Case study: Analysis of the RNA-seq data from human cell lines H1975 and HCC827
Pedro L. Baldoni
24 February, 2023
Last updated: 2023-02-24
Checks: 7 0
Knit directory:
TranscriptDE-code/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20221115) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 7b4aa30. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: ._.DS_Store
Ignored: .gitignore
Ignored: TranscriptDE-code.Rproj
Ignored: analysis/simulation-complete_cache/
Ignored: analysis/simulation-paper_cache/
Ignored: code/.DS_Store
Ignored: code/._.DS_Store
Ignored: code/lung-se/data/slurm-10685114.out
Ignored: code/lung-se/salmon/.RData
Ignored: code/lung-se/salmon/runWasabi.Rout
Ignored: code/lung-se/salmon/slurm-10685171.out
Ignored: code/lung-se/salmon/slurm-10694099.out
Ignored: code/lung/data/slurm-10678225.out
Ignored: code/lung/index/slurm-10679764.out
Ignored: code/lung/index/slurm-10679768.out
Ignored: code/lung/index/slurm-10684814.out
Ignored: code/lung/salmon/.RData
Ignored: code/lung/salmon/runWasabi.Rout
Ignored: code/lung/salmon/slurm-10681840.out
Ignored: code/lung/salmon/slurm-10681872.out
Ignored: code/lung/salmon/slurm-10684950.out
Ignored: code/lung/salmon/slurm-10694066.out
Ignored: code/pkg/.Rhistory
Ignored: code/pkg/.Rproj.user/
Ignored: code/pkg/pkg.Rproj
Ignored: code/pkg/src/RcppExports.o
Ignored: code/pkg/src/pkg.so
Ignored: code/pkg/src/rcpparma_hello_world.o
Ignored: data/.DS_Store
Ignored: data/._.DS_Store
Ignored: data/annotation/.DS_Store
Ignored: data/annotation/._.DS_Store
Ignored: data/annotation/hg38/
Ignored: data/annotation/mm39/
Ignored: data/annotation/sequins/._rnasequin_annotation_2.4.gtf
Ignored: data/annotation/sequins/._rnasequin_decoychr_2.4.fa
Ignored: data/annotation/sequins/._rnasequin_decoychr_2.4.fa.fai
Ignored: data/annotation/sequins/._rnasequin_genes_2.4.tsv
Ignored: data/annotation/sequins/._rnasequin_isoforms_2.4.tsv
Ignored: data/annotation/sequins/._rnasequin_sequences_2.4.fa
Ignored: data/lung-se/.DS_Store
Ignored: data/lung-se/._.DS_Store
Ignored: data/lung-se/fastq/
Ignored: data/lung-se/misc/._filereport_read_run_PRJNA341465_tsv.txt
Ignored: data/lung/.DS_Store
Ignored: data/lung/._.DS_Store
Ignored: data/lung/fastq/
Ignored: data/lung/index/
Ignored: data/lung/misc/._filereport_read_run_PRJNA723287_tsv.txt
Ignored: ignore/
Ignored: misc/.DS_Store
Ignored: misc/._.DS_Store
Ignored: misc/simulation-paper.Rmd/._figure2.png
Ignored: misc/simulation-paper.Rmd/._figure5.png
Ignored: output/lung-se/
Ignored: output/lung/
Ignored: output/quasi_poisson/
Ignored: output/simulation/
Unstaged changes:
Modified: analysis/_site.yml
Modified: analysis/simulation-complete.Rmd
Modified: analysis/simulation-paper.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/casestudy.Rmd) and HTML
(docs/casestudy.html) files. If you’ve configured a remote
Git repository (see ?wflow_git_remote), click on the
hyperlinks in the table below to view the files as they were in that
past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 1839ef1 | Pedro Baldoni | 2023-02-23 | Adding case study pages |
| html | 1839ef1 | Pedro Baldoni | 2023-02-23 | Adding case study pages |
Introduction
In this page, we present the analysis of the RNA-seq experiments from the human cancer cell lines H1975 and HCC827, namely the paired-end Illumina short read RNA-seq data (GSE172421), the single-end Illumina short read RNA-seq data (GSE86337) and the long read Oxford Nanopore Technologies (ONT) data (GSE172421). This report is divided in three main parts, one for each dataset. In the end, we summarize all the results and generate the plots and tables presented in the main and supplementary materials text from the edgeR with count scaling paper.
The analysis presented in this report begins with the experimental
data already quantified by Salmon. Please refer to the
script files located in the GitHub repository of this page under the
directory paths ./code/lung, ./code/lung-se,
and ./data/lung-ont, which show the commands (or the
already prepared DGEList object for ONT data) used to
quantify the RNA-seq reads from these experiments. The
targets files from these experiments are located in the
directory paths ./data/lung, ./data/lung-se,
and ./data/lung-ont, respectively.
Setup
We begin this report by setting up some options for rendering this webpage and loading the necessary libraries.
knitr::opts_chunk$set(dev = "png",
dpi = 300,
dev.args = list(type = "cairo-png"),
root.dir = '.')library(edgeR)
library(plyr)
library(AnnotationHub)
library(data.table)
library(tximeta)
library(SummarizedExperiment)
library(fishpond)
library(sleuth)
library(patchwork)
library(grid)
library(ComplexHeatmap)
library(tibble)
library(ragg)
library(ggplot2)
library(magrittr)
library(kableExtra)Paths used in this report are specified below.
path.misc <- file.path('../misc',knitr::current_input())
# dir.create(path.misc,recursive = TRUE,showWarnings = FALSE)
path.data.paired <- '../data/lung'
path.quant.paired <- '../output/lung'
path.data.single <- '../data/lung-se'
path.quant.single <- '../output/lung-se'
path.data.long <- '../data/lung-ont'Data loading
First, we load the targets data for all experiments we analyze in this report.
df.targets.short.paired <-
read.delim(file.path(path.data.paired,'misc/targets.txt'))
df.targets.short.paired$Color <-
mapvalues(df.targets.short.paired$Group,
from = c('H1975','HCC827'),to = c('blue','red'))df.targets.short.single <-
read.delim(file.path(path.data.single,'misc/targets.txt'))
df.targets.short.single$Color <-
mapvalues(df.targets.short.single$Group,
from = c('H1975','HCC827'),to = c('blue','red'))df.targets.long <-
read.delim(file.path(path.data.long,'misc/targets.txt'))
df.targets.long$Color <-
mapvalues(df.targets.long$Group,
from = c('H1975','HCC827'),to = c('blue','red'))Next, we load the output from Salmon with
catchSalmon. The ONT long read data have already been
stored in a DGEList object and is available for download in
the GitHub repository of this report.
catch.short.paired <-
catchSalmon(list.dirs(file.path(path.quant.paired,'salmon'),recursive = FALSE))Reading ../output/lung/salmon/GSM5255695, 227223 transcripts, 100 bootstraps
Reading ../output/lung/salmon/GSM5255696, 227223 transcripts, 100 bootstraps
Reading ../output/lung/salmon/GSM5255697, 227223 transcripts, 100 bootstraps
Reading ../output/lung/salmon/GSM5255704, 227223 transcripts, 100 bootstraps
Reading ../output/lung/salmon/GSM5255705, 227223 transcripts, 100 bootstraps
Reading ../output/lung/salmon/GSM5255706, 227223 transcripts, 100 bootstrapscatch.short.paired$annotation$TranscriptSymbol <-
strsplit2(rownames(catch.short.paired$counts),"\\|")[,5]
catch.short.paired$annotation$GeneID <-
strsplit2(rownames(catch.short.paired$counts),"\\|")[,2]
catch.short.paired$annotation$GeneSymbol <-
strsplit2(rownames(catch.short.paired$counts),"\\|")[,6]
catch.short.paired$samples <-
df.targets.short.paired[match(basename(colnames(catch.short.paired$counts)),
strsplit2(df.targets.short.paired$File1,"_")[,1]),]
colnames(catch.short.paired$counts) <- catch.short.paired$samples$Sample
rownames(catch.short.paired$counts) <-
rownames(catch.short.paired$annotation) <-
strsplit2(rownames(catch.short.paired$counts),"\\|")[,1]
dte.short.paired <-
DGEList(counts = catch.short.paired$counts,
group = strsplit2(colnames(catch.short.paired$counts),"\\.")[,1],
genes = catch.short.paired$annotation,
samples = catch.short.paired$samples)catch.short.single <-
catchSalmon(list.dirs(file.path(path.quant.single,'salmon'),recursive = FALSE))Reading ../output/lung-se/salmon/GSM2300448, 227223 transcripts, 100 bootstraps
Reading ../output/lung-se/salmon/GSM2300449, 227223 transcripts, 100 bootstraps
Reading ../output/lung-se/salmon/GSM2300450, 227223 transcripts, 100 bootstraps
Reading ../output/lung-se/salmon/GSM2300451, 227223 transcripts, 100 bootstrapscatch.short.single$annotation$TranscriptSymbol <-
strsplit2(rownames(catch.short.single$counts),"\\|")[,5]
catch.short.single$annotation$GeneID <-
strsplit2(rownames(catch.short.single$counts),"\\|")[,2]
catch.short.single$annotation$GeneSymbol <-
strsplit2(rownames(catch.short.single$counts),"\\|")[,6]
catch.short.single$samples <-
df.targets.short.single[match(basename(colnames(catch.short.single$counts)),
strsplit2(df.targets.short.single$File,"_")[,1]),]
colnames(catch.short.single$counts) <- catch.short.single$samples$Sample
rownames(catch.short.single$counts) <-
rownames(catch.short.single$annotation) <-
strsplit2(rownames(catch.short.single$counts),"\\|")[,1]
dte.short.single <-
DGEList(counts = catch.short.single$counts,
group = strsplit2(colnames(catch.short.single$counts),"\\.")[,1],
genes = catch.short.single$annotation,
samples = catch.short.single$samples)For the ONT data, we disregard the in-silico mixtures and only keep the pure cell lines data.
dte.long <- readRDS(file.path(path.data.long,'220928_dge.rds'))
# Keeping only pure cell lines
dte.long <- dte.long[,grepl('barcode',colnames(dte.long))]
dte.long$samples <-
cbind(dte.long$samples,
df.targets.long[match(basename(colnames(dte.long)),df.targets.long$File),])
colnames(dte.long$counts) <-
rownames(dte.long$samples) <-
dte.long$samples$Sample
dte.long$samples$group <- dte.long$samples$Group
dte.long$genes$TranscriptSymbol <- strsplit2(rownames(dte.long$genes),"\\|")[,5]
dte.long$genes$GeneID <- strsplit2(rownames(dte.long$genes),"\\|")[,2]
dte.long$genes$GeneSymbol <- strsplit2(rownames(dte.long$genes),"\\|")[,6]
rownames(dte.long$counts) <-
rownames(dte.long$genes) <- strsplit2(rownames(dte.long$counts),"\\|")[,1]The ONT long read data contain the 849 duplicated transcripts that
exist in the Gencode version 33 of the human genome hg38 (Ensembl
annotation version 99). This is because the ONT data was processed with
Salmon while keeping such duplicates in the transcriptome
index. I exclude such duplicates below.
stopifnot(nrow(dte.short.paired) == nrow(dte.short.single))
stopifnot(nrow(dte.long) - nrow(dte.short.paired) == 849)
stopifnot(rownames(dte.short.paired) %in% rownames(dte.long))
dte.long <- dte.long[rownames(dte.long) %in% rownames(dte.short.paired),]
stopifnot(rownames(dte.long) == rownames(dte.short.paired))Design
Now, we can create the design matrices (and the appropriate contrast) that will be used when assessing differential expression, both at the transcript-level and at the gene-level.
design.short.paired <- model.matrix(~0+group,data = dte.short.paired$samples)
colnames(design.short.paired) <- gsub("group","",colnames(design.short.paired))
design.short.single <- model.matrix(~0+group,data = dte.short.single$samples)
colnames(design.short.single) <- gsub("group","",colnames(design.short.single))
design.long <- model.matrix(~0+group,data = dte.long$samples)
colnames(design.long) <- gsub("group","",colnames(design.long))
contr <- makeContrasts(HCC827vsH1975 = HCC827 - H1975,levels = design.short.paired)Transcriptome annotation
Now, I will load the complete Ensemble annotation from
AnnotationHub to bring in some extra information that will
be used throughout this report. Again, all three datasets (paired-end
reads, single-end reads, and ONT long reads) were quantified with
Salmon using the Ensembl-oriented Gencode annotation
version 33 of the human genome hg38 (Ensembl version 99). We select
genes of interest for this analysis: only protein-coding and lncRNA
genes with an associated Entrez ID.
ah <- AnnotationHub()snapshotDate(): 2022-10-31edb <- ah[['AH78783']] # Ensembl version 99 relative to Gencode version 33loading from cacherequire("ensembldb")edb.names <- c("TXIDVERSION","SYMBOL","TXBIOTYPE",
"GENEIDVERSION","GENENAME","GENEBIOTYPE","ENTREZID")
dt.anno <- as.data.table(select(edb,keys(edb),edb.names))
dt.anno.gene <-
dt.anno[,.(NTranscriptPerGene = .N),
by = c('GENEIDVERSION','GENEBIOTYPE','ENTREZID','GENENAME')]
dt.anno.gene[,GeneOfInterest :=
GENEBIOTYPE %in% c('protein_coding','lncRNA') & !is.na(ENTREZID)]
key.anno <- match(dte.long$genes$GeneID,dt.anno.gene$GENEIDVERSION)
dte.short.paired$genes$EntrezID <- dt.anno.gene$ENTREZID[key.anno]
dte.short.single$genes$EntrezID <- dt.anno.gene$ENTREZID[key.anno]
dte.long$genes$EntrezID <- dt.anno.gene$ENTREZID[key.anno]
dte.short.paired$genes$GeneType <- dt.anno.gene$GENEBIOTYPE[key.anno]
dte.short.single$genes$GeneType <- dt.anno.gene$GENEBIOTYPE[key.anno]
dte.long$genes$GeneType <- dt.anno.gene$GENEBIOTYPE[key.anno]
dte.short.paired$genes$GeneOfInterest <- dt.anno.gene$GeneOfInterest[key.anno]
dte.short.single$genes$GeneOfInterest <- dt.anno.gene$GeneOfInterest[key.anno]
dte.long$genes$GeneOfInterest <- dt.anno.gene$GeneOfInterest[key.anno]
dte.short.paired$genes$GeneOfInterest[is.na(dte.short.paired$genes$GeneOfInterest)] <- FALSE
dte.short.single$genes$GeneOfInterest[is.na(dte.short.single$genes$GeneOfInterest)] <- FALSE
dte.long$genes$GeneOfInterest[is.na(dte.long$genes$GeneOfInterest)] <- FALSE
dte.short.paired$genes$NTranscriptPerGene <- dt.anno.gene$NTranscriptPerGene[key.anno]
dte.short.single$genes$NTranscriptPerGene <- dt.anno.gene$NTranscriptPerGene[key.anno]
dte.long$genes$NTranscriptPerGene <- dt.anno.gene$NTranscriptPerGene[key.anno]Analysis of Illumina paired-end short reads
The analysis of paired-end data is divided in two parts. First, we present the analysis at the transcript-level. Second, we present a gene-level analysis. All results discussed in the main paper regarding these experiments are presented in this report.
Differential transcript expression analysis
edgeR with count scaling
Below we run edgeR with scaled counts using the QL
pipeline.
dte.short.paired.edger.scaled <- dte.short.paired
dte.short.paired.edger.scaled$counts <-
dte.short.paired.edger.scaled$counts/dte.short.paired.edger.scaled$genes$Overdispersion
keep.short.paired.edger.scaled <-
filterByExpr(dte.short.paired.edger.scaled) &
(dte.short.paired.edger.scaled$genes$GeneOfInterest == TRUE)
dte.short.paired.edger.scaled.filtr <-
dte.short.paired.edger.scaled[keep.short.paired.edger.scaled,, keep.lib.sizes = FALSE]
dte.short.paired.edger.scaled.filtr <-
calcNormFactors(dte.short.paired.edger.scaled.filtr)
dte.short.paired.edger.scaled.filtr <-
estimateDisp(dte.short.paired.edger.scaled.filtr,
design = design.short.paired,robust = TRUE)
fit.short.paired.edger.scaled <-
glmQLFit(dte.short.paired.edger.scaled.filtr,design.short.paired,robust = TRUE)
qlf.short.paired.edger.scaled <-
glmQLFTest(fit.short.paired.edger.scaled,contrast = contr)
out.short.paired.edger.scaled <- topTags(qlf.short.paired.edger.scaled,n = Inf)
summary(decideTests(qlf.short.paired.edger.scaled)) -1*H1975 1*HCC827
Down 9620
NotSig 8667
Up 9079edgeR with raw counts
Below we run edgeR with raw counts using the QL
pipeline.
dte.short.paired.edger.raw <- dte.short.paired
keep.short.paired.edger.raw <-
filterByExpr(dte.short.paired.edger.raw) &
(dte.short.paired.edger.raw$genes$GeneOfInterest == TRUE)
dte.short.paired.edger.raw.filtr <-
dte.short.paired.edger.raw[keep.short.paired.edger.raw,, keep.lib.sizes = FALSE]
dte.short.paired.edger.raw.filtr <-
calcNormFactors(dte.short.paired.edger.raw.filtr)
dte.short.paired.edger.raw.filtr <-
estimateDisp(dte.short.paired.edger.raw.filtr,
design = design.short.paired,robust = TRUE)
fit.short.paired.edger.raw <-
glmQLFit(dte.short.paired.edger.raw.filtr,design.short.paired,robust = TRUE)
qlf.short.paired.edger.raw <-
glmQLFTest(fit.short.paired.edger.raw,contrast = contr)
out.short.paired.edger.raw <- topTags(qlf.short.paired.edger.raw,n = Inf)
summary(decideTests(qlf.short.paired.edger.raw)) -1*H1975 1*HCC827
Down 8722
NotSig 28799
Up 8234Swish
We then run Swish.
dt.swish <-
data.frame(paths = list.dirs(file.path(path.quant.paired,'salmon'),recursive = FALSE))
dt.swish$names <- df.targets.short.paired$Sample[match(basename(dt.swish$paths),strsplit2(df.targets.short.paired$File1,'_')[,1])]
dt.swish$files <- file.path(dt.swish$paths,'quant.sf')
dt.swish$group <-
factor(df.targets.short.paired$Group[match(basename(dt.swish$paths),
strsplit2(df.targets.short.paired$File1,'_')[,1])],levels = c("H1975","HCC827"))
dte.swish <- tximeta(coldata = dt.swish,type = 'salmon')importing quantificationsreading in files with read_tsv1 2 3 4 5 6
couldn't find matching transcriptome, returning non-ranged SummarizedExperimentmcols(dte.swish)$GeneID <- strsplit2(rownames(dte.swish),"\\|")[,2]
mcols(dte.swish)$GeneOfInterest <-
dt.anno.gene$GeneOfInterest[match(mcols(dte.swish)$GeneID ,dt.anno.gene$GENEIDVERSION)]
mcols(dte.swish)$GeneOfInterest[is.na(mcols(dte.swish)$GeneOfInterest)] <- FALSE
rownames(dte.swish) <- strsplit2(rownames(dte.swish),"\\|")[,1]
dte.swish <- scaleInfReps(dte.swish)
dte.swish <- labelKeep(dte.swish)
dte.swish <-
dte.swish[mcols(dte.swish)$keep & (mcols(dte.swish)$GeneOfInterest == TRUE),]
dte.swish <- swish(y = dte.swish, x = "group")
out.swish <- as.data.frame(mcols(dte.swish))sleuth with Wald
Now, we run sleuth with Wald test. For a fair comparison, we focus on genes of interest also with sleuth and Swish. In the code chunk below, we filter out genes that are lowly expressed (according to sleuth’s criteria, similar to what we have done with Swish) and genes that are not interesting (i.e., they are either not protein-coding or lncRNA gene, or do not have an associated Entrez ID).
dt.sleuth <-
data.frame(path = list.dirs(file.path(path.quant.paired,'salmon'),recursive = FALSE))
dt.sleuth$sample <-
df.targets.short.paired$Sample[match(basename(dt.sleuth$path),strsplit2(df.targets.short.paired$File1,'_')[,1])]
dt.sleuth$group <-
factor(df.targets.short.paired$Group[match(basename(dt.sleuth$path),strsplit2(df.targets.short.paired$File1,'_')[,1])],levels = c("H1975","HCC827"))
### Filtering for sleuth similarly to edgeR and swish, i.e. by expression (according to their own method) and only genes of interest
### (see https://github.com/pachterlab/sleuth/issues/184#issuecomment-397771403)
sleuth.matrix <-
sleuth_prep(sample_to_covariates = dt.sleuth,normalize = FALSE,filter_fun = function(x){TRUE})Warning in check_num_cores(num_cores): It appears that you are running Sleuth from within Rstudio.
Because of concerns with forking processes from a GUI, 'num_cores' is being set to 1.
If you wish to take advantage of multiple cores, please consider running sleuth from the command line.reading in kallisto resultsdropping unused factor levels......
227223 targets passed the filtersleuth.matrix <-
sleuth_to_matrix(sleuth.matrix, "obs_raw", "est_counts")Warning: `select_()` was deprecated in dplyr 0.7.0.
ℹ Please use `select()` instead.
ℹ The deprecated feature was likely used in the sleuth package.
Please report the issue at <]8;;https://github.com/pachterlab/sleuth/issueshttps://github.com/pachterlab/sleuth/issues]8;;>.Warning: `spread_()` was deprecated in tidyr 1.2.0.
ℹ Please use `spread()` instead.
ℹ The deprecated feature was likely used in the sleuth package.
Please report the issue at <]8;;https://github.com/pachterlab/sleuth/issueshttps://github.com/pachterlab/sleuth/issues]8;;>.sleuth.rownames <- strsplit2(rownames(sleuth.matrix),"\\|")[,2]
keep.sleuth <-
apply(sleuth.matrix,1,basic_filter) &
dt.anno.gene$GeneOfInterest[match(sleuth.rownames ,dt.anno.gene$GENEIDVERSION)]
dte.sleuth.wald <-
sleuth_prep(sample_to_covariates = dt.sleuth,
full_model = ~ group,
filter_target_id = rownames(sleuth.matrix)[keep.sleuth])Warning in check_num_cores(num_cores): It appears that you are running Sleuth from within Rstudio.
Because of concerns with forking processes from a GUI, 'num_cores' is being set to 1.
If you wish to take advantage of multiple cores, please consider running sleuth from the command line.reading in kallisto results
dropping unused factor levels
......
normalizing est_counts
A list of target IDs for filtering was found. Using this for filtering
57131 targets passed the filter
normalizing tpm
merging in metadata
summarizing bootstraps
......dte.sleuth.wald <- sleuth_fit(obj = dte.sleuth.wald, fit_name = 'full')fitting measurement error models
shrinkage estimation
3 NA values were found during variance shrinkage estimation due to mean observation values outside of the range used for the LOESS fit.
The LOESS fit will be repeated using exact computation of the fitted surface to extrapolate the missing values.
These are the target ids with NA values: ENST00000409702.1|ENSG00000150722.10|OTTHUMG00000154326.4|OTTHUMT00000334876.1|PPP1R1C-203|PPP1R1C|1157|protein_coding|, ENST00000432789.1|ENSG00000100413.17|OTTHUMG00000150971.2|OTTHUMT00000320706.1|POLR3H-207|POLR3H|1598|nonsense_mediated_decay|, ENST00000547886.5|ENSG00000079387.14|OTTHUMG00000169896.4|OTTHUMT00000406473.1|SENP1-203|SENP1|965|processed_transcript|
computing variance of betasdte.sleuth.wald <- sleuth_wt(obj = dte.sleuth.wald,which_beta = 'groupHCC827',which_model = 'full')
out.sleuth.wald <-
sleuth_results(obj = dte.sleuth.wald,test = 'groupHCC827',
test_type = 'wald',show_all = FALSE)sleuth with LRT
We proceed similarly with sleuth using LRT.
dte.sleuth.lrt <-
sleuth_prep(sample_to_covariates = dt.sleuth,
full_model = ~ group,
filter_target_id = rownames(sleuth.matrix)[keep.sleuth])Warning in check_num_cores(num_cores): It appears that you are running Sleuth from within Rstudio.
Because of concerns with forking processes from a GUI, 'num_cores' is being set to 1.
If you wish to take advantage of multiple cores, please consider running sleuth from the command line.reading in kallisto resultsdropping unused factor levels......
normalizing est_counts
A list of target IDs for filtering was found. Using this for filtering
57131 targets passed the filter
normalizing tpm
merging in metadata
summarizing bootstraps
......dte.sleuth.lrt <- sleuth_fit(obj = dte.sleuth.lrt, fit_name = 'full')fitting measurement error models
shrinkage estimation
3 NA values were found during variance shrinkage estimation due to mean observation values outside of the range used for the LOESS fit.
The LOESS fit will be repeated using exact computation of the fitted surface to extrapolate the missing values.
These are the target ids with NA values: ENST00000409702.1|ENSG00000150722.10|OTTHUMG00000154326.4|OTTHUMT00000334876.1|PPP1R1C-203|PPP1R1C|1157|protein_coding|, ENST00000432789.1|ENSG00000100413.17|OTTHUMG00000150971.2|OTTHUMT00000320706.1|POLR3H-207|POLR3H|1598|nonsense_mediated_decay|, ENST00000547886.5|ENSG00000079387.14|OTTHUMG00000169896.4|OTTHUMT00000406473.1|SENP1-203|SENP1|965|processed_transcript|
computing variance of betasdte.sleuth.lrt <-
sleuth_fit(obj = dte.sleuth.lrt,formula = ~ 1,fit_name = 'reduced')fitting measurement error models
shrinkage estimation
3 NA values were found during variance shrinkage estimation due to mean observation values outside of the range used for the LOESS fit.
The LOESS fit will be repeated using exact computation of the fitted surface to extrapolate the missing values.
These are the target ids with NA values: ENST00000409702.1|ENSG00000150722.10|OTTHUMG00000154326.4|OTTHUMT00000334876.1|PPP1R1C-203|PPP1R1C|1157|protein_coding|, ENST00000432789.1|ENSG00000100413.17|OTTHUMG00000150971.2|OTTHUMT00000320706.1|POLR3H-207|POLR3H|1598|nonsense_mediated_decay|, ENST00000547886.5|ENSG00000079387.14|OTTHUMG00000169896.4|OTTHUMT00000406473.1|SENP1-203|SENP1|965|processed_transcript|
computing variance of betasdte.sleuth.lrt <-
sleuth_lrt(obj = dte.sleuth.lrt,null_model = 'reduced',alt_model = 'full')
out.sleuth.lrt <-
sleuth_results(obj = dte.sleuth.lrt,
test = 'reduced:full',test_type = 'lrt',show_all = FALSE)Differential gene expression analysis
Now, we perform a gene-level analysis of the same data.
The function below computes estimates the mapping ambiguity
overdispersion parameter at the level of gene-wise counts. It implements
the exact same formula from catchSalmon, but it instead
uses the aggregated counts at the gene-level from
tximport::summarizeToGene.
## Function with catchSalmon's estimator for gene-level counts
## To be used only for exploratory purposes
geneLevelCatchSalmon <- function(x) {
NSamples <- ncol(x)
NBoot <- sum(grepl('infRep', assayNames(x)))
NTx <- nrow(x)
DF <- rep_len(0L, NTx)
OverDisp <- rep_len(0, NTx)
for (i.samples in 1:NSamples) {
Boot <- lapply(1:NBoot, function(i.boot) {
assay(x, paste0('infRep', i.boot))[, i.samples]
})
Boot <- do.call(cbind, Boot)
M <- rowMeans(Boot)
i <- (M > 0)
OverDisp[i] <- OverDisp[i] + rowSums((Boot[i,] - M[i]) ^ 2) / M[i]
DF[i] <- DF[i] + NBoot - 1L
}
i <- (DF > 0L)
OverDisp[i] <- OverDisp[i] / DF[i]
DFMedian <- median(DF[i])
DFPrior <- 3
OverDispPrior <-
median(OverDisp[i]) / qf(0.5, df1 = DFMedian, df2 = DFPrior)
if (OverDispPrior < 1) {
OverDispPrior <- 1
}
OverDisp[i] <-
(DFPrior * OverDispPrior + DF[i] * OverDisp[i]) / (DFPrior + DF[i])
OverDisp <- pmax(OverDisp, 1)
OverDisp[!i] <- OverDispPrior
rowData(x)$Overdispersion <- OverDisp
return(x)
}edgeR
We summarize counts to the gene-level below and run the standard
edgeR QL pipeline. We note here that the reason why
tximeta Love et al. (2020)
reports 160 missing transcripts is because we quantified such RNA-seq
dataset with the spiked-in sequins transcripts added to the
transcriptomic index (see here),
which are not present in the Ensembl annotation we get from
AnnotationHub. We are ignoring such transcripts in this
report.
txm <- tximeta(coldata = dt.swish[,c('files','names')],
tx2gene = dt.anno[,c('TXIDVERSION','GENEIDVERSION')],
txOut = FALSE, skipMeta = TRUE, ignoreAfterBar = TRUE)reading in files with read_tsv1 2 3 4 5 6
removing duplicated transcript rows from tx2gene
transcripts missing from tx2gene: 160
summarizing abundance
summarizing counts
summarizing length
summarizing inferential replicatestxm <- geneLevelCatchSalmon(txm)
dge <- DGEList(counts = assay(txm,'counts'),
samples = dt.swish,
genes = as.data.frame(rowData(txm)))
key.gene <- match(rownames(dge),dt.anno.gene$GENEIDVERSION)
dge$genes$EntrezID <- dt.anno.gene$ENTREZID[key.gene]
dge$genes$GeneName <- dt.anno.gene$GENENAME[key.gene]
dge$genes$GeneType <- dt.anno.gene$GENEBIOTYPE[key.gene]
dge$genes$GeneOfInterest <- dt.anno.gene$GeneOfInterest[key.gene]
keep.gene <- filterByExpr(dge) & dge$genes$GeneOfInterest == TRUE
dge.filtr <- dge[keep.gene, , keep.lib.sizes = FALSE]
dge.filtr <- calcNormFactors(dge.filtr)
dge.filtr <- estimateDisp(dge.filtr, design = design.short.paired, robust = TRUE)
fit.gene <- glmQLFit(dge.filtr,design.short.paired, robust = TRUE)
qlf.gene <- glmQLFTest(fit.gene,contrast = contr)
out.gene <- topTags(qlf.gene,n = Inf)
summary(decideTests(qlf.gene)) -1*H1975 1*HCC827
Down 5634
NotSig 2739
Up 5459Analysis of Illumina single-end short reads
Similarly to the paired-end read data, we now run a transcript-level DE analysis using the single-end read data. We note that such dataset only contains 2 biological replicates per group, whereas the paired-end data had 3 biological replicates per group.
Differential transcript expression analysis
edgeR with count scaling
dte.short.single.edger.scaled <- dte.short.single
dte.short.single.edger.scaled$counts <-
dte.short.single.edger.scaled$counts/dte.short.single.edger.scaled$genes$Overdispersion
keep.short.single.edger.scaled <-
filterByExpr(dte.short.single.edger.scaled) &
(dte.short.single.edger.scaled$genes$GeneOfInterest == TRUE)
dte.short.single.edger.scaled.filtr <-
dte.short.single.edger.scaled[keep.short.single.edger.scaled,, keep.lib.sizes = FALSE]
dte.short.single.edger.scaled.filtr <-
calcNormFactors(dte.short.single.edger.scaled.filtr)
dte.short.single.edger.scaled.filtr <-
estimateDisp(dte.short.single.edger.scaled.filtr,design = design.short.single,robust = TRUE)
fit.short.single.edger.scaled <-
glmQLFit(dte.short.single.edger.scaled.filtr,design.short.single,robust = TRUE)
qlf.short.single.edger.scaled <-
glmQLFTest(fit.short.single.edger.scaled,contrast = contr)
out.short.single.edger.scaled <-
topTags(qlf.short.single.edger.scaled,n = Inf)
summary(decideTests(qlf.short.single.edger.scaled)) -1*H1975 1*HCC827
Down 5401
NotSig 17587
Up 5067Analysis of ONT long reads
Finally, we run a transcript-level analysis with ONT long read data
using raw counts from Salmon.
Differential transcript expression analysis
edgeR
keep.long <- filterByExpr(dte.long) & dte.long$genes$GeneOfInterest == TRUE
dte.long.filtr <- dte.long[keep.long,, keep.lib.sizes = FALSE]
dte.long.filtr <- calcNormFactors(dte.long.filtr)
dte.long.filtr <- estimateDisp(dte.long.filtr,design = design.long,robust = TRUE)
fit.long <- glmQLFit(dte.long.filtr,design.long,robust = TRUE)
qlf.long <- glmQLFTest(fit.long,contrast = contr)
out.long <- topTags(qlf.long,n = Inf)
summary(decideTests(qlf.long)) -1*H1975 1*HCC827
Down 13671
NotSig 23593
Up 14146Results
Comparison of DTE methods using long reads as gold-standard
We first put all the results in a single data frame.
df.bench <- data.table(TranscriptID = rownames(out.long),
GeneID = out.long$table$GeneID,
GeneSymbol = out.long$table$GeneSymbol,
LongRead.FDR = out.long$table$FDR)
df.bench$edgeR.SE.Scaled.FDR <-
out.short.single.edger.scaled$table$FDR[match(df.bench$TranscriptID,rownames(out.short.single.edger.scaled))]
df.bench$edgeR.PE.Scaled.FDR <-
out.short.paired.edger.scaled$table$FDR[match(df.bench$TranscriptID,rownames(out.short.paired.edger.scaled))]
df.bench$edgeR.PE.Raw.FDR <-
out.short.paired.edger.raw$table$FDR[match(df.bench$TranscriptID,rownames(out.short.paired.edger.raw))]
df.bench$swish.FDR <-
out.swish$qvalue[match(df.bench$TranscriptID,rownames(out.swish))]
df.bench$sleuth.wald.FDR <-
out.sleuth.wald$qval[match(df.bench$TranscriptID,strsplit2(out.sleuth.wald$target_id,"\\|")[,1])]
df.bench$sleuth.lrt.FDR <- out.sleuth.lrt$qval[match(df.bench$TranscriptID,strsplit2(out.sleuth.lrt$target_id,"\\|")[,1])]
df.bench[is.na(df.bench)] <- 1Then, we check how many DE transcripts called using ONT long read data are also called by the DTE methods using short reads data.
foo.power <- function(target,reference,FDR = 0.05){
tb <- table(reference < FDR,target < FDR)
c(tb[2,2],tb[2,2]/sum(tb[2,]))
}
df.bench[,lapply(.SD,foo.power,reference = df.bench$LongRead.FDR),
.SDcols = c('edgeR.SE.Scaled.FDR','edgeR.PE.Scaled.FDR',
'edgeR.PE.Raw.FDR','swish.FDR',
'sleuth.wald.FDR','sleuth.lrt.FDR')] edgeR.SE.Scaled.FDR edgeR.PE.Scaled.FDR edgeR.PE.Raw.FDR swish.FDR
1: 5734.0000000 1.17800e+04 1.114800e+04 7836.0000000
2: 0.2061329 4.23482e-01 4.007621e-01 0.2816982
sleuth.wald.FDR sleuth.lrt.FDR
1: 1.020600e+04 1.07960e+04
2: 3.668979e-01 3.88108e-01Comparison of short and long read mapping ambiguity overdispersions
We begin by assessing the mapping ambiguity overdispersion parameter of each transcript (y-axis) according the number of expressed transcripts associated with their parent gene (x-axis). This is Figure 1 of the main paper.
dt.mao.plot <- as.data.table(dte.short.paired.edger.scaled.filtr$genes)
dt.mao.plot$TranscriptID <- rownames(dte.short.paired.edger.scaled.filtr)
dt.mao.plot$LR.Overdispersion <-
dte.long$genes$Overdispersion[match(dt.mao.plot$TranscriptID,rownames(dte.long$genes))]
dt.mao.plot[,NTranscriptPerGeneTrunc :=
ifelse(NTranscriptPerGene<10,NTranscriptPerGene,paste0('>=10'))]
dt.mao.plot$NTranscriptPerGeneTrunc %<>% factor(levels = paste0(c(1:10,'>=10')))
# Number of transcripts from single-transcript genes
dt.mao.plot[NTranscriptPerGene == 1,
.(.N,sum(NTranscriptPerGene == 1 & Overdispersion>(1/0.9))/sum(NTranscriptPerGene == 1))] N V2
1: 813 0.1057811# Number of transcripts from multi-transcript genes
dt.mao.plot[NTranscriptPerGene > 1,
.(.N,sum(NTranscriptPerGene > 1 & Overdispersion>(1/0.9))/sum(NTranscriptPerGene > 1))] N V2
1: 26553 0.9001243# Number of transcripts from transcript-rich genes (#tx>10)
dt.mao.plot[NTranscriptPerGene >= 10,
.(NGeneID = length(unique(GeneID)),
NTranscriptID = .N,mean(Overdispersion))] NGeneID NTranscriptID V3
1: 4687 13527 6.752402dt.mao.plot.long <-
melt(dt.mao.plot,
id.vars = c('TranscriptID','NTranscriptPerGeneTrunc'),
measure.vars = c('Overdispersion','LR.Overdispersion'),
variable.name = 'Type',value.name = 'Overdispersion')
dt.mao.plot.long$Type %<>%
mapvalues(from = c('Overdispersion','LR.Overdispersion'),
to = c('Illumina short reads','ONT long reads'))
plot.mao <-
ggplot(data = dt.mao.plot.long,aes(x = NTranscriptPerGeneTrunc,y = log10(Overdispersion))) +
geom_smooth(aes(group = Type,color = Type),se = FALSE,span = 0.8,method = 'loess',show.legend = FALSE) +
geom_boxplot(aes(fill = Type),outlier.alpha = 0.2,col = 'black',alpha = 0.5) +
labs(x = 'Number of expressed transcripts per gene', y = 'Overdispersion (log10 scale)') +
scale_y_continuous(limits = c(0,2.5),breaks = seq(0,3,0.5)) +
scale_fill_manual(values = c('Illumina short reads' = 'red','ONT long reads' = 'blue')) +
scale_color_manual(values = c('Illumina short reads' = 'red','ONT long reads' = 'blue')) +
theme_bw(base_size = 8,base_family = 'sans') +
theme(panel.grid = element_blank(),
axis.text = element_text(colour = 'black', size = 8),
legend.title = element_blank(),
legend.position = c(0.175, 0.925),
legend.text = element_text(colour = 'black', size = 8),
legend.background = element_rect(fill = alpha("white", 0)))
plot.mao`geom_smooth()` using formula = 'y ~ x'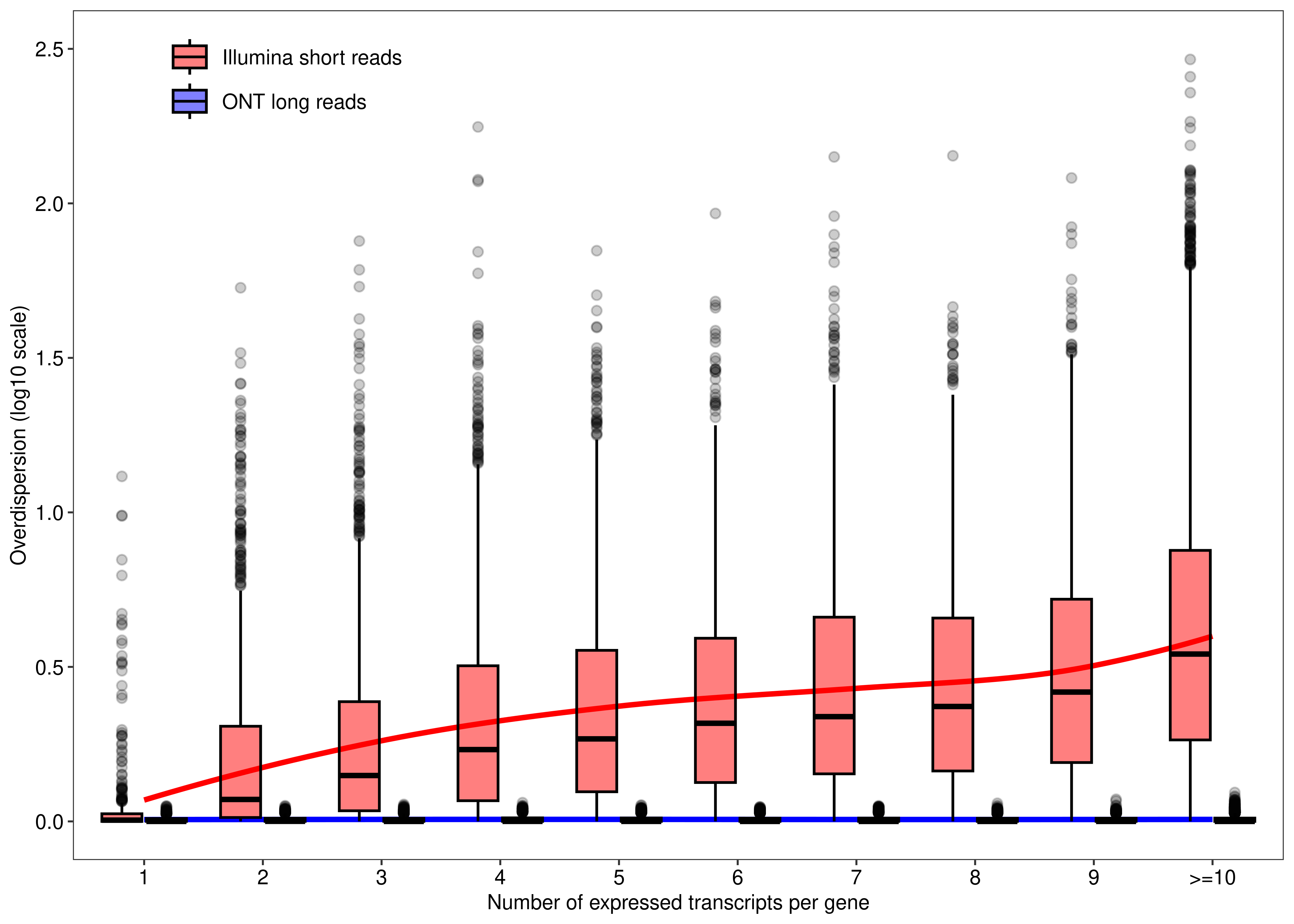
| Version | Author | Date |
|---|---|---|
| 1839ef1 | Pedro Baldoni | 2023-02-23 |
agg_png(filename = file.path(path.misc,"figure1.png"),
width = 5,height = 5,units = 'in',res = 300)
plot.mao`geom_smooth()` using formula = 'y ~ x'dev.off()png
2 Comparison of transcript-level and gene-level results using paired-end read data
Then, we generate Figure 6 of the main paper below. First we generate panel (a).
# plotMDS returns invisible(), we need to manually export the plot (code from limma::plotMDS.MDS)
foo.mds <- function(x,fontsize = 8){
obj.mds <- plotMDS(x,col = x$samples$Color,main = NULL)
par(mar = c(5, 4, 2, 2))
labels <- colnames(obj.mds$distance.matrix.squared)
StringRadius <- 0.15 * 1 * nchar(labels)
left.x <- obj.mds$x - StringRadius
right.x <- obj.mds$x + StringRadius
Perc <- round(obj.mds$var.explained * 100)
xlab <- paste(obj.mds$axislabel, 1)
ylab <- paste(obj.mds$axislabel, 2)
xlab <- paste0(xlab, " (", Perc[1], "%)")
ylab <- paste0(ylab, " (", Perc[2], "%)")
plot(c(-3.5, 3.5), c(-1, 1.5),
type = "n",xlab = xlab,ylab = ylab,
cex.lab = fontsize/12,
cex.axis = fontsize/12)
text(obj.mds$x, obj.mds$y, labels = labels, cex = fontsize/12,col = x$samples$Color)
}
plot.mds <- wrap_elements(full = ~foo.mds(dte.short.paired.edger.scaled.filtr))
plot.mds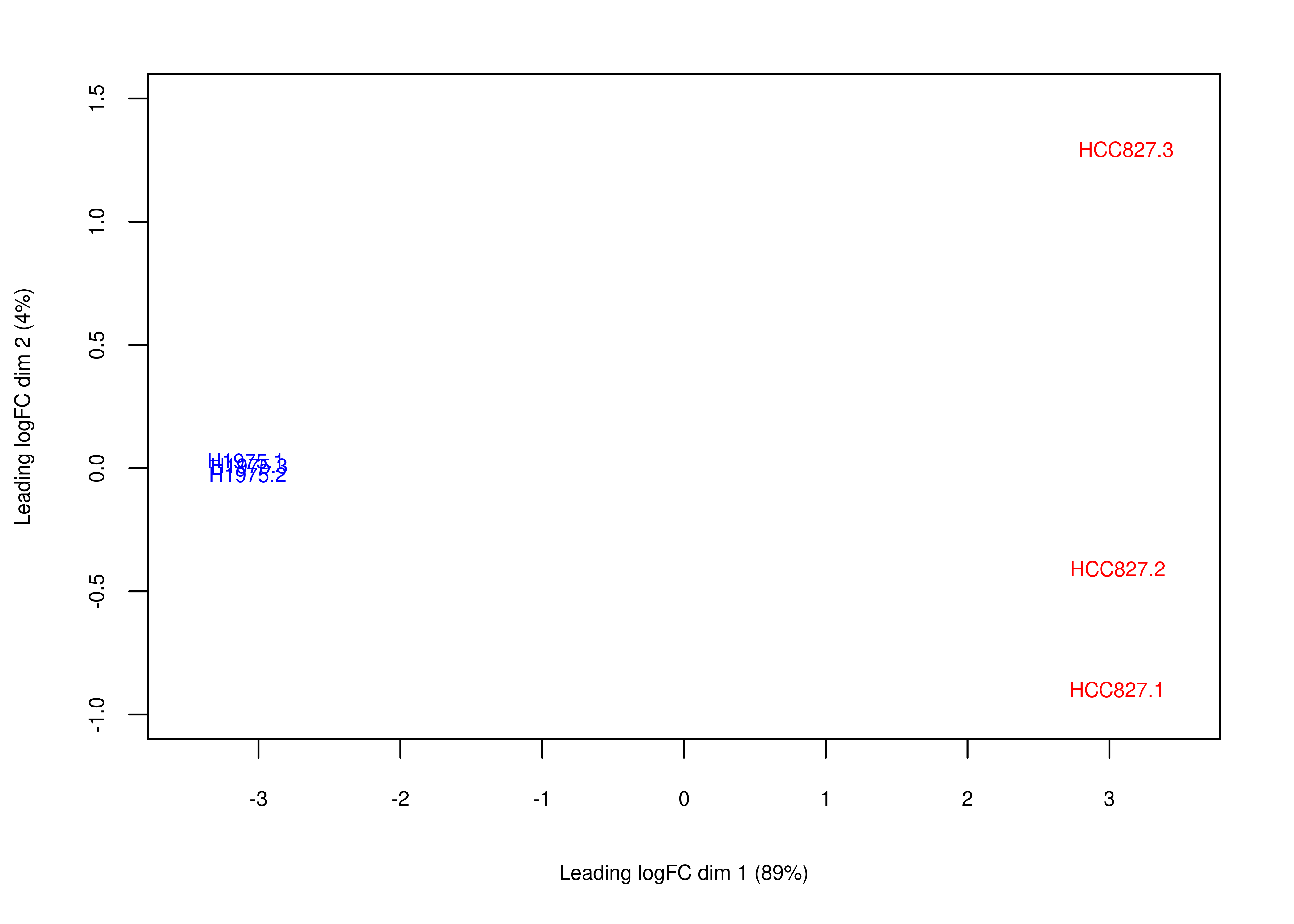
| Version | Author | Date |
|---|---|---|
| 1839ef1 | Pedro Baldoni | 2023-02-23 |
Next, we generate panel (b).
# plotMD does not return invisible(), so we just use wrap_elements
foo.md <- function(x,fontsize = 8){
par(mar = c(5, 4, 2, 2))
plotMD(x,
main = NULL,cex = 0.5,legend = FALSE,
cex.lab = fontsize/12,
cex.axis = fontsize/12)
legend('topright',
legend = c('NotSig','Up','Down'),
pch = rep(16,3),
col = c('black','red','blue'),
cex = fontsize/12,
pt.cex = c(0.3,0.5,0.5))
}
plot.md <-
wrap_elements(full = ~foo.md(qlf.short.paired.edger.scaled))
plot.md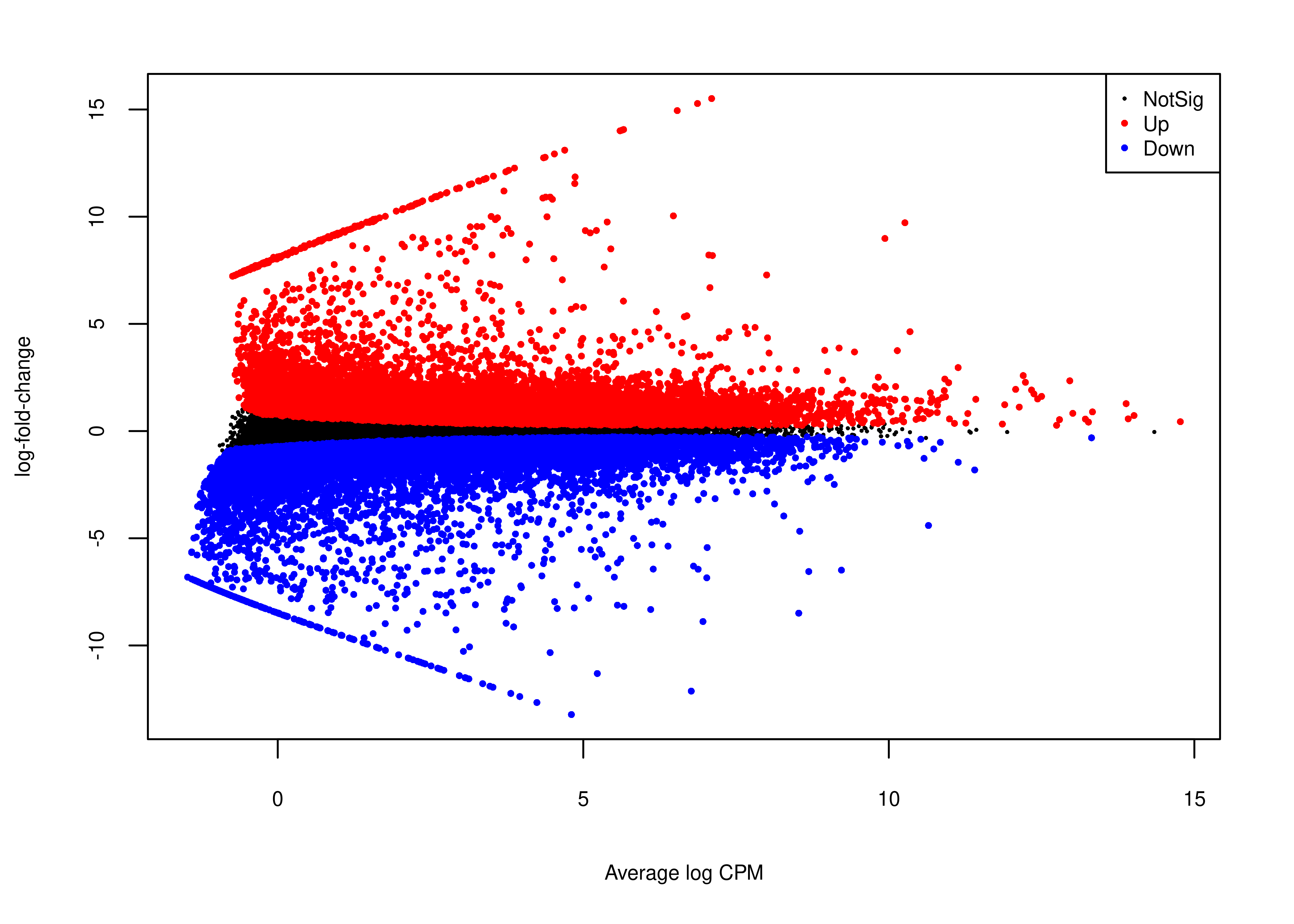
| Version | Author | Date |
|---|---|---|
| 1839ef1 | Pedro Baldoni | 2023-02-23 |
Panel (c) is created below.
foo.lolipop <-
function(symbols,gene.table,transcript.table, fontsize = 8,
legend = c(0.9,0.9),
colornames = c('NotSig' = 'black','Up' = 'red','Down' = 'blue')){
ls.loli <- lapply(symbols,function(x){
df.loli <-
rbind(as.data.table(transcript.table$table[transcript.table$table$GeneSymbol == x,c('TranscriptSymbol','FDR','logFC')]),
as.data.table(gene.table$table[gene.table$table$GeneName == x,c("FDR","logFC")]),fill = TRUE)
df.loli[is.na(df.loli)] <- x
df.loli$Color <- ifelse(df.loli$FDR > 0.05,'NotSig',ifelse(df.loli$logFC < 0,'Down','Up'))
df.loli <- df.loli[order(df.loli$TranscriptSymbol),]
df.loli$Gene <- x
return(df.loli)
})
ls.loli <- do.call(rbind,ls.loli)
ls.loli$Color <- factor(ls.loli$Color,levels = names(colornames))
ggplot(data = ls.loli,aes(x = TranscriptSymbol,xend = TranscriptSymbol,yend = 0,y = logFC)) +
facet_wrap('Gene',nrow = 1,scales = 'free_x') +
geom_segment(color = 'black') +
geom_point(size = 2,aes(color = Color)) +
geom_hline(yintercept = 0) +
geom_vline(xintercept = 1.5,linetype = 'dashed',color = 'gray') +
scale_color_manual(values = colornames) +
labs(x = NULL) +
theme_bw(base_size = fontsize,base_family = 'sans') +
theme(panel.grid = element_blank(),
strip.background = element_blank(),
strip.text = element_text(face = 'bold'),
axis.text = element_text(colour = 'black',size = fontsize),
legend.position = legend,
axis.text.x = element_text(angle = 90, vjust = 0.5, hjust=1),
legend.title = element_blank(),
legend.text = element_text(colour = 'black',size = fontsize),
legend.background=element_rect(fill = alpha("white", 0))) +
guides(colour = guide_legend(keywidth=0.1,
keyheight=0.1,
default.unit="inch",
override.aes = list(size = 0.75)))
}
plot.lolipop <- wrap_elements(foo.lolipop(symbols = c('EGLN3','RAF1','MET','AKT1','BCL2L1'),
gene.table = out.gene,
transcript.table = out.short.paired.edger.scaled,legend = c(0.95,0.875)))
plot.lolipop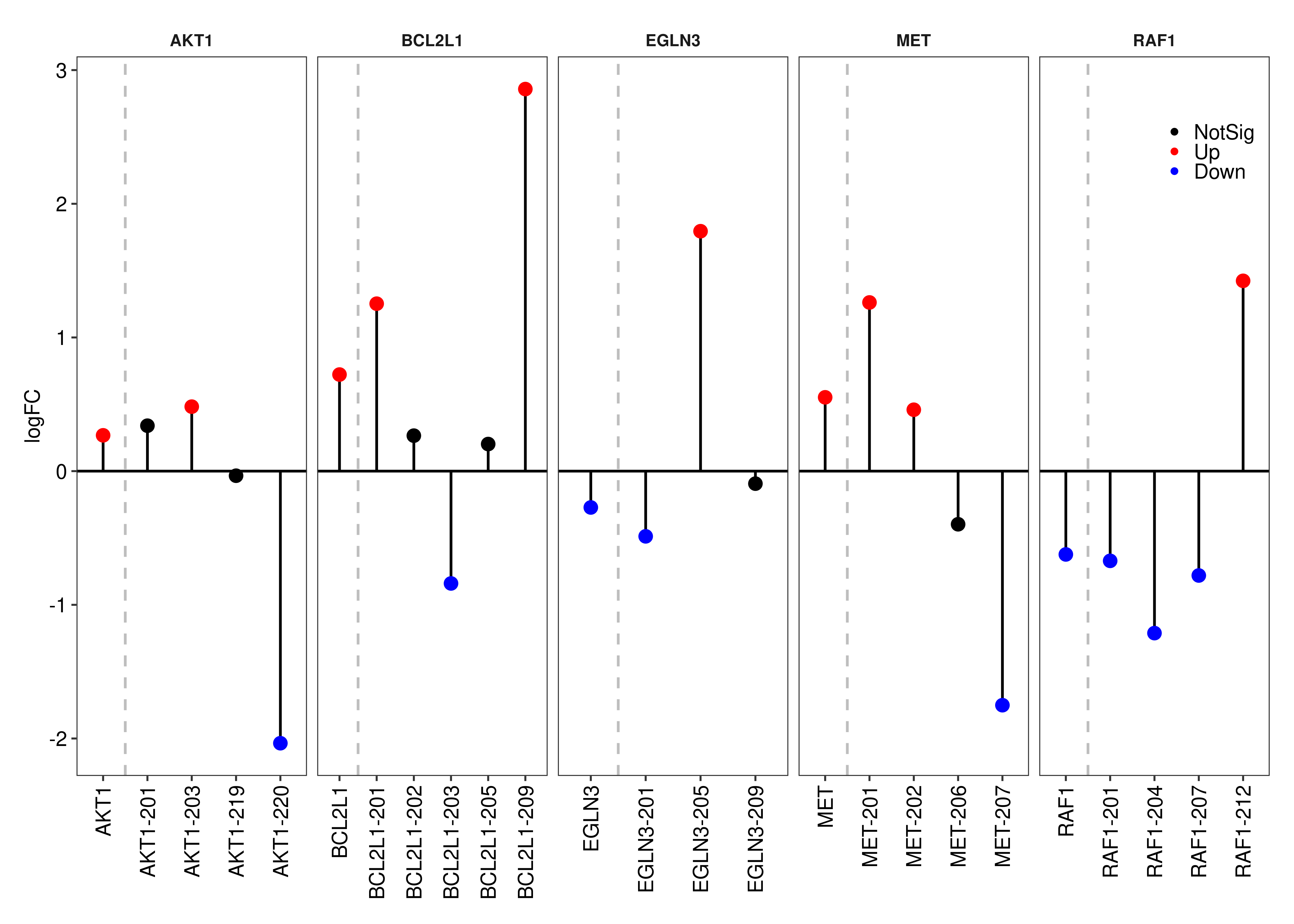
| Version | Author | Date |
|---|---|---|
| 1839ef1 | Pedro Baldoni | 2023-02-23 |
Finally, we create panel (d). We use that package
ComplexHeatmap Gu, Eils, and
Schlesner (2016) to create the heatmap plot.
cpm.scaled <- cpm(dte.short.paired.edger.scaled.filtr,log = TRUE)
cpm.scaled <- t(scale(t(cpm.scaled)))
## Here I want to check non-significant genes associated with cancer pathways that have at least 1 DE transcript
df.tx.gene <- out.short.paired.edger.scaled$table
df.tx.gene$FDR.Gene <-
out.gene$table$FDR[match(df.tx.gene$GeneID,rownames(out.gene$table))]
interestGenes.ns <-
unique(df.tx.gene[df.tx.gene$FDR.Gene > 0.05 &
df.tx.gene$FDR < 0.05 &
df.tx.gene$GeneOfInterest == TRUE,"EntrezID"])
# Number of non-significant genes for which at least one of their transcripts is DE
length(interestGenes.ns)[1] 841# Running KEGG analysis
GK <- getGeneKEGGLinks(species.KEGG = "hsa")
interestGenes.ns.cancer <-
interestGenes.ns[interestGenes.ns %in%
GK$GeneID[GK$PathwayID %in% c('path:hsa05222','path:hsa05223','path:hsa05200')]]
# Number of non-significant genes for which at least one of their transcripts is DE
length(interestGenes.ns.cancer)[1] 24df.tx.gene.cancer <-
df.tx.gene[df.tx.gene$EntrezID %in% interestGenes.ns.cancer,]
df.tx.gene.cancer <-
df.tx.gene.cancer[df.tx.gene.cancer$FDR < 0.05,]
cpm.scaled.cancer <- cpm.scaled[rownames(df.tx.gene.cancer),]
rownames(cpm.scaled.cancer) <-
dte.short.paired.edger.scaled.filtr$genes$TranscriptSymbol[match(rownames(cpm.scaled.cancer),rownames(dte.short.paired.edger.scaled.filtr))]
foo.heat <- function(x,cluster_rows = TRUE, fontsize = 8){
Heatmap(matrix = x,
heatmap_legend_param = list(title = 'Expression',
title_position = 'topcenter',
direction = "horizontal",
at = seq(-3,3,1),
title_position = 'topcenter',
title_gp = gpar(fontsize = fontsize),
labels_gp = gpar(fontsize = fontsize)),
cluster_rows = cluster_rows,
row_names_gp = gpar(fontsize = fontsize),
column_names_gp = gpar(fontsize = fontsize),
col = c("blue", "white", "red")) %>%
draw(heatmap_legend_side = "top")
}
plot.heat.cancer <-
wrap_elements(grid.grabExpr(draw(foo.heat(t(cpm.scaled.cancer)))))Finally, we put all these plots together with the package
patchwork Pedersen
(2022).
plot.design <- c(area(1, 1),area(1,2),area(2,1,2,2),area(3,1,3,2))
fig.panel <- wrap_plots(A = plot.mds,
B = plot.md,
C = plot.lolipop,
D = plot.heat.cancer,
design = plot.design,
heights = c(0.3,0.35,0.35)) +
plot_annotation(tag_levels = 'a')
fig.panel <- fig.panel &
theme(plot.tag = element_text(size = 8))
agg_png(filename = file.path(path.misc,"figure6.png"),
width = 10,height = 10,units = 'in',res = 300)
fig.panel
dev.off()png
2 For the genes presented in panel (b), below we present the results from our DE analysis at the gene-level.
tb.gene <-
out.gene$table[out.gene$table$GeneName %in% c('EGLN3','RAF1','MET','AKT1','BCL2L1'),]
tb.gene$EnsemblID <- rownames(tb.gene)
tb.gene <- data.frame(tb.gene[,c('EnsemblID','GeneName','EntrezID','GeneType',
'logFC','logCPM','F','PValue','FDR')],row.names = NULL)
setnames(tb.gene,
old = c('EnsemblID','GeneName','EntrezID','GeneType'),
new = c('Ensembl ID','Symbol','Entrez ID','Biotype'))
tb.gene$Biotype <- gsub("_"," ",tb.gene$Biotype)
tb.gene$logFC <- formatC(round(tb.gene$logFC,3),digits = 3,format = 'f')
tb.gene$logCPM <- formatC(round(tb.gene$logCPM,3),digits = 3,format = 'f')
tb.gene$`F` <- formatC(round(tb.gene$`F`,3),digits = 3,format = 'f')
tb.gene$PValue <- formatC(tb.gene$PValue,digits = 3,format = 'e')
tb.gene$FDR <- formatC(tb.gene$FDR,digits = 3,format = 'e')
tb.gene$Biotype <- NULL
cap.kb.gene <-
paste('edgeR results from a DE analysis at the gene-level for',
'a set of cancer-related genes comparing cell lines H1975 and HCC827.',
'Data from the paired-end RNA-seq experiment of the the human cell lines (GSE172421).',
'Such genes have at least one its transcripts differentially expressed between cell lines',
'(nominal FDR 0.05 at the transcript-level).')
kb.gene <-
kbl(tb.gene,
escape = FALSE,
format = 'latex',
booktabs = TRUE,
caption = cap.kb.gene,
align = 'llcccccc') %>%
kable_styling(font_size = 10)
save_kable(kb.gene,file = file.path(path.misc,"supptable_gene.tex"))We can also compare the distribution of the overdispersion estimates between gene- and transcript-level analyses.
df.output.gene <-
as.data.table(dge$genes[,c('GeneName','EntrezID','GeneType','Overdispersion')])
setnames(df.output.gene,
old = c('GeneName','EntrezID','GeneType','Overdispersion'),
new = c('Symbol','EntrezID','Type','Overdispersion'))
df.output.gene[,Level := 'Gene']
df.output.gene$EnsemblID <- rownames(dge)
## Transcript-level
df.output.tx <-
data.table(Symbol = dte.short.paired.edger.scaled$genes$TranscriptSymbol,
EntrezID = dte.short.paired.edger.scaled$genes$EntrezID,
Type = dte.short.paired.edger.scaled$genes$GeneType,
Overdispersion = catch.short.paired$annotation$Overdispersion,
Level = 'Transcript',
EnsemblID = rownames(dte.short.paired.edger.scaled$genes))
df.output.gene.tx <- rbindlist(list(df.output.gene,df.output.tx))
fig.mao.gene.tx <- ggplot(df.output.gene.tx,aes(x = log10(Overdispersion),y = Level,fill = Level)) +
geom_boxplot(outlier.alpha = 0.25,fill = "#bebebe",col = 'black') +
labs(y = NULL,x = 'Mapping ambiguity overdispersion (log10 scale)') +
theme_bw(base_size = 8,base_family = 'sans') +
theme(legend.position = 'none',
panel.grid = element_blank(),
axis.text = element_text(colour = 'black',size = 8))
agg_png(filename = file.path(path.misc,"suppfigure_overdispersion.png"),
width = 7.5,height = 5,units = 'in',res = 300)
fig.mao.gene.tx
dev.off()png
2 Below we have a table of the top genes with highest mapping ambiguity estimated overdispersion. Most of such genes do not have an associated Entrez ID.
df.output.gene.top <-
df.output.gene[order(-Overdispersion),][1:100,c('EnsemblID','Symbol','Type','EntrezID','Overdispersion')]
df.output.gene.top$Type <- gsub("_"," ",df.output.gene.top$Type)
df.output.gene.top$EntrezID[is.na(df.output.gene.top$EntrezID)] <- "-"
cap.mao <- paste("Top 100 genes with largest mapping ambiguity overdispersion.",
"Data from the RNA-seq experiment of the human cell lines",
"generated with paired-end reads (GSE172421).")
kb.output.gene.top <-
kbl(df.output.gene.top,
longtable = TRUE,
escape = FALSE,
format = 'latex',
booktabs = TRUE,
caption = cap.mao,
digits = 2,
align = c('lllcc'),
col.names = c('Ensembl ID','Symbol','Entrez ID','Biotype','Overdispersion')) %>%
kable_styling(latex_options = c("scale_down","repeat_header"),font_size = 10)Warning in styling_latex_scale_down(out, table_info): Longtable cannot be
resized.save_kable(kb.output.gene.top,file = file.path(path.misc,"supptable_overdispersion.tex"))Comparison between analyses using paired- and single-end reads
Finally, we can compute how many transcripts are being called as DE using both paired- and single-end Illumina short reads. We add the transcript-level results from ONT long read data as a comparison.
tb.tx.genes <- dte.short.paired$genes
tb.tx.genes$FDR.SE <-
out.short.single.edger.scaled$table$FDR[match(rownames(tb.tx.genes),rownames(out.short.single.edger.scaled))]
tb.tx.genes$FDR.PE <-
out.short.paired.edger.scaled$table$FDR[match(rownames(tb.tx.genes),rownames(out.short.paired.edger.scaled))]
tb.tx.genes$FDR.LR <- out.long$table$FDR[match(rownames(tb.tx.genes),rownames(out.long))]
tb.tx.genes <- (tb.tx.genes[,c('FDR.SE','FDR.PE','FDR.LR')] < 0.05)*1L
tb.tx.genes[is.na(tb.tx.genes)] <- 0L
agg_png(filename = file.path(path.misc,"suppfigure_venn.png"),
width = 6,height = 6,units = 'in',res = 300)
limma::vennDiagram(tb.tx.genes[,c('FDR.SE','FDR.PE','FDR.LR')],
names = c('Illumina\nsingle-end reads',
'Illumina\npaired-end reads',
'ONT\nlong reads'),
circle.col = c('salmon','red','blue'),cex = c(12,10,8)/12)
dev.off()png
2 And the MA plot highlighting DE transcripts called with single-end Illumina short reads is presented below.
agg_png(filename = file.path(path.misc,"suppfigure_maplot.png"),
width = 6,height = 6,units = 'in',res = 300)
plotMD(qlf.short.single.edger.scaled,
main = NULL,cex = 0.5,legend = FALSE,
cex.lab = 8/12,
cex.axis = 8/12)
legend('topright',
legend = c('NotSig','Up','Down'),
pch = rep(16,3),
col = c('black','red','blue'),
cex = 8/12,
pt.cex = c(0.3,0.5,0.5))
dev.off()png
2
sessionInfo()R version 4.2.1 (2022-06-23)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: CentOS Linux 7 (Core)
Matrix products: default
BLAS: /stornext/System/data/apps/R/R-4.2.1/lib64/R/lib/libRblas.so
LAPACK: /stornext/System/data/apps/R/R-4.2.1/lib64/R/lib/libRlapack.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] grid stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] ensembldb_2.22.0 AnnotationFilter_1.22.0
[3] GenomicFeatures_1.50.4 AnnotationDbi_1.60.0
[5] kableExtra_1.3.4 magrittr_2.0.3
[7] ggplot2_3.4.1 ragg_1.2.5
[9] tibble_3.1.8 ComplexHeatmap_2.14.0
[11] patchwork_1.1.2 sleuth_0.30.0
[13] fishpond_2.4.1 SummarizedExperiment_1.28.0
[15] Biobase_2.58.0 GenomicRanges_1.50.2
[17] GenomeInfoDb_1.34.9 IRanges_2.32.0
[19] S4Vectors_0.36.1 MatrixGenerics_1.10.0
[21] matrixStats_0.63.0 tximeta_1.16.1
[23] data.table_1.14.6 AnnotationHub_3.6.0
[25] BiocFileCache_2.6.0 dbplyr_2.3.0
[27] BiocGenerics_0.44.0 plyr_1.8.8
[29] edgeR_3.40.2 limma_3.54.1
[31] workflowr_1.7.0
loaded via a namespace (and not attached):
[1] circlize_0.4.15 systemfonts_1.0.4
[3] lazyeval_0.2.2 splines_4.2.1
[5] BiocParallel_1.32.5 digest_0.6.31
[7] foreach_1.5.2 htmltools_0.5.4
[9] fansi_1.0.4 memoise_2.0.1
[11] svMisc_1.2.3 cluster_2.1.4
[13] doParallel_1.0.17 tzdb_0.3.0
[15] readr_2.1.4 Biostrings_2.66.0
[17] vroom_1.6.1 svglite_2.1.1
[19] prettyunits_1.1.1 colorspace_2.1-0
[21] rvest_1.0.3 blob_1.2.3
[23] rappdirs_0.3.3 textshaping_0.3.6
[25] xfun_0.37 dplyr_1.1.0
[27] callr_3.7.3 crayon_1.5.2
[29] RCurl_1.98-1.10 jsonlite_1.8.4
[31] tximport_1.26.1 iterators_1.0.14
[33] glue_1.6.2 gtable_0.3.1
[35] zlibbioc_1.44.0 XVector_0.38.0
[37] webshot_0.5.4 GetoptLong_1.0.5
[39] DelayedArray_0.24.0 Rhdf5lib_1.20.0
[41] shape_1.4.6 SingleCellExperiment_1.20.0
[43] abind_1.4-5 scales_1.2.1
[45] DBI_1.1.3 Rcpp_1.0.10
[47] viridisLite_0.4.1 xtable_1.8-4
[49] progress_1.2.2 clue_0.3-64
[51] gridGraphics_0.5-1 bit_4.0.5
[53] httr_1.4.4 RColorBrewer_1.1-3
[55] ellipsis_0.3.2 farver_2.1.1
[57] pkgconfig_2.0.3 XML_3.99-0.13
[59] sass_0.4.1 locfit_1.5-9.7
[61] utf8_1.2.3 labeling_0.4.2
[63] reshape2_1.4.4 tidyselect_1.2.0
[65] rlang_1.0.6 later_1.3.0
[67] munsell_0.5.0 BiocVersion_3.16.0
[69] tools_4.2.1 cachem_1.0.6
[71] cli_3.6.0 generics_0.1.3
[73] RSQLite_2.2.20 evaluate_0.20
[75] stringr_1.5.0 fastmap_1.1.0
[77] yaml_2.3.7 processx_3.8.0
[79] knitr_1.42 bit64_4.0.5
[81] fs_1.6.1 purrr_1.0.1
[83] KEGGREST_1.38.0 nlme_3.1-162
[85] whisker_0.4.1 mime_0.12
[87] xml2_1.3.3 biomaRt_2.54.0
[89] compiler_4.2.1 rstudioapi_0.14
[91] filelock_1.0.2 curl_5.0.0
[93] png_0.1-8 interactiveDisplayBase_1.36.0
[95] statmod_1.5.0 bslib_0.4.2
[97] stringi_1.7.12 highr_0.10
[99] ps_1.7.2 lattice_0.20-45
[101] ProtGenerics_1.30.0 Matrix_1.5-3
[103] vctrs_0.5.2 pillar_1.8.1
[105] lifecycle_1.0.3 rhdf5filters_1.10.0
[107] BiocManager_1.30.19 jquerylib_0.1.4
[109] GlobalOptions_0.1.2 bitops_1.0-7
[111] qvalue_2.30.0 httpuv_1.6.5
[113] rtracklayer_1.58.0 R6_2.5.1
[115] BiocIO_1.8.0 promises_1.2.0.1
[117] codetools_0.2-19 gtools_3.9.4
[119] assertthat_0.2.1 rhdf5_2.42.0
[121] rprojroot_2.0.3 rjson_0.2.21
[123] withr_2.5.0 GenomicAlignments_1.34.0
[125] Rsamtools_2.14.0 GenomeInfoDbData_1.2.9
[127] mgcv_1.8-41 parallel_4.2.1
[129] hms_1.1.2 tidyr_1.3.0
[131] rmarkdown_2.20 Cairo_1.6-0
[133] git2r_0.31.0 getPass_0.2-2
[135] shiny_1.7.4 restfulr_0.0.15