simulation
Pedro Baldoni
19 January, 2023
Last updated: 2023-01-19
Checks: 5 2
Knit directory: wf-TranscriptDE/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of
the R Markdown file created these results, you’ll want to first commit
it to the Git repo. If you’re still working on the analysis, you can
ignore this warning. When you’re finished, you can run
wflow_publish to commit the R Markdown file and build the
HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20221115) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
- simulation-paper_data_load
To ensure reproducibility of the results, delete the cache directory
simulation-paper_cache and re-run the analysis. To have
workflowr automatically delete the cache directory prior to building the
file, set delete_cache = TRUE when running
wflow_build() or wflow_publish().
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version ca7c822. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: .gitignore
Ignored: analysis/simulation-complete_cache/
Ignored: analysis/simulation-paper_cache/
Ignored: code/mouse/single-end/salmon/slurm-9574761.out
Ignored: code/pkg/.Rhistory
Ignored: code/pkg/.Rproj.user/
Ignored: code/pkg/src/RcppExports.o
Ignored: code/pkg/src/pkg.so
Ignored: code/pkg/src/rcpparma_hello_world.o
Ignored: data/annotation/mm39/
Ignored: data/mouse/paired-end/fastq/
Ignored: data/mouse/single-end/fastq/
Ignored: output/mouse/paired-end/
Ignored: output/mouse/single-end/
Ignored: output/quasi_poisson/
Ignored: output/simulation/
Ignored: renv/
Untracked files:
Untracked: misc/simulation-paper.Rmd/figure2.png
Untracked: misc/simulation-paper.Rmd/figure3.png
Untracked: misc/simulation-paper.Rmd/figure4.png
Untracked: misc/simulation-paper.Rmd/figure5.png
Untracked: misc/simulation-paper.Rmd/table1.tex
Unstaged changes:
Modified: analysis/mouse.Rmd
Modified: analysis/simulation-paper.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/simulation-paper.Rmd) and
HTML (docs/simulation-paper.html) files. If you’ve
configured a remote Git repository (see ?wflow_git_remote),
click on the hyperlinks in the table below to view the files as they
were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 4276bfc | Pedro Baldoni | 2023-01-06 | Organizing output of latex table |
| html | 4276bfc | Pedro Baldoni | 2023-01-06 | Organizing output of latex table |
| Rmd | a8c51af | Pedro Baldoni | 2023-01-05 | Updating simulation-paper report |
| html | a8c51af | Pedro Baldoni | 2023-01-05 | Updating simulation-paper report |
| Rmd | d34d4e6 | Pedro Baldoni | 2022-11-24 | Adding simulation-paper-report |
| html | d34d4e6 | Pedro Baldoni | 2022-11-24 | Adding simulation-paper-report |
Introduction
Setup
knitr::opts_chunk$set(dev = "png",
dpi = 300,
dev.args = list(type = "cairo-png"),
root.dir = '.',
autodep = TRUE)
options(knitr.kable.NA = "-")library(data.table)
library(ggplot2)
library(thematic)
library(plyr)
library(magrittr)
library(limma)
library(edgeR)
library(BiocParallel)
library(devtools)
library(purrr)
library(readr)
library(ggpubr)
library(kableExtra)
library(patchwork)
load_all('../code/pkg/')
BPPARAM <- MulticoreParam(workers = 16,progressbar = TRUE)
register(BPPARAM)cleanPlot <- function(x,fig){
if (x == max(seq_along(fig))) {
y <- fig[[x]]
} else{
y <- fig[[x]] + theme(axis.title.x = element_blank(),
axis.text.x = element_blank(),
axis.ticks.x = element_blank())
}
if (x > 1) {
y <- y + theme(strip.background.x = element_blank(),
strip.text.x = element_blank())
}
return(y)
}
subsetDT <- function(x,scenario,panel = NULL,tx.per.gene = NULL, plot = TRUE){
if(isTRUE(plot)){
if(panel %in% c('A','B')){
out <- x[Genome == scenario['genome'] &
FC == ifelse(panel == 'A','fc2','fc1') &
Length == scenario['length'] &
Reads == scenario['read'] &
Quantifier == scenario['quantifier'] &
Scenario == scenario['scenario'],]
} else{
out <- x[Genome == scenario['genome'] &
FC == 'fc1' &
Length == scenario['length'] &
Reads == scenario['read'] &
Quantifier == scenario['quantifier'] &
Scenario == scenario['scenario'] &
TxPerGene == tx.per.gene ,]
}
} else{
out <- x[Genome == scenario['genome'] &
FC == 'fc2' &
Quantifier == scenario['quantifier'] &
TxPerGene == scenario['txpergene'],]
}
return(out)
}Analysis
Data wrangling
path.misc <- file.path('../misc',knitr::current_input())
dir.create(path.misc,recursive = TRUE,showWarnings = FALSE)
path.fdr <-
list.files('../output/simulation/summary','fdr.tsv.gz',recursive = TRUE,full.names = TRUE)
path.metrics <-
list.files('../output/simulation/summary','metrics.tsv.gz',recursive = TRUE,full.names = TRUE)
path.time <-
list.files('../output/simulation/summary','time.tsv.gz',recursive = TRUE,full.names = TRUE)
path.quantile <-
list.files('../output/simulation/summary','quantile.tsv.gz',recursive = TRUE,full.names = TRUE)
path.pvalue <-
list.files('../output/simulation/summary','pvalue.tsv.gz',recursive = TRUE,full.names = TRUE)
path.overdispersion <-
list.files('../output/simulation/summary','overdispersion.tsv.gz',recursive = TRUE,full.names = TRUE)# Loading datasets
dt.fdr <- do.call(rbind,lapply(path.fdr,fread))
dt.metrics <- do.call(rbind,lapply(path.metrics,fread))
dt.time <- do.call(rbind,lapply(path.time,fread))
dt.quantile <- do.call(rbind,lapply(path.quantile,fread))
dt.pvalue <- do.call(rbind,lapply(path.pvalue,fread))
dt.overdispersion <- do.call(rbind,lapply(path.overdispersion,fread))# Changing labels
dt.fdr$TxPerGene %<>%
mapvalues(from = paste0(c(2, 3, 4, 5, 9999), 'TxPerGene'),
to = c(paste0("#Tx/Gene = ", c(2, 3, 4, 5)), 'All Transcripts'))
dt.fdr$LibsPerGroup %<>%
mapvalues(from = paste0(c(3, 5), 'libsPerGroup'),
to = paste0('#Lib/Group = ', c(3, 5)))
dt.fdr$Quantifier %<>% mapvalues(from = 'salmon', to = 'Salmon')
dt.fdr$Length %<>% mapvalues(from = paste0('readlen-', seq(50, 150, 25)),
to = paste0(seq(50, 150, 25), 'bp'))
dt.metrics$TxPerGene %<>%
mapvalues(from = paste0(c(2, 3, 4, 5, 9999), 'TxPerGene'),
to = c(paste0("#Tx/Gene = ", c(2, 3, 4, 5)), 'All Transcripts'))
dt.metrics$LibsPerGroup %<>%
mapvalues(from = paste0(c(3, 5), 'libsPerGroup'),
to = paste0('#Lib/Group = ', c(3, 5)))
dt.metrics$Quantifier %<>% mapvalues(from = 'salmon', to = 'Salmon')
dt.metrics$Length %<>% mapvalues(from = paste0('readlen-', seq(50, 150, 25)),
to = paste0(seq(50, 150, 25), 'bp'))
dt.time$TxPerGene %<>%
mapvalues(from = paste0(c(2, 3, 4, 5, 9999), 'TxPerGene'),
to = c(paste0("#Tx/Gene = ", c(2, 3, 4, 5)), 'All Transcripts'))
dt.time$LibsPerGroup %<>%
mapvalues(from = paste0(c(3, 5), 'libsPerGroup'),
to = paste0('#Lib/Group = ', c(3, 5)))
dt.time$Quantifier %<>% mapvalues(from = 'salmon', to = 'Salmon')
dt.time$Length %<>% mapvalues(from = paste0('readlen-', seq(50, 150, 25)),
to = paste0(seq(50, 150, 25), 'bp'))
dt.quantile$TxPerGene %<>%
mapvalues(from = paste0(c(2, 3, 4, 5, 9999), 'TxPerGene'),
to = c(paste0("#Tx/Gene = ", c(2, 3, 4, 5)), 'All Transcripts'))
dt.quantile$LibsPerGroup %<>%
mapvalues(from = paste0(c(3, 5), 'libsPerGroup'),
to = paste0('#Lib/Group = ', c(3, 5)))
dt.quantile$Quantifier %<>% mapvalues(from = 'salmon', to = 'Salmon')
dt.quantile$Length %<>% mapvalues(from = paste0('readlen-', seq(50, 150, 25)),
to = paste0(seq(50, 150, 25), 'bp'))
dt.pvalue$TxPerGene %<>%
mapvalues(from = paste0(c(2, 3, 4, 5, 9999), 'TxPerGene'),
to = c(paste0("#Tx/Gene = ", c(2, 3, 4, 5)), 'All Transcripts'))
dt.pvalue$LibsPerGroup %<>%
mapvalues(from = paste0(c(3, 5), 'libsPerGroup'),
to = paste0('#Lib/Group = ', c(3, 5)))
dt.pvalue$Quantifier %<>% mapvalues(from = 'salmon', to = 'Salmon')
dt.pvalue$Length %<>% mapvalues(from = paste0('readlen-', seq(50, 150, 25)),
to = paste0(seq(50, 150, 25), 'bp'))
dt.overdispersion$TxPerGene %<>%
mapvalues(from = paste0(c(2, 3, 4, 5, 9999), 'TxPerGene'),
to = c(paste0("#Tx/Gene = ", c(2, 3, 4, 5)), 'All Transcripts'))
dt.overdispersion$LibsPerGroup %<>%
mapvalues(from = paste0(c(3, 5), 'libsPerGroup'),
to = paste0('#Lib/Group = ', c(3, 5)))
dt.overdispersion$Quantifier %<>% mapvalues(from = 'salmon', to = 'Salmon')
dt.overdispersion$Length %<>% mapvalues(from = paste0('readlen-', seq(50, 150, 25)),
to = paste0(seq(50, 150, 25), 'bp'))dt.scenario <- expand.grid('genome' = 'mm39',
'length' = c('50bp','75bp','100bp','125bp','150bp'),
'read' = c('single-end','paired-end'),
'quantifier' = c('Salmon','kallisto'),
'scenario' = c('balanced','unbalanced'),
stringsAsFactors = FALSE)
dt.scenario <- as.data.table(dt.scenario)Power plot & False discovery rate
scenario.balanced <- as.character(dt.scenario[length == '100bp' &
read == 'paired-end' &
quantifier == 'Salmon' &
scenario == 'balanced',])
scenario.unbalanced <- as.character(dt.scenario[length == '100bp' &
read == 'paired-end' &
quantifier == 'Salmon' &
scenario == 'unbalanced',])
names(scenario.balanced) <- colnames(dt.scenario)
names(scenario.unbalanced) <- colnames(dt.scenario)
dt.power <- rbind(subsetDT(dt.metrics,scenario.balanced,'A'),
subsetDT(dt.metrics,scenario.unbalanced,'A'))
dt.power$LibsPerGroup %<>% mapvalues(from = paste0('#Lib/Group = ', c(3, 5)),
to = paste0(c(3,5),' samples per group'))
dt.power$Scenario %<>%
mapvalues(from = c('balanced','unbalanced'),
to = c('Equal library sizes','Unequal library sizes'))
dt.power[, FDR := roundPretty(ifelse((FP+TP) == 0,NA,100*FP/(FP+TP)),1)]
dt.power <- dt.power[TxPerGene == 'All Transcripts',]
sub.byvar <-
colnames(dt.power)[-which(colnames(dt.power) %in% c('P.SIG','TP','FP'))]
gap <- 0.05*max(dt.power$TP + dt.power$FP)
x.melt <- melt(dt.power,id.vars = sub.byvar,
measure.vars = c('TP','FP'),
variable.name = 'Type',
value.name = 'Value')
x.melt$Type <-
factor(x.melt$Type,
levels = c('FP','TP'),
labels = c('False Positive','True Positive'))
plot.power <- function(df.bar,df.txt,scenario,library,legend = FALSE){
tb.bar <- df.bar[Scenario == scenario & LibsPerGroup == library,]
tb.txt <- df.txt[Scenario == scenario & LibsPerGroup == library,][FDR != 'NA',]
ggplot(tb.bar,aes(x = Method,y = Value,fill = Type)) +
geom_col(colour = 'black') +
geom_text(aes(x = Method,y = (TP + FP) + gap,label = FDR),
inherit.aes = FALSE,vjust = 0,data = tb.txt) +
theme_bw() +
scale_fill_manual(values = c('#ff0000','#bebebe')) +
labs(x = NULL,y = paste('# DE Transcripts')) +
scale_y_continuous(limits = c(0,3000)) +
theme(panel.grid = element_blank(),
legend.position = c(0.75,0.90),
legend.title = element_blank(),
axis.text.x = element_text(angle = 90),
axis.text = element_text(colour = 'black')) +
if (legend == TRUE) theme(legend.background = element_rect(fill = alpha('white', 0))) else theme(legend.position = 'none')
}
fig.power.a <- plot.power(df.bar = x.melt,df.txt = dt.power,scenario = 'Equal library sizes',library = '3 samples per group')
fig.power.b <- plot.power(df.bar = x.melt,df.txt = dt.power,scenario = 'Unequal library sizes',library = '3 samples per group',legend = TRUE)
fig.power.c <- plot.power(df.bar = x.melt,df.txt = dt.power,scenario = 'Equal library sizes',library = '5 samples per group')
fig.power.d <- plot.power(df.bar = x.melt,df.txt = dt.power,scenario = 'Unequal library sizes',library = '5 samples per group')
fig.power <- (fig.power.a + fig.power.b) / (fig.power.c + fig.power.d) +
plot_annotation(tag_levels = 'a')
png(filename = file.path(path.misc,"figure2.png"),width = 7,height = 7,units = 'in',res = 300)
fig.power
dev.off()png
2 fig.power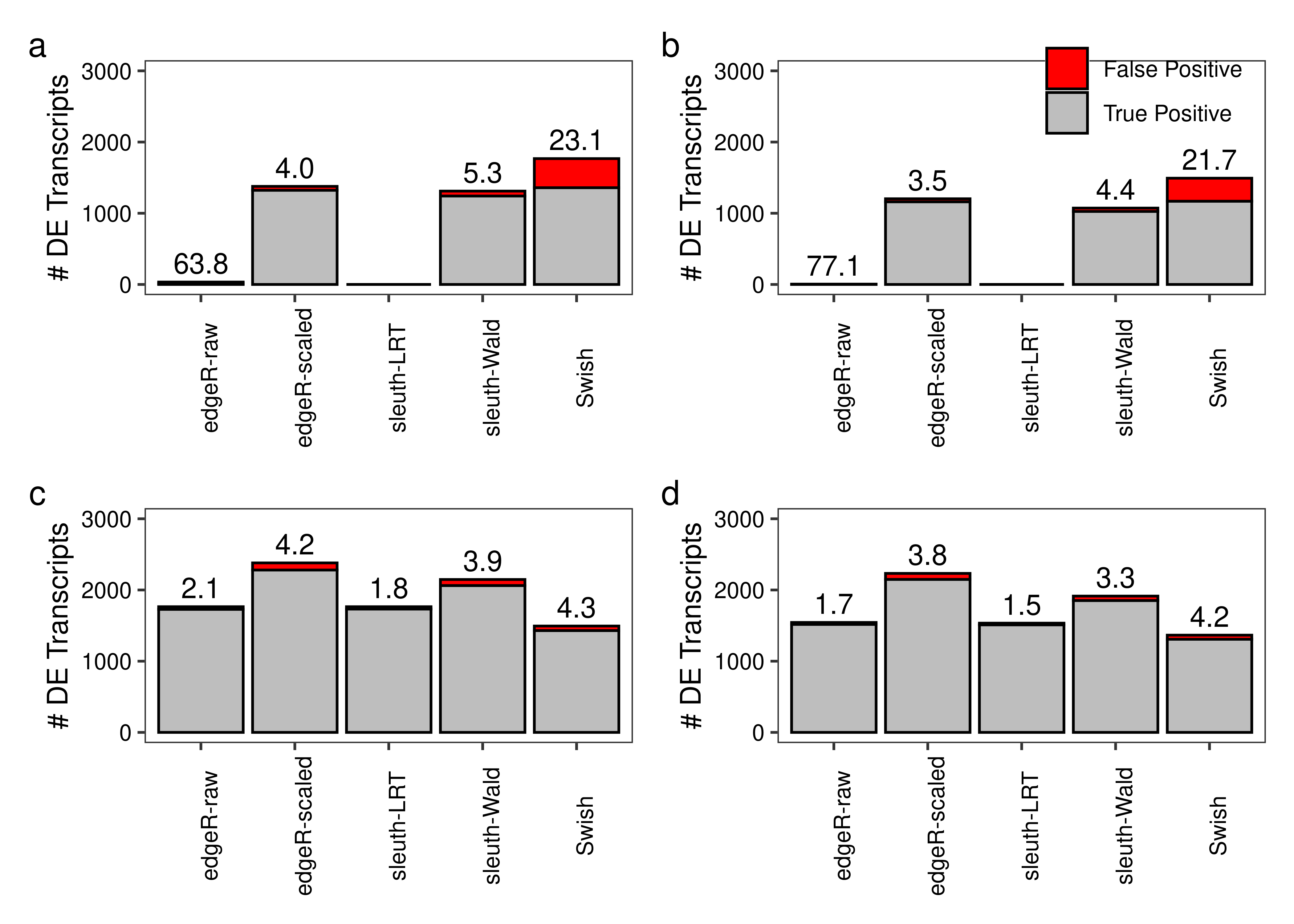
dt.fdr.plot <- rbind(subsetDT(dt.fdr,scenario.balanced,'A'),
subsetDT(dt.fdr,scenario.unbalanced,'A'))
dt.fdr.plot$LibsPerGroup %<>%
mapvalues(from = paste0('#Lib/Group = ', c(3, 5)),
to = paste0(c(3,5),' samples per group'))
dt.fdr.plot$Scenario %<>%
mapvalues(from = c('balanced','unbalanced'),
to = c('Equal library sizes','Unequal library sizes'))
dt.fdr.plot <- dt.fdr.plot[TxPerGene == 'All Transcripts',]
plot.fdr <- function(df.line,scenario,library,legend = FALSE){
tb.bar <- df.line[Scenario == scenario & LibsPerGroup == library,]
ggplot(tb.bar,aes(x = N,y = FDR,color = Method,group = Method)) +
geom_line(linewidth = 1) +
theme_bw() +
scale_color_manual(values = methodsNames()$color) +
scale_y_continuous(limits = c(0,1250)) +
labs(y = 'False discoveries',x = 'Transcripts chosen') +
theme(panel.grid = element_blank(),
legend.direction = 'vertical',
legend.position = c(0.25,0.75),
legend.title = element_blank(),
axis.text = element_text(colour = 'black')) +
if (legend == TRUE) theme(legend.background = element_rect(fill = alpha('white', 0))) else theme(legend.position = 'none')
}
fig.fdr.a <- plot.fdr(df.line = dt.fdr.plot,scenario = 'Equal library sizes',library = '3 samples per group')
fig.fdr.b <- plot.fdr(df.line = dt.fdr.plot,scenario = 'Unequal library sizes',library = '3 samples per group',legend = TRUE)
fig.fdr.c <- plot.fdr(df.line = dt.fdr.plot,scenario = 'Equal library sizes',library = '5 samples per group')
fig.fdr.d <- plot.fdr(df.line = dt.fdr.plot,scenario = 'Unequal library sizes',library = '5 samples per group')
fig.fdr <- (fig.fdr.a + fig.fdr.b) / (fig.fdr.c + fig.fdr.d) +
plot_annotation(tag_levels = 'a')
png(filename = file.path(path.misc,"figure3.png"),width = 7,height = 7,units = 'in',res = 300)
fig.fdr
dev.off()png
2 plot.fdrfunction(df.line,scenario,library,legend = FALSE){
tb.bar <- df.line[Scenario == scenario & LibsPerGroup == library,]
ggplot(tb.bar,aes(x = N,y = FDR,color = Method,group = Method)) +
geom_line(linewidth = 1) +
theme_bw() +
scale_color_manual(values = methodsNames()$color) +
scale_y_continuous(limits = c(0,1250)) +
labs(y = 'False discoveries',x = 'Transcripts chosen') +
theme(panel.grid = element_blank(),
legend.direction = 'vertical',
legend.position = c(0.25,0.75),
legend.title = element_blank(),
axis.text = element_text(colour = 'black')) +
if (legend == TRUE) theme(legend.background = element_rect(fill = alpha('white', 0))) else theme(legend.position = 'none')
}
<bytecode: 0x8be76378>Type 1 error
dt.type1error <- rbind(subsetDT(dt.metrics,scenario.balanced,'B'),
subsetDT(dt.metrics,scenario.unbalanced,'B'))
dt.type1error$LibsPerGroup %<>%
mapvalues(from = paste0('#Lib/Group = ', c(3, 5)),
to = paste0(c(3,5),' samples per group'))
dt.type1error$Scenario %<>%
mapvalues(from = c('balanced','unbalanced'),
to = c('Equal library sizes','Unequal library sizes'))
dt.type1error[, FDR := roundPretty(ifelse((FP+TP) == 0,NA,100*FP/(FP+TP)),1)]
dt.type1error <- dt.type1error[TxPerGene == 'All Transcripts',]
sub.byvar <-
colnames(dt.type1error)[-which(colnames(dt.type1error) %in% c('P.SIG','TP','FP'))]
x.melt <-
melt(dt.type1error,id.vars = sub.byvar,
measure.vars = c('P.SIG'),variable.name = 'Type',value.name = 'Value')
plot.type1error <- function(df.bar,scenario,library,legend = FALSE){
tb.bar <- df.bar[Scenario == scenario & LibsPerGroup == library,]
ggplot(tb.bar,aes(x = Method,y = Value)) +
geom_col(fill = "#bebebe",col = 'black') +
theme_bw() +
geom_hline(yintercept = 0.05,color = '#ff0000',linetype = 'dashed') +
labs(x = NULL,y = paste('Proportion of p-values < 0.05')) +
scale_y_continuous(limits = c(0,0.06),breaks = c(0,0.02,0.04,0.06)) +
theme(panel.grid = element_blank(),
axis.text.x = element_text(angle = 90),
axis.text = element_text(colour = 'black'))
}
fig.type1error.a <- plot.type1error(df.bar = x.melt,scenario = 'Equal library sizes',library = '3 samples per group')
fig.type1error.b <- plot.type1error(df.bar = x.melt,scenario = 'Unequal library sizes',library = '3 samples per group')
fig.type1error.c <- plot.type1error(df.bar = x.melt,scenario = 'Equal library sizes',library = '5 samples per group')
fig.type1error.d <- plot.type1error(df.bar = x.melt,scenario = 'Unequal library sizes',library = '5 samples per group')
fig.type1error <- (fig.type1error.a + fig.type1error.b) / (fig.type1error.c + fig.type1error.d) +
plot_annotation(tag_levels = 'a')
png(filename = file.path(path.misc,"figure4.png"),width = 7,height = 7,units = 'in',res = 300)
fig.type1error
dev.off()png
2 fig.type1error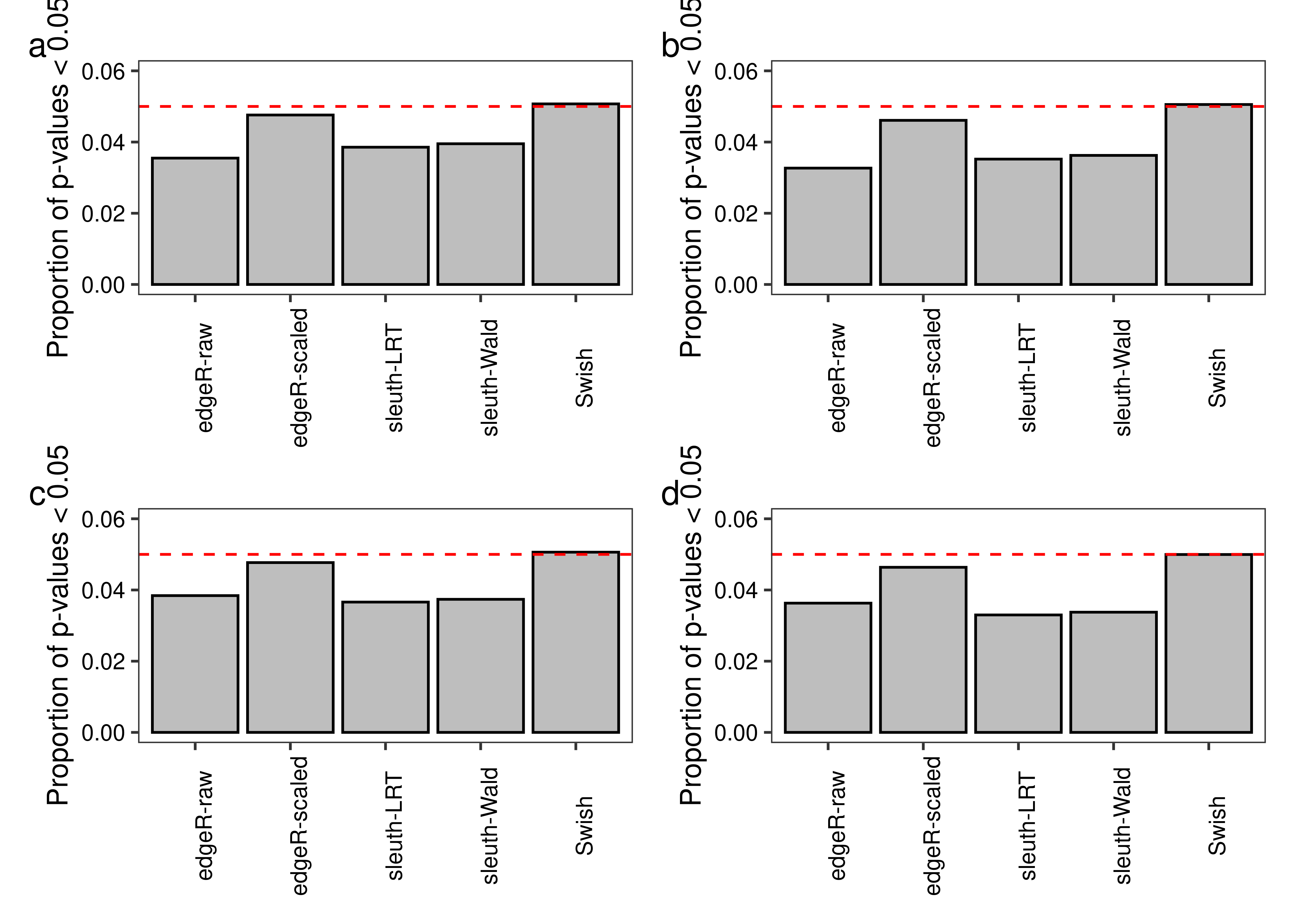
dt.pvalue.plot <- subsetDT(dt.pvalue,scenario.unbalanced,'C','All Transcripts')
dt.pvalue.plot <- dt.pvalue.plot[LibsPerGroup == '#Lib/Group = 5',]
plot.hist <- function(df.hist,method,legend = FALSE){
tb.bar <- df.hist[Method == method,]
ggplot(data = tb.bar,aes(x = PValue,y = Density.Avg)) +
geom_col(fill = "#bebebe",col = 'black',position = position_dodge(),width = 0.75) +
geom_hline(yintercept = 1,col = '#ff0000',linetype = 'dashed') +
scale_x_discrete(breaks = c("(0.00-0.05]","(0.50-0.55]","(0.95-1.00]"),
labels = c(0.00,0.50,1.00)) +
labs(x = 'P-values',y = 'Density') +
theme_bw() +
theme(panel.grid = element_blank(),
axis.text = element_text(colour = 'black'))
}
fig.hist.a <- plot.hist(df.hist = dt.pvalue.plot,method = 'edgeR-scaled')
fig.hist.b <- plot.hist(df.hist = dt.pvalue.plot,method = 'edgeR-raw')
fig.hist.c <- plot.hist(df.hist = dt.pvalue.plot,method = 'sleuth-LRT')
fig.hist.d <- plot.hist(df.hist = dt.pvalue.plot,method = 'sleuth-Wald')
fig.hist.e <- plot.hist(df.hist = dt.pvalue.plot,method = 'Swish')
design <- c(
area(1,1),area(1,2),
area(2,1),area(2,2),
area(3,1)
)
fig.hist <- fig.hist.a + fig.hist.b + fig.hist.c + fig.hist.d + fig.hist.e +
plot_layout(design = design) +
plot_annotation(tag_levels = 'a')
png(filename = file.path(path.misc,"figure5.png"),width = 7,height = 10.5,units = 'in',res = 300)
fig.hist
dev.off()png
2 fig.type1error
Read type
# Overdispersion fold-change
dt.sigma2 <- dt.overdispersion[TxPerGene == 'All Transcripts' &
Quantifier == 'Salmon' &
Scenario == 'unbalanced' &
FC == 'fc2',]
dt.sigma2 <- dt.sigma2[,-c(1,3,5,6,8,10:15)]
dt.sigma2.150.PE <- dt.sigma2[Length == '150bp' & Reads == 'paired-end',][,-c(1,2)]
setnames(dt.sigma2.150.PE,old = 'Mean',new = 'Mean.150.PE')
dt.sigma2 <- merge(dt.sigma2,dt.sigma2.150.PE,by = c('LibsPerGroup'),
all.x=TRUE,sort = FALSE)
dt.sigma2[,FC := Mean - Mean.150.PE]
dt.sigma2.3 <- dt.sigma2[LibsPerGroup == '#Lib/Group = 3',]
dt.sigma2.5 <- dt.sigma2[LibsPerGroup == '#Lib/Group = 5',]
dt.sigma2.3 <- dcast(dt.sigma2.3,LibsPerGroup + Length ~ Reads,value.var = 'FC')
dt.sigma2.5 <- dcast(dt.sigma2.5,LibsPerGroup + Length ~ Reads,value.var = 'FC')
setnames(dt.sigma2.3,
old = c('paired-end','single-end'),
new = c('FC.PE','FC.SE'))
setnames(dt.sigma2.5,
old = c('paired-end','single-end'),
new = c('FC.PE','FC.SE'))
setcolorder(dt.sigma2.3,neworder = c('LibsPerGroup','Length','FC.PE','FC.SE'))
setcolorder(dt.sigma2.5,neworder = c('LibsPerGroup','Length','FC.PE','FC.SE'))
dt.sigma2.long <- rbind(dt.sigma2.3,dt.sigma2.5)
dt.sigma2.long$LibsPerGroup %<>%
mapvalues(from = c('#Lib/Group = 3','#Lib/Group = 5'),to = c(3,5))
dt.sigma2.long$Length %<>% factor(levels = paste0(seq(50,150,25),'bp'))
dt.sigma2.long <- dt.sigma2.long[order(LibsPerGroup,Length),]
# Power and FDR
dt.scenario.table <-
expand.grid('genome' = 'mm39',
'quantifier' = c('Salmon','kallisto'),
'txpergene' = c(paste0('#Tx/Gene = ',2:5),'All Transcripts'),
stringsAsFactors = FALSE)
dt.scenario.table <- as.data.table(dt.scenario.table)
scenario.table <-
dt.scenario.table[quantifier == 'Salmon' & txpergene == 'All Transcripts',]
scenario.table <- as.character(scenario.table)
names(scenario.table) <- colnames(dt.scenario.table)
dt.table <- subsetDT(dt.metrics,scenario = scenario.table,plot = FALSE)
dt.table <- dt.table[Method == 'edgeR-scaled' &
Scenario == 'unbalanced',]
dt.table[,Power := TP/3000]
dt.table[,FDR := ifelse((FP+TP) == 0,NA,FP/(FP+TP))]
dt.table.3 <- dt.table[LibsPerGroup == '#Lib/Group = 3',][,-c(1,3,5,6,8:12)]
dt.table.5 <- dt.table[LibsPerGroup == '#Lib/Group = 5',][,-c(1,3,5,6,8:12)]
dt.table.3 <- dcast(dt.table.3,LibsPerGroup + Length ~ Reads,value.var = c('Power','FDR'))
dt.table.5 <- dcast(dt.table.5,LibsPerGroup + Length ~ Reads,value.var = c('Power','FDR'))
setnames(dt.table.3,
old = c('Power_paired-end','Power_single-end','FDR_paired-end','FDR_single-end'),
new = c('Power.PE','Power.SE','FDR.PE','FDR.SE'))
setnames(dt.table.5,
old = c('Power_paired-end','Power_single-end','FDR_paired-end','FDR_single-end'),
new = c('Power.PE','Power.SE','FDR.PE','FDR.SE'))
setcolorder(dt.table.3,neworder = c('LibsPerGroup','Length','Power.SE','FDR.SE','Power.PE','FDR.PE'))
setcolorder(dt.table.5,neworder = c('LibsPerGroup','Length','Power.SE','FDR.SE','Power.PE','FDR.PE'))
dt.table.long <- rbind(dt.table.3,dt.table.5)
dt.table.long$LibsPerGroup %<>% mapvalues(from = c('#Lib/Group = 3','#Lib/Group = 5'),to = c(3,5))
dt.table.long$Length %<>% factor(levels = paste0(seq(50,150,25),'bp'))
dt.table.long <- dt.table.long[order(LibsPerGroup,Length),]
# Organizing tables
dt.table.sigma2 <-
merge(dt.table.long,dt.sigma2.long,
all.x = TRUE,by = c('LibsPerGroup','Length'),sort = FALSE)
setcolorder(dt.table.sigma2,
neworder = c('LibsPerGroup','Length',
'FC.SE','Power.SE','FDR.SE',
'FC.PE','Power.PE','FDR.PE'))
dt.table.sigma2[,Length := gsub('bp','',Length)]
dt.table.sigma2$LibsPerGroup %<>% mapvalues(from = c(3,5),to = c('Three','Five'))
tb <- kbl(dt.table.sigma2,digits = 3,format = 'latex',escape = FALSE,booktabs = TRUE,
align = c('c','r',rep('r',6)),
col.names = linebreak(c('Samples per\ngroup','Read Length\n(bp)',
'Mapping Ambiguity\nFold Change','Power','FDR',
'Mapping Ambiguity\nFold Change','Power','FDR'),align = "c")) %>%
add_header_above(c(" " = 2, "Single-end Read" = 3, "Paired-end Read" = 3)) %>%
collapse_rows(1, latex_hline = 'major')
save_kable(tb,file = file.path(path.misc,"table1.tex"))
sessionInfo()R version 4.2.1 (2022-06-23)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: CentOS Linux 7 (Core)
Matrix products: default
BLAS: /stornext/System/data/apps/R/R-4.2.1/lib64/R/lib/libRblas.so
LAPACK: /stornext/System/data/apps/R/R-4.2.1/lib64/R/lib/libRlapack.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] pkg_1.0 patchwork_1.1.2 kableExtra_1.3.4
[4] ggpubr_0.5.0 readr_2.1.3 purrr_0.3.5
[7] devtools_2.4.5 usethis_2.1.6 BiocParallel_1.32.3
[10] edgeR_3.40.0 limma_3.54.0 magrittr_2.0.3
[13] plyr_1.8.8 thematic_0.1.2.1 ggplot2_3.4.0
[16] data.table_1.14.6 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] utf8_1.2.2 tidyselect_1.2.0
[3] RSQLite_2.2.19 AnnotationDbi_1.60.0
[5] htmlwidgets_1.5.4 grid_4.2.1
[7] munsell_0.5.0 codetools_0.2-18
[9] miniUI_0.1.1.1 withr_2.5.0
[11] colorspace_2.0-3 Biobase_2.58.0
[13] filelock_1.0.2 highr_0.9
[15] knitr_1.41 rstudioapi_0.14
[17] stats4_4.2.1 SingleCellExperiment_1.20.0
[19] ggsignif_0.6.4 Rsubread_2.12.0
[21] labeling_0.4.2 MatrixGenerics_1.10.0
[23] git2r_0.30.1 tximport_1.26.0
[25] GenomeInfoDbData_1.2.9 bit64_4.0.5
[27] farver_2.1.1 rhdf5_2.42.0
[29] rprojroot_2.0.3 vctrs_0.5.1
[31] generics_0.1.3 xfun_0.35
[33] BiocFileCache_2.6.0 fishpond_2.4.0
[35] R6_2.5.1 GenomeInfoDb_1.34.3
[37] locfit_1.5-9.6 AnnotationFilter_1.22.0
[39] bitops_1.0-7 rhdf5filters_1.10.0
[41] cachem_1.0.6 DelayedArray_0.24.0
[43] assertthat_0.2.1 promises_1.2.0.1
[45] BiocIO_1.8.0 scales_1.2.1
[47] gtable_0.3.1 processx_3.8.0
[49] ensembldb_2.22.0 rlang_1.0.6
[51] systemfonts_1.0.4 splines_4.2.1
[53] rtracklayer_1.58.0 rstatix_0.7.1
[55] lazyeval_0.2.2 broom_1.0.1
[57] BiocManager_1.30.19 yaml_2.3.6
[59] abind_1.4-5 GenomicFeatures_1.50.2
[61] backports_1.4.1 httpuv_1.6.6
[63] sleuth_0.30.0 wasabi_1.0.1
[65] tools_4.2.1 ellipsis_0.3.2
[67] jquerylib_0.1.4 BiocGenerics_0.44.0
[69] sessioninfo_1.2.2 Rcpp_1.0.9
[71] progress_1.2.2 zlibbioc_1.44.0
[73] RCurl_1.98-1.9 ps_1.7.2
[75] prettyunits_1.1.1 urlchecker_1.0.1
[77] S4Vectors_0.36.0 SummarizedExperiment_1.28.0
[79] fs_1.5.2 svMisc_1.2.3
[81] whisker_0.4 ProtGenerics_1.30.0
[83] matrixStats_0.63.0 pkgload_1.3.2
[85] hms_1.1.2 mime_0.12
[87] evaluate_0.18 xtable_1.8-4
[89] XML_3.99-0.12 IRanges_2.32.0
[91] compiler_4.2.1 biomaRt_2.54.0
[93] tibble_3.1.8 crayon_1.5.2
[95] htmltools_0.5.3 later_1.3.0
[97] tzdb_0.3.0 tidyr_1.2.1
[99] DBI_1.1.3 dbplyr_2.2.1
[101] rappdirs_0.3.3 Matrix_1.5-3
[103] car_3.1-1 cli_3.4.1
[105] parallel_4.2.1 GenomicRanges_1.50.1
[107] pkgconfig_2.0.3 getPass_0.2-2
[109] GenomicAlignments_1.34.0 xml2_1.3.3
[111] svglite_2.1.0 bslib_0.4.1
[113] webshot_0.5.4 XVector_0.38.0
[115] rvest_1.0.3 stringr_1.4.1
[117] callr_3.7.3 digest_0.6.30
[119] Biostrings_2.66.0 rmarkdown_2.18
[121] tximeta_1.16.0 restfulr_0.0.15
[123] curl_4.3.3 shiny_1.7.3
[125] Rsamtools_2.14.0 gtools_3.9.3
[127] rjson_0.2.21 lifecycle_1.0.3
[129] jsonlite_1.8.3 Rhdf5lib_1.20.0
[131] carData_3.0-5 desc_1.4.2
[133] viridisLite_0.4.1 fansi_1.0.3
[135] pillar_1.8.1 lattice_0.20-45
[137] KEGGREST_1.38.0 fastmap_1.1.0
[139] httr_1.4.4 pkgbuild_1.3.1
[141] interactiveDisplayBase_1.36.0 glue_1.6.2
[143] remotes_2.4.2 png_0.1-7
[145] BiocVersion_3.16.0 bit_4.0.5
[147] stringi_1.7.8 sass_0.4.4
[149] profvis_0.3.7 blob_1.2.3
[151] AnnotationHub_3.6.0 memoise_2.0.1
[153] dplyr_1.0.10