f__Rhodobacteraceae | g__ | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Alphaproteobacteria;o__Rhodobacterales;f__Rhodobacteraceae;g;s | taxonomic novelty determined using RED | N/A | N/A | 32.64 | 11 | 0.8550519 | N/A | 2 | Amphibolis antarctica | Shoot/Leaf Biofilm | 28 |
| PAS1 | 10 | CHDPEFPL_01776 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Posidonia australis | Shoot/Leaf | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__Granulosicoccales | f__Granulosicoccaceae | g__Granulosicoccus | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__Granulosicoccales;f__Granulosicoccaceae;gGranulosicoccus;s | ANI | N/A | GCF_002215215.1, s__Granulosicoccus antarcticus, 95.0, 76.59, 0.05; GCA_013002205.1, s__Granulosicoccus sp013002205, 95.0, 76.29, 0.04; GCA_002746645.1, s__Granulosicoccus sp002746645, 95.0, 75.91, 0.01 | 68.04 | 11 | 0.9247789 | N/A | 1 | Posidonia australis | Shoot/Leaf | 13 |
| PAS2 | 2 | NCBGNEEP_04020 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 2 | Posidonia australis | Shoot/Leaf | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__Granulosicoccales | f__Granulosicoccaceae | g__Granulosicoccus | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__Granulosicoccales;f__Granulosicoccaceae;gGranulosicoccus;s | ANI | N/A | GCA_002746645.1, s__Granulosicoccus sp002746645, 95.0, 77.93, 0.01; GCF_002215215.1, s__Granulosicoccus antarcticus, 95.0, 76.62, 0.06; GCA_013002205.1, s__Granulosicoccus sp013002205, 95.0, 76.51, 0.06 | 63.17 | 11 | 0.9224254 | Genome has more than 17.5% of markers with multiple hits | 2 | Posidonia australis | Shoot/Leaf | 13 |
| PAS4 | 11 | BFPAMIHD_02736 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 4 | Posidonia australis | Shoot/Leaf | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__Granulosicoccales | f__Granulosicoccaceae | g__Granulosicoccus | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__Granulosicoccales;f__Granulosicoccaceae;gGranulosicoccus;s | ANI | N/A | GCA_002746645.1, s__Granulosicoccus sp002746645, 95.0, 76.81, 0.02; GCF_002215215.1, s__Granulosicoccus antarcticus, 95.0, 76.58, 0.06; GCA_013002205.1, s__Granulosicoccus sp013002205, 95.0, 76.37, 0.07 | 86.70 | 11 | 0.9211233 | N/A | 4 | Posidonia australis | Shoot/Leaf | 17 |
| PAS4 | 12 | DBFKPNEP_00192 | CDS | 1023 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 4 | Posidonia australis | Shoot/Leaf | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| PASB1 | 12 | FCNNIBFF_01476 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Posidonia australis | Shoot/Leaf Biofilm | d__Bacteria | p__Bacteroidota | c__Bacteroidia | o__Flavobacteriales | f__Flavobacteriaceae | g__Croceitalea | s__ | N/A | N/A | N/A | N/A | N/A | GCF_001306415.1 | 95.0 | d__Bacteria;p__Bacteroidota;c__Bacteroidia;o__Flavobacteriales;f__Flavobacteriaceae;g__Croceitalea;s__Croceitalea dokdonensis | 77.12 | 0.16 | d__Bacteria;p__Bacteroidota;c__Bacteroidia;o__Flavobacteriales;f__Flavobacteriaceae;gCroceitalea;s | taxonomic classification defined by topology and ANI | N/A | GCA_013001145.1, s__Croceitalea sp013001145, 95.0, 76.81, 0.2 | 91.24 | 11 | 0.9618021 | N/A | 1 | Posidonia australis | Shoot/Leaf Biofilm | 26 |
| PASB1 | 8 | APPEIGNM_00826 | CDS | 1044 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 1 | Posidonia australis | Shoot/Leaf Biofilm | d__Bacteria | p__Proteobacteria | c__Alphaproteobacteria | o__Rhodobacterales | f__Rhodobacteraceae | g__ | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Alphaproteobacteria;o__Rhodobacterales;f__Rhodobacteraceae;g;s | taxonomic classification fully defined by topology | N/A | N/A | 63.81 | 11 | 0.8362940 | N/A | 1 | Posidonia australis | Shoot/Leaf Biofilm | 26 |
| PASB3 | 5 | LBPDOBCG_01279 | CDS | 1092 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Posidonia australis | Shoot/Leaf Biofilm | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__Granulosicoccales | f__Granulosicoccaceae | g__Granulosicoccus | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__Granulosicoccales;f__Granulosicoccaceae;gGranulosicoccus;s | ANI | N/A | GCA_013002205.1, s__Granulosicoccus sp013002205, 95.0, 77.03, 0.04; GCF_002215215.1, s__Granulosicoccus antarcticus, 95.0, 76.75, 0.05; GCA_002746645.1, s__Granulosicoccus sp002746645, 95.0, 76.38, 0.02 | 76.89 | 11 | 0.9149777 | N/A | 3 | Posidonia australis | Shoot/Leaf Biofilm | 14 |
| PASB3 | 9 | PELECGGA_02313 | CDS | 1014 | acdS | NA | 3.5.99.7 | NA | 1-aminocyclopropane-1-carboxylate deaminase | 3 | Posidonia australis | Shoot/Leaf Biofilm | d__Bacteria | p__Proteobacteria | c__Gammaproteobacteria | o__UBA4575 | f__UBA4575 | g__JABDMD01 | s__ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | d__Bacteria;p__Proteobacteria;c__Gammaproteobacteria;o__UBA4575;f__UBA4575;g;s | taxonomic novelty determined using RED | N/A | N/A | 71.37 | 11 | 0.9234002 | N/A | 3 | Posidonia australis | Shoot/Leaf Biofilm | 14 |
Lots of unknown taxonomies :(
genessplit |> filter(gene == 'acdS') |> left_join(amph_pos_tax, by=c('sample', 'MAG'), multiple = 'any') |>
select(-c(Tissue.y, Sample_species.y)) |>
rename(Tissue = Tissue.x, Sample_species = Sample_species.x) |>
plot_orders_gtdb(group_remainder = FALSE)`summarise()` has grouped output by 'sample', 'Tissue', 'Sample_species', 'n'.
You can override using the `.groups` argument.
Adding missing grouping variables: `Tissue`, `Sample_species`
Joining with `by = join_by(sample)`Warning: Removed 3 rows containing missing values (`position_stack()`).
#select(sample, MAG, domain:species)genessplit |> filter(gene == 'acdS') |>
left_join(amph_pos_tax_cat, by=c('sample', 'MAG')) |>
select(-c(Tissue.y, Sample_species.y)) |>
rename(Tissue = Tissue.x, Sample_species = Sample_species.x) |>
plot_orders_cat(group_remainder = FALSE) #Warning: Expected 2 pieces. Additional pieces discarded in 19 rows [1, 7, 8, 12, 16, 17,
18, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31].`summarise()` has grouped output by 'sample', 'Tissue', 'Sample_species', 'n',
'counted_orders'. You can override using the `.groups` argument.
Joining with `by = join_by(sample)`
| Version | Author | Date |
|---|---|---|
| b5757ac | Philipp Bayer | 2023-11-20 |
#select(sample, MAG, Superkingdom:Species) |> Let’s chekc for nitrogen fixation genes (nif) - hypothesis is that Amphibolis roots contain more of these
genessplit |> filter(str_detect(gene, 'nifH') | str_detect(gene, 'acdS') | str_detect(gene, 'sctC')) |>
mutate(gene_class = case_when(str_detect(gene, 'nifH') ~ '*nifH*',
str_detect(gene, 'acdS') ~ '*acdS*',
str_detect(gene, 'sctC') ~ '*sctC*')) |>
#group_by(Sample_species, Tissue, Sample_replicate, MAG) |>
#summarise(gene_class = paste0(sort(unique(gene_class)), collapse = ' & ')) |>
group_by(Sample_species, Tissue, Sample_replicate) |>
count(gene_class) |>
mutate(Sample_species = trunc_str(Sample_species)) |>
mutate(Sample_species = paste0('*', Sample_species, '*')) |>
ggplot(aes(x=factor(Sample_species, levels=rev(c(unique(Sample_species)))), y=n, fill=gene_class)) +
geom_boxplot(outlier.shape = NA) +
facet_wrap(~factor(Tissue, levels=rev(c(unique(Tissue))))) +
geom_point(position = position_jitterdodge(), aes(color=gene_class)) +
coord_flip() +
theme_minimal() +
labs(color='MAG gene content', fill='MAG gene content') +
theme(axis.text.y = element_markdown(),legend.text = element_markdown()) +
ylab('Count of MAGs') + xlab('Species')
| Version | Author | Date |
|---|---|---|
| b5757ac | Philipp Bayer | 2023-11-20 |
Can we make a simple association test?
tmp <- genessplit |> filter(str_detect(gene, 'nifH') | str_detect(gene, 'acdS') | str_detect(gene, 'sctC')) |>
mutate(gene_class = case_when(str_detect(gene, 'nifH') ~ '*nifH*',
str_detect(gene, 'acdS') ~ '*acdS*',
str_detect(gene, 'sctC') ~ '*sctC*')) |>
group_by(Sample_species, Sample_replicate, Tissue) |>
count(gene_class) |>
mutate(Sample_species = factor(Sample_species, levels = c('Posidonia australis', 'Amphibolis antarctica')))
summary(lm(n ~ gene_class + Sample_species + Sample_replicate + Tissue, data=tmp))
Call:
lm(formula = n ~ gene_class + Sample_species + Sample_replicate +
Tissue, data = tmp)
Residuals:
Min 1Q Median 3Q Max
-25.496 -7.571 -1.483 4.858 44.164
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) -7.1173 6.5738 -1.083 0.28343
gene_class*nifH* 8.7515 5.1233 1.708 0.09295 .
gene_class*sctC* 20.1394 4.1926 4.804 1.14e-05 ***
Sample_speciesAmphibolis antarctica 10.1763 3.5162 2.894 0.00535 **
Sample_replicate -0.6348 1.5468 -0.410 0.68300
TissueRoot 13.5423 4.3568 3.108 0.00291 **
TissueShoot/Leaf -3.0185 5.0868 -0.593 0.55523
TissueShoot/Leaf Biofilm 5.2019 5.2435 0.992 0.32528
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 13.59 on 58 degrees of freedom
Multiple R-squared: 0.4475, Adjusted R-squared: 0.3809
F-statistic: 6.712 on 7 and 58 DF, p-value: 7.885e-06OK! So we have an association with gene_class, which is obvious, but we also have an association with species (great!) and root-tissue (also great!)
sessionInfo()R version 4.3.2 (2023-10-31)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Ubuntu 20.04.6 LTS
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/blas/libblas.so.3.9.0
LAPACK: /usr/lib/x86_64-linux-gnu/lapack/liblapack.so.3.9.0
locale:
[1] LC_CTYPE=en_AU.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_AU.UTF-8 LC_COLLATE=en_AU.UTF-8
[5] LC_MONETARY=en_AU.UTF-8 LC_MESSAGES=en_AU.UTF-8
[7] LC_PAPER=en_AU.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_AU.UTF-8 LC_IDENTIFICATION=C
time zone: Australia/Perth
tzcode source: system (glibc)
attached base packages:
[1] stats graphics grDevices datasets utils methods base
other attached packages:
[1] scales_1.2.1 ggtext_0.1.2 microshades_1.11 lubridate_1.9.2
[5] forcats_1.0.0 stringr_1.5.0 dplyr_1.1.2 purrr_1.0.1
[9] readr_2.1.4 tidyr_1.3.0 tibble_3.2.1 ggplot2_3.4.2
[13] tidyverse_2.0.0 workflowr_1.7.1
loaded via a namespace (and not attached):
[1] gtable_0.3.3 xfun_0.39 bslib_0.4.2
[4] processx_3.8.1 callr_3.7.3 tzdb_0.4.0
[7] vctrs_0.6.2 tools_4.3.2 ps_1.7.5
[10] generics_0.1.3 parallel_4.3.2 fansi_1.0.4
[13] highr_0.10 pkgconfig_2.0.3 lifecycle_1.0.3
[16] farver_2.1.1 compiler_4.3.2 git2r_0.32.0
[19] munsell_0.5.0 getPass_0.2-2 httpuv_1.6.11
[22] htmltools_0.5.5 sass_0.4.6 yaml_2.3.7
[25] crayon_1.5.2 later_1.3.1 pillar_1.9.0
[28] jquerylib_0.1.4 whisker_0.4.1 cachem_1.0.8
[31] commonmark_1.9.0 tidyselect_1.2.0 digest_0.6.31
[34] stringi_1.7.12 labeling_0.4.2 cowplot_1.1.1
[37] rprojroot_2.0.3 fastmap_1.1.1 grid_4.3.2
[40] colorspace_2.1-0 cli_3.6.1 magrittr_2.0.3
[43] utf8_1.2.3 withr_2.5.0 promises_1.2.0.1
[46] bit64_4.0.5 timechange_0.2.0 rmarkdown_2.21
[49] httr_1.4.6 bit_4.0.5 hms_1.1.3
[52] evaluate_0.21 knitr_1.42 markdown_1.11
[55] rlang_1.1.1 gridtext_0.1.5 Rcpp_1.0.10
[58] glue_1.6.2 BiocManager_1.30.20 xml2_1.3.4
[61] renv_1.0.2 rstudioapi_0.14 vroom_1.6.3
[64] jsonlite_1.8.4 R6_2.5.1 fs_1.6.2