SCZ 2018 - Brain_Putamen_basal_ganglia
sheng Qian
2021-2-6
Last updated: 2022-05-12
Checks: 5 2
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 011327d. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .ipynb_checkpoints/
Ignored: data/AF/
Untracked files:
Untracked: G_list.RData
Untracked: Rplot.png
Untracked: SCZ_annotation.xlsx
Untracked: analysis/.ipynb_checkpoints/
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_2014_EUR_out/
Untracked: code/SCZ_2018_S_out/
Untracked: code/SCZ_2018_out/
Untracked: code/SCZ_2020_Single_out/
Untracked: code/SCZ_2020_out/
Untracked: code/SCZ_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/process_scz_2018_snps.R
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2014_EUR_analysis.sbatch
Untracked: code/run_SCZ_2014_EUR_analysis.sh
Untracked: code/run_SCZ_2014_EUR_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_analysis.sbatch
Untracked: code/run_SCZ_2018_analysis.sh
Untracked: code/run_SCZ_2018_analysis_S.sbatch
Untracked: code/run_SCZ_2018_analysis_S.sh
Untracked: code/run_SCZ_2018_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2020_Single_analysis.sbatch
Untracked: code/run_SCZ_2020_Single_analysis.sh
Untracked: code/run_SCZ_2020_Single_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2020_analysis.sbatch
Untracked: code/run_SCZ_2020_analysis.sh
Untracked: code/run_SCZ_2020_ctwas_rss_LDR.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_analysis_S.sbatch
Untracked: code/run_SCZ_analysis_S.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_SCZ_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: code/wflow_build.R
Untracked: code/wflow_build.sbatch
Untracked: data/.ipynb_checkpoints/
Untracked: data/BMI/
Untracked: data/GO_Terms/
Untracked: data/PGC3_SCZ_wave3_public.v2.tsv
Untracked: data/SCZ/
Untracked: data/SCZ_2014_EUR/
Untracked: data/SCZ_2018/
Untracked: data/SCZ_2018_S/
Untracked: data/SCZ_2020/
Untracked: data/SCZ_2020_Single/
Untracked: data/SCZ_S/
Untracked: data/Supplementary Table 15 - MAGMA.xlsx
Untracked: data/Supplementary Table 20 - Prioritised Genes.xlsx
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/scz_2018.RDS
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Untracked: top_genes_32.txt
Untracked: top_genes_37.txt
Untracked: top_genes_43.txt
Untracked: top_genes_81.txt
Untracked: z_snp_pos_SCZ.RData
Untracked: z_snp_pos_SCZ_2014_EUR.RData
Untracked: z_snp_pos_SCZ_2018.RData
Untracked: z_snp_pos_SCZ_2020.RData
Unstaged changes:
Deleted: analysis/BMI_S_results.Rmd
Modified: analysis/SCZ_2018_Brain_Amygdala_S.Rmd
Modified: analysis/SCZ_2018_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/SCZ_2018_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellum_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cortex_S.Rmd
Modified: analysis/SCZ_2018_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hippocampus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hypothalamus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/SCZ_2018_Brain_Substantia_nigra_S.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_2018_Brain_Putamen_basal_ganglia_S.Rmd) and HTML (docs/SCZ_2018_Brain_Putamen_basal_ganglia_S.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| html | 011327d | sq-96 | 2022-05-12 | update |
| Rmd | 6c6abbd | sq-96 | 2022-05-12 | update |
library(reticulate)
use_python("/scratch/midway2/shengqian/miniconda3/envs/PythonForR/bin/python",required=T)Weight QC
#number of imputed weights
nrow(qclist_all)[1] 18714#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1715 1341 1135 747 753 935 1084 644 764 833 1148 1001 362 676 630 762
17 18 19 20 21 22
1314 271 1307 672 31 589 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 16516#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8825finish
Attaching package: 'dplyr'The following objects are masked from 'package:stats':
filter, lagThe following objects are masked from 'package:base':
intersect, setdiff, setequal, unionCheck convergence of parameters
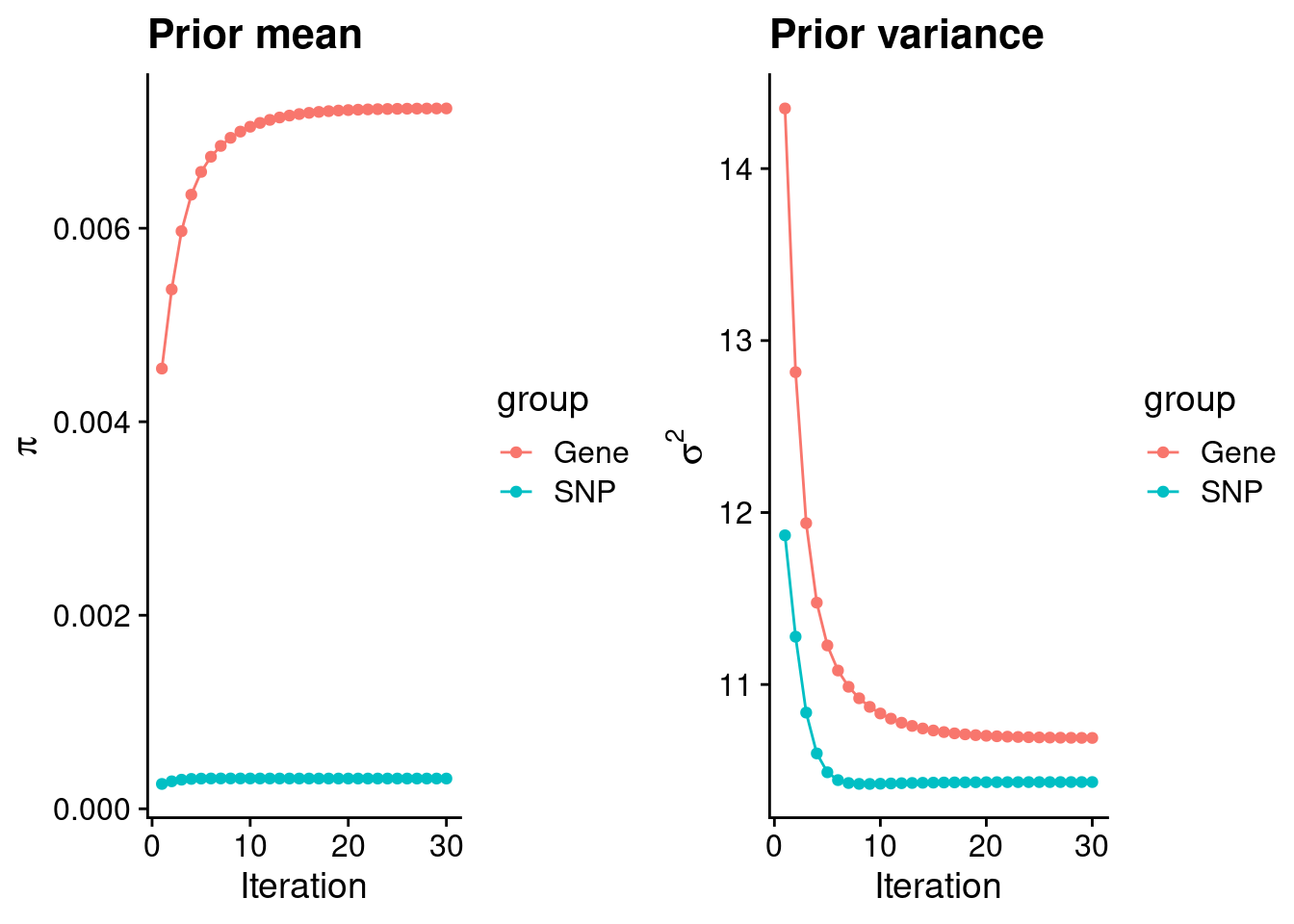
gene snp
0.0072380 0.0003126 gene snp
10.69 10.43 [1] 105318[1] 7109 6309950 gene snp
0.005222 0.195372 [1] 0.01435 1.07231Genes with highest PIPs
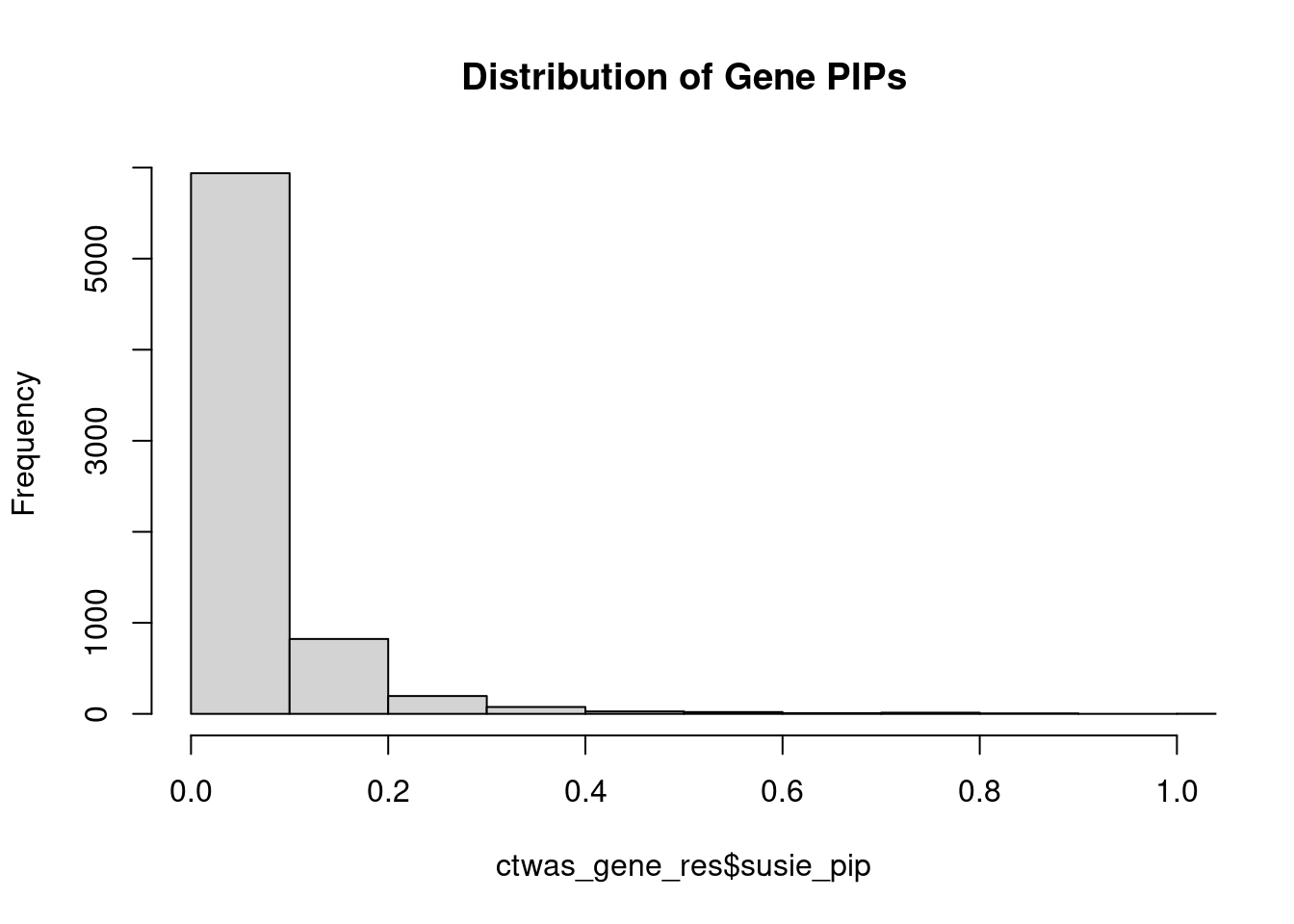
genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
3298 LRP8 1_33 1.1442 32.81 0.0003081 -4.820 6 6
3183 LINC00320 21_6 1.0821 28.61 0.0003052 -5.336 5 5
2119 FAM177A1 14_9 1.0113 23.94 0.0002035 -4.872 11 14
3112 LAMA5 20_36 0.8624 24.08 0.0001561 -4.335 9 12
242 ALG1L13P 8_11 0.8561 23.49 0.0001531 4.461 4 4
5379 SF3B1 2_117 0.8398 44.94 0.0002906 -7.053 4 4
107 ACTR1B 2_57 0.8262 19.35 0.0001232 -3.978 6 6
4477 PLCB2 15_14 0.8102 24.79 0.0001286 -4.470 5 5
1776 DPYSL3 5_86 0.7896 22.79 0.0001349 -4.157 1 1
1322 COA8 14_54 0.7860 46.84 0.0002658 -7.431 6 9
589 B3GAT1 11_84 0.7836 22.65 0.0001170 4.265 7 10
4839 PYROXD2 10_62 0.7830 21.51 0.0001164 3.718 10 11
228 AKT3 1_128 0.7781 34.91 0.0001934 -6.350 4 4
2967 KAT5 11_36 0.7775 24.18 0.0001362 4.491 7 7
4101 NTRK3 15_41 0.7498 23.92 0.0001194 -4.457 3 3
2398 GIGYF1 7_62 0.7342 27.41 0.0001384 5.266 2 2
4088 NT5C2 10_66 0.7314 47.24 0.0002278 -8.668 9 11
3357 LY6H 8_94 0.7233 22.36 0.0001059 -4.186 4 4
4294 PCSK6 15_50 0.7178 22.06 0.0001061 -3.967 2 3
5292 SDCCAG8 1_128 0.7027 27.40 0.0001204 5.377 7 11Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
3298 LRP8 1_33 1.1442 32.81 0.0003081 -4.820 6 6
3183 LINC00320 21_6 1.0821 28.61 0.0003052 -5.336 5 5
5379 SF3B1 2_117 0.8398 44.94 0.0002906 -7.053 4 4
1322 COA8 14_54 0.7860 46.84 0.0002658 -7.431 6 9
4088 NT5C2 10_66 0.7314 47.24 0.0002278 -8.668 9 11
2119 FAM177A1 14_9 1.0113 23.94 0.0002035 -4.872 11 14
228 AKT3 1_128 0.7781 34.91 0.0001934 -6.350 4 4
3112 LAMA5 20_36 0.8624 24.08 0.0001561 -4.335 9 12
242 ALG1L13P 8_11 0.8561 23.49 0.0001531 4.461 4 4
1646 DGKZ 11_28 0.5830 47.17 0.0001522 7.216 1 1
2398 GIGYF1 7_62 0.7342 27.41 0.0001384 5.266 2 2
2967 KAT5 11_36 0.7775 24.18 0.0001362 4.491 7 7
3380 MAD1L1 7_3 0.5571 54.63 0.0001359 7.478 4 4
1776 DPYSL3 5_86 0.7896 22.79 0.0001349 -4.157 1 1
4477 PLCB2 15_14 0.8102 24.79 0.0001286 -4.470 5 5
107 ACTR1B 2_57 0.8262 19.35 0.0001232 -3.978 6 6
5292 SDCCAG8 1_128 0.7027 27.40 0.0001204 5.377 7 11
4101 NTRK3 15_41 0.7498 23.92 0.0001194 -4.457 3 3
589 B3GAT1 11_84 0.7836 22.65 0.0001170 4.265 7 10
4839 PYROXD2 10_62 0.7830 21.51 0.0001164 3.718 10 11Comparing z scores and PIPs
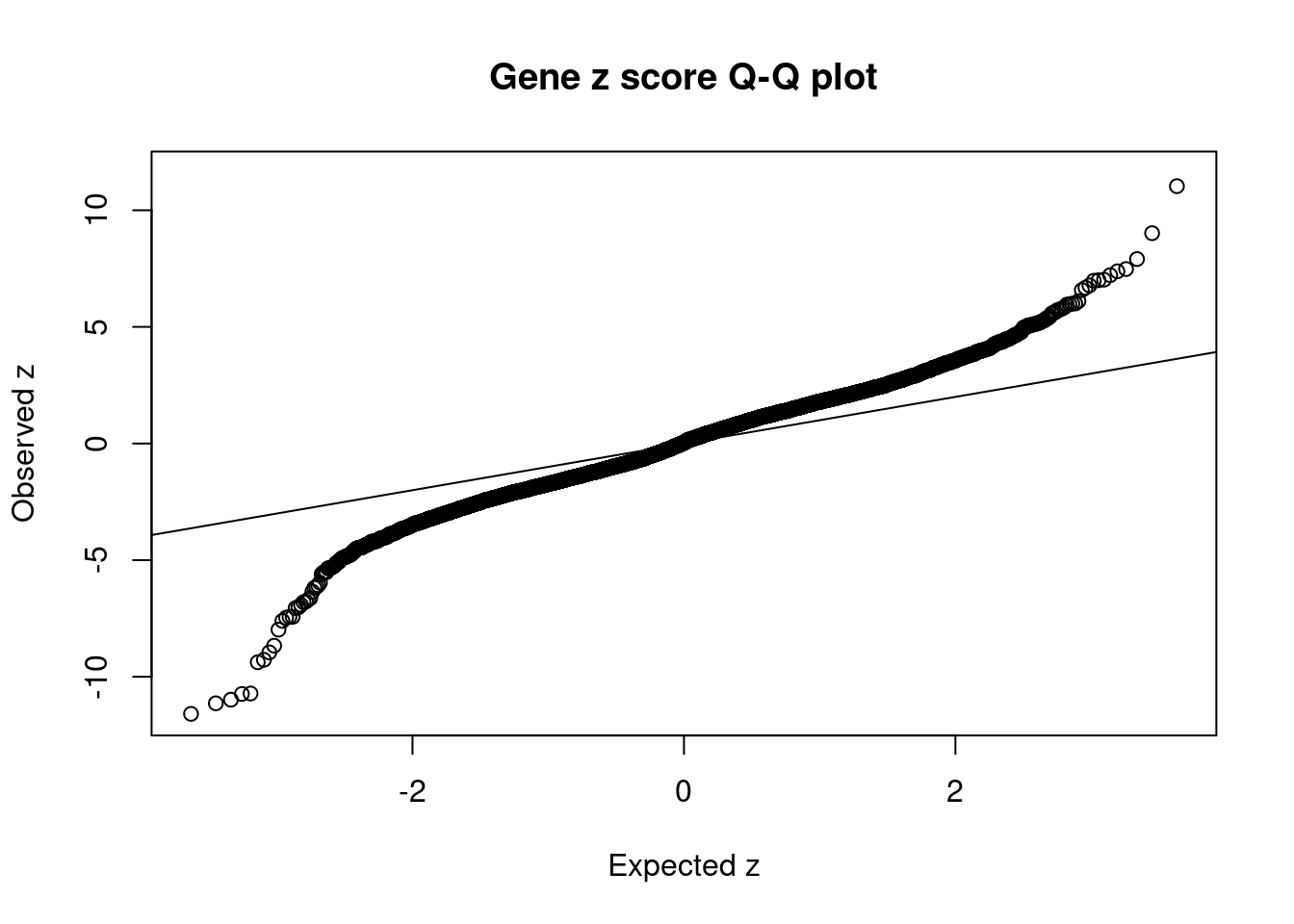
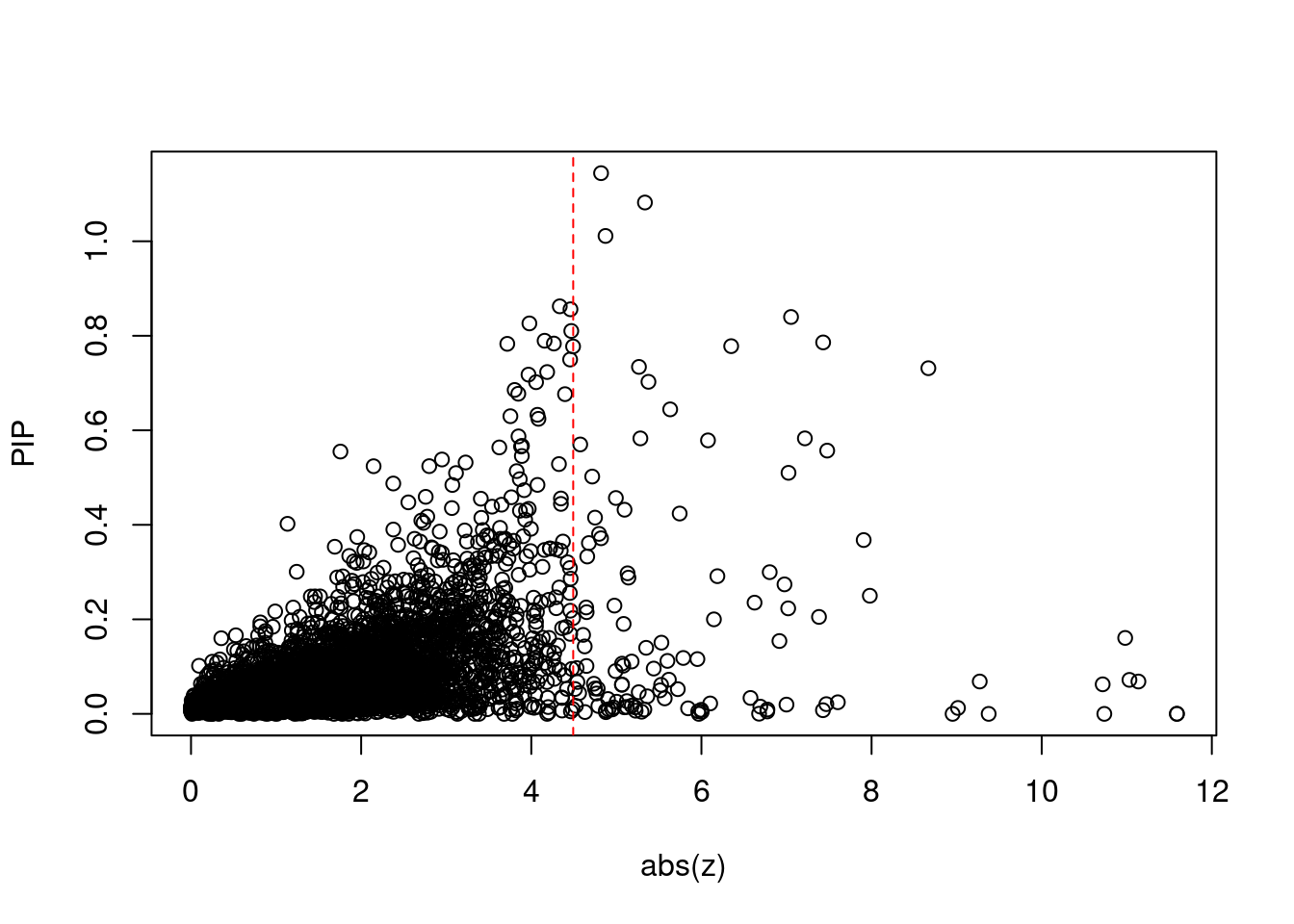
[1] 0.01618 genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
374 APOM 6_26 1.639e-04 215.80 5.484e-11 11.590 2 2
605 BAG6 6_26 1.164e-04 215.80 2.571e-11 -11.590 6 6
6621 VARS2 6_25 6.847e-02 101.95 4.538e-06 -11.137 1 1
819 C6orf136 6_24 7.194e-02 79.99 3.931e-06 11.031 2 2
2252 FLOT1 6_24 1.607e-01 78.65 1.917e-05 -10.981 6 6
1535 CYP21A2 6_26 3.944e-06 179.01 2.643e-14 -10.736 1 2
726 BTN3A2 6_20 6.252e-02 91.47 1.705e-06 -10.717 4 5
2519 GPSM3 6_26 3.863e-06 120.46 1.706e-14 -9.377 2 2
1006 CCHCR1 6_25 6.850e-02 61.89 1.309e-06 -9.272 10 15
1591 DDR1 6_25 1.253e-02 68.37 1.019e-07 9.016 1 1
1878 EGFL8 6_26 1.227e-05 121.04 1.708e-13 -8.953 2 3
4088 NT5C2 10_66 7.314e-01 47.24 2.278e-04 -8.668 9 11
3392 MAIP1 2_118 2.500e-01 44.44 2.637e-05 -7.980 1 1
472 AS3MT 10_66 3.678e-01 40.78 5.194e-05 7.907 4 5
7102 ZSCAN26 6_22 2.458e-02 37.09 1.450e-07 -7.603 3 4
3380 MAD1L1 7_3 5.571e-01 54.63 1.359e-04 7.478 4 4
7098 ZSCAN16 6_22 2.059e-02 53.72 1.087e-07 -7.468 2 2
1322 COA8 14_54 7.860e-01 46.84 2.658e-04 -7.431 6 9
6912 ZNF192P1 6_22 7.746e-03 53.28 3.035e-08 -7.429 1 1
3060 KLC1 14_54 2.052e-01 49.40 1.648e-05 7.382 6 6GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 48Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"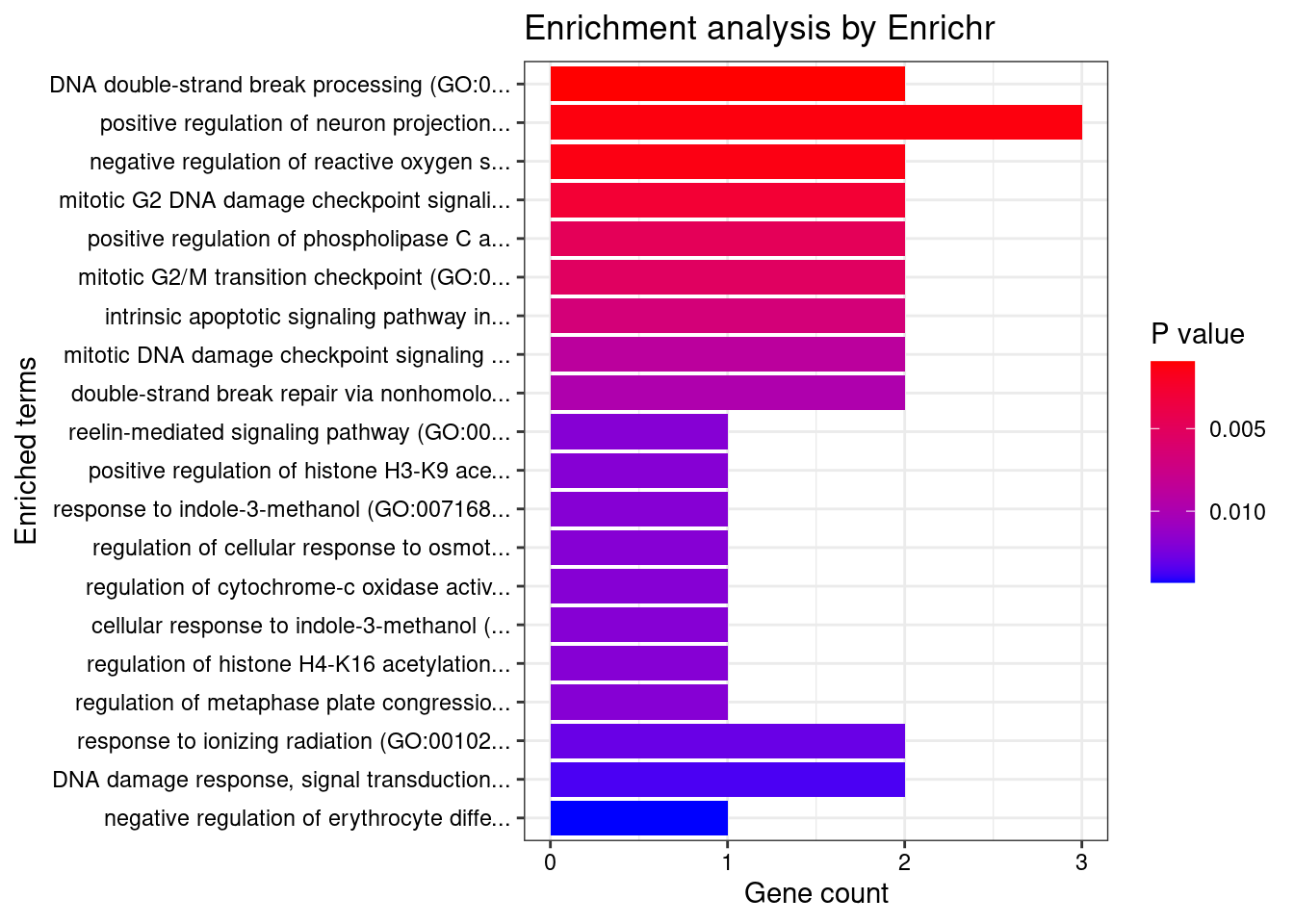
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Cellular_Component_2021"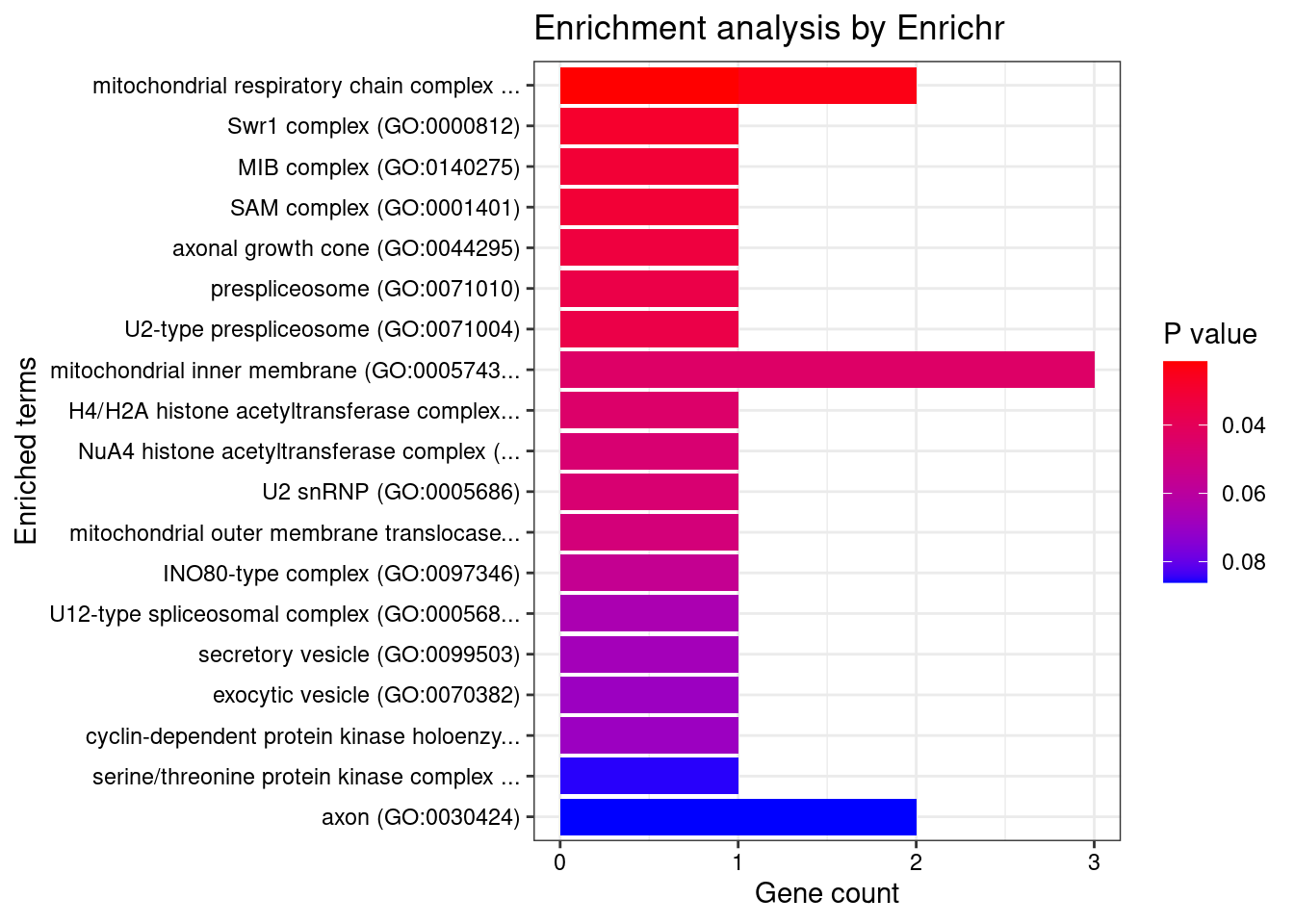
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"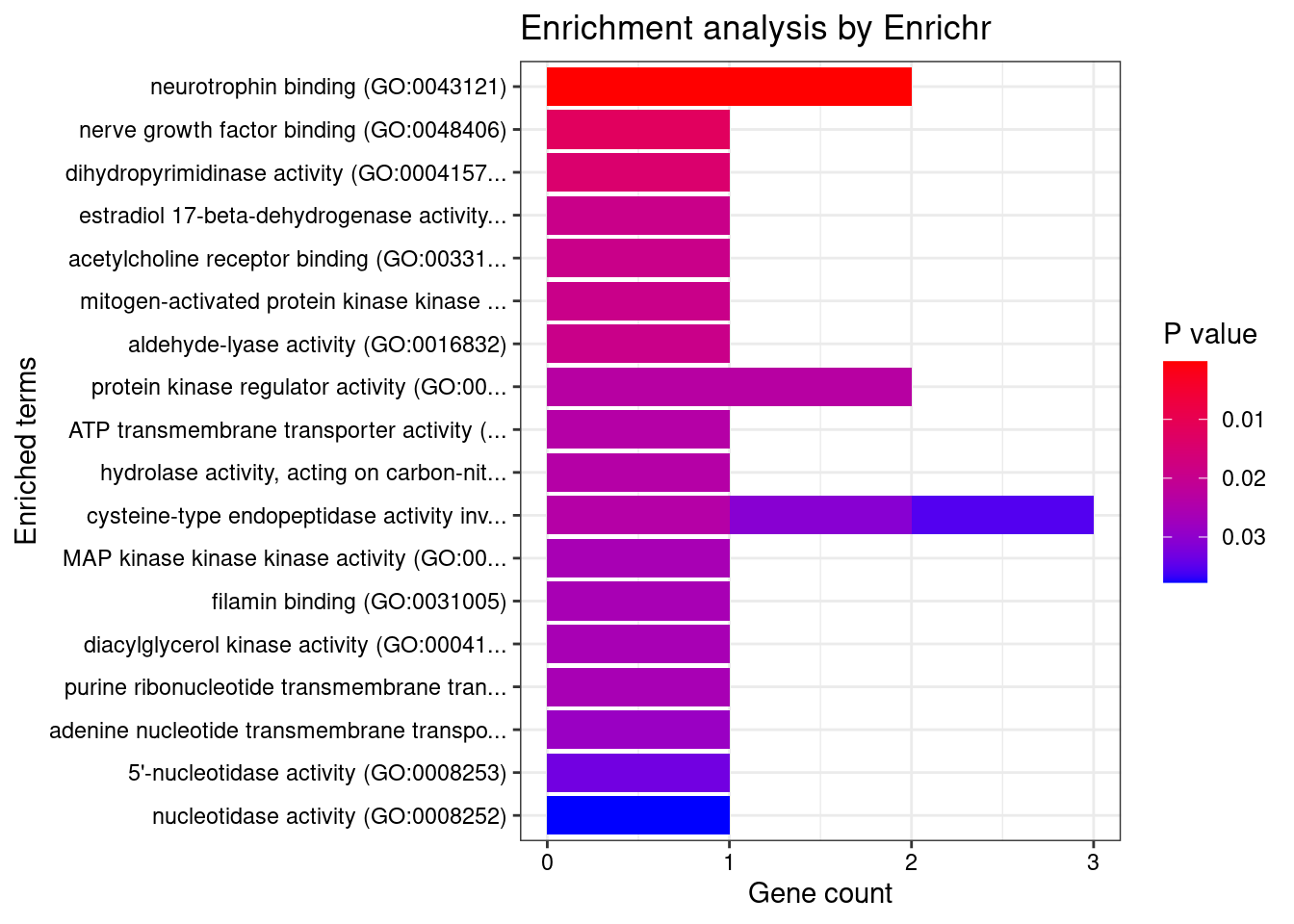
Term Overlap Adjusted.P.value Genes
1 neurotrophin binding (GO:0043121) 2/8 0.01252 NTRK3;PCSK6DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio
49 Schizophrenia 0.01231 9/22
7 Adenocarcinoma of prostate 0.01755 2/22
34 Measles 0.01755 1/22
56 Electroencephalogram abnormal 0.01755 1/22
125 Sporadic Breast Carcinoma 0.01755 1/22
128 Primary peritoneal carcinoma 0.01755 1/22
137 BREAST-OVARIAN CANCER, FAMILIAL, SUSCEPTIBILITY TO, 1 0.01755 1/22
138 BREAST CANCER, FAMILIAL, SUSCEPTIBILITY TO, 1 0.01755 1/22
139 OVARIAN CANCER, FAMILIAL, SUSCEPTIBILITY TO, 1 0.01755 1/22
141 HEMOLYTIC UREMIC SYNDROME, ATYPICAL, SUSCEPTIBILITY TO, 2 0.01755 1/22
BgRatio
49 883/9703
7 20/9703
34 1/9703
56 1/9703
125 1/9703
128 1/9703
137 1/9703
138 1/9703
139 1/9703
141 1/9703WebGestalt enrichment analysis for genes with PIP>0.5
Warning: replacing previous import 'lifecycle::last_warnings' by
'rlang::last_warnings' when loading 'hms'Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Warning: ggrepel: 13 unlabeled data points (too many overlaps). Consider
increasing max.overlaps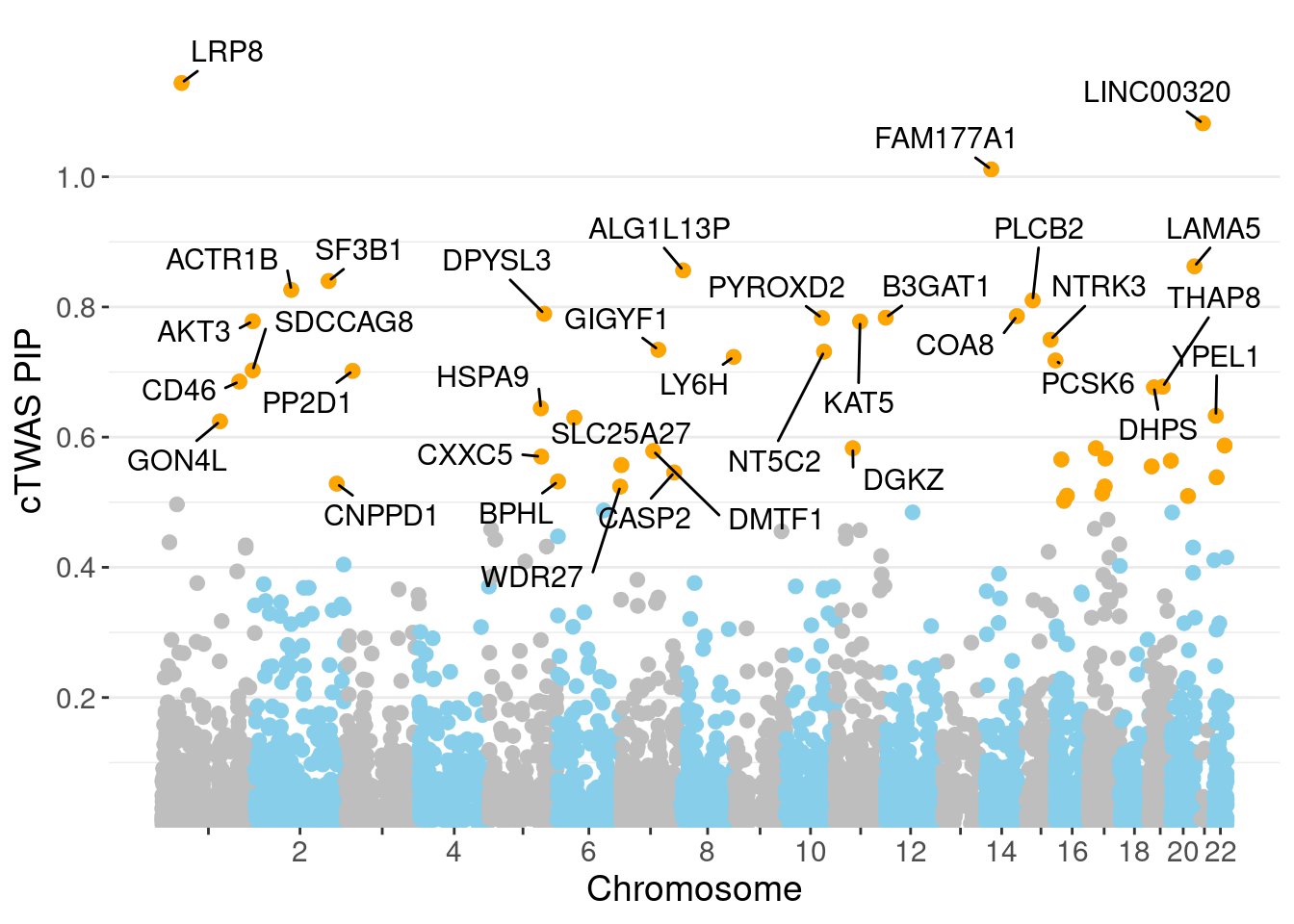
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 130#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 56#significance threshold for TWAS
print(sig_thresh)[1] 4.493#number of ctwas genes
length(ctwas_genes)[1] 8#number of TWAS genes
length(twas_genes)[1] 115#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
107 ACTR1B 2_57 0.8262 19.35 0.0001232 -3.978 6 6
242 ALG1L13P 8_11 0.8561 23.49 0.0001531 4.461 4 4
3112 LAMA5 20_36 0.8624 24.08 0.0001561 -4.335 9 12
4477 PLCB2 15_14 0.8102 24.79 0.0001286 -4.470 5 5#sensitivity / recall
print(sensitivity) ctwas TWAS
0.02308 0.10769 #specificity
print(specificity) ctwas TWAS
0.9993 0.9857 #precision / PPV
print(precision) ctwas TWAS
0.3750 0.1217
sessionInfo()R version 4.1.0 (2021-05-18)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.3.13-el7-x86_64/lib/libopenblas_haswellp-r0.3.13.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.4.0 forcats_0.5.1 stringr_1.4.0 purrr_0.3.4
[5] readr_1.4.0 tidyr_1.1.3 tidyverse_1.3.1 tibble_3.1.7
[9] WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0 cowplot_1.1.1
[13] ggplot2_3.3.5 dplyr_1.0.7 reticulate_1.20 workflowr_1.6.2
loaded via a namespace (and not attached):
[1] fs_1.5.0 lubridate_1.7.10 doParallel_1.0.16 httr_1.4.2
[5] rprojroot_2.0.2 tools_4.1.0 backports_1.2.1 doRNG_1.8.2
[9] bslib_0.2.5.1 utf8_1.2.1 R6_2.5.0 vipor_0.4.5
[13] DBI_1.1.1 colorspace_2.0-2 withr_2.4.2 ggrastr_1.0.1
[17] tidyselect_1.1.1 curl_4.3.2 compiler_4.1.0 git2r_0.28.0
[21] rvest_1.0.0 cli_3.0.0 Cairo_1.5-15 xml2_1.3.2
[25] labeling_0.4.2 sass_0.4.0 scales_1.1.1 systemfonts_1.0.4
[29] apcluster_1.4.9 digest_0.6.27 rmarkdown_2.9 svglite_2.0.0
[33] pkgconfig_2.0.3 htmltools_0.5.1.1 dbplyr_2.1.1 highr_0.9
[37] rlang_1.0.2 rstudioapi_0.13 jquerylib_0.1.4 farver_2.1.0
[41] generics_0.1.0 jsonlite_1.7.2 magrittr_2.0.1 Matrix_1.3-3
[45] ggbeeswarm_0.6.0 Rcpp_1.0.7 munsell_0.5.0 fansi_0.5.0
[49] lifecycle_1.0.0 stringi_1.6.2 whisker_0.4 yaml_2.2.1
[53] plyr_1.8.6 grid_4.1.0 ggrepel_0.9.1 parallel_4.1.0
[57] promises_1.2.0.1 crayon_1.4.1 lattice_0.20-44 haven_2.4.1
[61] hms_1.1.0 knitr_1.33 pillar_1.7.0 igraph_1.2.6
[65] rjson_0.2.20 rngtools_1.5 reshape2_1.4.4 codetools_0.2-18
[69] reprex_2.0.0 glue_1.4.2 evaluate_0.14 data.table_1.14.0
[73] modelr_0.1.8 png_0.1-7 vctrs_0.3.8 httpuv_1.6.1
[77] foreach_1.5.1 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[81] xfun_0.24 broom_0.7.8 later_1.2.0 iterators_1.0.13
[85] beeswarm_0.4.0 ellipsis_0.3.2