Atrial fibrillation - Heart Left Ventricle
sheng Qian
2021-12-18
Last updated: 2021-12-24
Checks: 6 1
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 762fd7f. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Untracked files:
Untracked: code/ctwas_config.R
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: data/AF/
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/summary_known_genes_annotations.xlsx
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/Atrial_Fibrillation_Heart_Left_Ventricle.Rmd) and HTML (docs/Atrial_Fibrillation_Heart_Left_Ventricle.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 762fd7f | sq-96 | 2021-12-24 | update |
| html | 1f785cf | sq-96 | 2021-12-22 | Build site. |
| html | 82c68fd | sq-96 | 2021-12-22 | Build site. |
| html | 9cf0e70 | sq-96 | 2021-12-22 | Build site. |
| html | 77de9fb | sq-96 | 2021-12-22 | Build site. |
| html | 6b46de7 | sq-96 | 2021-12-22 | Build site. |
| html | e22d0c9 | sq-96 | 2021-12-21 | Build site. |
| Rmd | a6c7890 | sq-96 | 2021-12-21 | Start my new project |
qclist_all <- list()
qc_files <- paste0(results_dir, "/", list.files(results_dir, pattern="exprqc.Rd"))
for (i in 1:length(qc_files)){
load(qc_files[i])
chr <- unlist(strsplit(rev(unlist(strsplit(qc_files[i], "_")))[1], "[.]"))[1]
qclist_all[[chr]] <- cbind(do.call(rbind, lapply(qclist,unlist)), as.numeric(substring(chr,4)))
}
qclist_all <- data.frame(do.call(rbind, qclist_all))
colnames(qclist_all)[ncol(qclist_all)] <- "chr"
rm(qclist, wgtlist, z_gene_chr)
#number of imputed weights
nrow(qclist_all)[1] 10150#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1003 711 570 412 451 607 495 367 384 411 592 556 186 332 344 494
17 18 19 20 21 22
639 150 783 300 110 253 #proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.6545813Load ctwas results
Check convergence of parameters
library(ggplot2)
library(cowplot)
********************************************************Note: As of version 1.0.0, cowplot does not change the default ggplot2 theme anymore. To recover the previous behavior, execute:
theme_set(theme_cowplot())********************************************************load(paste0(results_dir, "/", analysis_id, "_ctwas.s2.susieIrssres.Rd"))
df <- data.frame(niter = rep(1:ncol(group_prior_rec), 2),
value = c(group_prior_rec[1,], group_prior_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_rec)))
df$group <- as.factor(df$group)
df$value[df$group=="SNP"] <- df$value[df$group=="SNP"]*thin #adjust parameter to account for thin argument
p_pi <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(pi)) +
ggtitle("Prior mean") +
theme_cowplot()
df <- data.frame(niter = rep(1:ncol(group_prior_var_rec), 2),
value = c(group_prior_var_rec[1,], group_prior_var_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_var_rec)))
df$group <- as.factor(df$group)
p_sigma2 <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(sigma^2)) +
ggtitle("Prior variance") +
theme_cowplot()
plot_grid(p_pi, p_sigma2)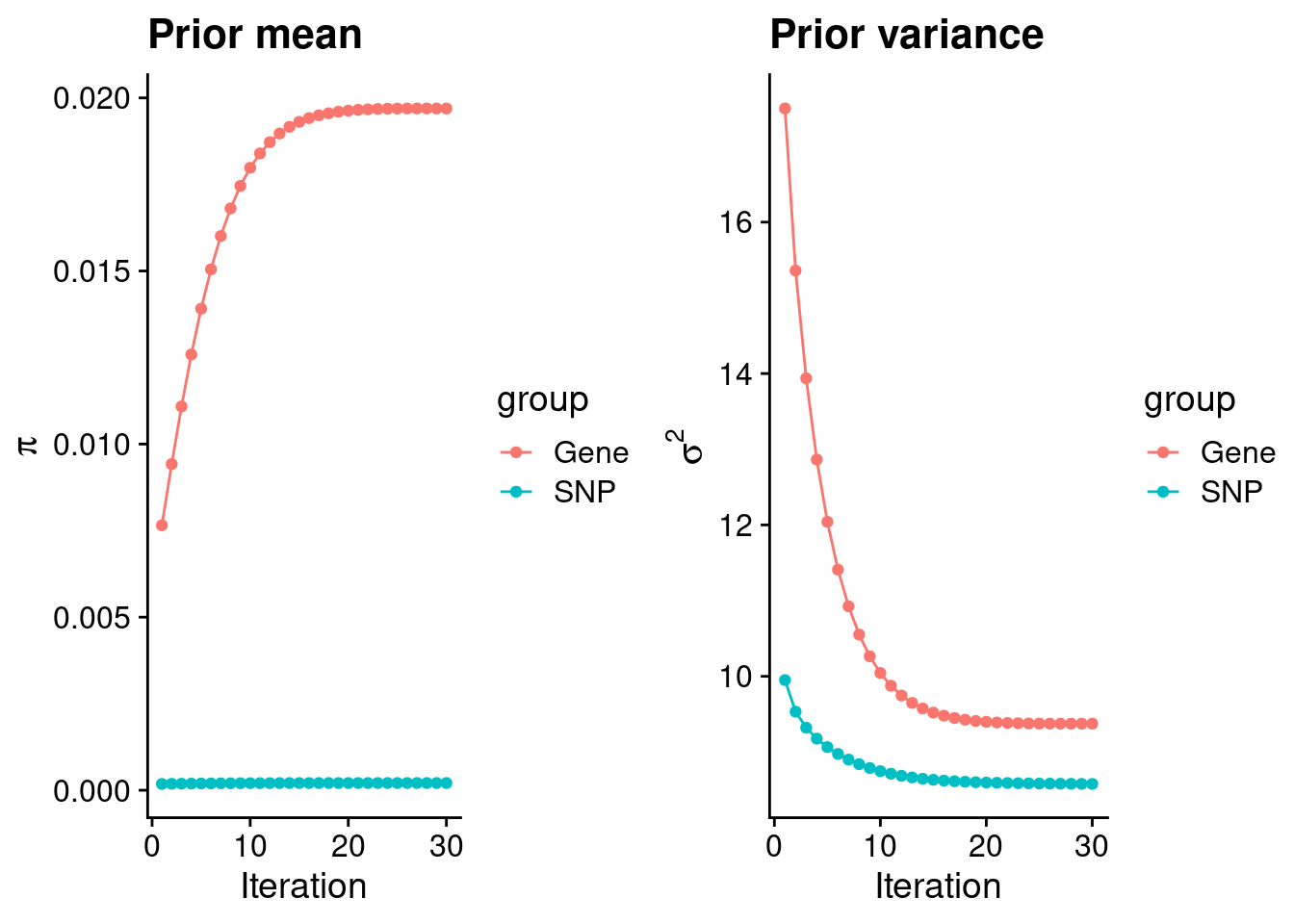
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0196935664 0.0002083061 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
9.374271 8.577991 #report sample size
print(sample_size)[1] 1030836#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 10150 6839050#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.001817768 0.011854791 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.01188393 0.13624409Genes with highest PIPs
#distribution of PIPs
hist(ctwas_gene_res$susie_pip, xlim=c(0,1), main="Distribution of Gene PIPs")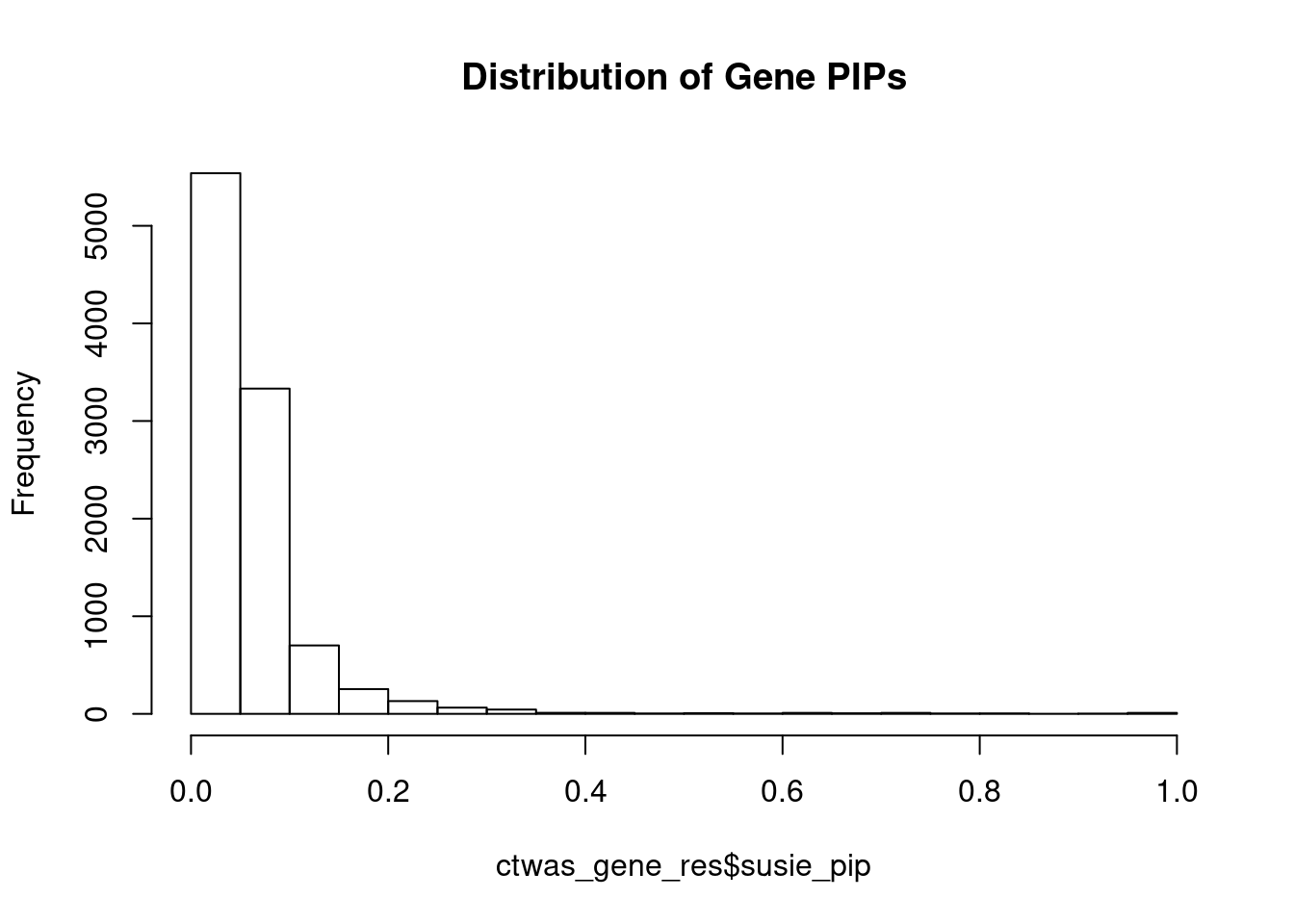
#genes with PIP>0.8 or 20 highest PIPs
head(ctwas_gene_res[order(-ctwas_gene_res$susie_pip),report_cols], max(sum(ctwas_gene_res$susie_pip>0.8), 20)) genename region_tag susie_pip mu2 PVE z num_eqtl
3169 PRRX1 1_84 0.9999999 153.79916 1.491985e-04 14.848088 2
3147 WIPF1 2_105 0.9993101 2124.21550 2.059251e-03 8.987351 2
2431 DNAJC12 10_44 0.9967458 850.85693 8.227187e-04 -5.328244 1
1214 GMCL1 2_46 0.9944249 60.60721 5.846645e-05 -8.434995 2
9840 NKX2-5 5_103 0.9942183 61.83714 5.964054e-05 -9.616279 2
10979 MSRB1 16_2 0.9667967 26.84161 2.517411e-05 5.929412 1
9417 PLEC 8_94 0.9666163 43.61991 4.090245e-05 3.093844 2
6663 JAM2 21_9 0.9633616 22.37976 2.091487e-05 4.569598 2
7794 KDM1B 6_14 0.9581444 64.33734 5.980046e-05 -9.000000 1
2987 GNB4 3_110 0.9537748 32.01351 2.962031e-05 -5.869048 1
11279 DPF3 14_34 0.9246749 30.95765 2.776946e-05 6.100000 1
2960 PCCB 3_84 0.9229236 849.82984 7.608659e-04 -5.382353 1
9960 PGP 16_2 0.9051693 28.48915 2.501611e-05 5.943820 1
1551 CCDC134 22_17 0.8683170 26.15261 2.202945e-05 -5.180556 1
12376 LINC01314 15_37 0.8300211 20.71969 1.668334e-05 -4.379164 4
4891 NPL 1_90 0.8251030 18.75569 1.501245e-05 -3.825175 1
5042 PSMB7 9_64 0.8211676 25.19921 2.007378e-05 -4.820896 1
8847 WASHC2C 10_31 0.8186904 19.80281 1.572740e-05 -3.972152 1
12133 LINC00964 8_82 0.8180590 22.20129 1.761867e-05 4.554265 2
3725 SSPN 12_18 0.7969230 42.42202 3.279579e-05 -6.791667 1Genes with largest effect sizes
#plot PIP vs effect size
plot(ctwas_gene_res$susie_pip, ctwas_gene_res$mu2, xlab="PIP", ylab="mu^2", main="Gene PIPs vs Effect Size")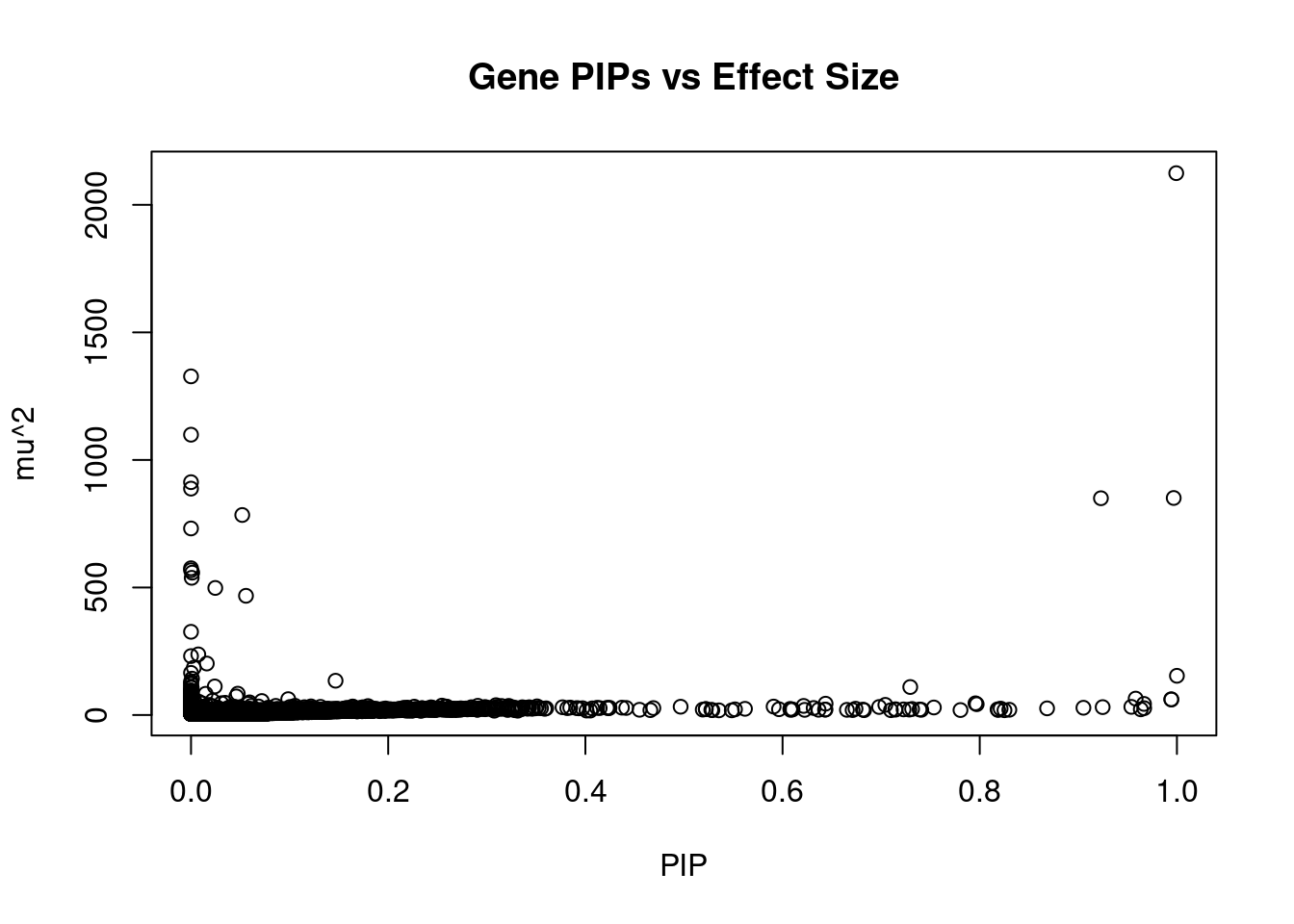
#genes with 20 largest effect sizes
head(ctwas_gene_res[order(-ctwas_gene_res$mu2),report_cols],20) genename region_tag susie_pip mu2 PVE z
3147 WIPF1 2_105 0.9993100519 2124.2155 2.059251e-03 8.987351
929 BAZ2A 12_35 0.0000000000 1327.6344 0.000000e+00 -5.942857
10471 ZNF292 6_59 0.0000000000 1098.8945 0.000000e+00 -5.216216
4819 CGA 6_59 0.0000000000 912.8138 0.000000e+00 4.780822
7442 GPR155 2_105 0.0000000000 887.5060 0.000000e+00 -4.929575
2431 DNAJC12 10_44 0.9967457594 850.8569 8.227187e-04 -5.328244
2960 PCCB 3_84 0.9229235511 849.8298 7.608659e-04 -5.382353
6231 HERC4 10_44 0.0519716850 784.1117 3.953258e-05 -5.368895
7690 SLC35A1 6_59 0.0000000000 731.3839 0.000000e+00 -4.906053
4816 ORC3 6_59 0.0000000000 575.4343 0.000000e+00 -4.305556
11646 AC010894.3 2_105 0.0000000000 567.5177 0.000000e+00 2.807292
2445 WNT3 17_28 0.0012204211 558.1877 6.608462e-07 -5.270588
10151 ARL17A 17_28 0.0006810977 537.9069 3.554078e-07 -4.159800
10265 MAPT 17_28 0.0246154818 498.1750 1.189599e-05 5.606451
5236 MYPN 10_44 0.0557487640 467.4182 2.527850e-05 4.754237
11600 MKRN2OS 3_9 0.0000000000 326.2847 0.000000e+00 -2.927621
1450 SIRT1 10_44 0.0073685535 237.7219 1.699268e-06 -2.641975
856 PPP2R3A 3_84 0.0000000000 231.0518 0.000000e+00 -2.690141
8296 PDHB 3_40 0.0160173130 201.8814 3.136869e-06 2.296671
11694 ARL17B 17_28 0.0028320351 185.4496 5.094893e-07 3.095544
num_eqtl
3147 2
929 1
10471 1
4819 1
7442 3
2431 1
2960 1
6231 2
7690 2
4816 1
11646 2
2445 1
10151 2
10265 2
5236 1
11600 2
1450 1
856 1
8296 2
11694 2Genes with highest PVE
#genes with 20 highest pve
head(ctwas_gene_res[order(-ctwas_gene_res$PVE),report_cols],20) genename region_tag susie_pip mu2 PVE z
3147 WIPF1 2_105 0.99931005 2124.21550 2.059251e-03 8.987351
2431 DNAJC12 10_44 0.99674576 850.85693 8.227187e-04 -5.328244
2960 PCCB 3_84 0.92292355 849.82984 7.608659e-04 -5.382353
3169 PRRX1 1_84 0.99999992 153.79916 1.491985e-04 14.848088
11458 ZSWIM8 10_49 0.72951933 109.78862 7.769705e-05 -11.216495
7794 KDM1B 6_14 0.95814438 64.33734 5.980046e-05 -9.000000
9840 NKX2-5 5_103 0.99421828 61.83714 5.964054e-05 -9.616279
1214 GMCL1 2_46 0.99442495 60.60721 5.846645e-05 -8.434995
9417 PLEC 8_94 0.96661634 43.61991 4.090245e-05 3.093844
6231 HERC4 10_44 0.05197169 784.11168 3.953258e-05 -5.368895
2927 NR3C1 5_84 0.79565655 45.98656 3.549498e-05 -8.392405
3725 SSPN 12_18 0.79692298 42.42202 3.279579e-05 -6.791667
2987 GNB4 3_110 0.95377479 32.01351 2.962031e-05 -5.869048
11279 DPF3 14_34 0.92467491 30.95765 2.776946e-05 6.100000
3396 CASQ2 1_72 0.64365572 44.46248 2.776244e-05 6.684932
2401 PITX3 10_65 0.70424642 39.87615 2.724258e-05 -6.793786
5236 MYPN 10_44 0.05574876 467.41822 2.527850e-05 4.754237
10979 MSRB1 16_2 0.96679672 26.84161 2.517411e-05 5.929412
9960 PGP 16_2 0.90516927 28.48915 2.501611e-05 5.943820
1551 CCDC134 22_17 0.86831704 26.15261 2.202945e-05 -5.180556
num_eqtl
3147 2
2431 1
2960 1
3169 2
11458 1
7794 1
9840 2
1214 2
9417 2
6231 2
2927 1
3725 1
2987 1
11279 1
3396 1
2401 2
5236 1
10979 1
9960 1
1551 1Genes with largest z scores
#genes with 20 largest z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
3169 PRRX1 1_84 9.999999e-01 153.79916 1.491985e-04 14.848088
7976 SYNPO2L 10_48 1.466304e-01 134.47936 1.912890e-05 -11.782609
11458 ZSWIM8 10_49 7.295193e-01 109.78862 7.769705e-05 -11.216495
7134 ZBTB7B 1_76 1.008001e-04 120.87046 1.181930e-08 10.638889
504 SYNE2 14_29 4.741777e-02 83.62062 3.846492e-06 10.029851
9840 NKX2-5 5_103 9.942183e-01 61.83714 5.964054e-05 -9.616279
7794 KDM1B 6_14 9.581444e-01 64.33734 5.980046e-05 -9.000000
3147 WIPF1 2_105 9.993101e-01 2124.21550 2.059251e-03 8.987351
3876 DEK 6_14 9.860627e-02 62.21941 5.951697e-06 -8.651764
1214 GMCL1 2_46 9.944249e-01 60.60721 5.846645e-05 -8.434995
7445 DCST2 1_76 6.958988e-04 82.23437 5.551494e-08 -8.408993
2927 NR3C1 5_84 7.956566e-01 45.98656 3.549498e-05 -8.392405
9371 MYOZ1 10_48 4.594225e-02 72.94692 3.251095e-06 8.282302
5279 HCN4 15_35 1.027118e-03 142.65541 1.421409e-07 7.582677
554 MXD1 2_46 5.931507e-02 49.69584 2.859536e-06 7.537313
2039 ASAH1 8_21 2.248119e-05 67.20598 1.465675e-09 -7.443420
2401 PITX3 10_65 7.042464e-01 39.87615 2.724258e-05 -6.793786
3725 SSPN 12_18 7.969230e-01 42.42202 3.279579e-05 -6.791667
7823 CFL2 14_9 2.408814e-02 112.05711 2.618504e-06 -6.728039
5803 ADAM15 1_76 7.026909e-04 51.73422 3.526571e-08 -6.717647
num_eqtl
3169 2
7976 1
11458 1
7134 1
504 1
9840 2
7794 1
3147 2
3876 3
1214 2
7445 2
2927 1
9371 2
5279 1
554 1
2039 2
2401 2
3725 1
7823 2
5803 1Comparing z scores and PIPs
#set nominal signifiance threshold for z scores
alpha <- 0.05
#bonferroni adjusted threshold for z scores
sig_thresh <- qnorm(1-(alpha/nrow(ctwas_gene_res)/2), lower=T)
#Q-Q plot for z scores
obs_z <- ctwas_gene_res$z[order(ctwas_gene_res$z)]
exp_z <- qnorm((1:nrow(ctwas_gene_res))/nrow(ctwas_gene_res))
plot(exp_z, obs_z, xlab="Expected z", ylab="Observed z", main="Gene z score Q-Q plot")
abline(a=0,b=1)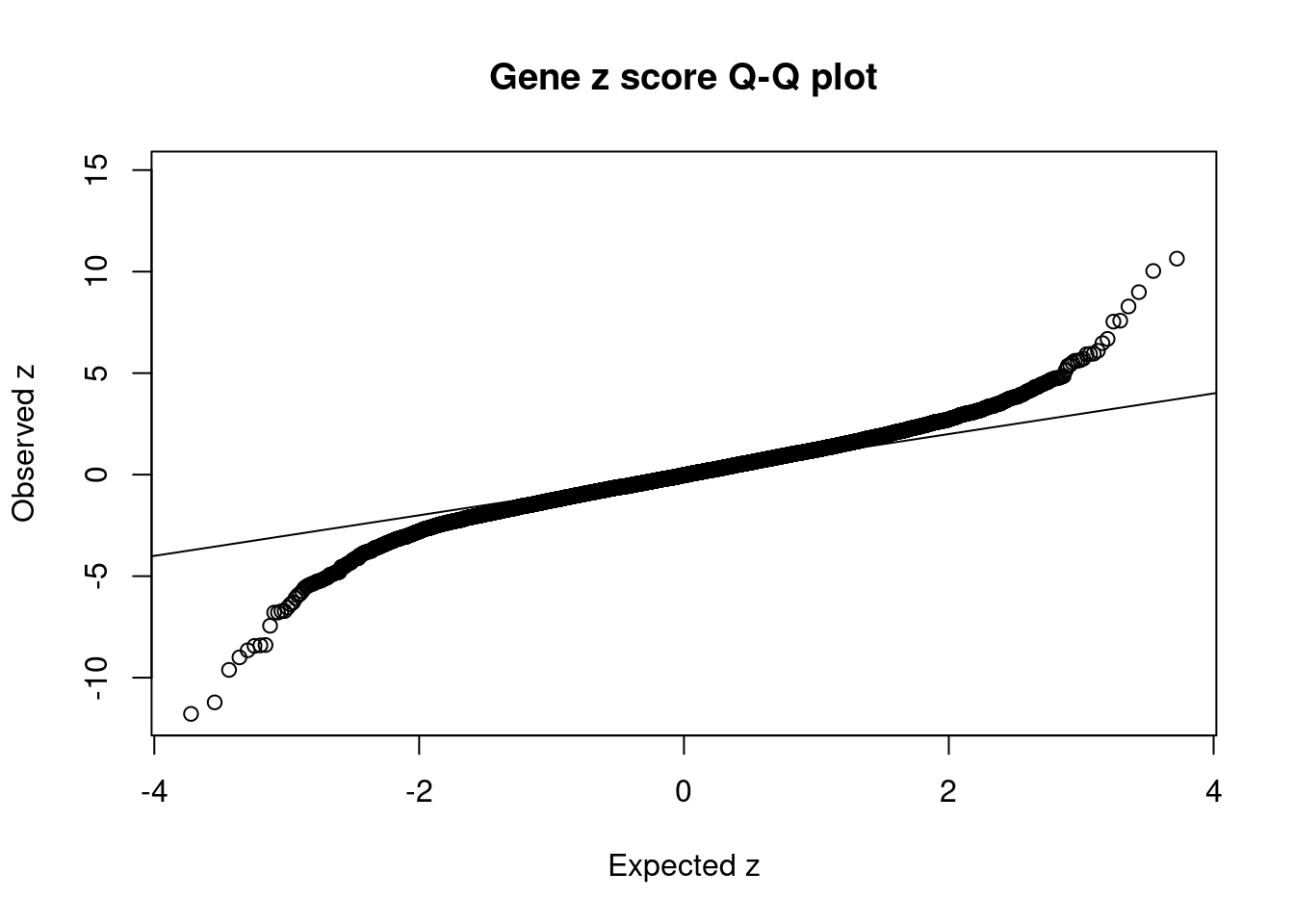
#plot z score vs PIP
plot(abs(ctwas_gene_res$z), ctwas_gene_res$susie_pip, xlab="abs(z)", ylab="PIP")
abline(v=sig_thresh, col="red", lty=2)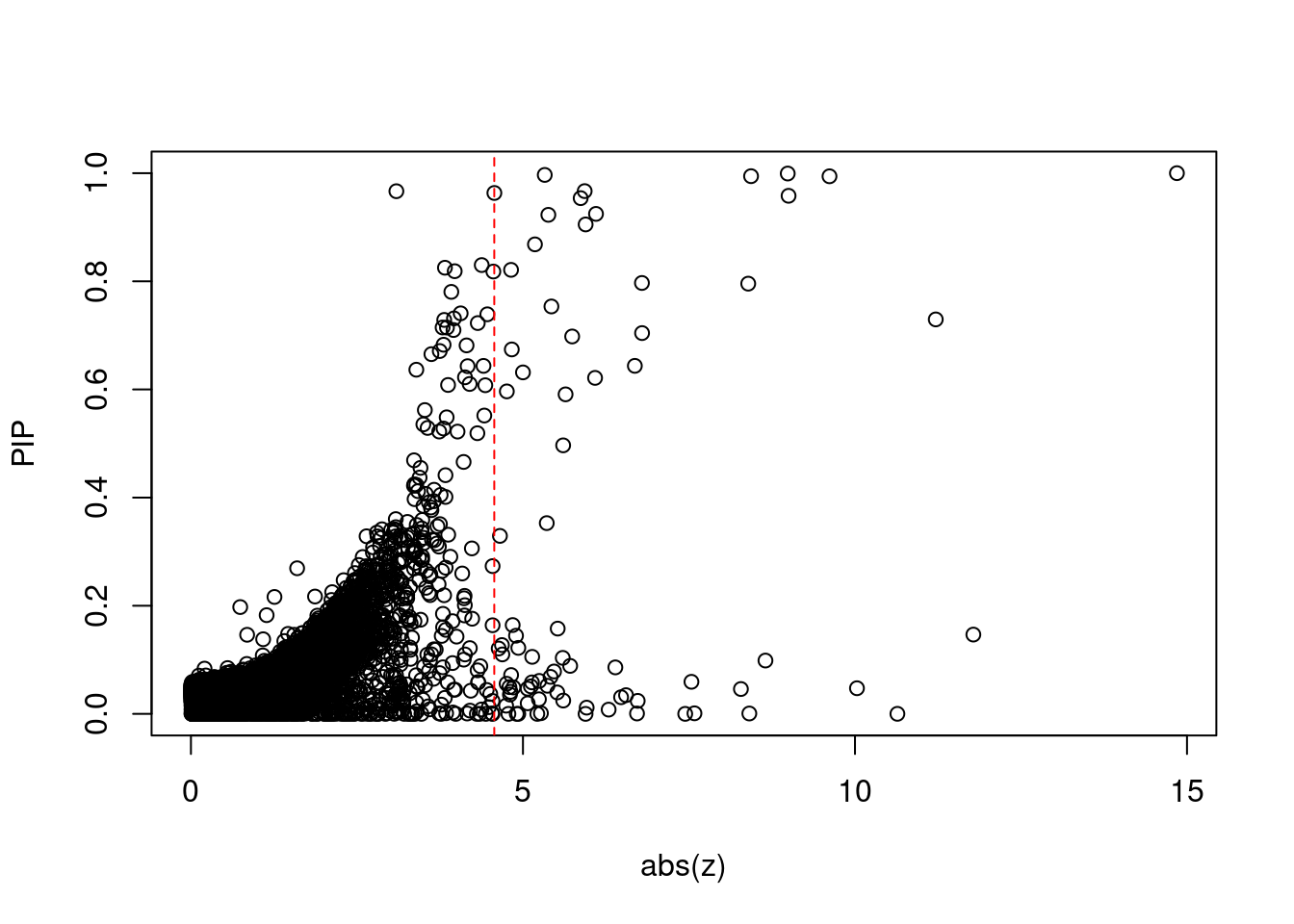
#proportion of significant z scores
mean(abs(ctwas_gene_res$z) > sig_thresh)[1] 0.007783251#genes with most significant z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
3169 PRRX1 1_84 9.999999e-01 153.79916 1.491985e-04 14.848088
7976 SYNPO2L 10_48 1.466304e-01 134.47936 1.912890e-05 -11.782609
11458 ZSWIM8 10_49 7.295193e-01 109.78862 7.769705e-05 -11.216495
7134 ZBTB7B 1_76 1.008001e-04 120.87046 1.181930e-08 10.638889
504 SYNE2 14_29 4.741777e-02 83.62062 3.846492e-06 10.029851
9840 NKX2-5 5_103 9.942183e-01 61.83714 5.964054e-05 -9.616279
7794 KDM1B 6_14 9.581444e-01 64.33734 5.980046e-05 -9.000000
3147 WIPF1 2_105 9.993101e-01 2124.21550 2.059251e-03 8.987351
3876 DEK 6_14 9.860627e-02 62.21941 5.951697e-06 -8.651764
1214 GMCL1 2_46 9.944249e-01 60.60721 5.846645e-05 -8.434995
7445 DCST2 1_76 6.958988e-04 82.23437 5.551494e-08 -8.408993
2927 NR3C1 5_84 7.956566e-01 45.98656 3.549498e-05 -8.392405
9371 MYOZ1 10_48 4.594225e-02 72.94692 3.251095e-06 8.282302
5279 HCN4 15_35 1.027118e-03 142.65541 1.421409e-07 7.582677
554 MXD1 2_46 5.931507e-02 49.69584 2.859536e-06 7.537313
2039 ASAH1 8_21 2.248119e-05 67.20598 1.465675e-09 -7.443420
2401 PITX3 10_65 7.042464e-01 39.87615 2.724258e-05 -6.793786
3725 SSPN 12_18 7.969230e-01 42.42202 3.279579e-05 -6.791667
7823 CFL2 14_9 2.408814e-02 112.05711 2.618504e-06 -6.728039
5803 ADAM15 1_76 7.026909e-04 51.73422 3.526571e-08 -6.717647
num_eqtl
3169 2
7976 1
11458 1
7134 1
504 1
9840 2
7794 1
3147 2
3876 3
1214 2
7445 2
2927 1
9371 2
5279 1
554 1
2039 2
2401 2
3725 1
7823 2
5803 1Locus plots for genes and SNPs
ctwas_gene_res_sortz <- ctwas_gene_res[order(-abs(ctwas_gene_res$z)),]
report_cols_region <- report_cols[!(report_cols %in% c("num_eqtl"))]
n_plots <- 5
for (region_tag_plot in head(unique(ctwas_gene_res_sortz$region_tag), n_plots)){
ctwas_res_region <- ctwas_res[ctwas_res$region_tag==region_tag_plot,]
start <- min(ctwas_res_region$pos)
end <- max(ctwas_res_region$pos)
ctwas_res_region <- ctwas_res_region[order(ctwas_res_region$pos),]
ctwas_res_region_gene <- ctwas_res_region[ctwas_res_region$type=="gene",]
ctwas_res_region_snp <- ctwas_res_region[ctwas_res_region$type=="SNP",]
#region name
print(paste0("Region: ", region_tag_plot))
#table of genes in region
print(ctwas_res_region_gene[,report_cols_region])
par(mfrow=c(4,1))
#gene z scores
plot(ctwas_res_region_gene$pos, abs(ctwas_res_region_gene$z), xlab="Position", ylab="abs(gene_z)", xlim=c(start,end),
ylim=c(0,max(sig_thresh, abs(ctwas_res_region_gene$z))),
main=paste0("Region: ", region_tag_plot))
abline(h=sig_thresh,col="red",lty=2)
#significance threshold for SNPs
alpha_snp <- 5*10^(-8)
sig_thresh_snp <- qnorm(1-alpha_snp/2, lower=T)
#snp z scores
plot(ctwas_res_region_snp$pos, abs(ctwas_res_region_snp$z), xlab="Position", ylab="abs(snp_z)",xlim=c(start,end),
ylim=c(0,max(sig_thresh_snp, max(abs(ctwas_res_region_snp$z)))))
abline(h=sig_thresh_snp,col="purple",lty=2)
#gene pips
plot(ctwas_res_region_gene$pos, ctwas_res_region_gene$susie_pip, xlab="Position", ylab="Gene PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
#snp pips
plot(ctwas_res_region_snp$pos, ctwas_res_region_snp$susie_pip, xlab="Position", ylab="SNP PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
}[1] "Region: 1_84"
genename region_tag susie_pip mu2 PVE z
3169 PRRX1 1_84 0.999999920 153.799161 1.491985e-04 14.8480881
12761 RP1-79C4.4 1_84 0.024341023 13.845716 3.269374e-07 -1.9030834
1412 FMO2 1_84 0.009387442 4.595372 4.184835e-08 0.1428671
195 FMO1 1_84 0.018074710 10.899980 1.911206e-07 -1.3032787
933 FMO4 1_84 0.009987054 5.516273 5.344334e-08 0.8144330
3311 PRRC2C 1_84 0.015542432 9.494343 1.431510e-07 -1.3333333
362 MYOC 1_84 0.026303197 14.058670 3.587263e-07 -1.5409457
174 METTL13 1_84 0.010143926 5.032091 4.951822e-08 -0.4696970
10837 DNM3 1_84 0.115781900 24.407332 2.741394e-06 -2.5074627
4893 PIGC 1_84 0.009503763 4.771745 4.399296e-08 0.2160346
9656 C1orf105 1_84 0.009994999 5.273780 5.113463e-08 -0.6575342
1413 SUCO 1_84 0.011032318 5.779488 6.185382e-08 0.3414128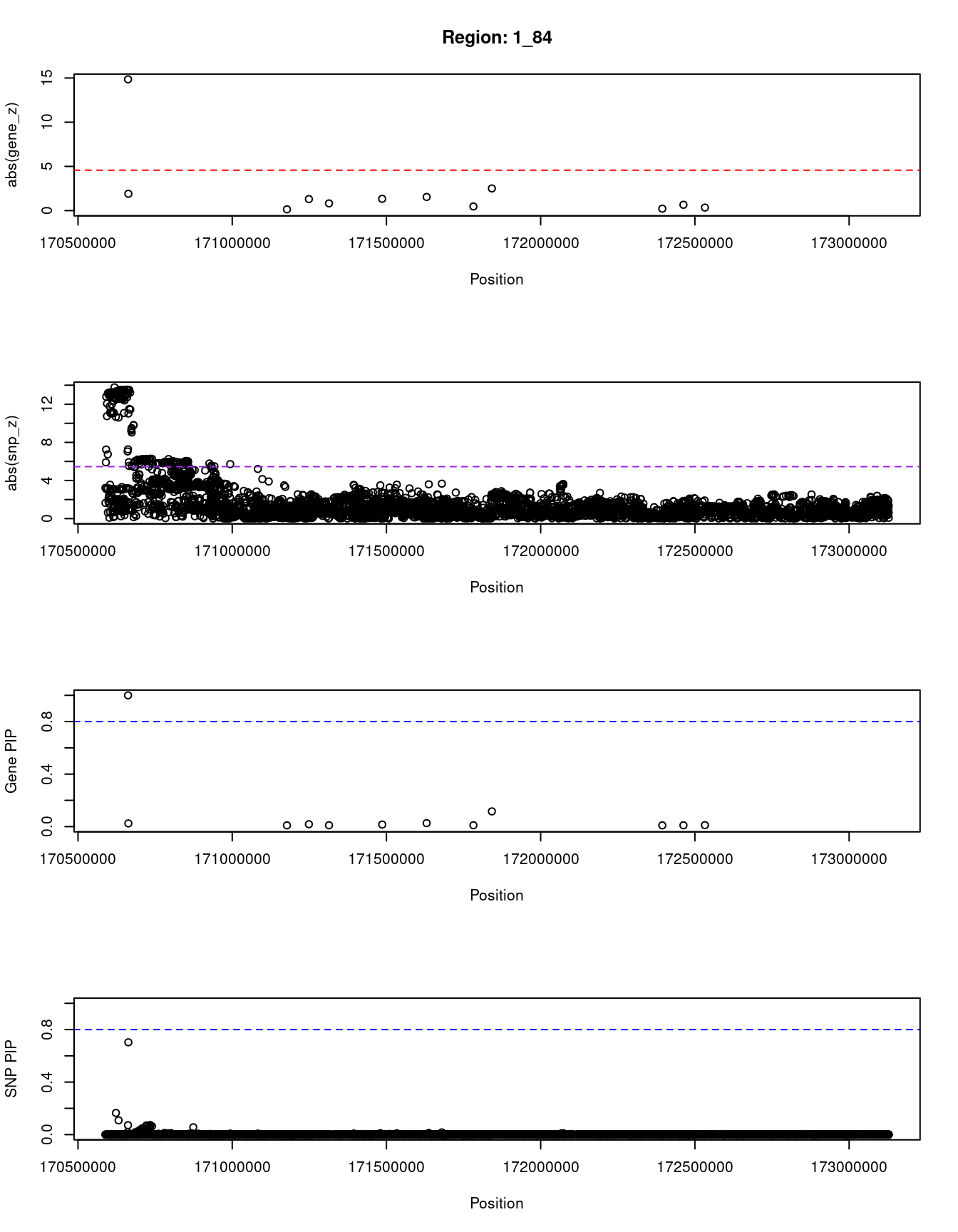
[1] "Region: 10_48"
genename region_tag susie_pip mu2 PVE z
2387 SPOCK2 10_48 0.04579962 5.431342 2.413123e-07 0.5000000
7973 ANAPC16 10_48 0.04811514 5.677193 2.649878e-07 0.3992052
5231 ASCC1 10_48 0.04682667 5.378961 2.443443e-07 0.2097134
6238 DNAJB12 10_48 0.04202769 5.549508 2.262562e-07 -1.2142857
6754 MCU 10_48 0.04926449 5.721392 2.734300e-07 0.6527778
12844 RP11-344N10.5 10_48 0.04398047 5.964501 2.544746e-07 1.0986301
7977 NUDT13 10_48 0.04474438 28.765465 1.248591e-06 -3.9779412
11372 DNAJC9 10_48 0.04424273 32.169184 1.380678e-06 -4.4538462
9745 MRPS16 10_48 0.10807981 16.127655 1.690932e-06 1.0818247
5229 ANXA7 10_48 0.04278973 29.926635 1.242247e-06 -4.2137405
7982 USP54 10_48 0.04948664 29.476819 1.415074e-06 -4.7964072
12864 RP11-464F9.22 10_48 0.04878309 29.880579 1.414063e-06 4.8562874
9371 MYOZ1 10_48 0.04594225 72.946922 3.251095e-06 8.2823023
7976 SYNPO2L 10_48 0.14663037 134.479362 1.912890e-05 -11.7826087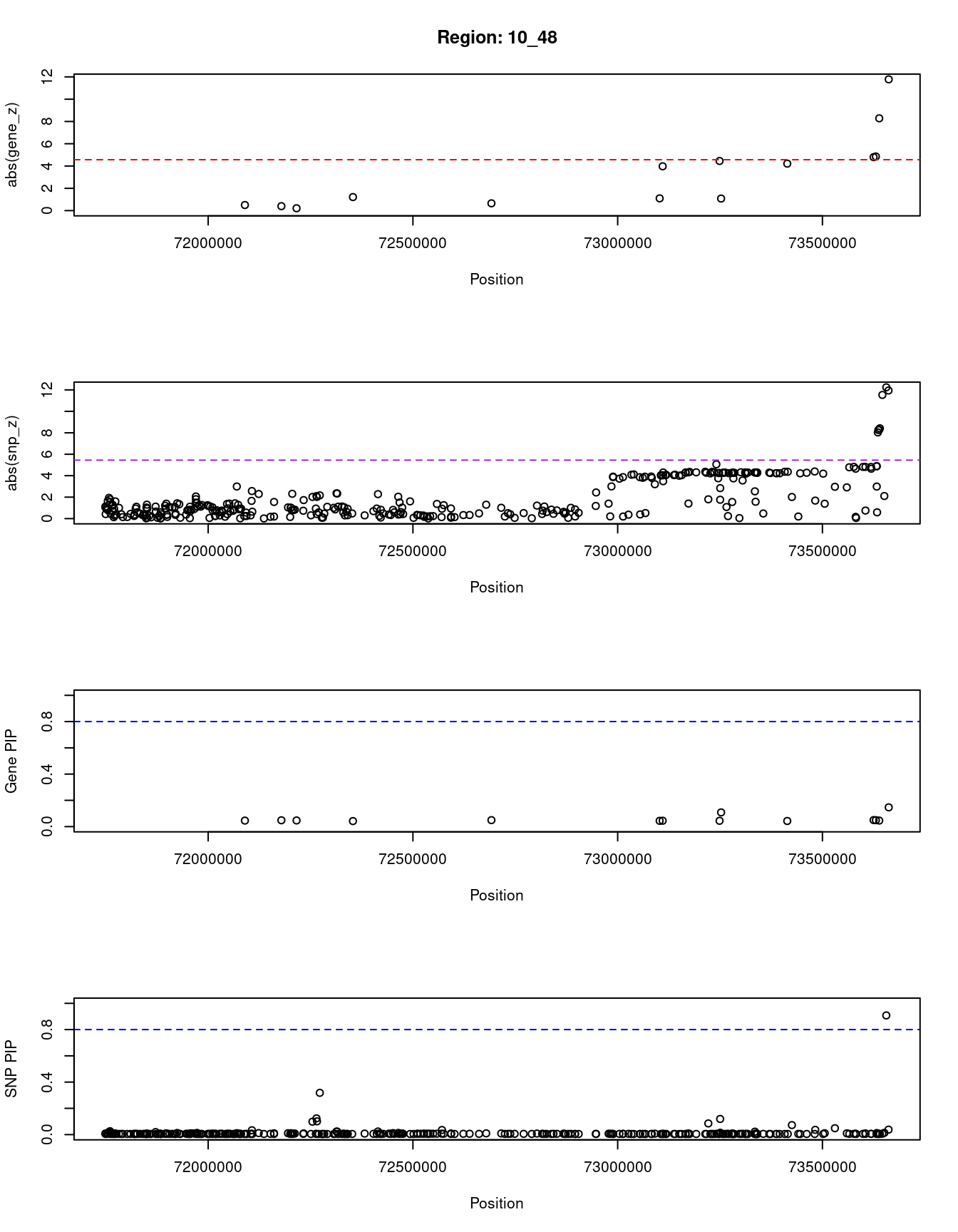
[1] "Region: 10_49"
genename region_tag susie_pip mu2 PVE z
12792 RP11-574K11.29 10_49 0.022990638 19.165704 4.274509e-07 -3.2007683
9287 SEC24C 10_49 0.030788946 46.259307 1.381670e-06 6.4776119
10671 FUT11 10_49 0.034603540 47.907740 1.608187e-06 -6.5522388
11458 ZSWIM8 10_49 0.729519326 109.788618 7.769705e-05 -11.2164948
3705 PLAU 10_49 0.008878220 5.750547 4.952739e-08 -0.9189189
11550 C10orf55 10_49 0.009058826 11.033338 9.695925e-08 2.8208955
365 VCL 10_49 0.093489730 20.671759 1.874786e-06 1.5128205
10041 AP3M1 10_49 0.035279184 15.591795 5.336114e-07 -1.7388852
10442 DUPD1 10_49 0.008429365 5.258110 4.299668e-08 -1.1529412
1016 DUSP13 10_49 0.100284705 31.460222 3.060602e-06 4.1086957
7858 VDAC2 10_49 0.088251394 21.153236 1.810960e-06 -4.3582090
12696 RP11-399K21.11 10_49 0.012183813 8.284501 9.791742e-08 -0.1303262
11619 ZNF503-AS1 10_49 0.010899216 10.034096 1.060923e-07 -2.1217391
6232 C10orf11 10_49 0.008899518 5.580033 4.817411e-08 -0.8573335
12851 RP11-399K21.14 10_49 0.010355302 6.113393 6.141232e-08 0.3968750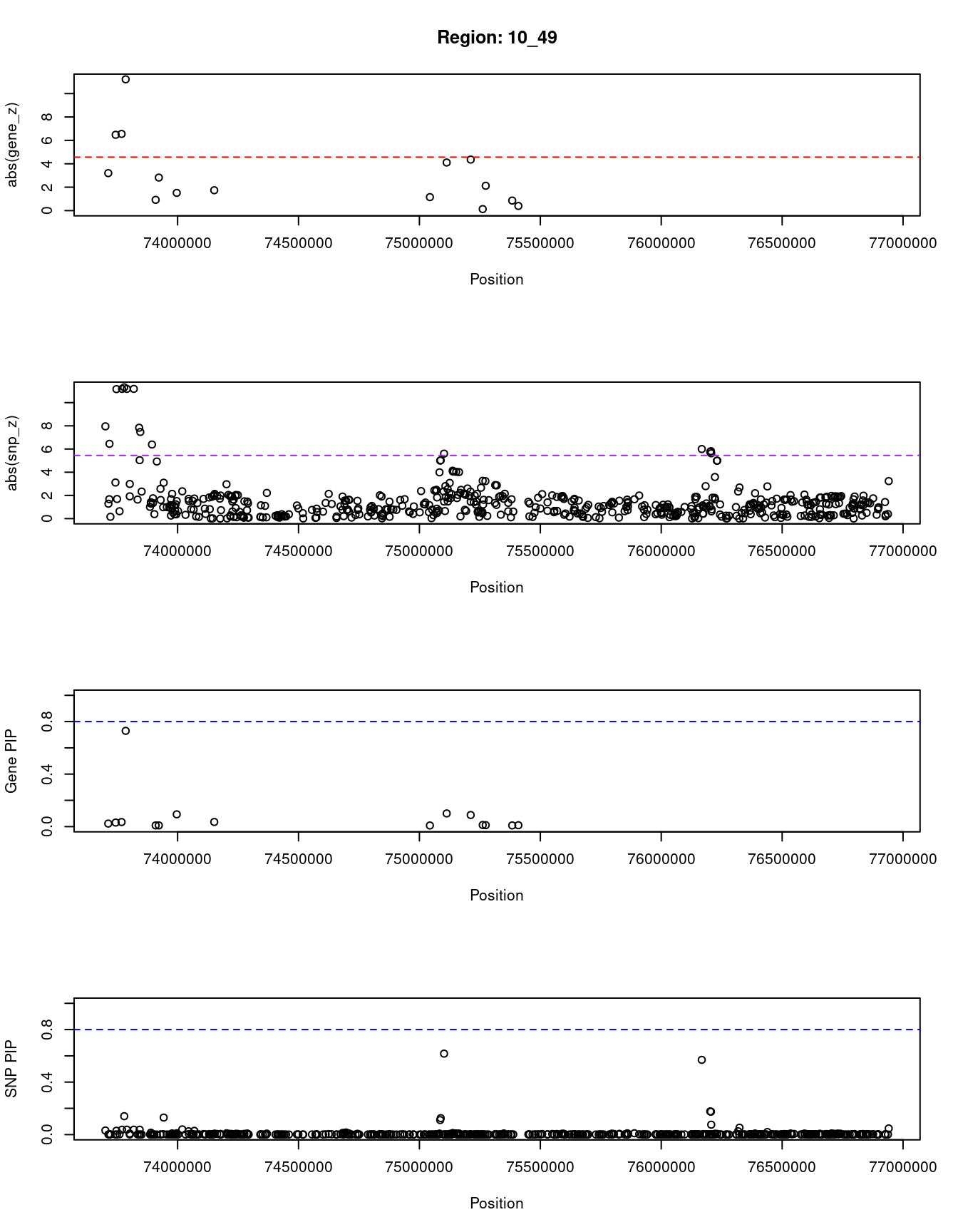
[1] "Region: 1_76"
genename region_tag susie_pip mu2 PVE z
7136 SHC1 1_76 0.0001071838 12.588788 1.308951e-09 -0.59459459
7134 ZBTB7B 1_76 0.0001008001 120.870457 1.181930e-08 10.63888889
7445 DCST2 1_76 0.0006958988 82.234374 5.551494e-08 -8.40899309
5803 ADAM15 1_76 0.0007026909 51.734219 3.526571e-08 -6.71764706
5811 EFNA3 1_76 0.0001139320 11.158293 1.233258e-09 -2.27241379
8429 EFNA1 1_76 0.0002737294 18.665656 4.956500e-09 -2.17756382
8428 SLC50A1 1_76 0.0001322459 14.952603 1.918269e-09 -3.06101695
10106 MUC1 1_76 0.0002616261 20.816785 5.283299e-09 -0.97169811
9349 GBA 1_76 0.0001241545 12.959234 1.560818e-09 2.56378222
3200 SCAMP3 1_76 0.0003015954 23.555275 6.891652e-09 -3.32894737
5817 HCN3 1_76 0.0003432258 23.447311 7.806986e-09 3.28000000
7144 FDPS 1_76 0.0008565642 32.876662 2.731858e-08 -3.73684211
4539 DAP3 1_76 0.0003781219 21.621249 7.930910e-09 -2.27139816
7447 YY1AP1 1_76 0.0011961594 36.920267 4.284146e-08 3.84291511
4546 SYT11 1_76 0.0025684400 41.313747 1.029377e-07 3.94520548
5815 RIT1 1_76 0.0002398255 20.210033 4.701893e-09 2.59154930
4540 KIAA0907 1_76 0.0001730573 8.076786 1.355935e-09 -0.01612903
3203 ARHGEF2 1_76 0.0026299911 38.288470 9.768609e-08 3.13235294
3204 LAMTOR2 1_76 0.0010573388 20.725806 2.125867e-08 0.07593099
10541 SEMA4A 1_76 0.0001058235 6.575695 6.750474e-10 -2.12384196
7148 SLC25A44 1_76 0.0001316434 8.333213 1.064197e-09 -0.95882039
11980 BGLAP 1_76 0.0000970415 8.094425 7.619982e-10 1.39902385
7147 PAQR6 1_76 0.0005254314 17.491144 8.915479e-09 -0.74698795
11050 SMG5 1_76 0.0001231042 12.776433 1.525783e-09 -2.65753425
10969 GLMP 1_76 0.0001350938 14.151121 1.854542e-09 -2.78082192
7465 CCT3 1_76 0.0001282575 13.369857 1.663489e-09 2.71621622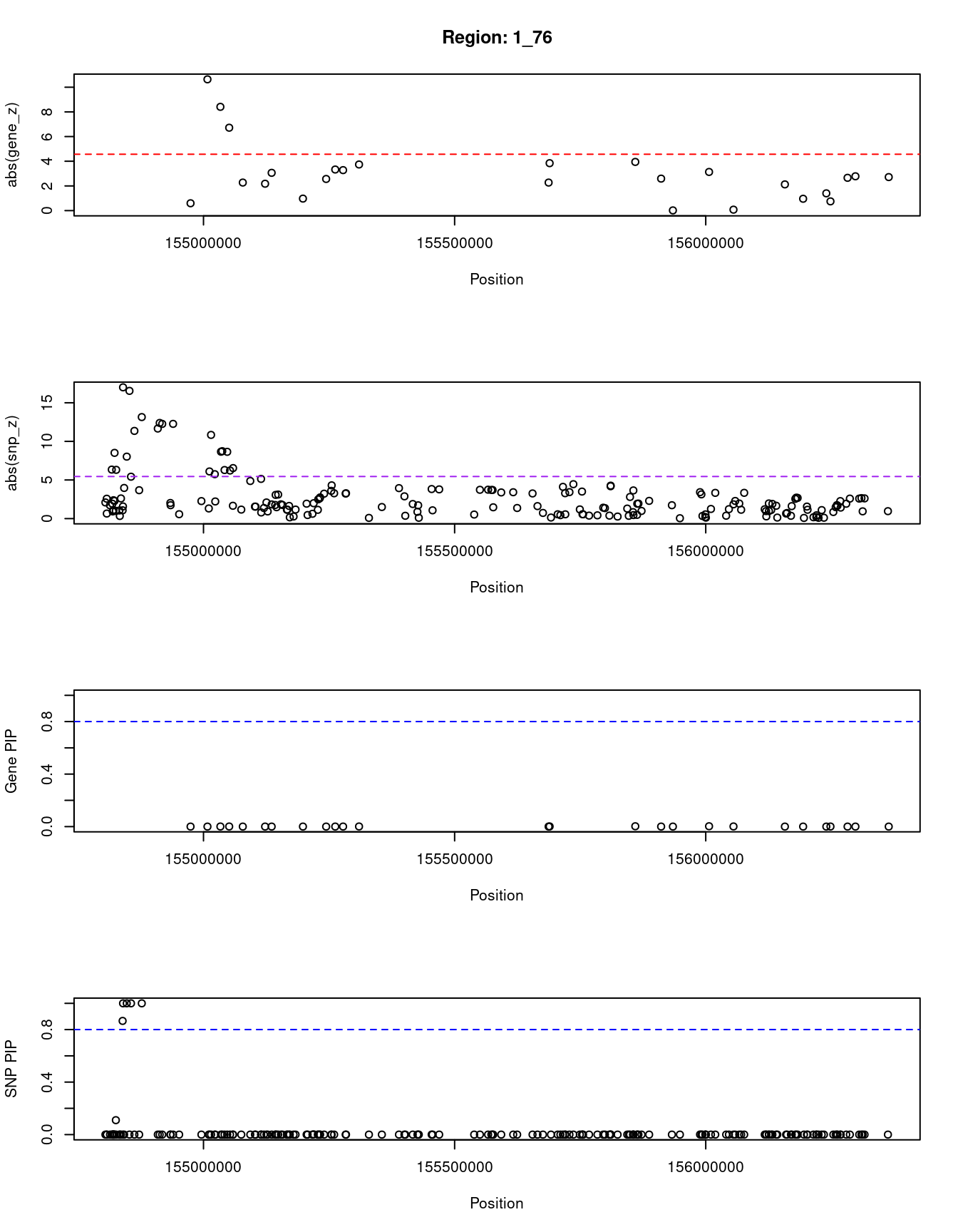
[1] "Region: 14_29"
genename region_tag susie_pip mu2 PVE z
6610 PPP2R5E 14_29 0.06366122 10.876680 6.717099e-07 1.45827521
5433 WDR89 14_29 0.05798355 9.024694 5.076305e-07 -0.14146341
504 SYNE2 14_29 0.04741777 83.620616 3.846492e-06 10.02985075
1668 MTHFD1 14_29 0.03157326 4.544419 1.391900e-07 0.04166667
4040 ZBTB1 14_29 0.11881769 23.264806 2.681581e-06 -3.68656716
4039 HSPA2 14_29 0.03598029 10.071122 3.515224e-07 -2.34567901
4043 PLEKHG3 14_29 0.05661913 10.958167 6.018822e-07 1.73972603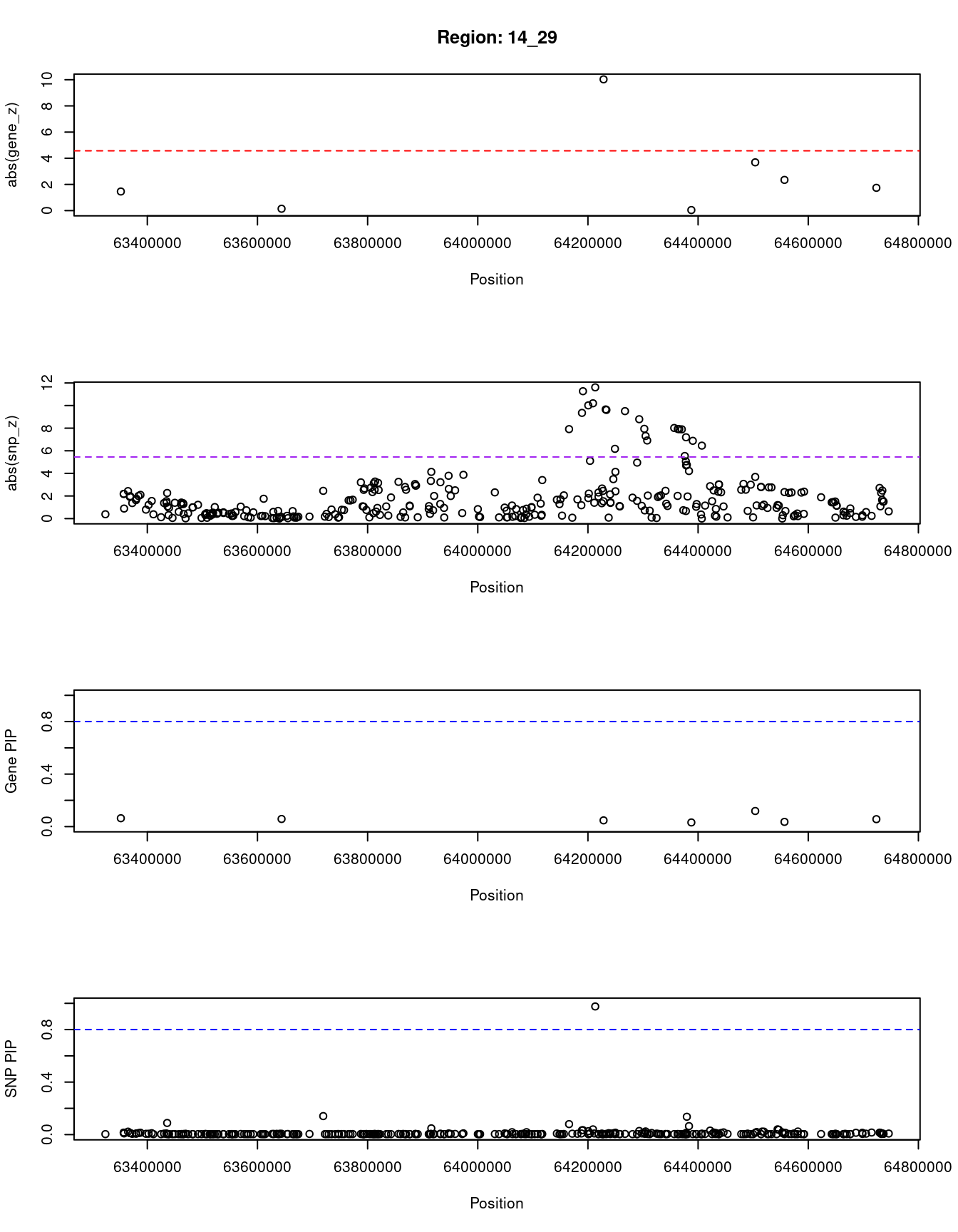
SNPs with highest PIPs
#snps with PIP>0.8 or 20 highest PIPs
head(ctwas_snp_res[order(-ctwas_snp_res$susie_pip),report_cols_snps],
max(sum(ctwas_snp_res$susie_pip>0.8), 20)) id region_tag susie_pip mu2 PVE z
28637 rs12404927 1_75 1.0000000 1404.55102 1.362536e-03 -1.5517241
28638 rs7536152 1_75 1.0000000 1439.64649 1.396582e-03 -6.4626866
28731 rs34515871 1_76 1.0000000 296.98405 2.881002e-04 17.0000000
32608 rs12142529 1_83 1.0000000 145.38857 1.410395e-04 12.0396040
32633 rs112797273 1_83 1.0000000 101.40197 9.836867e-05 9.4903846
183948 rs1906615 4_72 1.0000000 3299.17745 3.200487e-03 45.1604938
183953 rs7440714 4_72 1.0000000 1498.40640 1.453584e-03 -9.2183406
272791 rs111990232 6_59 1.0000000 1672.21316 1.622191e-03 0.5392670
472755 rs17287293 12_18 1.0000000 150.43066 1.459307e-04 -10.2947368
556238 rs140798119 15_35 1.0000000 282.62226 2.741680e-04 3.9950739
556241 rs74022964 15_35 1.0000000 340.33003 3.301495e-04 12.5777778
698783 rs4972703 2_105 1.0000000 3592.88497 3.485409e-03 -0.7842670
703489 rs114415025 3_9 1.0000000 2723.92507 2.642443e-03 1.2192513
728978 rs201862093 7_48 1.0000000 1114.36488 1.081030e-03 -0.8625000
765251 rs76106073 10_44 1.0000000 836.85538 8.118220e-04 0.6572238
768375 rs61937778 12_35 1.0000000 1898.73730 1.841939e-03 -1.3056995
28735 rs2878412 1_76 1.0000000 104.66388 1.015330e-04 5.4250000
423415 rs60469668 10_66 1.0000000 171.39264 1.662657e-04 16.9647059
28733 rs11576820 1_76 1.0000000 131.66128 1.277228e-04 8.0243902
592256 rs62056842 17_28 1.0000000 764.07813 7.412218e-04 1.1269036
733889 rs62643683 8_21 1.0000000 262.98617 2.551193e-04 0.8007519
183979 rs186546224 4_72 1.0000000 278.28019 2.699558e-04 -6.8398876
117614 rs7373492 3_28 1.0000000 57.12050 5.541183e-05 -7.6417323
323213 rs62469005 7_70 1.0000000 248.90606 2.414604e-04 -3.8636364
323204 rs28557111 7_70 0.9999995 268.50949 2.604773e-04 13.6818182
299063 rs12112152 7_15 0.9999995 45.05583 4.370803e-05 6.9358974
28738 rs10908445 1_76 0.9999993 192.70836 1.869436e-04 -13.1470588
576584 rs6499606 16_39 0.9999989 103.66865 1.005674e-04 12.6376812
785055 rs8005417 14_9 0.9999977 418.17010 4.056602e-04 0.9863946
323219 rs12669209 7_70 0.9999965 107.24435 1.040359e-04 -4.2937500
423413 rs7094488 10_66 0.9999961 105.12014 1.019752e-04 14.1060606
96493 rs1975584 2_118 0.9999837 71.18500 6.905447e-05 1.2311828
804323 rs140185678 16_2 0.9999825 44.60953 4.327434e-05 7.6100917
280210 rs11756438 6_79 0.9999688 47.48500 4.606312e-05 -8.2089552
117485 rs116202356 3_27 0.9999532 36.40320 3.531260e-05 6.0813559
176633 rs1458038 4_54 0.9998799 34.94748 3.389801e-05 6.0277778
661464 rs464901 22_3 0.9997751 44.32757 4.299190e-05 -7.0555556
121514 rs12330500 3_40 0.9996970 506.36533 4.910693e-04 0.5641026
397657 rs1886296 9_73 0.9995450 34.53850 3.349008e-05 5.9863014
734589 rs7508 8_21 0.9993753 79.71601 7.728312e-05 9.4800000
725018 rs3176326 6_29 0.9991706 50.71157 4.915381e-05 -7.3647059
117627 rs10428132 3_28 0.9991198 74.57949 7.228487e-05 9.2058824
586504 rs72811292 17_11 0.9982520 39.85061 3.859096e-05 -6.5233645
39384 rs12353975 1_102 0.9981456 35.67154 3.454031e-05 -4.3000000
184535 rs7700110 4_73 0.9978791 45.09924 4.365737e-05 7.0666667
117601 rs9824157 3_28 0.9976315 29.97394 2.900844e-05 3.8732394
734231 rs208757 8_21 0.9970887 239.59396 2.317502e-04 7.6629213
232614 rs199992924 5_68 0.9967799 1308.36140 1.265137e-03 -0.6309524
663223 rs133902 22_7 0.9963536 31.00376 2.996666e-05 6.1617647
232434 rs338623 5_68 0.9953355 66.77245 6.447290e-05 -7.9855072
40060 rs4951023 1_104 0.9951712 32.47858 3.135489e-05 5.5970149
39383 rs6427989 1_102 0.9941339 35.76701 3.449355e-05 -4.4142857
592472 rs75230966 17_28 0.9928717 43.15503 4.156569e-05 6.0582524
576592 rs876727 16_39 0.9925728 48.76988 4.695961e-05 -10.0000000
275859 rs9496567 6_67 0.9919760 26.48986 2.549126e-05 5.1125000
801039 rs12908004 15_37 0.9873706 57.56113 5.513405e-05 8.1333333
183968 rs4631108 4_72 0.9858853 278.72914 2.665748e-04 -17.6376812
576597 rs60602157 16_39 0.9858538 38.73318 3.704299e-05 10.4415584
526368 rs74968516 14_8 0.9857359 34.86384 3.333851e-05 -2.0924370
657485 rs7282237 21_16 0.9765780 24.87091 2.356183e-05 -4.7605634
534174 rs2738413 14_29 0.9761605 111.24337 1.053430e-04 -11.6119403
366949 rs7460121 8_88 0.9755516 24.08834 2.279647e-05 4.7500000
323211 rs4730742 7_70 0.9739413 148.25445 1.400719e-04 -5.3181818
576589 rs4788691 16_39 0.9737540 39.90042 3.769096e-05 -1.6900000
331163 rs35760656 7_93 0.9721535 35.73611 3.370176e-05 -6.3536585
284152 rs958747 6_89 0.9716551 24.32337 2.292695e-05 -4.9710145
2727 rs284278 1_7 0.9709039 35.05628 3.301813e-05 -6.0857143
280167 rs77435894 6_78 0.9646884 28.76469 2.691889e-05 6.1111111
785061 rs8011559 14_9 0.9644048 426.28574 3.988142e-04 5.6913580
561807 rs12898337 15_48 0.9627997 35.30067 3.297079e-05 -6.0579710
58545 rs7578482 2_15 0.9603813 32.19728 2.999669e-05 5.7435897
555608 rs745636 15_33 0.9584917 25.47962 2.369146e-05 -5.0740741
25981 rs4839174 1_69 0.9562825 33.83277 3.138588e-05 5.9459459
99263 rs35880620 2_125 0.9544729 39.72780 3.678482e-05 -6.2168675
610855 rs17794590 18_24 0.9544647 24.57014 2.274981e-05 -4.8242424
96468 rs11889306 2_118 0.9532842 45.60961 4.217831e-05 6.7391304
728771 rs74910854 7_48 0.9504108 30.92661 2.851373e-05 5.4878049
705484 rs9830653 3_84 0.9497164 834.68211 7.689985e-04 -0.9344262
784984 rs73241997 14_9 0.9463663 271.39025 2.491517e-04 7.8817204
183920 rs1823291 4_72 0.9443849 275.38267 2.522877e-04 -19.4520548
32655 rs7522387 1_83 0.9380199 59.33695 5.399427e-05 6.6375000
28693 rs906280 1_75 0.9352012 59.46409 5.394737e-05 7.9111111
492885 rs12425471 12_69 0.9315032 41.65186 3.763823e-05 -5.2500000
506242 rs1326122 13_21 0.9280182 24.16457 2.175434e-05 4.7543103
729019 rs112661041 7_48 0.9246584 1098.87113 9.856858e-04 4.6532258
280117 rs89107 6_78 0.9200337 47.40943 4.231350e-05 -9.5606061
372218 rs1594768 9_9 0.9191704 24.06779 2.146064e-05 -4.6911765
218017 rs114414434 5_30 0.9100084 25.52863 2.253634e-05 -4.9718310
416198 rs60632610 10_48 0.9078072 142.32178 1.253359e-04 -12.2395833
5627 rs10917072 1_15 0.9035741 33.32457 2.921048e-05 5.8235294
666612 rs11705586 22_15 0.9032885 25.71531 2.253350e-05 4.8988764
698793 rs1367220 2_105 0.9027873 3624.47361 3.174247e-03 7.2207792
239095 rs6894302 5_84 0.8962639 34.59893 3.008215e-05 -7.2933333
232607 rs4073838 5_68 0.8907151 1336.89003 1.155167e-03 -5.9545455
272788 rs371814 6_59 0.8887549 1684.18630 1.452053e-03 6.1911765
252811 rs73724866 6_13 0.8854526 93.92663 8.067974e-05 -10.4141414
556230 rs519946 15_34 0.8802637 34.42175 2.939383e-05 5.9242424
497493 rs6560886 12_82 0.8786081 28.83992 2.458101e-05 5.6666667
769560 rs71454237 12_43 0.8731783 45.03633 3.814840e-05 -7.3809524
28729 rs12128882 1_76 0.8660530 31.94687 2.684004e-05 1.0731707
469758 rs12821447 12_12 0.8604052 24.28342 2.026858e-05 4.5128205
622090 rs73919353 19_6 0.8573709 29.15094 2.424554e-05 5.4076433
455650 rs565449 11_54 0.8568684 28.25040 2.348276e-05 -5.2535211
67841 rs243080 2_40 0.8464105 23.94966 1.966486e-05 -4.5522388
245279 rs62377226 5_100 0.8419746 33.84803 2.764667e-05 5.8493151
583304 rs75329315 17_2 0.8321222 26.35764 2.127669e-05 -5.2460317
121517 rs6924 3_40 0.8238581 510.02479 4.076187e-04 4.2133333
498623 rs7321083 13_3 0.8169737 24.61195 1.950584e-05 4.8292683
304963 rs145593380 7_26 0.8132918 29.70683 2.343760e-05 5.3333333
151266 rs720389 3_114 0.8068588 23.55072 1.843368e-05 4.2898551SNPs with largest effect sizes
#plot PIP vs effect size
#plot(ctwas_snp_res$susie_pip, ctwas_snp_res$mu2, xlab="PIP", ylab="mu^2", main="SNP PIPs vs Effect Size")
#SNPs with 50 largest effect sizes
head(ctwas_snp_res[order(-ctwas_snp_res$mu2),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
698793 rs1367220 2_105 9.027873e-01 3624.474 3.174247e-03 7.220779
698794 rs1367219 2_105 8.971134e-02 3615.477 3.146469e-04 7.103896
698789 rs10168156 2_105 2.265398e-01 3615.000 7.944439e-04 7.233766
698787 rs6713018 2_105 5.859132e-02 3613.822 2.054047e-04 7.168831
698778 rs1864453 2_105 1.251599e-01 3612.780 4.386490e-04 7.207792
698770 rs2033315 2_105 5.844195e-02 3609.583 2.046408e-04 7.064935
698781 rs6707162 2_105 1.730083e-02 3603.368 6.047640e-05 7.090909
698782 rs6735680 2_105 1.150635e-02 3602.875 4.021584e-05 7.077922
698771 rs2033314 2_105 1.165286e-02 3602.717 4.072611e-05 7.155844
698773 rs28485554 2_105 2.399129e-03 3600.749 8.380248e-06 7.000000
698783 rs4972703 2_105 1.000000e+00 3592.885 3.485409e-03 -0.784267
698804 rs10803884 2_105 3.412607e-05 3550.073 1.175260e-07 7.285714
698812 rs6738901 2_105 6.033312e-05 3535.783 2.069435e-07 -7.272727
698797 rs12466643 2_105 1.495366e-05 3532.253 5.124007e-08 7.272727
698828 rs35368253 2_105 1.910799e-05 3515.570 6.516601e-08 -7.256410
698814 rs10197521 2_105 2.167801e-08 3505.736 7.372403e-11 -6.547619
698816 rs34661753 2_105 1.912728e-06 3466.123 6.431433e-09 -7.076923
698767 rs7590328 2_105 1.204450e-07 3454.423 4.036218e-10 6.784810
183948 rs1906615 4_72 1.000000e+00 3299.177 3.200487e-03 45.160494
698765 rs6433497 2_105 2.090339e-11 2907.446 5.895746e-14 6.345679
698766 rs2115874 2_105 2.801248e-11 2905.297 7.895008e-14 6.419753
698758 rs1430185 2_105 1.876388e-12 2842.067 5.173296e-15 6.185185
698745 rs1991601 2_105 5.335732e-13 2821.002 1.460185e-15 5.950617
703494 rs56204325 3_9 7.189264e-01 2763.521 1.927337e-03 7.865672
703475 rs1969154 3_9 3.789176e-01 2761.449 1.015061e-03 7.850746
703474 rs6799179 3_9 3.766987e-01 2761.418 1.009106e-03 7.850746
703484 rs12714880 3_9 1.905204e-01 2760.752 5.102457e-04 7.820896
703442 rs6763619 3_9 6.794745e-03 2742.502 1.807717e-05 7.776119
703441 rs13320486 3_9 6.774412e-03 2742.480 1.802293e-05 7.776119
703443 rs9883561 3_9 5.623830e-04 2740.038 1.494855e-06 7.676471
703469 rs9821066 3_9 3.249060e-05 2729.436 8.602823e-08 7.588235
703464 rs9872103 3_9 1.785730e-04 2728.262 4.726201e-07 7.686567
703465 rs9871991 3_9 3.800932e-04 2727.319 1.005626e-06 7.716418
703470 rs9877165 3_9 4.469283e-05 2727.313 1.182451e-07 7.626866
703476 rs1985428 3_9 3.173254e-05 2727.176 8.395150e-08 7.611940
703489 rs114415025 3_9 1.000000e+00 2723.925 2.642443e-03 1.219251
703461 rs6791647 3_9 2.233448e-05 2720.842 5.895080e-08 7.611940
703456 rs4447735 3_9 3.120521e-05 2720.726 8.236113e-08 7.626866
703462 rs6767504 3_9 7.609822e-06 2717.065 2.005788e-08 7.597015
703459 rs4321514 3_9 7.424475e-06 2716.792 1.956737e-08 7.597015
703457 rs4299468 3_9 7.118141e-06 2716.475 1.875783e-08 7.597015
703452 rs3901665 3_9 1.258296e-05 2715.352 3.314511e-08 7.626866
703488 rs56082700 3_9 0.000000e+00 2702.262 0.000000e+00 2.696970
703471 rs9822382 3_9 0.000000e+00 2678.591 0.000000e+00 4.277228
703447 rs9870070 3_9 0.000000e+00 2676.505 0.000000e+00 4.516129
703445 rs9850214 3_9 0.000000e+00 2676.317 0.000000e+00 4.483871
703448 rs9850227 3_9 0.000000e+00 2675.871 0.000000e+00 4.397849
703558 rs3732675 3_9 1.173251e-08 2674.016 3.043446e-11 7.735294
703446 rs9850480 3_9 0.000000e+00 2664.144 0.000000e+00 4.505376
703492 rs11706068 3_9 2.610084e-06 2600.801 6.585245e-09 7.970588SNPs with highest PVE
#SNPs with 50 highest pve
head(ctwas_snp_res[order(-ctwas_snp_res$PVE),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
698783 rs4972703 2_105 1.00000000 3592.8850 0.0034854089 -0.7842670
183948 rs1906615 4_72 1.00000000 3299.1775 0.0032004872 45.1604938
698793 rs1367220 2_105 0.90278725 3624.4736 0.0031742475 7.2207792
703489 rs114415025 3_9 1.00000000 2723.9251 0.0026424427 1.2192513
703494 rs56204325 3_9 0.71892638 2763.5212 0.0019273369 7.8656716
768375 rs61937778 12_35 1.00000000 1898.7373 0.0018419393 -1.3056995
272791 rs111990232 6_59 1.00000000 1672.2132 0.0016221913 0.5392670
183953 rs7440714 4_72 1.00000000 1498.4064 0.0014535837 -9.2183406
272788 rs371814 6_59 0.88875486 1684.1863 0.0014520532 6.1911765
28638 rs7536152 1_75 1.00000000 1439.6465 0.0013965815 -6.4626866
703515 rs4642101 3_9 0.71504872 1969.7326 0.0013663229 10.2318841
28637 rs12404927 1_75 1.00000000 1404.5510 0.0013625359 -1.5517241
768315 rs2860482 12_35 0.72039239 1945.5816 0.0013596558 -7.1052632
232614 rs199992924 5_68 0.99677991 1308.3614 0.0012651366 -0.6309524
232607 rs4073838 5_68 0.89071507 1336.8900 0.0011551674 -5.9545455
768370 rs7313074 12_35 0.59547601 1953.4234 0.0011284208 -6.9868421
728978 rs201862093 7_48 1.00000000 1114.3649 0.0010810302 -0.8625000
703475 rs1969154 3_9 0.37891760 2761.4487 0.0010150611 7.8507463
703474 rs6799179 3_9 0.37669868 2761.4183 0.0010091059 7.8507463
729019 rs112661041 7_48 0.92465841 1098.8711 0.0009856858 4.6532258
765251 rs76106073 10_44 1.00000000 836.8554 0.0008118220 0.6572238
698789 rs10168156 2_105 0.22653976 3615.0005 0.0007944439 7.2337662
705484 rs9830653 3_84 0.94971640 834.6821 0.0007689985 -0.9344262
768373 rs4759256 12_35 0.40562812 1952.8889 0.0007684507 -6.9736842
592256 rs62056842 17_28 1.00000000 764.0781 0.0007412218 1.1269036
272792 rs384318 6_59 0.37973072 1682.6223 0.0006198303 6.1029412
728888 rs12154789 7_48 0.51476523 1098.6922 0.0005486504 4.4923077
703514 rs7650482 3_9 0.28023466 1973.5020 0.0005365002 10.1571429
703484 rs12714880 3_9 0.19052042 2760.7520 0.0005102457 7.8208955
121514 rs12330500 3_40 0.99969702 506.3653 0.0004910693 0.5641026
704796 rs199808025 3_84 0.56221627 819.6038 0.0004470106 0.3170732
698778 rs1864453 2_105 0.12515989 3612.7802 0.0004386490 7.2077922
272793 rs1145714 6_59 0.26668394 1679.0994 0.0004343939 6.1029412
121517 rs6924 3_40 0.82385812 510.0248 0.0004076187 4.2133333
785055 rs8005417 14_9 0.99999767 418.1701 0.0004056602 0.9863946
785061 rs8011559 14_9 0.96440477 426.2857 0.0003988142 5.6913580
232606 rs4235764 5_68 0.29404637 1335.1711 0.0003808581 -5.8787879
768310 rs7978685 12_35 0.19327209 1942.9081 0.0003642770 -7.0657895
556241 rs74022964 15_35 1.00000000 340.3300 0.0003301495 12.5777778
698794 rs1367219 2_105 0.08971134 3615.4774 0.0003146469 7.1038961
232609 rs4235768 5_68 0.24023863 1335.2967 0.0003111939 -5.8030303
272750 rs9444476 6_59 0.19310251 1660.7317 0.0003110984 -6.3088235
28731 rs34515871 1_76 1.00000000 296.9840 0.0002881002 17.0000000
272775 rs9444488 6_59 0.17207131 1665.5818 0.0002780256 -6.2647059
556238 rs140798119 15_35 1.00000000 282.6223 0.0002741680 3.9950739
183979 rs186546224 4_72 0.99999999 278.2802 0.0002699558 -6.8398876
183968 rs4631108 4_72 0.98588526 278.7291 0.0002665748 -17.6376812
323204 rs28557111 7_70 0.99999953 268.5095 0.0002604773 13.6818182
733889 rs62643683 8_21 0.99999999 262.9862 0.0002551193 0.8007519
183920 rs1823291 4_72 0.94438487 275.3827 0.0002522877 -19.4520548SNPs with largest z scores
#histogram of (abs) SNP z scores
hist(abs(ctwas_snp_res$z))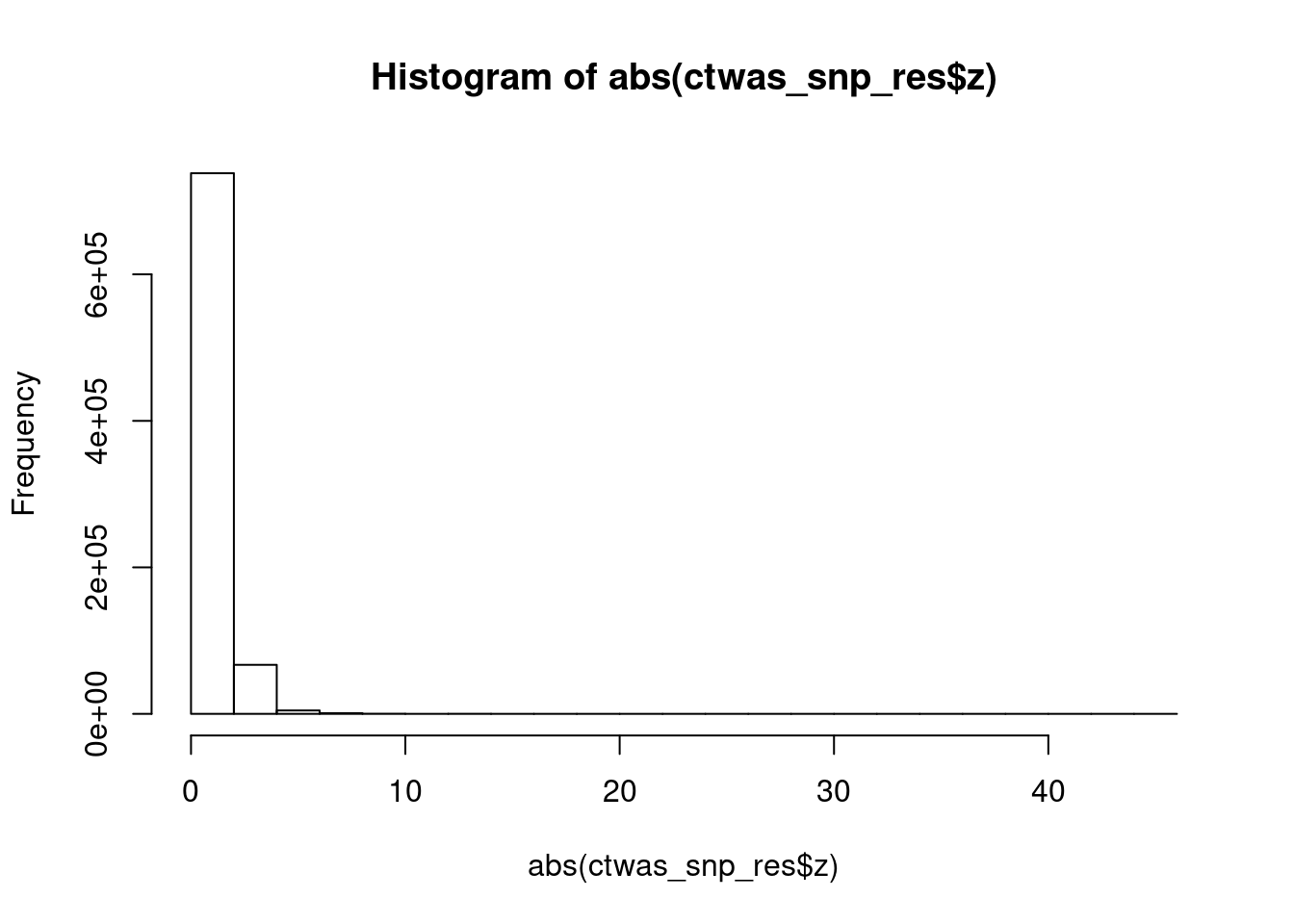
#SNPs with 50 largest z scores
head(ctwas_snp_res[order(-abs(ctwas_snp_res$z)),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
183948 rs1906615 4_72 1.000000e+00 3299.1775 3.200487e-03 45.16049
183949 rs75725917 4_72 0.000000e+00 1501.2534 0.000000e+00 42.33663
183947 rs1906611 4_72 0.000000e+00 1386.7531 0.000000e+00 40.97143
183946 rs28521134 4_72 0.000000e+00 1323.8641 0.000000e+00 40.22772
183942 rs10019689 4_72 0.000000e+00 1314.7532 0.000000e+00 40.11000
183943 rs76013973 4_72 0.000000e+00 1309.8604 0.000000e+00 40.04902
183945 rs12639820 4_72 0.000000e+00 1308.0495 0.000000e+00 40.03922
183941 rs12647393 4_72 0.000000e+00 1303.9357 0.000000e+00 39.98020
183944 rs74496596 4_72 0.000000e+00 1284.8290 0.000000e+00 39.75728
183940 rs12647316 4_72 0.000000e+00 1271.3853 0.000000e+00 39.55882
183939 rs4529121 4_72 0.000000e+00 1275.2573 0.000000e+00 39.55340
183933 rs12650829 4_72 0.000000e+00 605.3700 0.000000e+00 31.54444
183956 rs3866831 4_72 0.000000e+00 1596.6468 0.000000e+00 -30.10000
183955 rs6533530 4_72 0.000000e+00 1580.2990 0.000000e+00 -29.91429
183938 rs12644107 4_72 0.000000e+00 465.5759 0.000000e+00 25.98851
183936 rs2723318 4_72 0.000000e+00 813.2565 0.000000e+00 -25.45833
183931 rs2218698 4_72 0.000000e+00 771.3648 0.000000e+00 -25.09589
183934 rs2197814 4_72 0.000000e+00 772.0146 0.000000e+00 24.59722
183932 rs1448799 4_72 0.000000e+00 770.9787 0.000000e+00 24.56944
183930 rs112927894 4_72 0.000000e+00 741.1342 0.000000e+00 24.50685
183920 rs1823291 4_72 9.443849e-01 275.3827 2.522877e-04 -19.45205
183924 rs2723296 4_72 5.561070e-02 266.6973 1.438757e-05 -19.02740
183929 rs11724067 4_72 0.000000e+00 654.4966 0.000000e+00 -18.94059
183923 rs2044674 4_72 4.429420e-06 242.9736 1.044038e-09 18.58108
183968 rs4631108 4_72 9.858853e-01 278.7291 2.665748e-04 -17.63768
183967 rs1906613 4_72 1.411474e-02 268.6140 3.678003e-06 17.34783
28731 rs34515871 1_76 1.000000e+00 296.9840 2.881002e-04 17.00000
423415 rs60469668 10_66 1.000000e+00 171.3926 1.662657e-04 16.96471
183928 rs13111704 4_72 0.000000e+00 700.2351 0.000000e+00 -16.91743
28734 rs12058931 1_76 2.023295e-04 220.3706 4.325369e-08 16.54054
183952 rs4124159 4_72 0.000000e+00 1664.4211 0.000000e+00 -16.14414
183950 rs1906606 4_72 0.000000e+00 1659.0494 0.000000e+00 -16.02703
183957 rs4032974 4_72 0.000000e+00 1649.6039 0.000000e+00 -15.86726
183977 rs17513625 4_72 0.000000e+00 196.2481 0.000000e+00 15.71130
183954 rs10006881 4_72 0.000000e+00 1641.6919 0.000000e+00 -15.68750
423411 rs12572965 10_66 2.787622e-04 132.8475 3.592507e-08 15.27059
423410 rs56965730 10_66 2.796755e-04 131.7056 3.573296e-08 15.21176
183922 rs12642151 4_72 7.894574e-12 227.0161 1.738585e-15 14.14286
423413 rs7094488 10_66 9.999961e-01 105.1201 1.019752e-04 14.10606
672306 rs577676 1_84 4.329934e-04 124.1225 5.213653e-08 -13.77612
323204 rs28557111 7_70 9.999995e-01 268.5095 2.604773e-04 13.68182
672415 rs680084 1_84 6.024752e-04 136.4064 7.972312e-08 -13.53731
672386 rs608930 1_84 1.253762e-03 138.0822 1.679436e-07 -13.52239
672422 rs629234 1_84 5.624740e-04 135.5991 7.398942e-08 -13.52239
672340 rs598993 1_84 9.849966e-04 137.8588 1.317285e-07 -13.51515
672343 rs541557 1_84 9.796191e-04 137.8182 1.309707e-07 -13.51515
672429 rs525489 1_84 1.190958e-03 137.5496 1.589156e-07 -13.49254
672430 rs503706 1_84 1.177181e-03 137.4686 1.569846e-07 -13.49254
672350 rs539045 1_84 8.984718e-04 136.7722 1.192100e-07 -13.48485
672356 rs580487 1_84 8.877917e-04 136.6820 1.177153e-07 -13.48485Gene set enrichment for genes with PIP>0.8
#GO enrichment analysis
library(enrichR)Welcome to enrichR
Checking connection ... Enrichr ... Connection is Live!
FlyEnrichr ... Connection is available!
WormEnrichr ... Connection is available!
YeastEnrichr ... Connection is available!
FishEnrichr ... Connection is available!dbs <- c("GO_Biological_Process_2021", "GO_Cellular_Component_2021", "GO_Molecular_Function_2021")
genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
#number of genes for gene set enrichment
length(genes)[1] 19if (length(genes)>0){
GO_enrichment <- enrichr(genes, dbs)
for (db in dbs){
print(db)
df <- GO_enrichment[[db]]
print(plotEnrich(GO_enrichment[[db]]))
df <- df[df$Adjusted.P.value<0.05,c("Term", "Overlap", "Adjusted.P.value", "Genes")]
print(df)
}
#DisGeNET enrichment
# devtools::install_bitbucket("ibi_group/disgenet2r")
library(disgenet2r)
disgenet_api_key <- get_disgenet_api_key(
email = "wesleycrouse@gmail.com",
password = "uchicago1" )
Sys.setenv(DISGENET_API_KEY= disgenet_api_key)
res_enrich <-disease_enrichment(entities=genes, vocabulary = "HGNC",
database = "CURATED" )
df <- res_enrich@qresult[1:10, c("Description", "FDR", "Ratio", "BgRatio")]
print(df)
#WebGestalt enrichment
library(WebGestaltR)
background <- ctwas_gene_res$genename
#listGeneSet()
databases <- c("pathway_KEGG", "disease_GLAD4U", "disease_OMIM")
enrichResult <- WebGestaltR(enrichMethod="ORA", organism="hsapiens",
interestGene=genes, referenceGene=background,
enrichDatabase=databases, interestGeneType="genesymbol",
referenceGeneType="genesymbol", isOutput=F)
print(enrichResult[,c("description", "size", "overlap", "FDR", "database", "userId")])
}Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
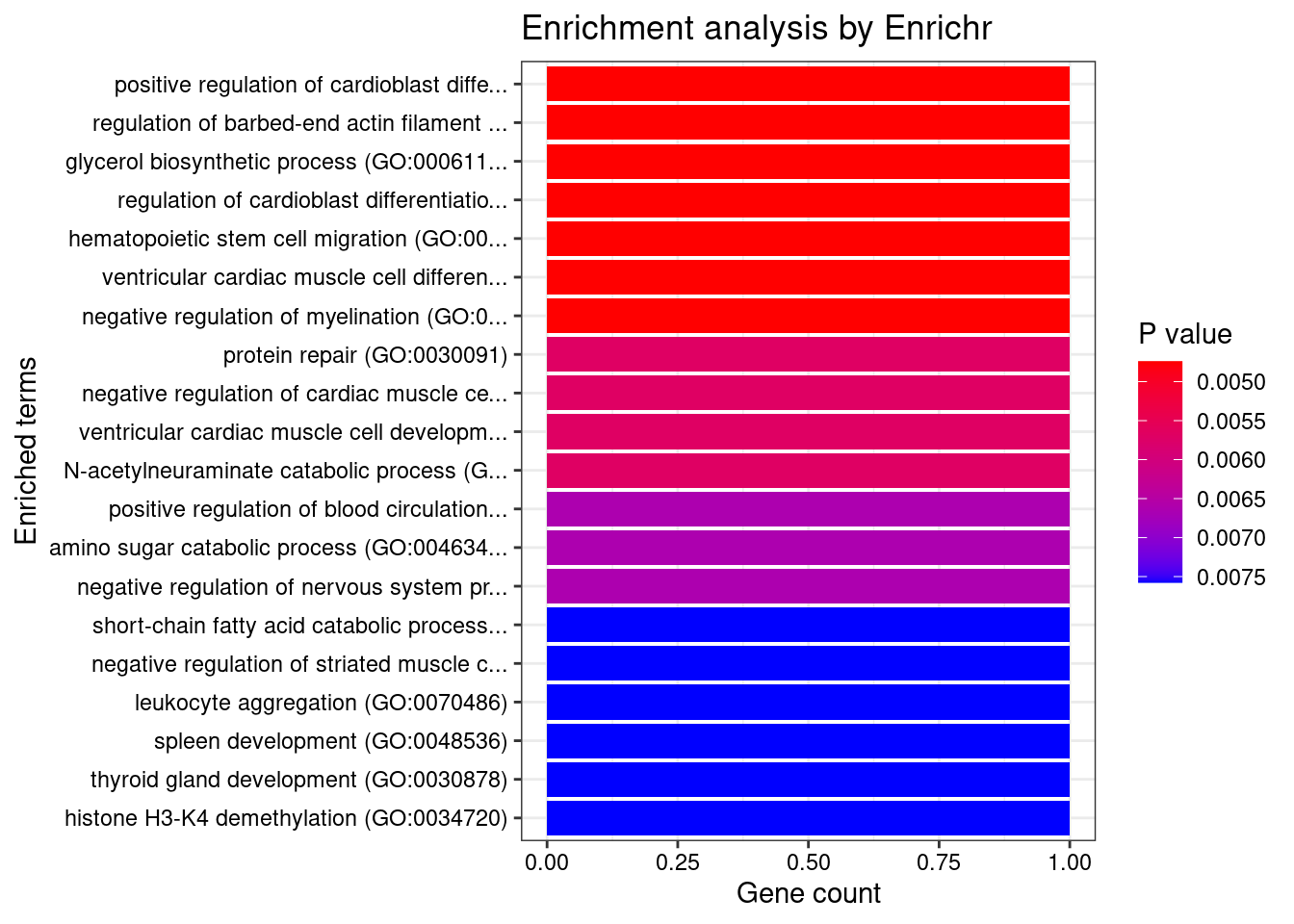
[1] "GO_Biological_Process_2021"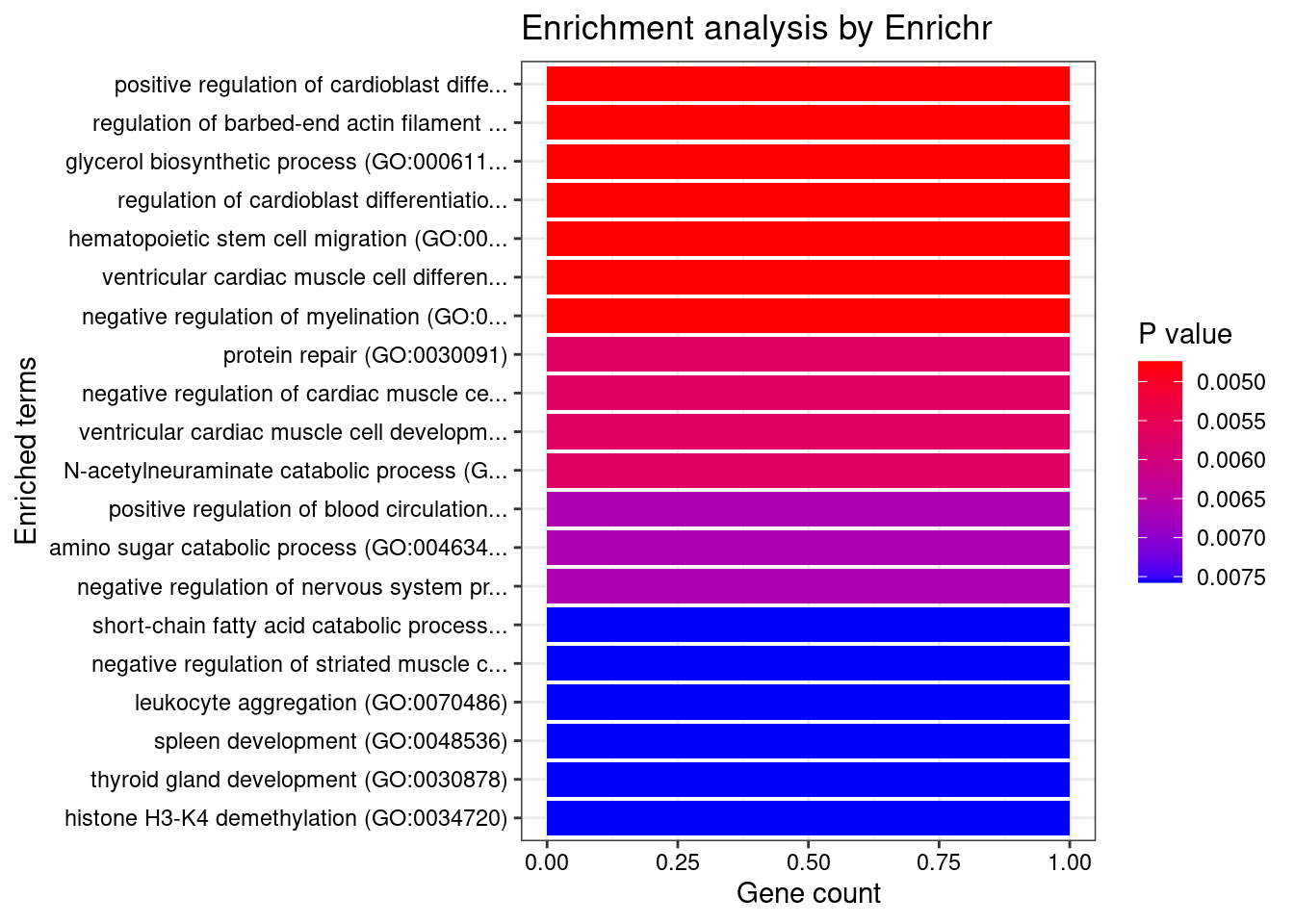
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Cellular_Component_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021" Term
1 zinc ion binding (GO:0008270)
2 nucleotide phosphatase activity, acting on free nucleotides (GO:0098519)
3 oxidoreductase activity, acting on a sulfur group of donors, disulfide as acceptor (GO:0016671)
4 oxo-acid-lyase activity (GO:0016833)
5 CoA carboxylase activity (GO:0016421)
6 transition metal ion binding (GO:0046914)
7 histone demethylase activity (H3-K4 specific) (GO:0032453)
Overlap Adjusted.P.value Genes
1 3/336 0.04663435 KDM1B;DPF3;MSRB1
2 1/5 0.04663435 PGP
3 1/5 0.04663435 MSRB1
4 1/5 0.04663435 NPL
5 1/6 0.04663435 PCCB
6 3/445 0.04989809 KDM1B;DPF3;MSRB1
7 1/9 0.04989809 KDM1BLINC00964 gene(s) from the input list not found in DisGeNET CURATEDJAM2 gene(s) from the input list not found in DisGeNET CURATEDGMCL1 gene(s) from the input list not found in DisGeNET CURATEDWASHC2C gene(s) from the input list not found in DisGeNET CURATEDLINC01314 gene(s) from the input list not found in DisGeNET CURATED Description
1 Atrial Fibrillation
27 Paroxysmal atrial fibrillation
56 Persistent atrial fibrillation
68 familial atrial fibrillation
19 Congenital retrognathism
38 Epidermolysis bullosa simplex, Ogna type
57 HYPOTHYROIDISM, CONGENITAL, NONGOITROUS, 5 (disorder)
60 Epidermolysa bullosa simplex and limb girdle muscular dystrophy
63 MUSCULAR DYSTROPHY, LIMB-GIRDLE, TYPE 2Q
64 ATRIAL SEPTAL DEFECT 7 WITH OR WITHOUT ATRIOVENTRICULAR CONDUCTION DEFECTS
FDR Ratio BgRatio
1 1.865793e-08 7/14 160/9703
27 1.865793e-08 7/14 156/9703
56 1.865793e-08 7/14 156/9703
68 1.865793e-08 7/14 156/9703
19 5.378460e-03 1/14 1/9703
38 5.378460e-03 1/14 1/9703
57 5.378460e-03 1/14 1/9703
60 5.378460e-03 1/14 1/9703
63 5.378460e-03 1/14 1/9703
64 5.378460e-03 1/14 1/9703******************************************
* *
* Welcome to WebGestaltR ! *
* *
******************************************Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!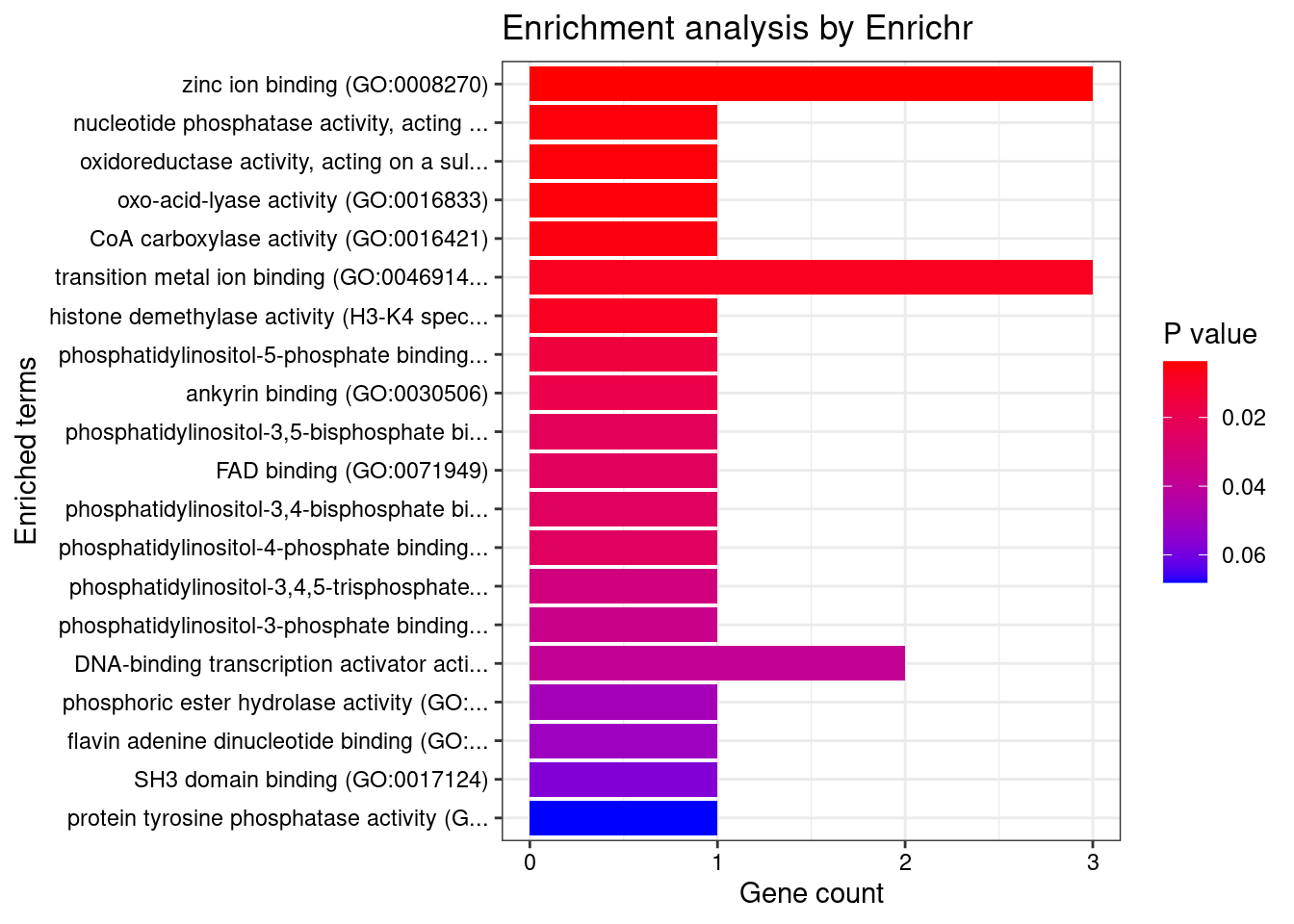
NULLSensitivity, specificity and precision for silver standard genes
library("readxl")
known_annotations <- read_xlsx("data/summary_known_genes_annotations.xlsx", sheet="LDL")New names:
* `` -> ...4
* `` -> ...5known_annotations <- unique(known_annotations$`Gene Symbol`)
unrelated_genes <- ctwas_gene_res$genename[!(ctwas_gene_res$genename %in% known_annotations)]
#number of genes in known annotations
print(length(known_annotations))[1] 69#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 37#assign ctwas, TWAS, and bystander genes
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>sig_thresh]
novel_genes <- ctwas_genes[!(ctwas_genes %in% twas_genes)]
#significance threshold for TWAS
print(sig_thresh)[1] 4.56791#number of ctwas genes
length(ctwas_genes)[1] 19#number of TWAS genes
length(twas_genes)[1] 79#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_eqtl
4891 NPL 1_90 0.8251030 18.75569 1.501245e-05 -3.825175 1
12133 LINC00964 8_82 0.8180590 22.20129 1.761867e-05 4.554265 2
9417 PLEC 8_94 0.9666163 43.61991 4.090245e-05 3.093844 2
8847 WASHC2C 10_31 0.8186904 19.80281 1.572740e-05 -3.972152 1
12376 LINC01314 15_37 0.8300211 20.71969 1.668334e-05 -4.379164 4#sensitivity / recall
sensitivity <- rep(NA,2)
names(sensitivity) <- c("ctwas", "TWAS")
sensitivity["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
sensitivity["TWAS"] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
sensitivityctwas TWAS
0 0 #specificity
specificity <- rep(NA,2)
names(specificity) <- c("ctwas", "TWAS")
specificity["ctwas"] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
specificity["TWAS"] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
specificity ctwas TWAS
0.9981212 0.9921883 #precision / PPV
precision <- rep(NA,2)
names(precision) <- c("ctwas", "TWAS")
precision["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
precision["TWAS"] <- sum(twas_genes %in% known_annotations)/length(twas_genes)
precisionctwas TWAS
0 0 #ROC curves
pip_range <- (0:1000)/1000
sensitivity <- rep(NA, length(pip_range))
specificity <- rep(NA, length(pip_range))
for (index in 1:length(pip_range)){
pip <- pip_range[index]
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>=pip]
sensitivity[index] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
}
plot(1-specificity, sensitivity, type="l", xlim=c(0,1), ylim=c(0,1))
sig_thresh_range <- seq(from=0, to=max(abs(ctwas_gene_res$z)), length.out=length(pip_range))
for (index in 1:length(sig_thresh_range)){
sig_thresh_plot <- sig_thresh_range[index]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>=sig_thresh_plot]
sensitivity[index] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
}
lines(1-specificity, sensitivity, xlim=c(0,1), ylim=c(0,1), col="red", lty=2)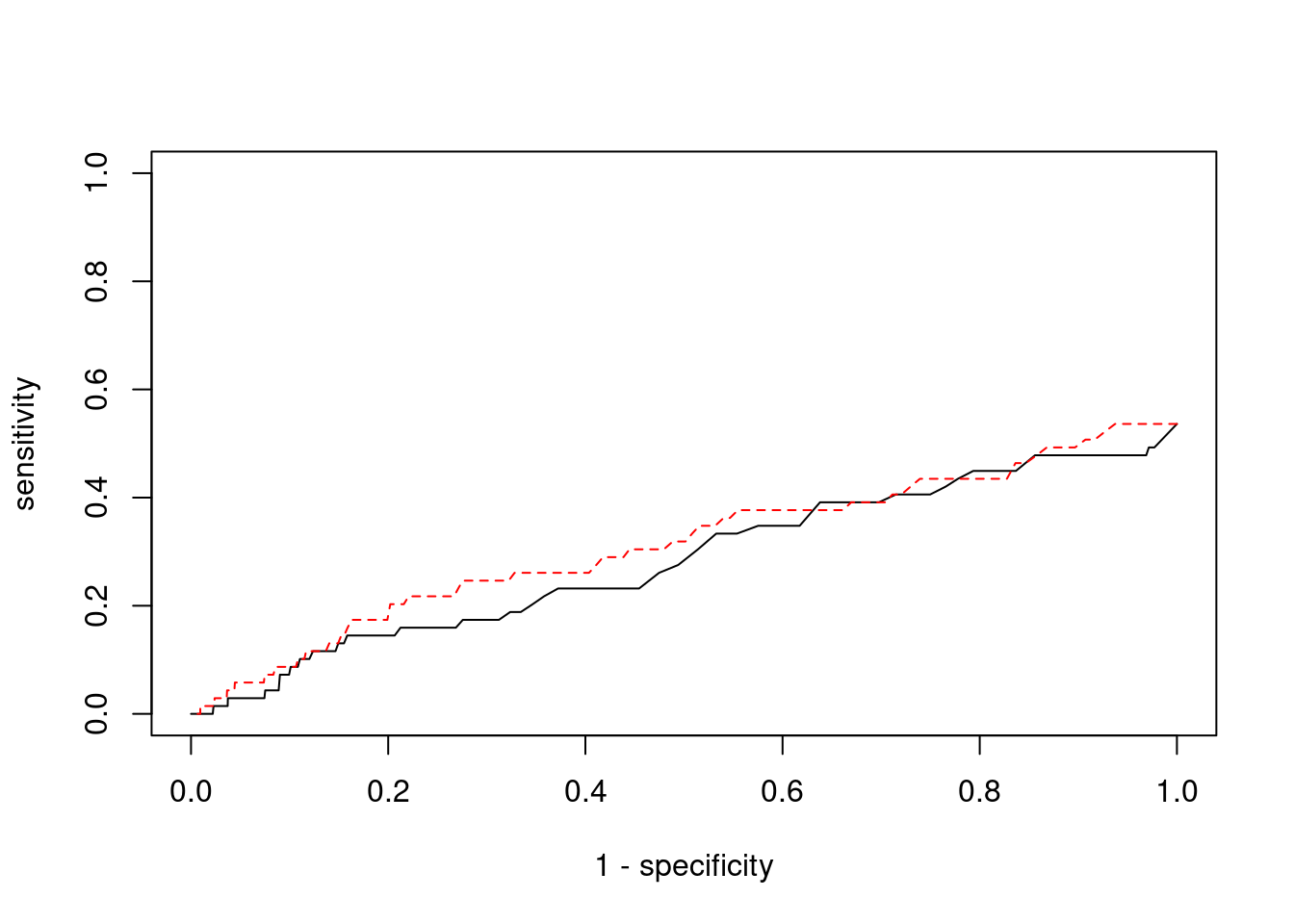
Sensitivity, specificity and precision for silver standard genes - bystanders only
This section first uses imputed silver standard genes to identify bystander genes within 1Mb. The bystander gene list is then subset to only genes with imputed expression in this analysis. Then, the ctwas and TWAS gene lists from this analysis are subset to only genes that are in the (subset) silver standard and bystander genes. These gene lists are then used to compute sensitivity, specificity and precision for ctwas and TWAS.
# library(biomaRt)
# library(GenomicRanges)
#
# ensembl <- useEnsembl(biomart="ENSEMBL_MART_ENSEMBL", dataset="hsapiens_gene_ensembl")
# G_list <- getBM(filters= "chromosome_name", attributes= c("hgnc_symbol","chromosome_name","start_position","end_position","gene_biotype"), values=1:22, mart=ensembl)
# G_list <- G_list[G_list$hgnc_symbol!="",]
# G_list <- G_list[G_list$gene_biotype %in% c("protein_coding","lncRNA"),]
# G_list$start <- G_list$start_position
# G_list$end <- G_list$end_position
# G_list_granges <- makeGRangesFromDataFrame(G_list, keep.extra.columns=T)
#
# #remove genes without imputed expression from gene lists
# known_annotations <- known_annotations[known_annotations %in% ctwas_gene_res$genename]
#
# known_annotations_positions <- G_list[G_list$hgnc_symbol %in% known_annotations,]
# half_window <- 1000000
# known_annotations_positions$start <- known_annotations_positions$start_position - half_window
# known_annotations_positions$end <- known_annotations_positions$end_position + half_window
# known_annotations_positions$start[known_annotations_positions$start<1] <- 1
# known_annotations_granges <- makeGRangesFromDataFrame(known_annotations_positions, keep.extra.columns=T)
#
# bystanders <- findOverlaps(known_annotations_granges,G_list_granges)
# bystanders <- unique(subjectHits(bystanders))
# bystanders <- G_list$hgnc_symbol[bystanders]
# bystanders <- unique(bystanders[!(bystanders %in% known_annotations)])
# unrelated_genes <- bystanders
#
# #save gene lists
# save(known_annotations, file=paste0(results_dir, "/known_annotations.Rd"))
# save(unrelated_genes, file=paste0(results_dir, "/bystanders.Rd"))
load(paste0(results_dir, "/known_annotations.Rd"))
load(paste0(results_dir, "/bystanders.Rd"))
#remove genes without imputed expression from bystander list
unrelated_genes <- unrelated_genes[unrelated_genes %in% ctwas_gene_res$genename]
#number of genes in known annotations (with imputed expression)
print(length(known_annotations))[1] 37#number of bystander genes (with imputed expression)
print(length(unrelated_genes))[1] 442#subset results to genes in known annotations or bystanders
ctwas_gene_res_subset <- ctwas_gene_res[ctwas_gene_res$genename %in% c(known_annotations, unrelated_genes),]
#assign ctwas and TWAS genes
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>0.8]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>sig_thresh]
#significance threshold for TWAS
print(sig_thresh)[1] 4.56791#number of ctwas genes (in known annotations or bystanders)
length(ctwas_genes)[1] 0#number of TWAS genes (in known annotations or bystanders)
length(twas_genes)[1] 5#sensitivity / recall
sensitivity <- rep(NA,2)
names(sensitivity) <- c("ctwas", "TWAS")
sensitivity["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
sensitivity["TWAS"] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
sensitivityctwas TWAS
0 0 #specificity / (1 - False Positive Rate)
specificity <- rep(NA,2)
names(specificity) <- c("ctwas", "TWAS")
specificity["ctwas"] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
specificity["TWAS"] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
specificity ctwas TWAS
1.0000000 0.9886878 #precision / PPV / (1 - False Discovery Rate)
precision <- rep(NA,2)
names(precision) <- c("ctwas", "TWAS")
precision["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
precision["TWAS"] <- sum(twas_genes %in% known_annotations)/length(twas_genes)
precisionctwas TWAS
NaN 0 #store sensitivity and specificity calculations for plots
sensitivity_plot <- sensitivity
specificity_plot <- specificity
#precision / PPV by PIP bin
pip_range <- c(0.2, 0.4, 0.6, 0.8, 1)
precision_range <- rep(NA, length(pip_range))
for (i in 1:length(pip_range)){
pip_upper <- pip_range[i]
if (i==1){
pip_lower <- 0
} else {
pip_lower <- pip_range[i-1]
}
#assign ctwas genes in PIP bin
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>=pip_lower & ctwas_gene_res_subset$susie_pip<pip_upper]
precision_range[i] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
}
names(precision_range) <- paste(c(0, pip_range[-length(pip_range)]), pip_range,sep=" - ")
barplot(precision_range, ylim=c(0,1), main="Precision by PIP Range", xlab="PIP Range", ylab="Precision")
abline(h=0.2, lty=2)
abline(h=0.4, lty=2)
abline(h=0.6, lty=2)
abline(h=0.8, lty=2)
barplot(precision_range, add=T, col="darkgrey")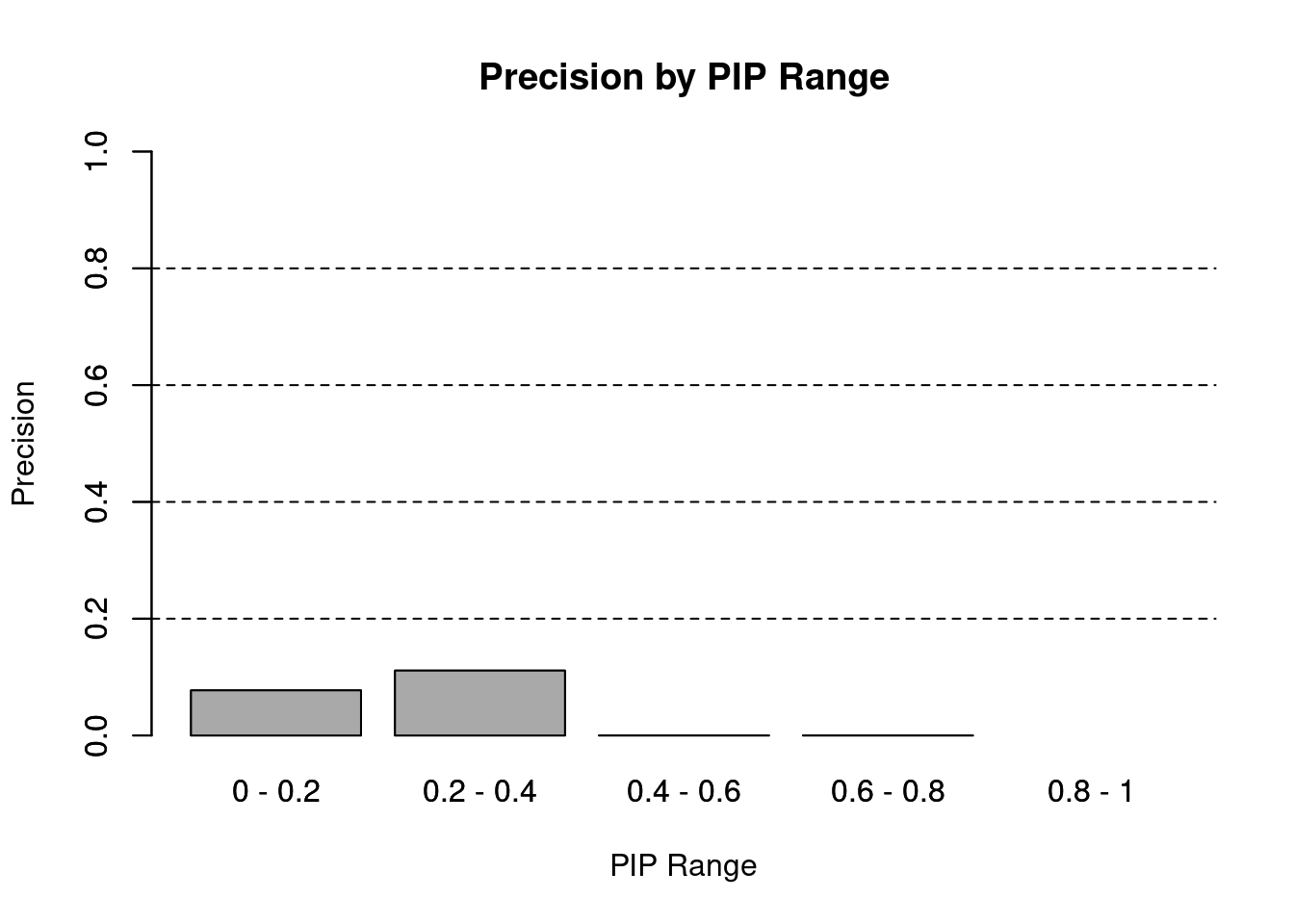
#precision / PPV by PIP threshold
#pip_range <- c(0.2, 0.4, 0.6, 0.8, 1)
pip_range <- c(0.5, 0.8, 1)
precision_range <- rep(NA, length(pip_range))
number_detected <- rep(NA, length(pip_range))
for (i in 1:length(pip_range)){
pip_upper <- pip_range[i]
if (i==1){
pip_lower <- 0
} else {
pip_lower <- pip_range[i-1]
}
#assign ctwas genes using PIP threshold
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>=pip_lower]
number_detected[i] <- length(ctwas_genes)
precision_range[i] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
}
names(precision_range) <- paste0(">= ", c(0, pip_range[-length(pip_range)]))
precision_range <- precision_range*100
precision_range <- c(precision_range, precision["TWAS"]*100)
names(precision_range)[4] <- "TWAS Bonferroni"
number_detected <- c(number_detected, length(twas_genes))
barplot(precision_range, ylim=c(0,100), main="Precision for Distinguishing Silver Standard and Bystander Genes", xlab="PIP Threshold for Detection", ylab="% of Detected Genes in Silver Standard")
abline(h=20, lty=2)
abline(h=40, lty=2)
abline(h=60, lty=2)
abline(h=80, lty=2)
xx <- barplot(precision_range, add=T, col=c(rep("darkgrey",3), "white"))
text(x = xx, y = rep(0, length(number_detected)), label = paste0(number_detected, " detected"), pos = 3, cex=0.8)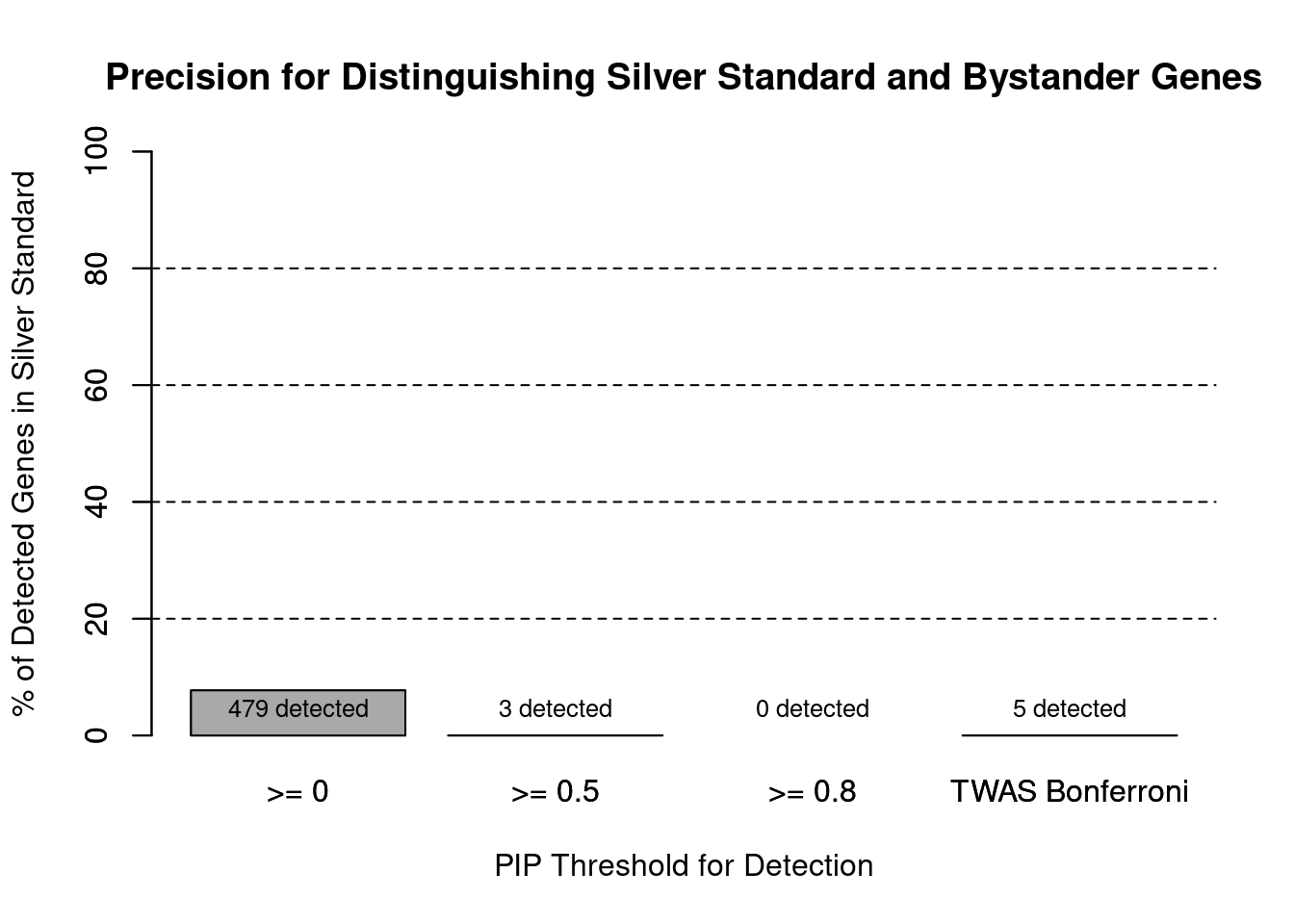
#text(x = xx, y = precision_range, label = paste0(round(precision_range,1), "%"), pos = 3, cex=0.8, offset = 1.5)
#false discovery rate by PIP threshold
barplot(100-precision_range, ylim=c(0,100), main="False Discovery Rate for Distinguishing Silver Standard and Bystander Genes", xlab="PIP Threshold for Detection", ylab="% Bystanders in Detected Genes")
abline(h=20, lty=2)
abline(h=40, lty=2)
abline(h=60, lty=2)
abline(h=80, lty=2)
xx <- barplot(100-precision_range, add=T, col=c(rep("darkgrey",3), "white"))
text(x = xx, y = rep(0, length(number_detected)), label = paste0(number_detected, " detected"), pos = 3, cex=0.8)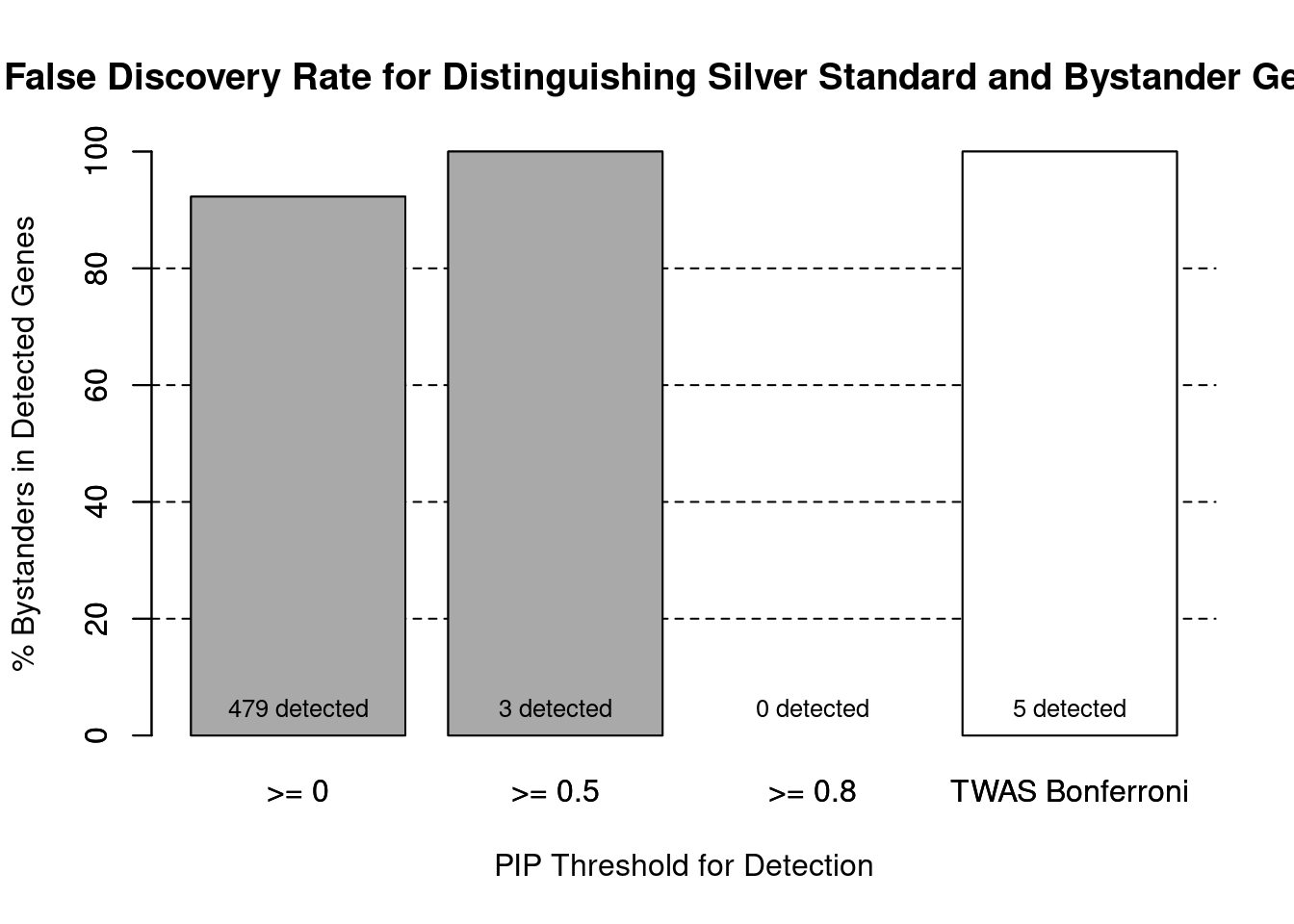
#text(x = xx, y = precision_range, label = paste0(round(precision_range,1), "%"), pos = 3, cex=0.8, offset = 1.5)
#ROC curves
pip_range <- (0:1000)/1000
sensitivity <- rep(NA, length(pip_range))
specificity <- rep(NA, length(pip_range))
for (index in 1:length(pip_range)){
pip <- pip_range[index]
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>=pip]
sensitivity[index] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
}
plot(1-specificity, sensitivity, type="l", xlim=c(0,1), ylim=c(0,1), main="", xlab="1 - Specificity", ylab="Sensitivity")
title(expression("ROC Curve for cTWAS (black) and TWAS (" * phantom("red") * ")"))
title(expression(phantom("ROC Curve for cTWAS (black) and TWAS (") * "red" * phantom(")")), col.main="red")
sig_thresh_range <- seq(from=0, to=max(abs(ctwas_gene_res_subset$z)), length.out=length(pip_range))
for (index in 1:length(sig_thresh_range)){
sig_thresh_plot <- sig_thresh_range[index]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>=sig_thresh_plot]
sensitivity[index] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
}
lines(1-specificity, sensitivity, xlim=c(0,1), ylim=c(0,1), col="red", lty=1)
abline(a=0,b=1,lty=3)
#add previously computed points from the analysis
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>0.8]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>sig_thresh]
points(1-specificity_plot["ctwas"], sensitivity_plot["ctwas"], pch=21, bg="black")
points(1-specificity_plot["TWAS"], sensitivity_plot["TWAS"], pch=21, bg="red")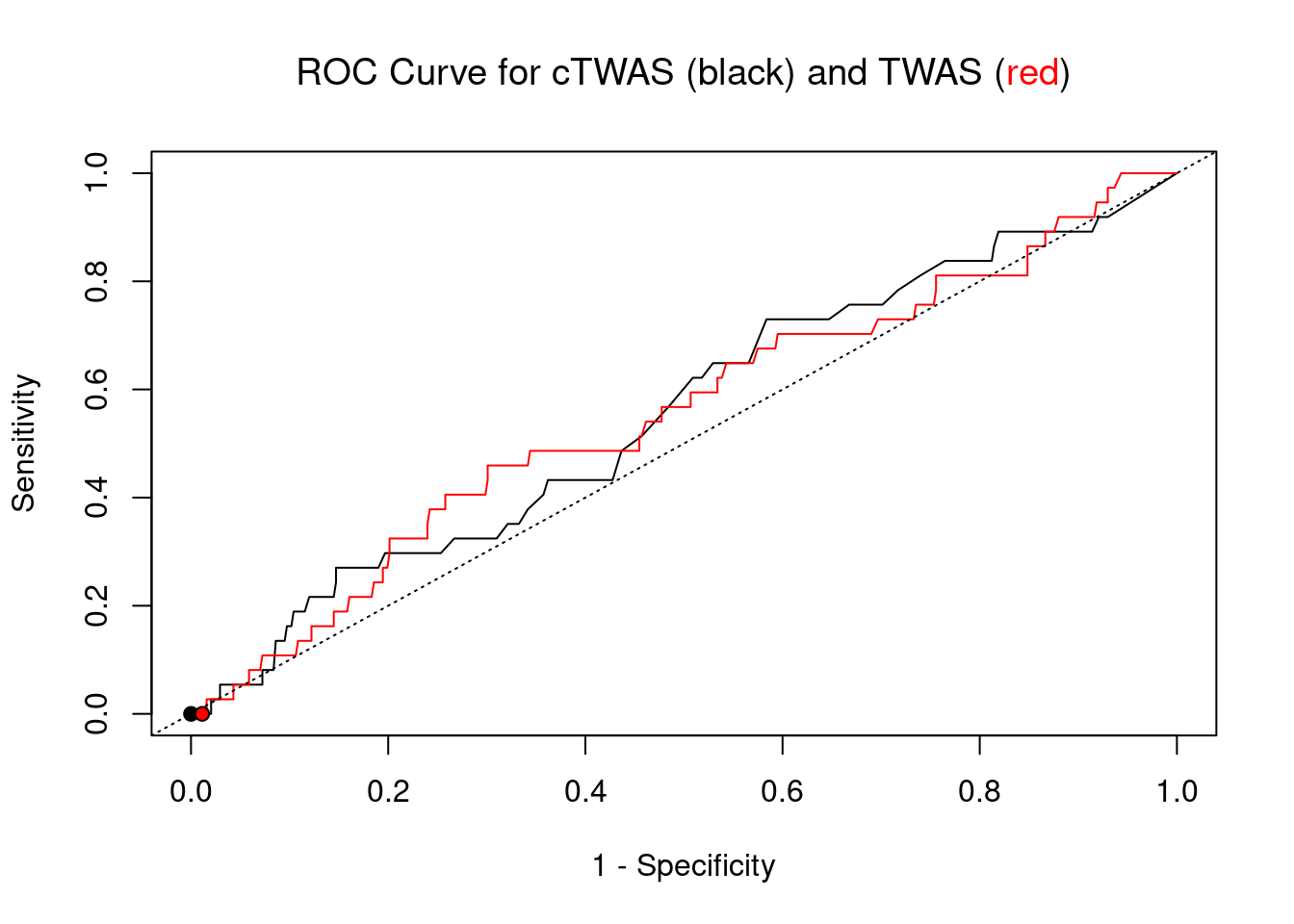
PIP Manhattan Plot
library(tibble)
library(tidyverse)── Attaching packages ─────────────────────────────────────── tidyverse 1.3.1 ──✔ tidyr 1.1.4 ✔ dplyr 1.0.7
✔ readr 2.1.1 ✔ stringr 1.4.0
✔ purrr 0.3.4 ✔ forcats 0.5.1── Conflicts ────────────────────────────────────────── tidyverse_conflicts() ──
✖ tidyr::extract() masks disgenet2r::extract()
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()full.gene.pip.summary <- data.frame(gene_name = ctwas_gene_res$genename,
gene_pip = ctwas_gene_res$susie_pip,
gene_id = ctwas_gene_res$id,
chr = as.integer(ctwas_gene_res$chrom),
start = ctwas_gene_res$pos / 1e3,
is_highlight = F, stringsAsFactors = F) %>% as_tibble()
full.gene.pip.summary$is_highlight <- full.gene.pip.summary$gene_pip > 0.80
don <- full.gene.pip.summary %>%
# Compute chromosome size
group_by(chr) %>%
summarise(chr_len=max(start)) %>%
# Calculate cumulative position of each chromosome
mutate(tot=cumsum(chr_len)-chr_len) %>%
dplyr::select(-chr_len) %>%
# Add this info to the initial dataset
left_join(full.gene.pip.summary, ., by=c("chr"="chr")) %>%
# Add a cumulative position of each SNP
arrange(chr, start) %>%
mutate( BPcum=start+tot)
axisdf <- don %>% group_by(chr) %>% summarize(center=( max(BPcum) + min(BPcum) ) / 2 )
x_axis_labels <- axisdf$chr
x_axis_labels[seq(1,21,2)] <- ""
ggplot(don, aes(x=BPcum, y=gene_pip)) +
# Show all points
ggrastr::geom_point_rast(aes(color=as.factor(chr)), size=2) +
scale_color_manual(values = rep(c("grey", "skyblue"), 22 )) +
# custom X axis:
# scale_x_continuous(label = axisdf$chr,
# breaks= axisdf$center,
# guide = guide_axis(n.dodge = 2)) +
scale_x_continuous(label = x_axis_labels,
breaks = axisdf$center) +
scale_y_continuous(expand = c(0, 0), limits = c(0,1.25), breaks=(1:5)*0.2, minor_breaks=(1:10)*0.1) + # remove space between plot area and x axis
# Add highlighted points
ggrastr::geom_point_rast(data=subset(don, is_highlight==T), color="orange", size=2) +
# Add label using ggrepel to avoid overlapping
ggrepel::geom_label_repel(data=subset(don, is_highlight==T),
aes(label=gene_name),
size=4,
min.segment.length = 0,
label.size = NA,
fill = alpha(c("white"),0)) +
# Custom the theme:
theme_bw() +
theme(
text = element_text(size = 14),
legend.position="none",
panel.border = element_blank(),
panel.grid.major.x = element_blank(),
panel.grid.minor.x = element_blank()
) +
xlab("Chromosome") +
ylab("cTWAS PIP")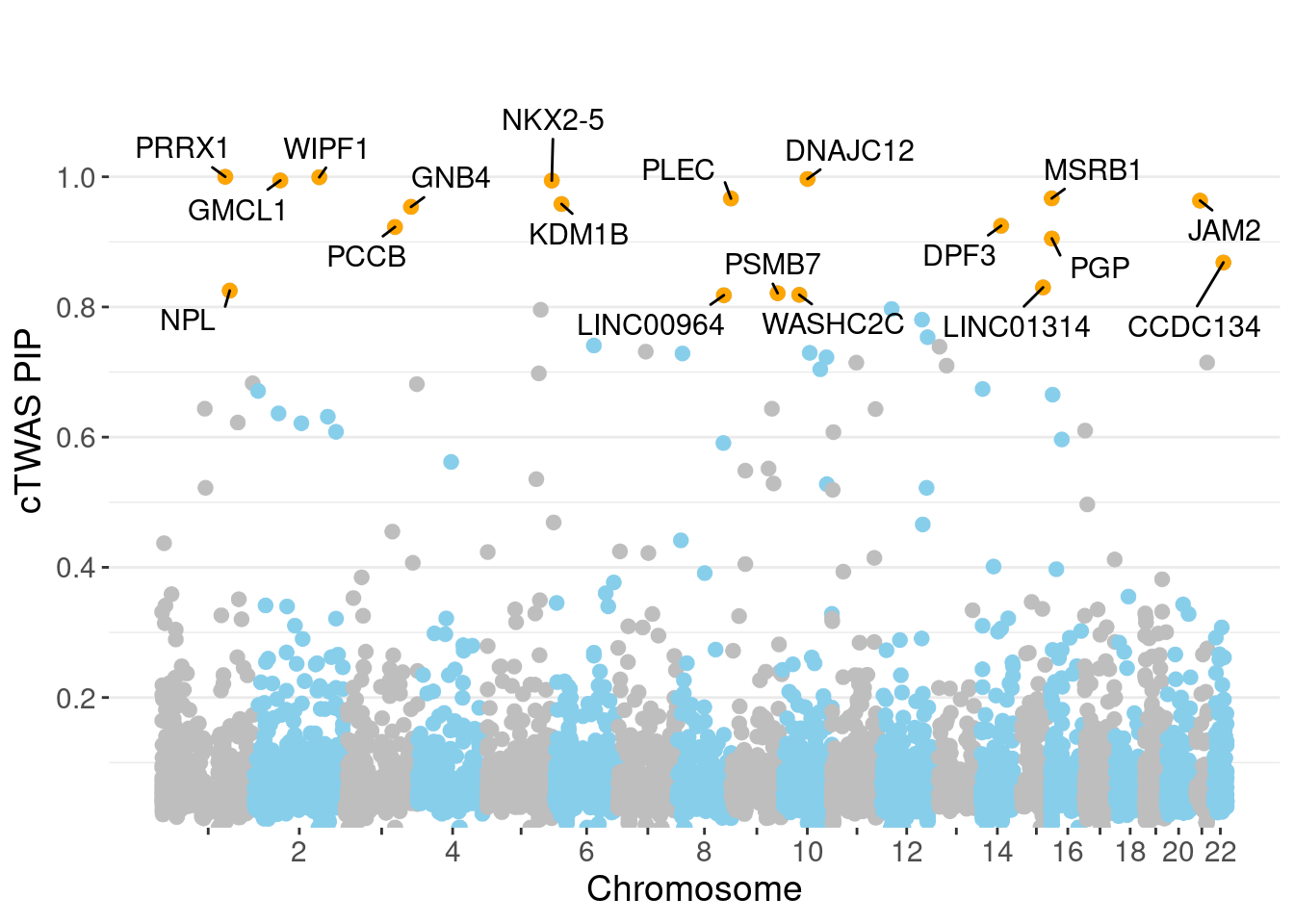
Locus Plots - 1_84
library(ctwas)
Attaching package: 'ctwas'The following object is masked _by_ '.GlobalEnv':
z_snplocus_plot <- function(region_tag, rerun_ctwas = F, plot_eqtl = T, label="cTWAS"){
region_tag1 <- unlist(strsplit(region_tag, "_"))[1]
region_tag2 <- unlist(strsplit(region_tag, "_"))[2]
a <- ctwas_res[ctwas_res$region_tag==region_tag,]
regionlist <- readRDS(paste0(results_dir, "/", analysis_id, "_ctwas.regionlist.RDS"))
region <- regionlist[[as.numeric(region_tag1)]][[region_tag2]]
R_snp_info <- do.call(rbind, lapply(region$regRDS, function(x){data.table::fread(paste0(tools::file_path_sans_ext(x), ".Rvar"))}))
if (isTRUE(rerun_ctwas)){
ld_exprfs <- paste0(results_dir, "/", analysis_id, "_expr_chr", 1:22, ".expr.gz")
temp_reg <- data.frame("chr" = paste0("chr",region_tag1), "start" = region$start, "stop" = region$stop)
write.table(temp_reg,
#file= paste0(results_dir, "/", analysis_id, "_ctwas.temp.reg.txt") ,
file= "temp_reg.txt",
row.names=F, col.names=T, sep="\t", quote = F)
load(paste0(results_dir, "/", analysis_id, "_expr_z_snp.Rd"))
z_gene_temp <- z_gene[z_gene$id %in% a$id[a$type=="gene"],]
z_snp_temp <- z_snp[z_snp$id %in% R_snp_info$id,]
ctwas_rss(z_gene_temp, z_snp_temp, ld_exprfs, ld_pgenfs = NULL,
ld_R_dir = dirname(region$regRDS)[1],
ld_regions_custom = "temp_reg.txt", thin = 1,
outputdir = ".", outname = "temp", ncore = 1, ncore.rerun = 1, prob_single = 0,
group_prior = estimated_group_prior, group_prior_var = estimated_group_prior_var,
estimate_group_prior = F, estimate_group_prior_var = F)
a <- data.table::fread("temp.susieIrss.txt", header = T)
rownames(z_snp_temp) <- z_snp_temp$id
z_snp_temp <- z_snp_temp[a$id[a$type=="SNP"],]
z_gene_temp <- z_gene_temp[a$id[a$type=="gene"],]
a$z <- NA
a$z[a$type=="SNP"] <- z_snp_temp$z
a$z[a$type=="gene"] <- z_gene_temp$z
}
a$ifcausal <- 0
focus <- a$id[a$type=="gene"][which.max(abs(a$z[a$type=="gene"]))]
a$ifcausal <- as.numeric(a$id==focus)
a$PVALUE <- (-log(2) - pnorm(abs(a$z), lower.tail=F, log.p=T))/log(10)
R_gene <- readRDS(region$R_g_file)
R_snp_gene <- readRDS(region$R_sg_file)
R_snp <- as.matrix(Matrix::bdiag(lapply(region$regRDS, readRDS)))
rownames(R_gene) <- region$gid
colnames(R_gene) <- region$gid
rownames(R_snp_gene) <- R_snp_info$id
colnames(R_snp_gene) <- region$gid
rownames(R_snp) <- R_snp_info$id
colnames(R_snp) <- R_snp_info$id
a$r2max <- NA
a$r2max[a$type=="gene"] <- R_gene[focus,a$id[a$type=="gene"]]
a$r2max[a$type=="SNP"] <- R_snp_gene[a$id[a$type=="SNP"],focus]
r2cut <- 0.4
colorsall <- c("#7fc97f", "#beaed4", "#fdc086")
layout(matrix(1:2, ncol = 1), widths = 1, heights = c(1.5,1.5), respect = FALSE)
par(mar = c(0, 4.1, 4.1, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 19, xlab=paste0("Chromosome ", region_tag1, " Position"),frame.plot=FALSE, col = "white", ylim= c(-0.1,1.1), ylab = "cTWAS PIP", xaxt = 'n')
grid()
points(a$pos[a$type=="SNP"], a$susie_pip[a$type == "SNP"], pch = 21, xlab="Genomic position", bg = colorsall[1])
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$susie_pip[a$type == "SNP" & a$r2max >r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$susie_pip[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$susie_pip[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$susie_pip[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$susie_pip[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
if (isTRUE(plot_eqtl)){
for (cgene in a[a$type=="gene" & a$ifcausal == 1, ]$id){
load(paste0(results_dir, "/",analysis_id, "_expr_chr", region_tag1, ".exprqc.Rd"))
eqtls <- rownames(wgtlist[[cgene]])
points(a[a$id %in% eqtls,]$pos, rep( -0.15, nrow(a[a$id %in% eqtls,])), pch = "|", col = "salmon", cex = 1.5)
}
}
legend(min(a$pos), y= 1.1 ,c("Gene", "SNP"), pch = c(22,21), title="Shape Legend", bty ='n', cex=0.6, title.adj = 0)
legend(min(a$pos), y= 0.7 ,c("Lead TWAS Gene", "R2 > 0.4", "R2 <= 0.4"), pch = 19, col = c("salmon", "purple", colorsall[1]), title="Color Legend", bty ='n', cex=0.6, title.adj = 0)
if (label=="cTWAS"){
text(a$pos[a$id==focus], a$susie_pip[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
par(mar = c(4.1, 4.1, 0.5, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 21, xlab=paste0("Chromosome ", region_tag1, " Position"), frame.plot=FALSE, bg = colorsall[1], ylab = "TWAS -log10(p value)", panel.first = grid(), ylim =c(0, max(a$PVALUE)*1.2))
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$PVALUE[a$type == "SNP" & a$r2max > r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$PVALUE[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$PVALUE[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$PVALUE[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$PVALUE[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
abline(h=-log10(alpha/nrow(ctwas_gene_res)), col ="red", lty = 2)
if (label=="TWAS"){
text(a$pos[a$id==focus], a$PVALUE[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
}
#locus_plot("1_84", label="TWAS")locus_plot4 <- function(region_tag, rerun_ctwas = F, plot_eqtl = T, label="cTWAS"){
region_tag1 <- unlist(strsplit(region_tag, "_"))[1]
region_tag2 <- unlist(strsplit(region_tag, "_"))[2]
a <- ctwas_res[ctwas_res$region_tag==region_tag,]
regionlist <- readRDS(paste0(results_dir, "/", analysis_id, "_ctwas.regionlist.RDS"))
region <- regionlist[[as.numeric(region_tag1)]][[region_tag2]]
R_snp_info <- do.call(rbind, lapply(region$regRDS, function(x){data.table::fread(paste0(tools::file_path_sans_ext(x), ".Rvar"))}))
if (isTRUE(rerun_ctwas)){
ld_exprfs <- paste0(results_dir, "/", analysis_id, "_expr_chr", 1:22, ".expr.gz")
temp_reg <- data.frame("chr" = paste0("chr",region_tag1), "start" = region$start, "stop" = region$stop)
write.table(temp_reg,
#file= paste0(results_dir, "/", analysis_id, "_ctwas.temp.reg.txt") ,
file= "temp_reg.txt",
row.names=F, col.names=T, sep="\t", quote = F)
load(paste0(results_dir, "/", analysis_id, "_expr_z_snp.Rd"))
z_gene_temp <- z_gene[z_gene$id %in% a$id[a$type=="gene"],]
z_snp_temp <- z_snp[z_snp$id %in% R_snp_info$id,]
ctwas_rss(z_gene_temp, z_snp_temp, ld_exprfs, ld_pgenfs = NULL,
ld_R_dir = dirname(region$regRDS)[1],
ld_regions_custom = "temp_reg.txt", thin = 1,
outputdir = ".", outname = "temp", ncore = 1, ncore.rerun = 1, prob_single = 0,
group_prior = estimated_group_prior, group_prior_var = estimated_group_prior_var,
estimate_group_prior = F, estimate_group_prior_var = F)
a <- data.table::fread("temp.susieIrss.txt", header = T)
rownames(z_snp_temp) <- z_snp_temp$id
z_snp_temp <- z_snp_temp[a$id[a$type=="SNP"],]
z_gene_temp <- z_gene_temp[a$id[a$type=="gene"],]
a$z <- NA
a$z[a$type=="SNP"] <- z_snp_temp$z
a$z[a$type=="gene"] <- z_gene_temp$z
}
a$ifcausal <- 0
focus <- a$id[a$type=="gene"][which.max(abs(a$z[a$type=="gene"]))]
a$ifcausal <- as.numeric(a$id==focus)
a$PVALUE <- (-log(2) - pnorm(abs(a$z), lower.tail=F, log.p=T))/log(10)
R_gene <- readRDS(region$R_g_file)
R_snp_gene <- readRDS(region$R_sg_file)
R_snp <- as.matrix(Matrix::bdiag(lapply(region$regRDS, readRDS)))
rownames(R_gene) <- region$gid
colnames(R_gene) <- region$gid
rownames(R_snp_gene) <- R_snp_info$id
colnames(R_snp_gene) <- region$gid
rownames(R_snp) <- R_snp_info$id
colnames(R_snp) <- R_snp_info$id
a$r2max <- NA
a$r2max[a$type=="gene"] <- R_gene[focus,a$id[a$type=="gene"]]
a$r2max[a$type=="SNP"] <- R_snp_gene[a$id[a$type=="SNP"],focus]
r2cut <- 0.4
colorsall <- c("#7fc97f", "#beaed4", "#fdc086")
layout(matrix(1:2, ncol = 1), widths = 1, heights = c(1.5,1.5), respect = FALSE)
par(mar = c(0, 4.1, 4.1, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 19, xlab=paste0("Chromosome ", region_tag1, " Position"),frame.plot=FALSE, col = "white", ylim= c(-0.1,1.1), ylab = "cTWAS PIP", xaxt = 'n')
grid()
points(a$pos[a$type=="SNP"], a$susie_pip[a$type == "SNP"], pch = 21, xlab="Genomic position", bg = colorsall[1])
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$susie_pip[a$type == "SNP" & a$r2max >r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$susie_pip[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$susie_pip[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$susie_pip[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$susie_pip[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
if (isTRUE(plot_eqtl)){
for (cgene in a[a$type=="gene" & a$ifcausal == 1, ]$id){
load(paste0(results_dir, "/",analysis_id, "_expr_chr", region_tag1, ".exprqc.Rd"))
eqtls <- rownames(wgtlist[[cgene]])
points(a[a$id %in% eqtls,]$pos, rep( -0.15, nrow(a[a$id %in% eqtls,])), pch = "|", col = "salmon", cex = 1.5)
}
}
#legend(min(a$pos), y= 1.1 ,c("Gene", "SNP"), pch = c(22,21), title="Shape Legend", bty ='n', cex=0.6, title.adj = 0)
#legend(min(a$pos), y= 0.7 ,c("Lead TWAS Gene", "R2 > 0.4", "R2 <= 0.4"), pch = 19, col = c("salmon", "purple", colorsall[1]), title="Color Legend", bty ='n', cex=0.6, title.adj = 0)
legend(max(a$pos)-0.2*(max(a$pos)-min(a$pos)), y= 1.1 ,c("Gene", "SNP"), pch = c(22,21), title="Shape Legend", bty ='n', cex=0.6, title.adj = 0)
legend(max(a$pos)-0.2*(max(a$pos)-min(a$pos)), y= 0.7 ,c("Lead TWAS Gene", "R2 > 0.4", "R2 <= 0.4"), pch = 19, col = c("salmon", "purple", colorsall[1]), title="Color Legend", bty ='n', cex=0.6, title.adj = 0)
if (label=="cTWAS"){
text(a$pos[a$id==focus], a$susie_pip[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
par(mar = c(4.1, 4.1, 0.5, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 21, xlab=paste0("Chromosome ", region_tag1, " Position"), frame.plot=FALSE, bg = colorsall[1], ylab = "TWAS -log10(p value)", panel.first = grid(), ylim =c(0, max(a$PVALUE)*1.2))
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$PVALUE[a$type == "SNP" & a$r2max > r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$PVALUE[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$PVALUE[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$PVALUE[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$PVALUE[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
abline(h=-log10(alpha/nrow(ctwas_gene_res)), col ="red", lty = 2)
if (label=="TWAS"){
text(a$pos[a$id==focus], a$PVALUE[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
}
#locus_plot4("10_48", label="cTWAS")locus_plot5 <- function(region_tag, rerun_ctwas = F, plot_eqtl = T, label="cTWAS", focus){
region_tag1 <- unlist(strsplit(region_tag, "_"))[1]
region_tag2 <- unlist(strsplit(region_tag, "_"))[2]
a <- ctwas_res[ctwas_res$region_tag==region_tag,]
regionlist <- readRDS(paste0(results_dir, "/", analysis_id, "_ctwas.regionlist.RDS"))
region <- regionlist[[as.numeric(region_tag1)]][[region_tag2]]
R_snp_info <- do.call(rbind, lapply(region$regRDS, function(x){data.table::fread(paste0(tools::file_path_sans_ext(x), ".Rvar"))}))
if (isTRUE(rerun_ctwas)){
ld_exprfs <- paste0(results_dir, "/", analysis_id, "_expr_chr", 1:22, ".expr.gz")
temp_reg <- data.frame("chr" = paste0("chr",region_tag1), "start" = region$start, "stop" = region$stop)
write.table(temp_reg,
#file= paste0(results_dir, "/", analysis_id, "_ctwas.temp.reg.txt") ,
file= "temp_reg.txt",
row.names=F, col.names=T, sep="\t", quote = F)
load(paste0(results_dir, "/", analysis_id, "_expr_z_snp.Rd"))
z_gene_temp <- z_gene[z_gene$id %in% a$id[a$type=="gene"],]
z_snp_temp <- z_snp[z_snp$id %in% R_snp_info$id,]
ctwas_rss(z_gene_temp, z_snp_temp, ld_exprfs, ld_pgenfs = NULL,
ld_R_dir = dirname(region$regRDS)[1],
ld_regions_custom = "temp_reg.txt", thin = 1,
outputdir = ".", outname = "temp", ncore = 1, ncore.rerun = 1, prob_single = 0,
group_prior = estimated_group_prior, group_prior_var = estimated_group_prior_var,
estimate_group_prior = F, estimate_group_prior_var = F)
a <- data.table::fread("temp.susieIrss.txt", header = T)
rownames(z_snp_temp) <- z_snp_temp$id
z_snp_temp <- z_snp_temp[a$id[a$type=="SNP"],]
z_gene_temp <- z_gene_temp[a$id[a$type=="gene"],]
a$z <- NA
a$z[a$type=="SNP"] <- z_snp_temp$z
a$z[a$type=="gene"] <- z_gene_temp$z
}
a$ifcausal <- 0
focus <- a$id[which(a$genename==focus)]
a$ifcausal <- as.numeric(a$id==focus)
a$PVALUE <- (-log(2) - pnorm(abs(a$z), lower.tail=F, log.p=T))/log(10)
R_gene <- readRDS(region$R_g_file)
R_snp_gene <- readRDS(region$R_sg_file)
R_snp <- as.matrix(Matrix::bdiag(lapply(region$regRDS, readRDS)))
rownames(R_gene) <- region$gid
colnames(R_gene) <- region$gid
rownames(R_snp_gene) <- R_snp_info$id
colnames(R_snp_gene) <- region$gid
rownames(R_snp) <- R_snp_info$id
colnames(R_snp) <- R_snp_info$id
a$r2max <- NA
a$r2max[a$type=="gene"] <- R_gene[focus,a$id[a$type=="gene"]]
a$r2max[a$type=="SNP"] <- R_snp_gene[a$id[a$type=="SNP"],focus]
r2cut <- 0.4
colorsall <- c("#7fc97f", "#beaed4", "#fdc086")
layout(matrix(1:2, ncol = 1), widths = 1, heights = c(1.5,1.5), respect = FALSE)
par(mar = c(0, 4.1, 4.1, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 19, xlab=paste0("Chromosome ", region_tag1, " Position"),frame.plot=FALSE, col = "white", ylim= c(-0.1,1.1), ylab = "cTWAS PIP", xaxt = 'n')
grid()
points(a$pos[a$type=="SNP"], a$susie_pip[a$type == "SNP"], pch = 21, xlab="Genomic position", bg = colorsall[1])
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$susie_pip[a$type == "SNP" & a$r2max >r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$susie_pip[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$susie_pip[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$susie_pip[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$susie_pip[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
if (isTRUE(plot_eqtl)){
for (cgene in a[a$type=="gene" & a$ifcausal == 1, ]$id){
load(paste0(results_dir, "/",analysis_id, "_expr_chr", region_tag1, ".exprqc.Rd"))
eqtls <- rownames(wgtlist[[cgene]])
points(a[a$id %in% eqtls,]$pos, rep( -0.15, nrow(a[a$id %in% eqtls,])), pch = "|", col = "salmon", cex = 1.5)
}
}
legend(max(a$pos)-0.2*(max(a$pos)-min(a$pos)), y= 1.1 ,c("Gene", "SNP"), pch = c(22,21), title="Shape Legend", bty ='n', cex=0.6, title.adj = 0)
legend(max(a$pos)-0.2*(max(a$pos)-min(a$pos)), y= 0.7 ,c("Focal Gene", "R2 > 0.4", "R2 <= 0.4"), pch = 19, col = c("salmon", "purple", colorsall[1]), title="Color Legend", bty ='n', cex=0.6, title.adj = 0)
if (label=="cTWAS"){
text(a$pos[a$id==focus], a$susie_pip[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
par(mar = c(4.1, 4.1, 0.5, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 21, xlab=paste0("Chromosome ", region_tag1, " Position"), frame.plot=FALSE, bg = colorsall[1], ylab = "TWAS -log10(p value)", panel.first = grid(), ylim =c(0, max(a$PVALUE)*1.2))
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$PVALUE[a$type == "SNP" & a$r2max > r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$PVALUE[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$PVALUE[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$PVALUE[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$PVALUE[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
abline(h=-log10(alpha/nrow(ctwas_gene_res)), col ="red", lty = 2)
if (label=="TWAS"){
text(a$pos[a$id==focus], a$PVALUE[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
}locus_plot3 <- function(region_tag, rerun_ctwas = F, plot_eqtl = T, label="cTWAS", focus){
region_tag1 <- unlist(strsplit(region_tag, "_"))[1]
region_tag2 <- unlist(strsplit(region_tag, "_"))[2]
a <- ctwas_res[ctwas_res$region_tag==region_tag,]
regionlist <- readRDS(paste0(results_dir, "/", analysis_id, "_ctwas.regionlist.RDS"))
region <- regionlist[[as.numeric(region_tag1)]][[region_tag2]]
R_snp_info <- do.call(rbind, lapply(region$regRDS, function(x){data.table::fread(paste0(tools::file_path_sans_ext(x), ".Rvar"))}))
if (isTRUE(rerun_ctwas)){
ld_exprfs <- paste0(results_dir, "/", analysis_id, "_expr_chr", 1:22, ".expr.gz")
temp_reg <- data.frame("chr" = paste0("chr",region_tag1), "start" = region$start, "stop" = region$stop)
write.table(temp_reg,
#file= paste0(results_dir, "/", analysis_id, "_ctwas.temp.reg.txt") ,
file= "temp_reg.txt",
row.names=F, col.names=T, sep="\t", quote = F)
load(paste0(results_dir, "/", analysis_id, "_expr_z_snp.Rd"))
z_gene_temp <- z_gene[z_gene$id %in% a$id[a$type=="gene"],]
z_snp_temp <- z_snp[z_snp$id %in% R_snp_info$id,]
ctwas_rss(z_gene_temp, z_snp_temp, ld_exprfs, ld_pgenfs = NULL,
ld_R_dir = dirname(region$regRDS)[1],
ld_regions_custom = "temp_reg.txt", thin = 1,
outputdir = ".", outname = "temp", ncore = 1, ncore.rerun = 1, prob_single = 0,
group_prior = estimated_group_prior, group_prior_var = estimated_group_prior_var,
estimate_group_prior = F, estimate_group_prior_var = F)
a <- data.table::fread("temp.susieIrss.txt", header = T)
rownames(z_snp_temp) <- z_snp_temp$id
z_snp_temp <- z_snp_temp[a$id[a$type=="SNP"],]
z_gene_temp <- z_gene_temp[a$id[a$type=="gene"],]
a$z <- NA
a$z[a$type=="SNP"] <- z_snp_temp$z
a$z[a$type=="gene"] <- z_gene_temp$z
}
a$ifcausal <- 0
focus <- a$id[which(a$genename==focus)]
a$ifcausal <- as.numeric(a$id==focus)
a$PVALUE <- (-log(2) - pnorm(abs(a$z), lower.tail=F, log.p=T))/log(10)
R_gene <- readRDS(region$R_g_file)
R_snp_gene <- readRDS(region$R_sg_file)
R_snp <- as.matrix(Matrix::bdiag(lapply(region$regRDS, readRDS)))
rownames(R_gene) <- region$gid
colnames(R_gene) <- region$gid
rownames(R_snp_gene) <- R_snp_info$id
colnames(R_snp_gene) <- region$gid
rownames(R_snp) <- R_snp_info$id
colnames(R_snp) <- R_snp_info$id
a$r2max <- NA
a$r2max[a$type=="gene"] <- R_gene[focus,a$id[a$type=="gene"]]
a$r2max[a$type=="SNP"] <- R_snp_gene[a$id[a$type=="SNP"],focus]
r2cut <- 0.4
colorsall <- c("#7fc97f", "#beaed4", "#fdc086")
layout(matrix(1:2, ncol = 1), widths = 1, heights = c(1.5,1.5), respect = FALSE)
par(mar = c(0, 4.1, 4.1, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 19, xlab=paste0("Chromosome ", region_tag1, " Position"),frame.plot=FALSE, col = "white", ylim= c(-0.1,1.1), ylab = "cTWAS PIP", xaxt = 'n')
grid()
points(a$pos[a$type=="SNP"], a$susie_pip[a$type == "SNP"], pch = 21, xlab="Genomic position", bg = colorsall[1])
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$susie_pip[a$type == "SNP" & a$r2max >r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$susie_pip[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$susie_pip[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$susie_pip[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$susie_pip[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
if (isTRUE(plot_eqtl)){
for (cgene in a[a$type=="gene" & a$ifcausal == 1, ]$id){
load(paste0(results_dir, "/",analysis_id, "_expr_chr", region_tag1, ".exprqc.Rd"))
eqtls <- rownames(wgtlist[[cgene]])
points(a[a$id %in% eqtls,]$pos, rep( -0.15, nrow(a[a$id %in% eqtls,])), pch = "|", col = "salmon", cex = 1.5)
}
}
legend(min(a$pos), y= 1.1 ,c("Gene", "SNP"), pch = c(22,21), title="Shape Legend", bty ='n', cex=0.6, title.adj = 0)
legend(min(a$pos), y= 0.7 ,c("Lead TWAS Gene", "R2 > 0.4", "R2 <= 0.4"), pch = 19, col = c("salmon", "purple", colorsall[1]), title="Color Legend", bty ='n', cex=0.6, title.adj = 0)
if (label=="cTWAS"){
text(a$pos[a$id==focus], a$susie_pip[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
par(mar = c(4.1, 4.1, 0.5, 2.1))
plot(a$pos[a$type=="SNP"], a$PVALUE[a$type == "SNP"], pch = 21, xlab=paste0("Chromosome ", region_tag1, " Position"), frame.plot=FALSE, bg = colorsall[1], ylab = "TWAS -log10(p value)", panel.first = grid(), ylim =c(0, max(a$PVALUE)*1.2))
points(a$pos[a$type=="SNP" & a$r2max > r2cut], a$PVALUE[a$type == "SNP" & a$r2max > r2cut], pch = 21, bg = "purple")
points(a$pos[a$type=="SNP" & a$ifcausal == 1], a$PVALUE[a$type == "SNP" & a$ifcausal == 1], pch = 21, bg = "salmon")
points(a$pos[a$type=="gene"], a$PVALUE[a$type == "gene"], pch = 22, bg = colorsall[1], cex = 2)
points(a$pos[a$type=="gene" & a$r2max > r2cut], a$PVALUE[a$type == "gene" & a$r2max > r2cut], pch = 22, bg = "purple", cex = 2)
points(a$pos[a$type=="gene" & a$ifcausal == 1], a$PVALUE[a$type == "gene" & a$ifcausal == 1], pch = 22, bg = "salmon", cex = 2)
abline(h=-log10(alpha/nrow(ctwas_gene_res)), col ="red", lty = 2)
if (label=="TWAS"){
text(a$pos[a$id==focus], a$PVALUE[a$id==focus], labels=ctwas_gene_res$genename[ctwas_gene_res$id==focus], pos=3, cex=0.6)
}
}load(paste0(results_dir, "/known_annotations.Rd"))
load(paste0(results_dir, "/bystanders.Rd"))
for (i in 1:length(known_annotations)){
focus <- known_annotations[i]
region_tag <- ctwas_res$region_tag[which(ctwas_res$genename==focus)]
locus_plot3(region_tag, focus=focus)
mtext(text=region_tag)
print(focus)
print(region_tag)
print(ctwas_gene_res[ctwas_gene_res$region_tag==region_tag,report_cols,])
}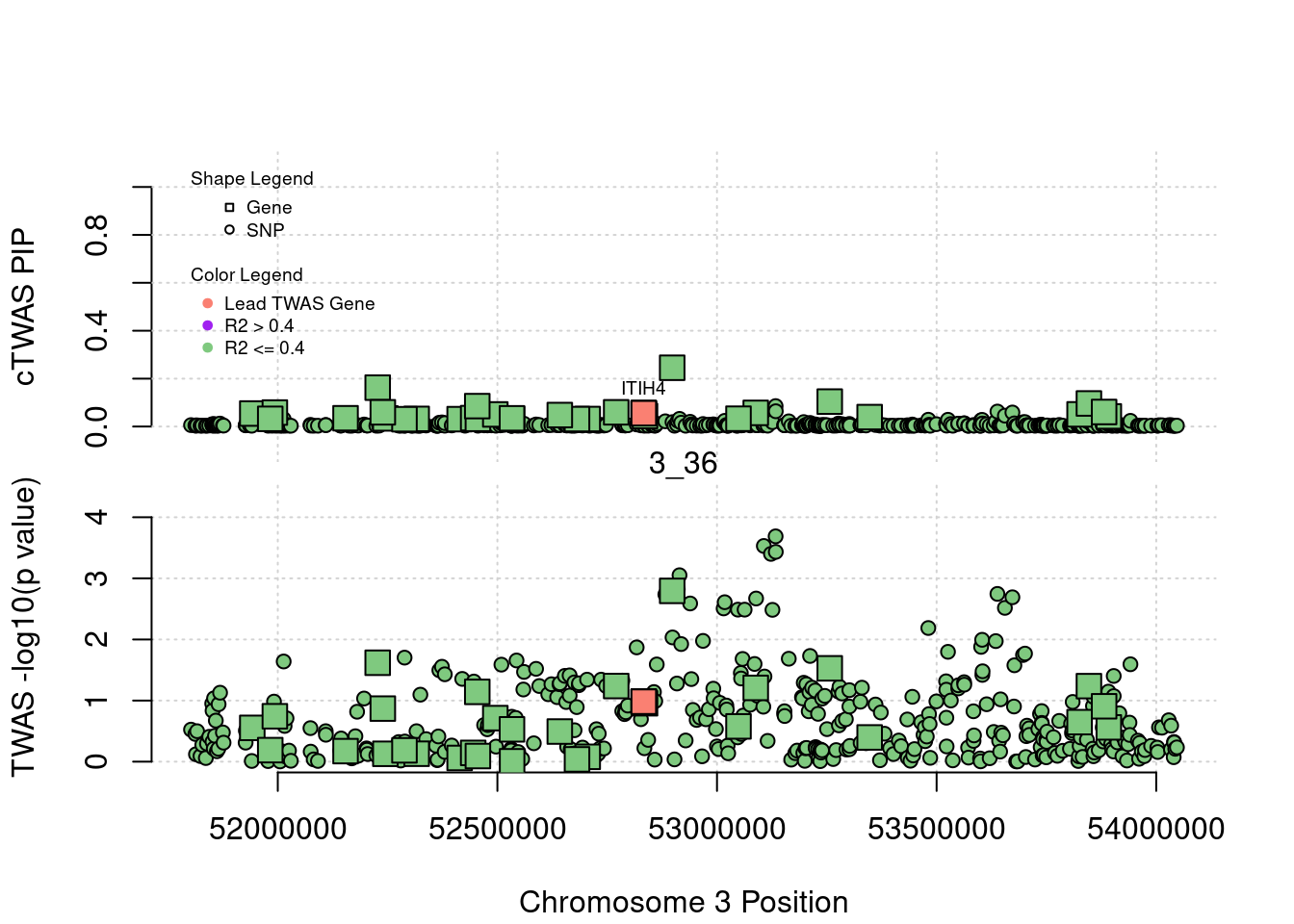
[1] "ITIH4"
[1] "3_36"
genename region_tag susie_pip mu2 PVE z
181 PHF7 3_36 0.03088976 4.615967 1.383208e-07 0.16374269
182 SEMA3G 3_36 0.03502755 5.767093 1.959644e-07 -0.36057692
183 NISCH 3_36 0.03205134 4.953846 1.540278e-07 0.24038462
184 STAB1 3_36 0.05050992 9.131302 4.474245e-07 -1.29936306
255 CHDH 3_36 0.05077506 9.180983 4.522203e-07 -1.21195804
257 GLT8D1 3_36 0.03128338 4.731850 1.436002e-07 0.21088172
521 ITIH4 3_36 0.05569229 10.035020 5.421554e-07 1.62424242
527 IL17RB 3_36 0.09631607 15.141119 1.414709e-06 1.89507924
2950 SELENOK 3_36 0.04225325 7.488869 3.069635e-07 -1.09160305
2951 ACTR8 3_36 0.06071920 10.834779 6.381996e-07 1.53764919
3014 RRP9 3_36 0.05429349 9.799640 5.161409e-07 -1.08015267
3022 DNAH1 3_36 0.03189817 4.909838 1.519299e-07 -0.32954545
3025 TNNC1 3_36 0.08528015 13.996033 1.157879e-06 -1.79878049
3029 NEK4 3_36 0.05944421 10.638362 6.134720e-07 1.89705882
7266 RPL29 3_36 0.05844021 10.480473 5.941596e-07 1.33884298
7573 TKT 3_36 0.10408770 15.873090 1.602770e-06 2.17391304
7574 RFT1 3_36 0.05699405 10.248647 5.666391e-07 1.86567164
7575 SFMBT1 3_36 0.03277893 5.159485 1.640633e-07 -1.11538462
7576 GNL3 3_36 0.03094543 4.632390 1.390631e-07 0.10404624
7577 PBRM1 3_36 0.04784020 8.631905 4.005992e-07 0.99111111
7616 POC1A 3_36 0.03468540 5.677120 1.910228e-07 -0.42222222
7617 PPM1M 3_36 0.03387693 5.461144 1.794726e-07 -0.32684355
7619 WDR82 3_36 0.03056377 4.518898 1.339831e-07 -0.45070423
8289 NT5DC2 3_36 0.03143065 4.774743 1.455840e-07 0.01714286
8290 SMIM4 3_36 0.03430308 5.575767 1.855445e-07 1.04477612
11371 TMEM110 3_36 0.24537681 24.212753 5.763524e-06 -3.15524405
11922 TLR9 3_36 0.16240825 20.125488 3.170771e-06 -2.25595238
12027 ACY1 3_36 0.03204571 4.952252 1.539512e-07 0.45679012
12087 TWF2 3_36 0.05954270 10.653671 6.153727e-07 -1.49316098
12840 MUSTN1 3_36 0.04396227 7.853739 3.349400e-07 -1.59493671
12877 DCP1A 3_36 0.03936580 6.838519 2.611509e-07 0.83905021
num_eqtl
181 1
182 1
183 1
184 1
255 4
257 2
521 1
527 2
2950 1
2951 2
3014 1
3022 1
3025 1
3029 1
7266 1
7573 1
7574 1
7575 1
7576 1
7577 1
7616 1
7617 3
7619 1
8289 1
8290 1
11371 2
11922 1
12027 1
12087 2
12840 1
12877 2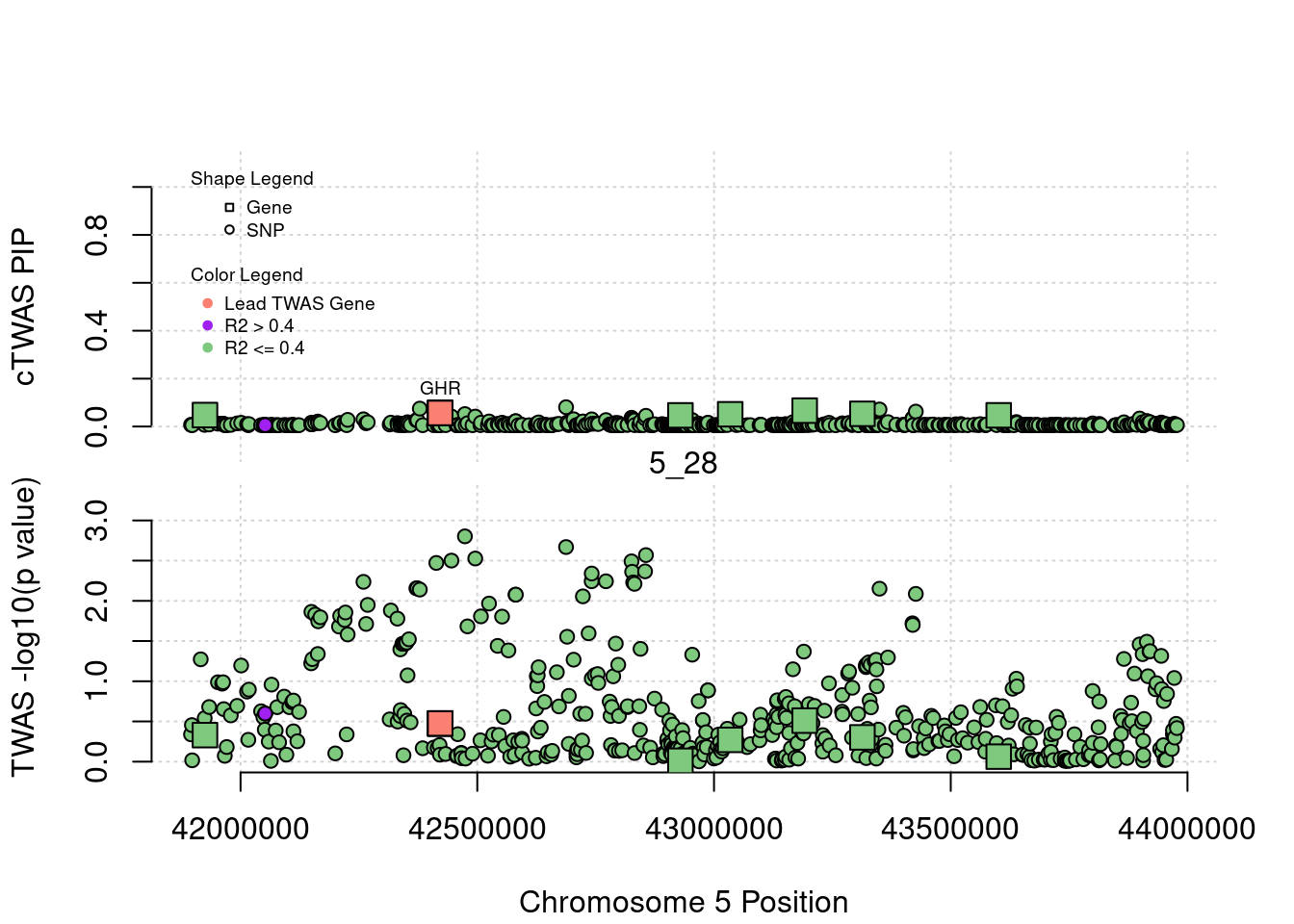
[1] "GHR"
[1] "5_28"
genename region_tag susie_pip mu2 PVE z
2871 GHR 5_28 0.05662180 6.295470 3.457979e-07 0.96376812
2872 HMGCS1 5_28 0.05317255 5.714465 2.947633e-07 0.67639191
2875 NNT 5_28 0.04675584 4.528240 2.053883e-07 0.16804150
6447 FBXO4 5_28 0.04783224 4.737969 2.198484e-07 -0.72633910
9328 NIM1K 5_28 0.06647410 7.782454 5.018564e-07 1.01775148
12160 SELENOP 5_28 0.04670773 4.518757 2.047473e-07 -0.02985075
12817 CTD-2035E11.4 5_28 0.05127003 5.378057 2.674850e-07 0.61951220
num_eqtl
2871 1
2872 2
2875 2
6447 2
9328 1
12160 1
12817 1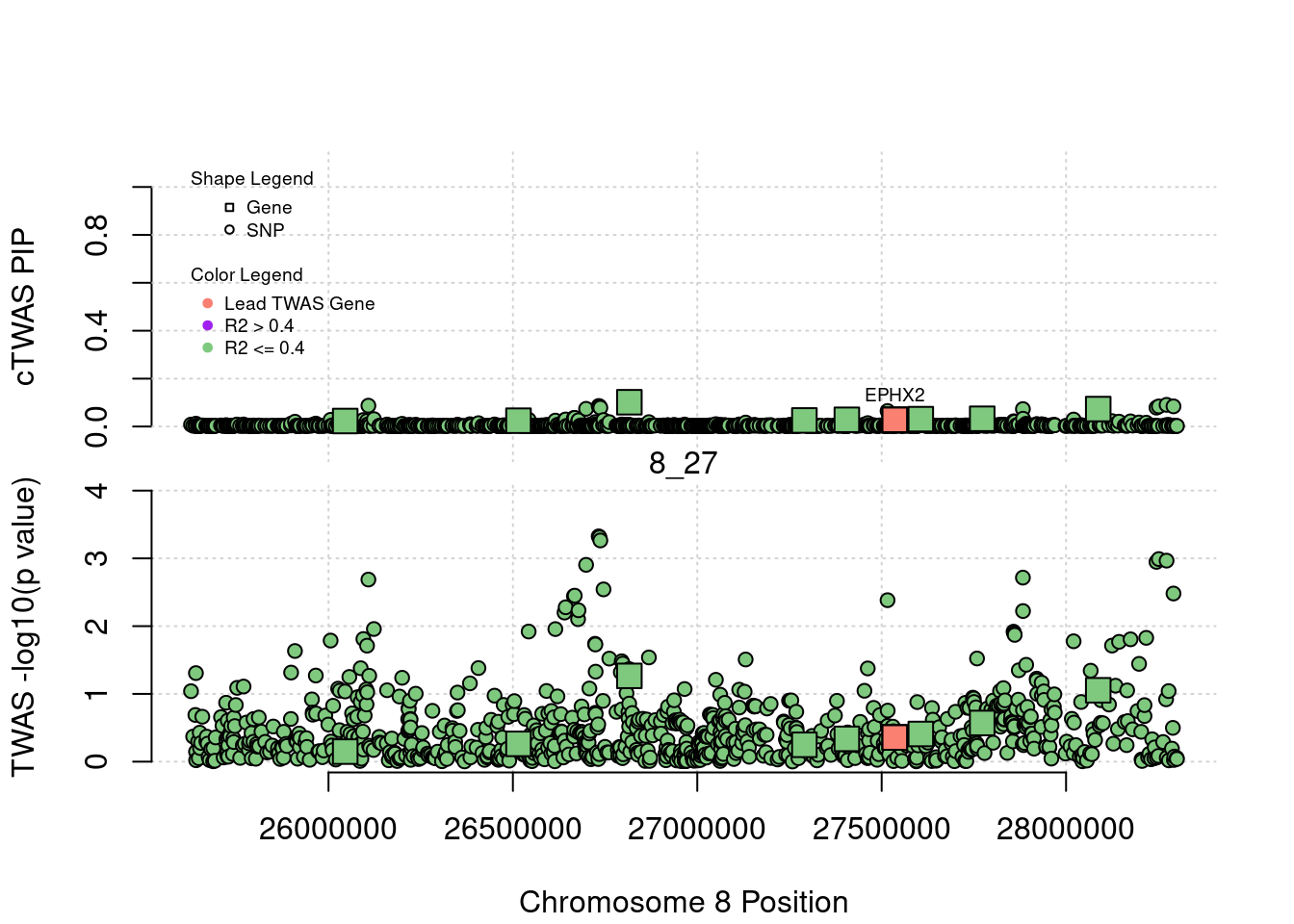
[1] "EPHX2"
[1] "8_27"
genename region_tag susie_pip mu2 PVE z num_eqtl
3581 ADRA1A 8_27 0.10143196 18.692023 1.839253e-06 -1.9251055 3
11520 EBF2 8_27 0.02311643 5.031026 1.128204e-07 0.3706897 1
11937 PNMA2 8_27 0.02435414 5.506897 1.301038e-07 0.6029412 1
1986 TRIM35 8_27 0.02533988 5.869059 1.442725e-07 -0.5765401 2
3578 CLU 8_27 0.03081260 7.656246 2.288519e-07 -0.8593986 2
3580 PTK2B 8_27 0.02814020 6.826559 1.863543e-07 0.7368421 1
3584 EPHX2 8_27 0.02795738 6.766985 1.835279e-07 0.7717391 1
4662 ELP3 8_27 0.07296783 15.605213 1.104617e-06 -1.7035831 1
6136 CCDC25 8_27 0.03246217 8.133609 2.561364e-07 -1.1060606 1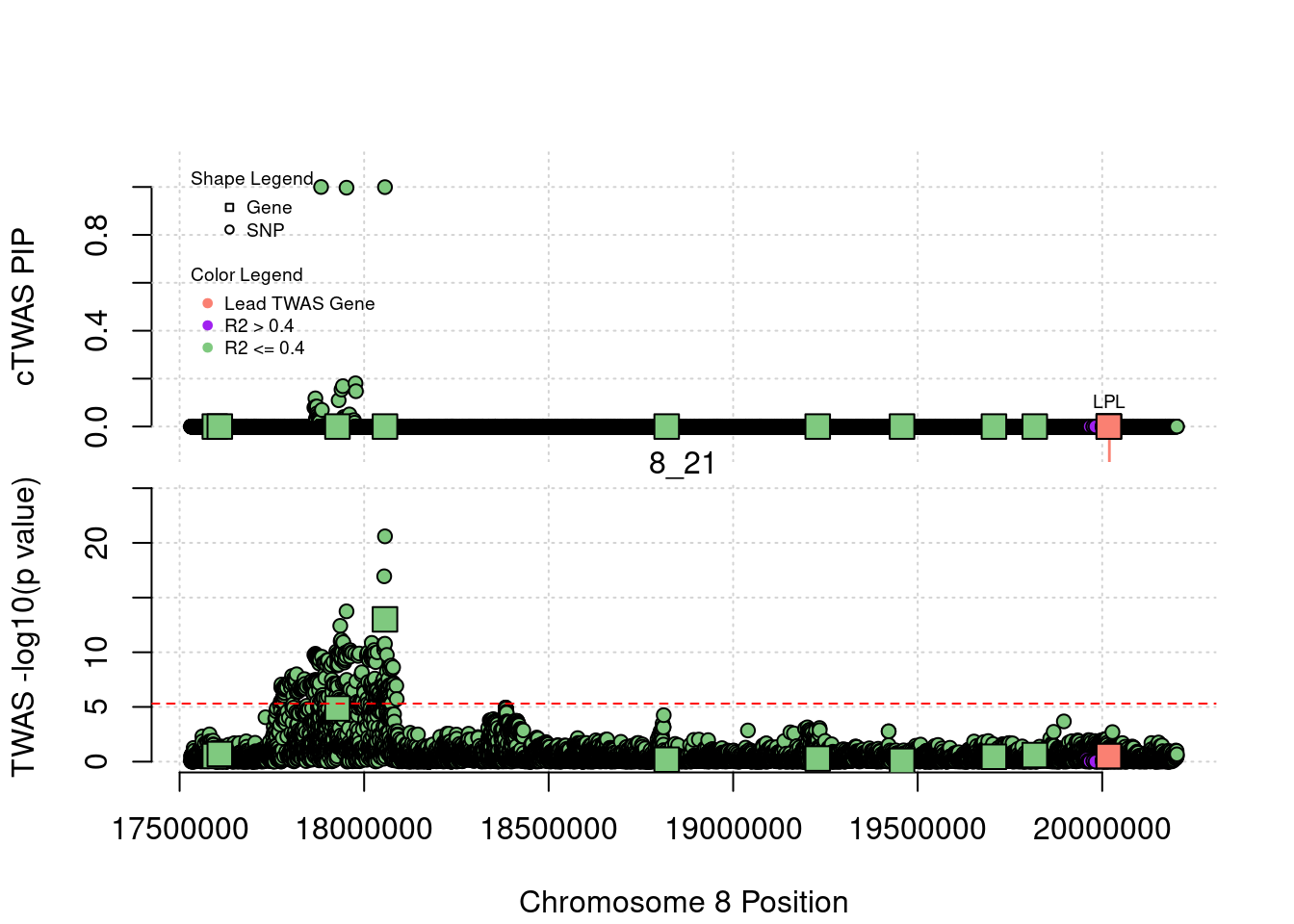
[1] "LPL"
[1] "8_21"
genename region_tag susie_pip mu2 PVE z
995 PCM1 8_21 1.585694e-05 91.963533 1.414639e-09 -4.3484471
1983 PDGFRL 8_21 8.970799e-10 7.706520 6.706560e-15 1.0256850
4228 MTUS1 8_21 2.167432e-09 7.886506 1.658213e-14 1.2790698
2022 SH2D4A 8_21 9.619676e-10 5.897348 5.503356e-15 0.6404494
2039 ASAH1 8_21 2.248119e-05 67.205978 1.465675e-09 -7.4434198
6134 CSGALNACT1 8_21 8.474523e-10 4.684407 3.851061e-15 -0.2163023
6753 PSD3 8_21 9.086929e-10 5.309504 4.680385e-15 -0.4411765
2023 INTS10 8_21 1.510797e-09 9.902528 1.451318e-14 -1.1484375
9143 LPL 8_21 1.325698e-09 8.721530 1.121625e-14 1.0147059
12196 RP11-1105O14.1 8_21 1.222229e-09 7.987228 9.470196e-15 0.9218264
num_eqtl
995 2
1983 2
4228 1
2022 1
2039 2
6134 3
6753 1
2023 1
9143 1
12196 2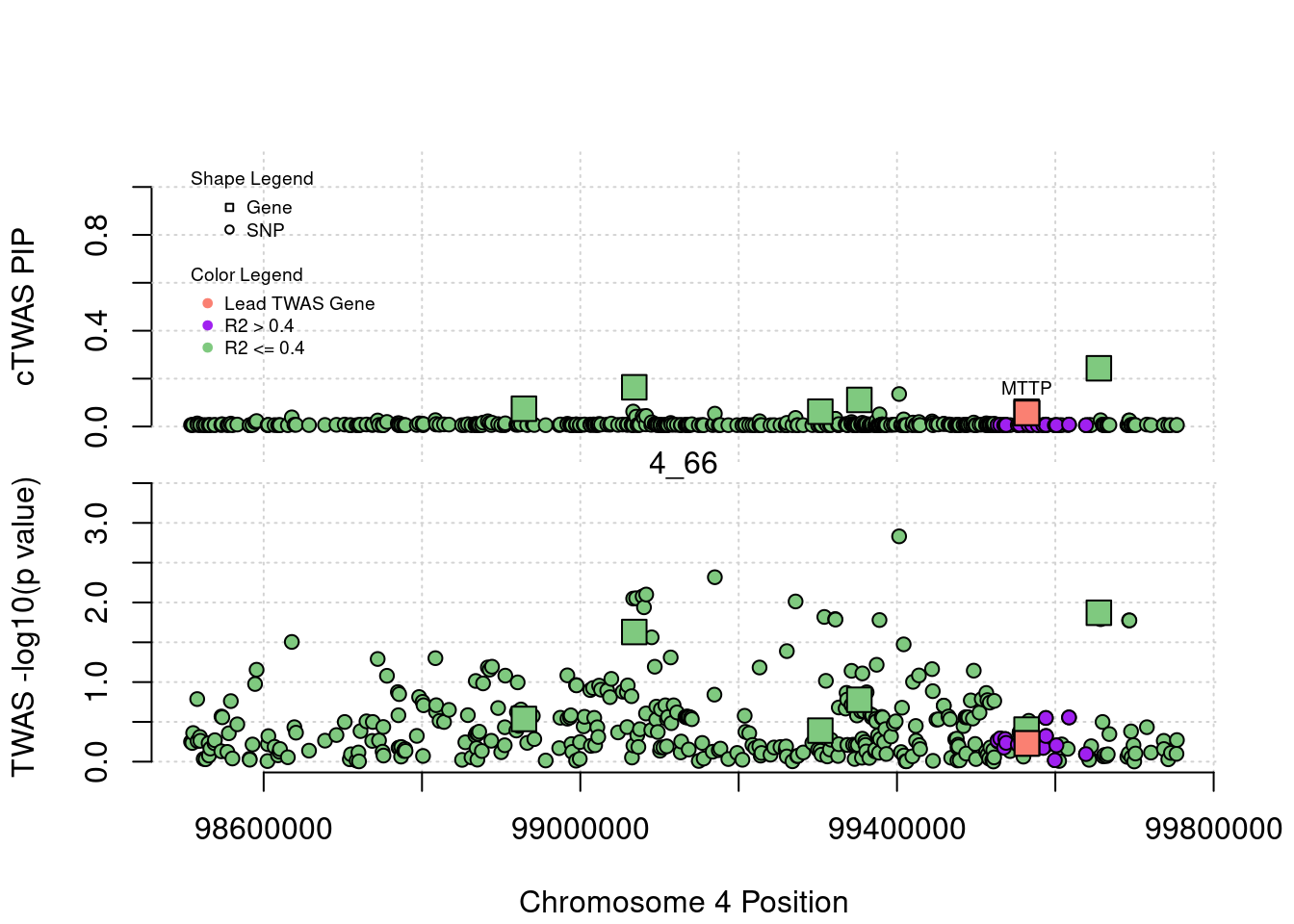
[1] "MTTP"
[1] "4_66"
genename region_tag susie_pip mu2 PVE z
5323 MTTP 4_66 0.05713053 5.733633 3.177669e-07 0.5394737
5984 TRMT10A 4_66 0.06199325 6.490226 3.903145e-07 0.8490721
6388 EIF4E 4_66 0.07451430 8.200439 5.927712e-07 -1.0566038
10617 ADH1B 4_66 0.11117753 11.959347 1.289837e-06 -1.3819444
12092 ADH1C 4_66 0.06215161 6.513876 3.927374e-07 0.8287844
12107 RP11-766F14.2 4_66 0.24311774 19.587066 4.619516e-06 -2.4715447
12861 RP11-571L19.8 4_66 0.16383078 15.676799 2.491514e-06 -2.2652120
num_eqtl
5323 1
5984 2
6388 1
10617 1
12092 3
12107 1
12861 2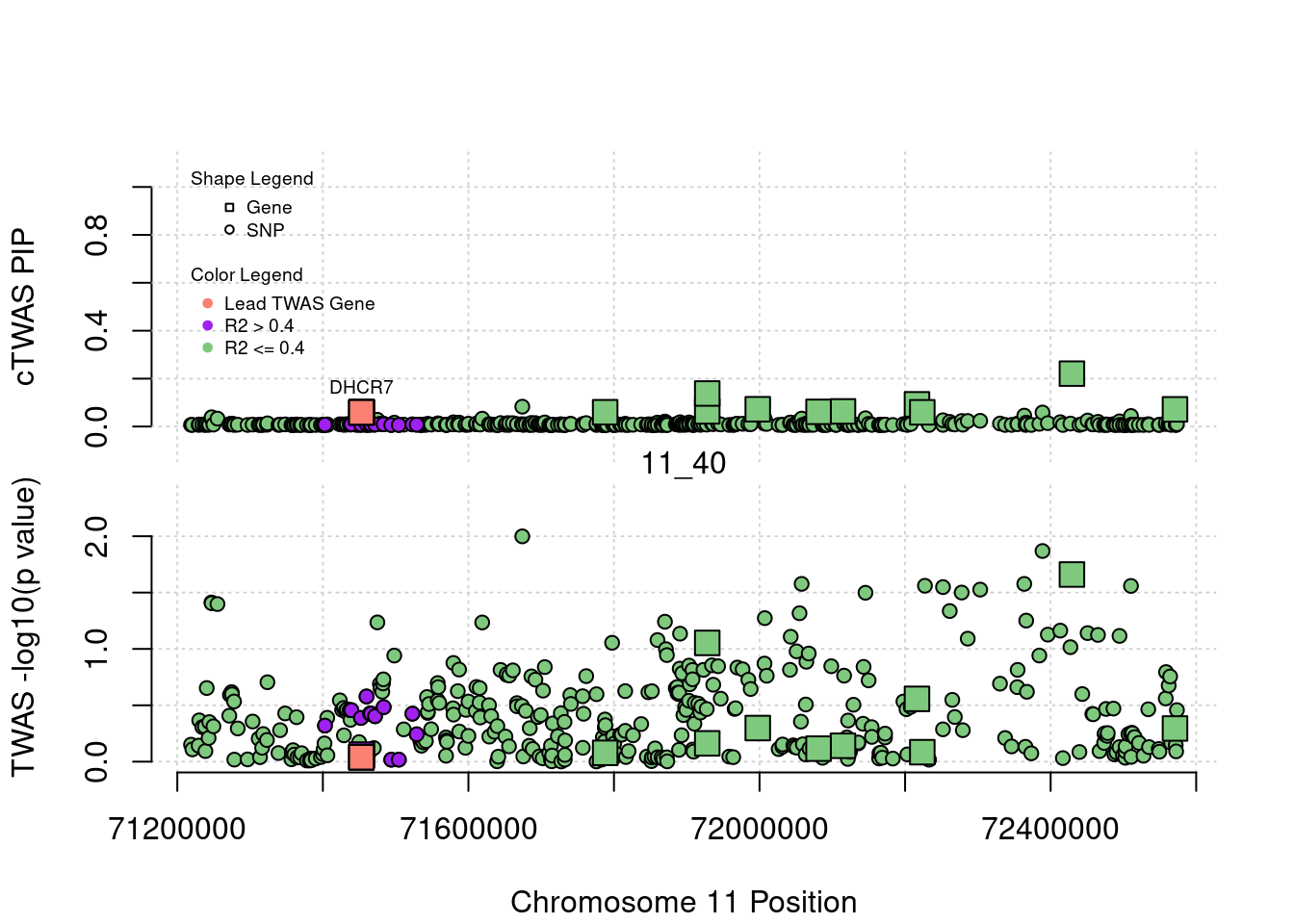
[1] "DHCR7"
[1] "11_40"
genename region_tag susie_pip mu2 PVE z
2602 FOLR3 11_40 0.06321706 5.176238 3.174381e-07 -0.3541236
5123 IL18BP 11_40 0.07221106 6.412588 4.492080e-07 -0.6694005
5124 NUMA1 11_40 0.06148379 4.918434 2.933580e-07 0.2965517
5130 RNF121 11_40 0.06333601 5.193677 3.191068e-07 0.4020962
6938 FAM86C1 11_40 0.05986088 4.670549 2.712199e-07 0.2075472
7254 CLPB 11_40 0.22082235 17.123207 3.668078e-06 2.2927426
7828 FOLR2 11_40 0.09264058 8.742876 7.857168e-07 1.0861244
7829 INPPL1 11_40 0.05951221 4.616439 2.665162e-07 0.2215389
8875 NADSYN1 11_40 0.05973022 4.650307 2.694549e-07 -0.1643836
8876 DHCR7 11_40 0.05928308 4.580713 2.634355e-07 -0.1081081
11662 LINC01537 11_40 0.07183761 6.364302 4.435199e-07 0.6622075
12242 RP11-849H4.2 11_40 0.13798992 12.524095 1.676502e-06 1.7038627
num_eqtl
2602 2
5123 2
5124 1
5130 2
6938 1
7254 2
7828 1
7829 2
8875 1
8876 1
11662 2
12242 1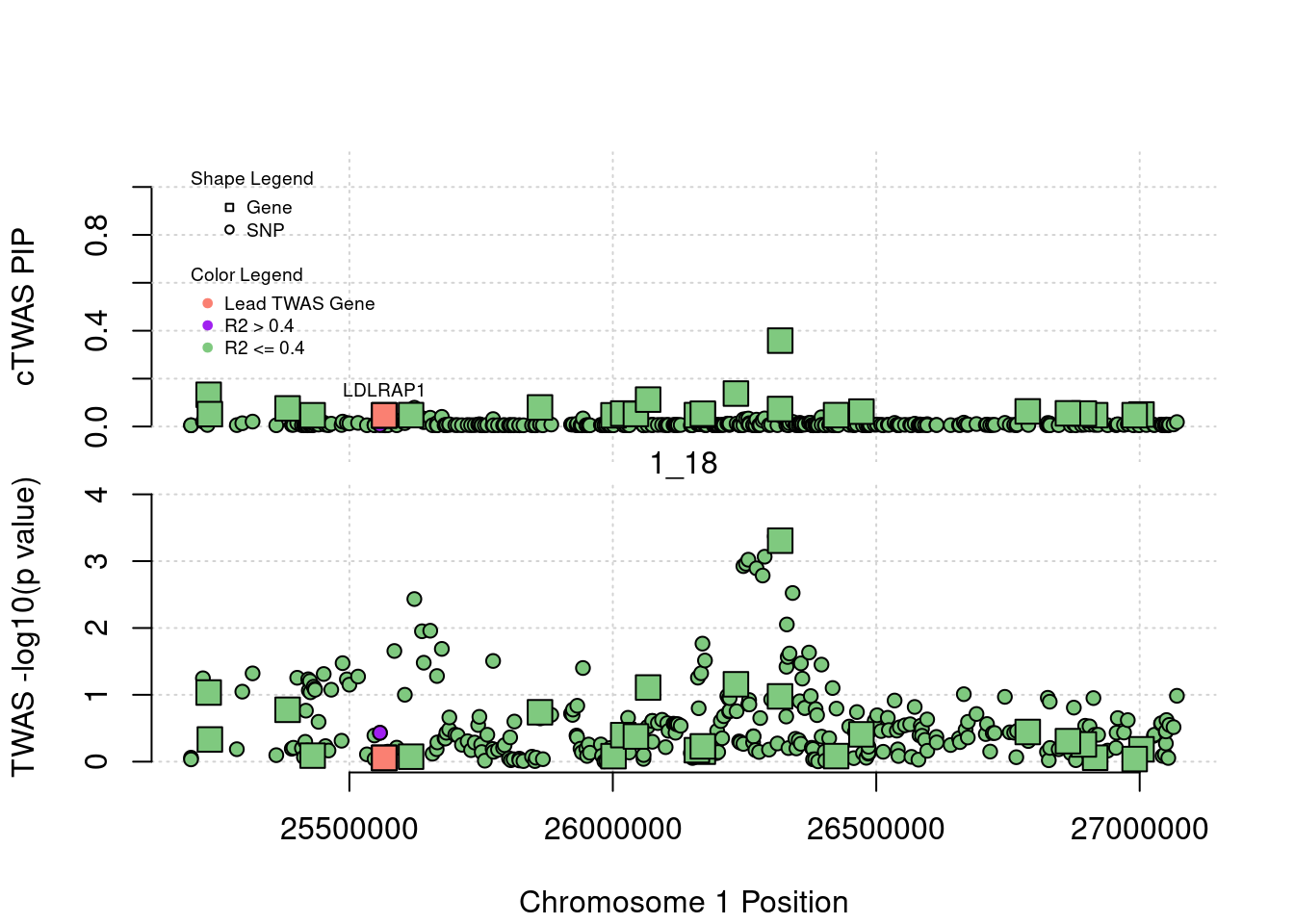
[1] "LDLRAP1"
[1] "1_18"
genename region_tag susie_pip mu2 PVE z
563 PIGV 1_18 0.06331853 7.366137 4.524609e-07 0.9159664
3323 SYF2 1_18 0.13264392 14.320449 1.842699e-06 -1.6825397
3326 MAN1C1 1_18 0.04774416 4.756187 2.202874e-07 0.1987179
3327 DHDDS 1_18 0.04757833 4.724125 2.180424e-07 0.2191845
4077 AUNIP 1_18 0.07980968 9.521010 7.371384e-07 -1.3333333
4336 CEP85 1_18 0.13764065 14.674907 1.959442e-06 -1.8148148
4456 NR0B2 1_18 0.04747773 4.704592 2.166818e-07 -0.1594203
5695 CNKSR1 1_18 0.04805156 4.815364 2.244642e-07 -0.3717949
5703 GPN2 1_18 0.05429167 5.942143 3.129585e-07 -0.5934066
6895 LDLRAP1 1_18 0.04652726 4.518310 2.039360e-07 -0.1515152
6898 PAFAH2 1_18 0.04675828 4.563940 2.070184e-07 0.2173913
6899 EXTL1 1_18 0.05432911 5.948515 3.135102e-07 -0.8405797
6900 SLC30A2 1_18 0.05252772 5.637007 2.872417e-07 0.8057856
6902 TRIM63 1_18 0.11242300 12.744786 1.389947e-06 -1.7641509
6906 UBXN11 1_18 0.35871762 24.332422 8.467369e-06 -3.4841270
6923 FAM46B 1_18 0.05027576 5.232552 2.552012e-07 0.4492187
8452 CD52 1_18 0.07315646 8.708947 6.180573e-07 1.6184211
9183 SFN 1_18 0.05511372 6.081000 3.251211e-07 0.6842105
10436 RHCE 1_18 0.07584166 9.044827 6.654548e-07 -1.3799902
10716 FAM110D 1_18 0.04767572 4.743000 2.193617e-07 -0.4202899
11006 HMGN2 1_18 0.06250445 7.246163 4.393691e-07 0.8588235
11107 TMEM57 1_18 0.04707952 4.627003 2.113208e-07 -0.2371968
11872 RP11-96L14.7 1_18 0.05360600 5.824729 3.029002e-07 -0.5374556
12200 TRNP1 1_18 0.04745212 4.699640 2.163370e-07 0.1078431
12822 RP3-465N24.6 1_18 0.05180862 5.509723 2.769123e-07 -0.7297297
num_eqtl
563 1
3323 1
3326 1
3327 2
4077 1
4336 1
4456 1
5695 1
5703 1
6895 1
6898 1
6899 1
6900 2
6902 1
6906 1
6923 1
8452 1
9183 1
10436 3
10716 1
11006 1
11107 1
11872 2
12200 1
12822 1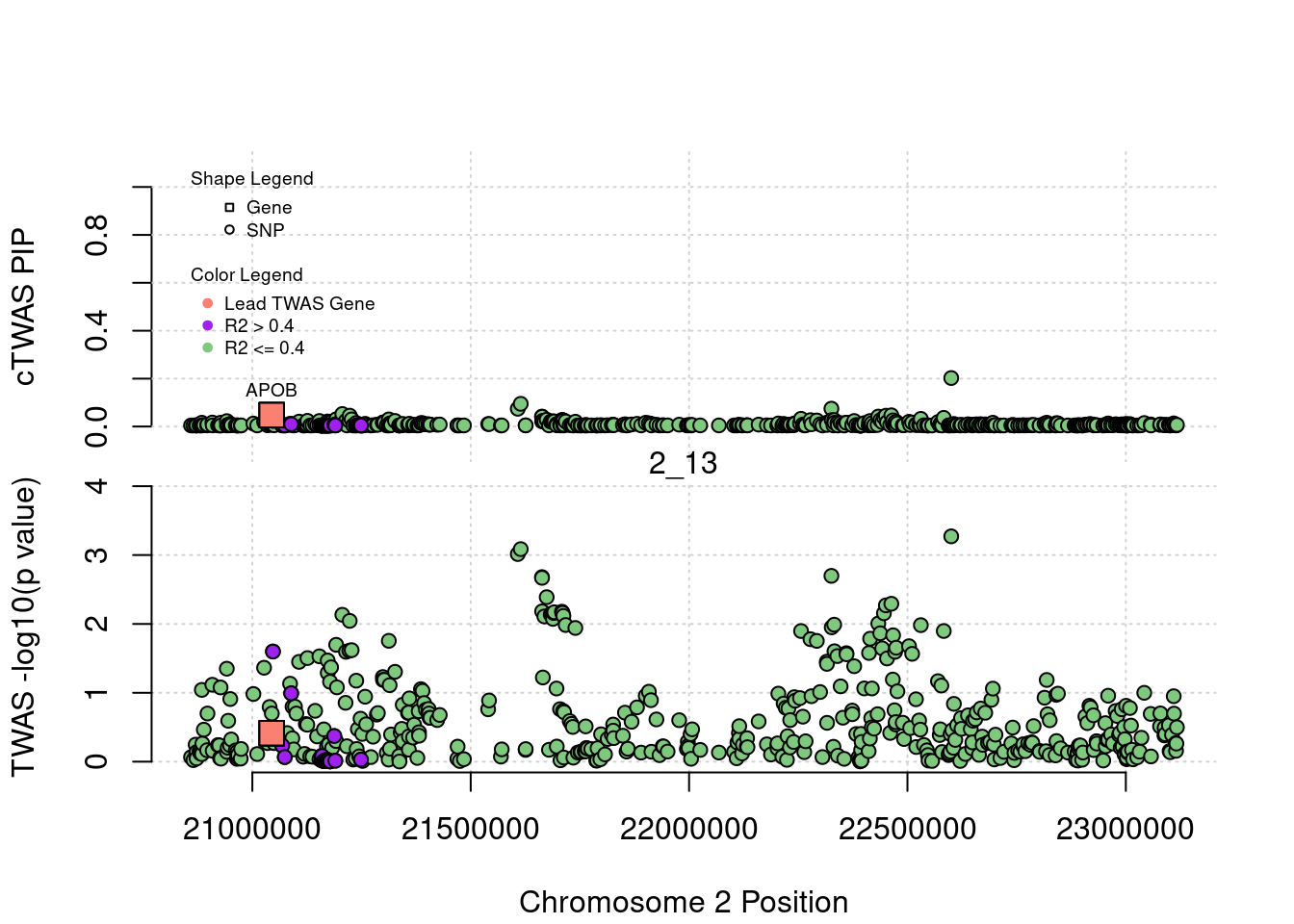
[1] "APOB"
[1] "2_13"
genename region_tag susie_pip mu2 PVE z num_eqtl
1135 APOB 2_13 0.04781908 6.177873 2.865831e-07 0.8658364 2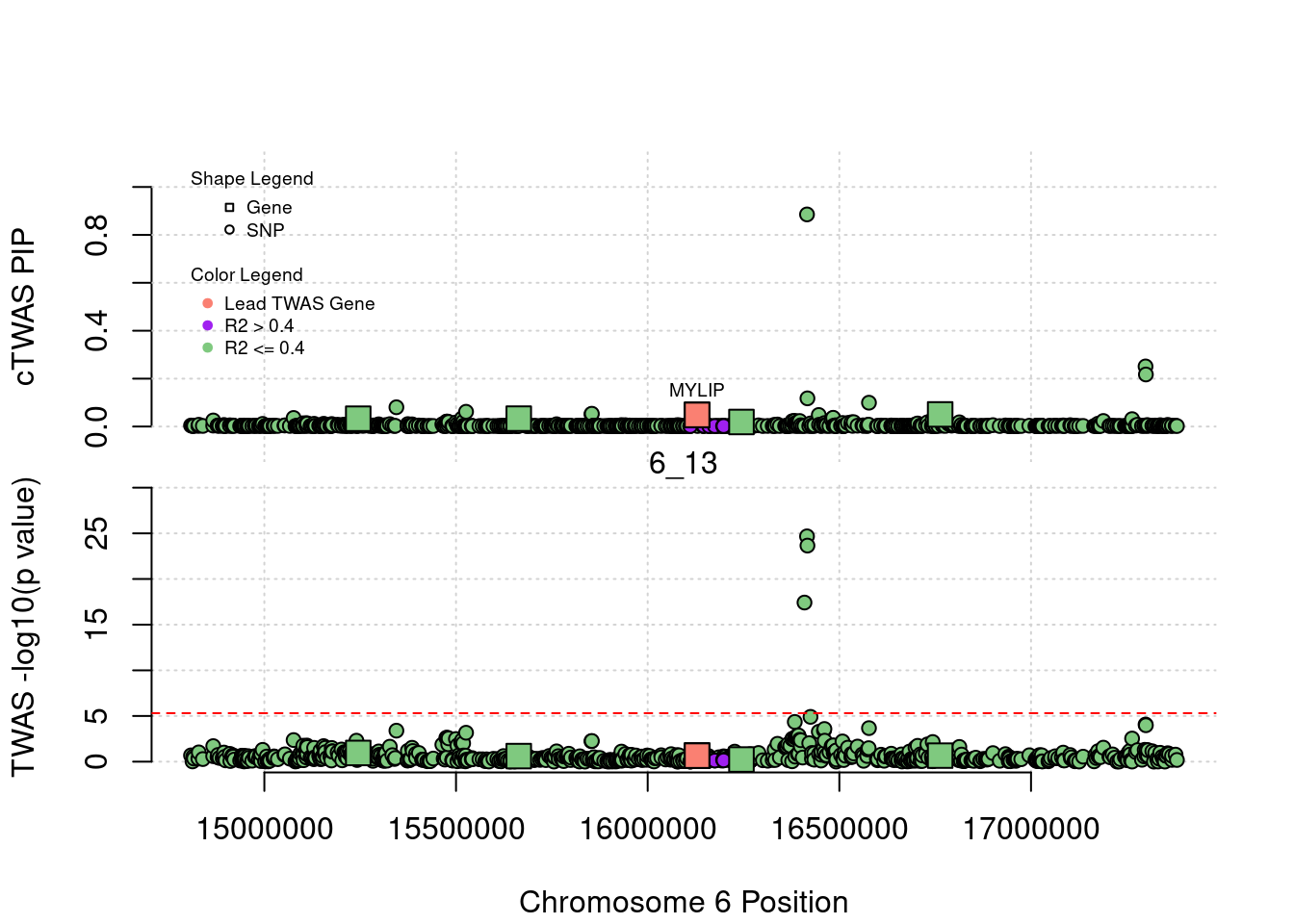
[1] "MYLIP"
[1] "6_13"
genename region_tag susie_pip mu2 PVE z
135 MYLIP 6_13 0.04846714 12.545013 5.898329e-07 1.2238806
427 DTNBP1 6_13 0.03284069 9.353887 2.979990e-07 1.1609007
5080 GMPR 6_13 0.01895961 4.784990 8.800775e-08 0.5505618
12767 RP11-560J1.2 6_13 0.03259631 9.698987 3.066939e-07 1.5871560
12809 RP1-151F17.2 6_13 0.05068457 12.830591 6.308598e-07 1.2287287
num_eqtl
135 1
427 2
5080 1
12767 1
12809 2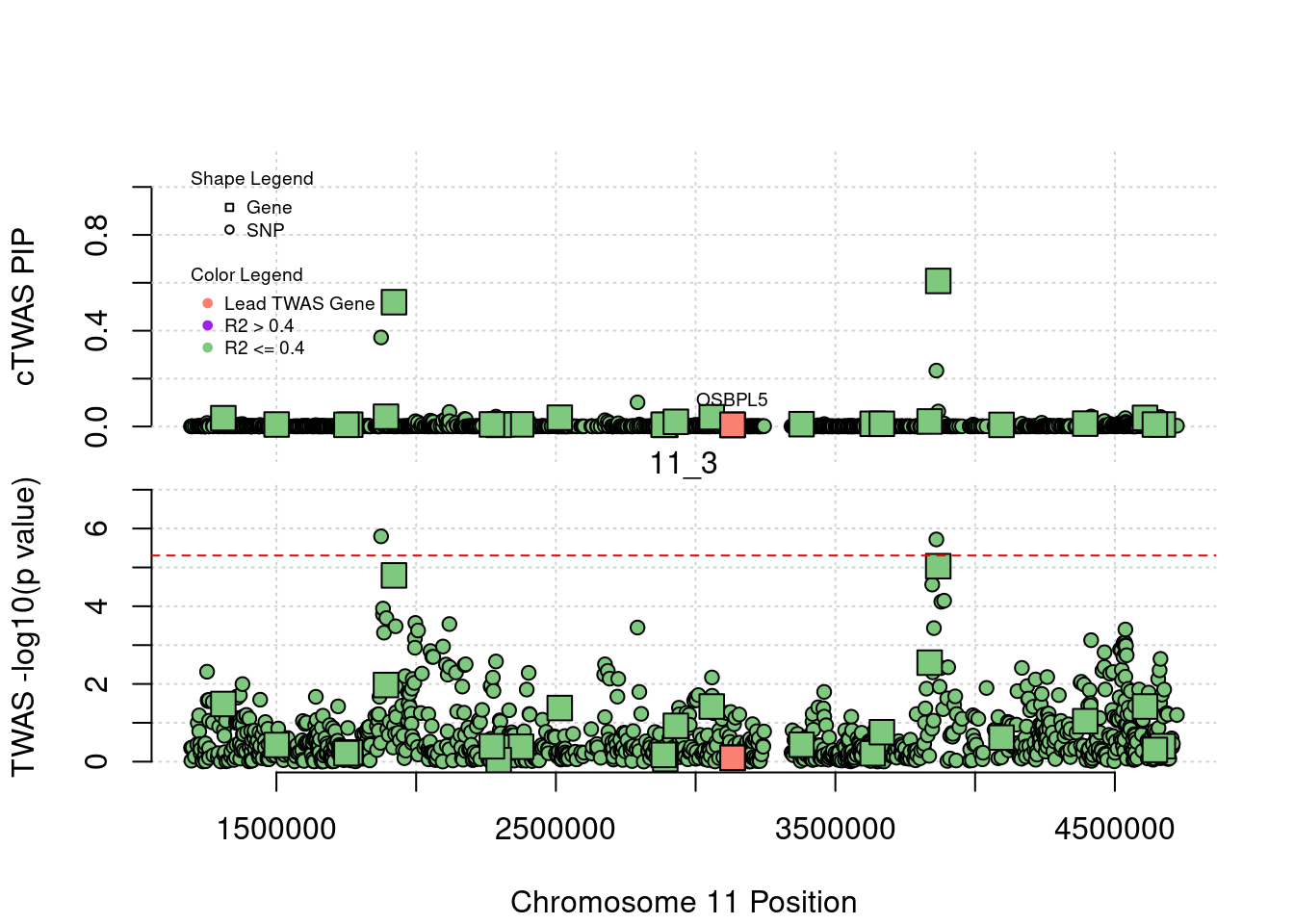
[1] "OSBPL5"
[1] "11_3"
genename region_tag susie_pip mu2 PVE z
77 ZNF195 11_3 0.009895187 7.863944 7.548747e-08 -0.9041096
281 OSBPL5 11_3 0.006968720 4.720008 3.190848e-08 0.2551020
492 KCNQ1 11_3 0.037004455 19.905379 7.145537e-07 -2.0366972
603 TSPAN32 11_3 0.009218940 7.258928 6.491781e-08 0.7857143
1003 TOLLIP 11_3 0.034819757 19.288799 6.515404e-07 2.1340206
2627 CARS 11_3 0.038923449 19.910910 7.518182e-07 2.0746269
2628 SLC22A18 11_3 0.006893503 4.621317 3.090410e-08 0.1736637
2629 CD81 11_3 0.009110689 7.021626 6.205822e-08 0.8191489
2631 C11orf21 11_3 0.006842856 4.556587 3.024736e-08 0.1133250
3341 CTSD 11_3 0.007786252 5.725131 4.324385e-08 0.5583333
4258 CDKN1C 11_3 0.007417506 5.266790 3.789783e-08 -0.4254144
4328 TNNT3 11_3 0.519052917 21.311832 1.073107e-05 4.3137700
8125 ART5 11_3 0.011541333 8.575870 9.601621e-08 0.5078098
9700 PHLDA2 11_3 0.019871210 14.016957 2.702019e-07 -1.5568182
9749 MOB2 11_3 0.008668060 6.798267 5.716505e-08 -0.8924187
9915 ASCL2 11_3 0.008825312 6.904973 5.911565e-08 0.8376502
12034 IFITM10 11_3 0.007203209 5.075247 3.546448e-08 -0.5141243
13194 PRR33 11_3 0.040955066 22.325154 8.869773e-07 2.5537634
4257 CHRNA10 11_3 0.011554834 9.661976 1.083029e-07 1.3576642
4472 TRIM21 11_3 0.012225836 10.363399 1.229111e-07 -1.6901408
8127 STIM1 11_3 0.607774446 24.072335 1.419290e-05 4.4328358
8128 RRM1 11_3 0.007008898 5.669799 3.855030e-08 -1.1740296
8129 OR51E2 11_3 0.008596400 6.686241 5.575824e-08 0.8419368
8130 TRIM68 11_3 0.036085737 19.084751 6.680862e-07 2.0740741
9301 RHOG 11_3 0.021091074 17.688036 3.619001e-07 2.9857143
9633 OR51E1 11_3 0.008342338 6.216940 5.031238e-08 -0.6761545
num_eqtl
77 1
281 1
492 1
603 1
1003 1
2627 1
2628 3
2629 1
2631 3
3341 1
4258 1
4328 2
8125 4
9700 1
9749 2
9915 2
12034 1
13194 1
4257 1
4472 1
8127 1
8128 2
8129 2
8130 1
9301 1
9633 5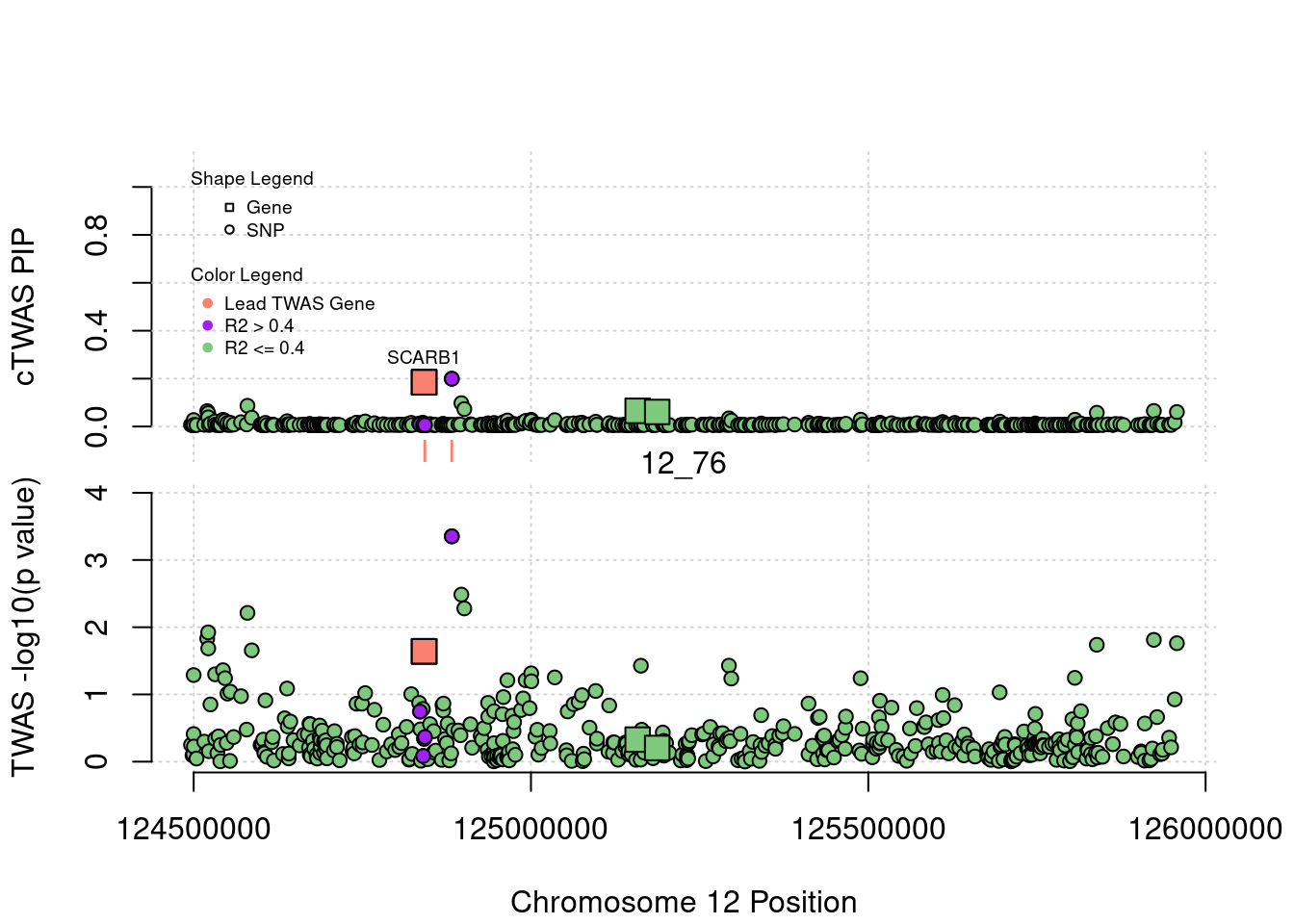
[1] "SCARB1"
[1] "12_76"
genename region_tag susie_pip mu2 PVE z num_eqtl
844 SCARB1 12_76 0.18474753 16.120470 2.889128e-06 2.2759188 2
1066 AACS 12_76 0.06555756 6.279105 3.993291e-07 0.7222222 1
5370 TMEM132B 12_76 0.06094059 5.601627 3.311550e-07 -0.4868290 2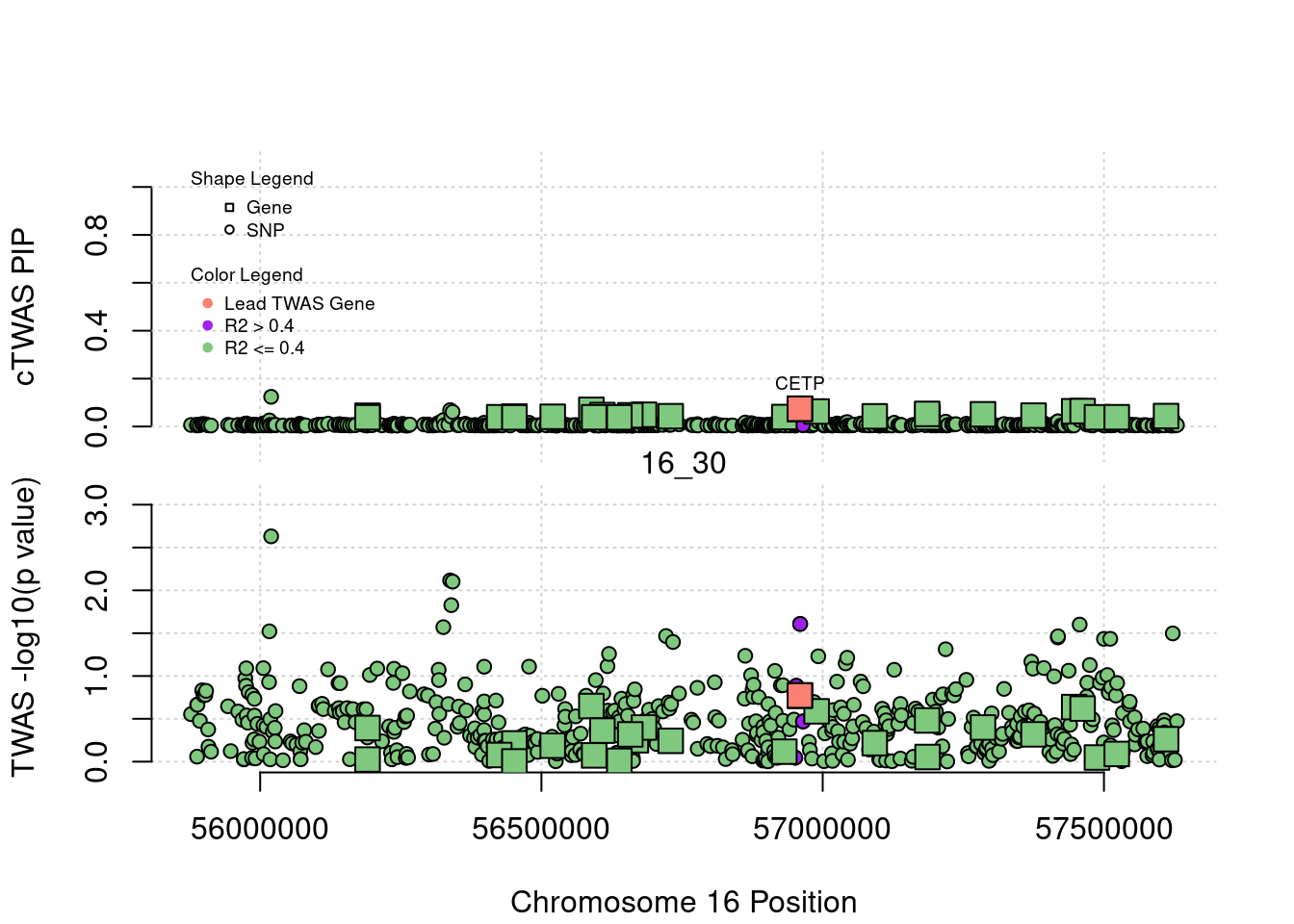
[1] "CETP"
[1] "16_30"
genename region_tag susie_pip mu2 PVE z
60 CIAPIN1 16_30 0.06372381 9.231049 5.706413e-07 -1.198426871
93 CX3CL1 16_30 0.04557558 6.133672 2.711835e-07 -0.705882353
470 HERPUD1 16_30 0.03957545 4.835657 1.856487e-07 -0.298507463
1201 CETP 16_30 0.07420553 10.647176 7.664452e-07 -1.375513239
1203 MT3 16_30 0.06992413 10.093756 6.846842e-07 -1.215047343
1205 GNAO1 16_30 0.04497389 6.011327 2.622656e-07 0.832698393
1206 OGFOD1 16_30 0.04149728 5.271319 2.122020e-07 0.506493506
1858 NUP93 16_30 0.04322186 5.645706 2.367185e-07 -0.565957447
1863 PLLP 16_30 0.05115035 7.197374 3.571356e-07 -0.842857143
1867 POLR2C 16_30 0.06447329 9.339562 5.841398e-07 -1.175182482
3889 BBS2 16_30 0.04070678 5.094572 2.011800e-07 -0.451777670
3890 MT1G 16_30 0.04391281 5.791604 2.467178e-07 -0.578947368
3891 MT2A 16_30 0.04785108 6.582470 3.055562e-07 -0.781609195
3894 DOK4 16_30 0.03865430 4.619412 1.732188e-07 -0.131313131
4877 CCDC102A 16_30 0.03884325 4.664180 1.757524e-07 -0.236261447
5518 CPNE2 16_30 0.04247781 5.486014 2.260630e-07 -0.512995403
5519 NLRC5 16_30 0.06281622 9.097971 5.544045e-07 1.130434783
7022 AMFR 16_30 0.03892499 4.683480 1.768511e-07 0.206060483
7025 RSPRY1 16_30 0.03860229 4.607051 1.725228e-07 -0.147619048
8084 NUDT21 16_30 0.03823239 4.518668 1.675916e-07 0.008012715
8861 FAM192A 16_30 0.05266633 7.467009 3.814961e-07 -0.973544974
10296 MT1X 16_30 0.04920084 6.838902 3.264144e-07 0.843243243
10915 MT1F 16_30 0.04682653 6.383045 2.899548e-07 -0.705263158
11252 ADGRG1 16_30 0.04379855 5.767633 2.450574e-07 0.602339181
11256 MT1A 16_30 0.03828804 4.532018 1.683314e-07 0.026315789
11258 MT1M 16_30 0.03870894 4.632379 1.739505e-07 -0.194690265
12072 RP11-461O7.1 16_30 0.03845842 4.572776 1.706011e-07 -0.069034092
num_eqtl
60 2
93 1
470 1
1201 2
1203 2
1205 2
1206 1
1858 1
1863 1
1867 1
3889 2
3890 1
3891 1
3894 1
4877 2
5518 2
5519 1
7022 2
7025 1
8084 2
8861 1
10296 1
10915 1
11252 1
11256 1
11258 1
12072 2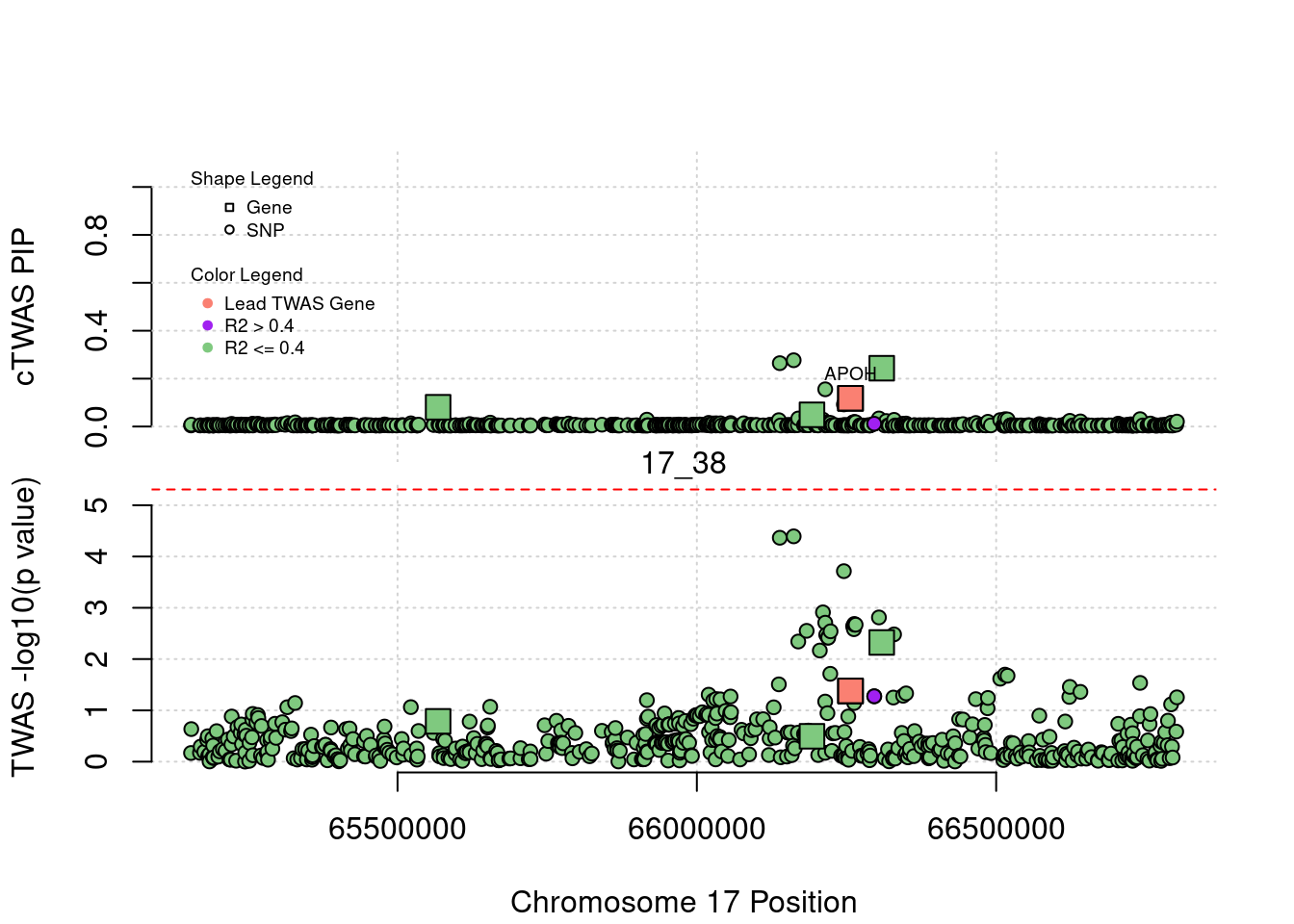
[1] "APOH"
[1] "17_38"
genename region_tag susie_pip mu2 PVE z num_eqtl
1354 APOH 17_38 0.11801095 14.851973 1.700266e-06 -2.0358974 1
6629 PRKCA 17_38 0.24348667 22.110347 5.222533e-06 -2.8239485 2
6631 CEP112 17_38 0.04911209 6.517311 3.105041e-07 0.9939394 1
8345 AXIN2 17_38 0.08057069 11.215542 8.766127e-07 1.3925234 1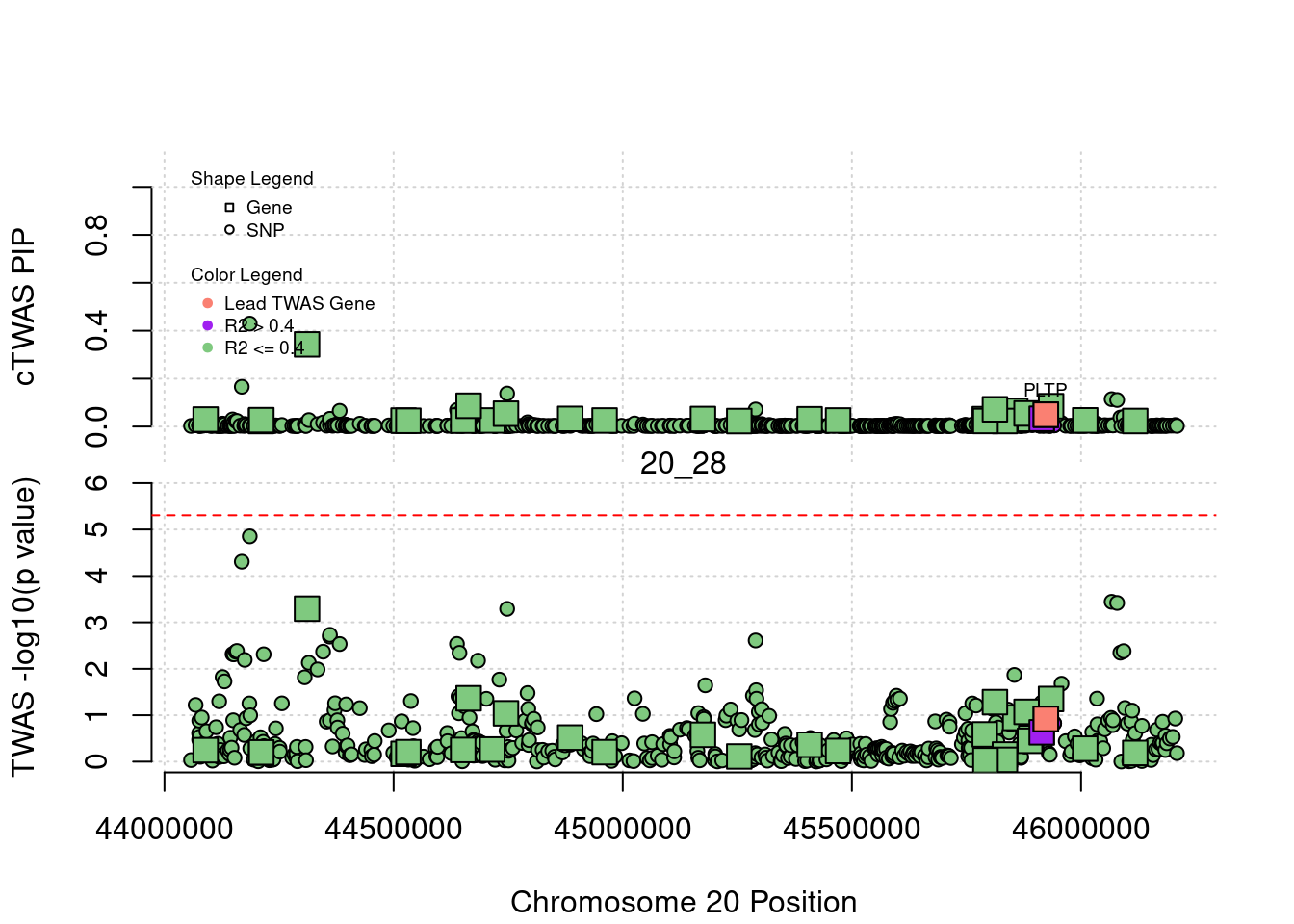
[1] "PLTP"
[1] "20_28"
genename region_tag susie_pip mu2 PVE z
313 TOMM34 20_28 0.02524516 5.492950 1.345223e-07 0.48837209
604 WISP2 20_28 0.02530617 5.558368 1.364533e-07 0.60139860
614 CTSA 20_28 0.03231449 7.721463 2.420513e-07 1.19117647
1704 PLTP 20_28 0.04939108 11.604702 5.560232e-07 1.55421687
1705 PCIF1 20_28 0.08493887 16.643262 1.371372e-06 -2.00769231
1707 MMP9 20_28 0.02582196 5.723032 1.433593e-07 0.63461538
1714 CD40 20_28 0.02293930 4.640313 1.032614e-07 0.45453638
1790 WFDC2 20_28 0.02526981 5.533380 1.356447e-07 -0.54087814
1795 DNTTIP1 20_28 0.03077945 7.292193 2.177356e-07 1.12815943
1799 TNNC2 20_28 0.02377720 4.970289 1.146444e-07 -0.38260870
1800 ACOT8 20_28 0.06515733 14.232829 8.996321e-07 1.58080246
3789 PI3 20_28 0.03122838 7.451401 2.257345e-07 1.11627907
3790 SNX21 20_28 0.02307338 4.702641 1.052600e-07 -0.10729654
3791 SLPI 20_28 0.02287167 4.616208 1.024221e-07 0.28846154
3792 WFDC3 20_28 0.02314523 4.738309 1.063887e-07 -0.08148941
3816 KCNK15 20_28 0.05367652 12.388371 6.450732e-07 -1.69068935
3817 TP53TG5 20_28 0.03020217 7.177909 2.103035e-07 -0.78564127
3820 NEURL2 20_28 0.03421084 8.308952 2.757531e-07 1.01552795
4557 OSER1 20_28 0.02290891 4.702150 1.044988e-07 -0.47546906
4558 SERINC3 20_28 0.02492153 5.393470 1.303927e-07 -0.41420118
6310 JPH2 20_28 0.02932188 6.806741 1.936161e-07 -0.57352941
6314 SPATA25 20_28 0.03158694 7.545802 2.312189e-07 0.92485549
8067 YWHAB 20_28 0.03196934 7.670595 2.378883e-07 -1.03090900
8342 ZSWIM1 20_28 0.05466044 12.558543 6.659211e-07 -1.72463768
8354 PKIG 20_28 0.02406997 5.105897 1.192225e-07 0.48837209
9096 UBE2C 20_28 0.07207160 15.108582 1.056327e-06 -1.94078947
10651 ADA 20_28 0.02458064 5.280983 1.259269e-07 0.58282209
10724 FITM2 20_28 0.34299801 30.562546 1.016931e-05 3.47633136
11569 OSER1-AS1 20_28 0.02649116 5.891855 1.514131e-07 -0.47898985
13188 RP11-445H22.3 20_28 0.08674289 16.923237 1.424058e-06 -2.02222222
num_eqtl
313 1
604 1
614 1
1704 1
1705 1
1707 1
1714 2
1790 2
1795 2
1799 1
1800 2
3789 1
3790 2
3791 1
3792 2
3816 2
3817 2
3820 1
4557 2
4558 1
6310 1
6314 1
8067 2
8342 1
8354 1
9096 1
10651 1
10724 1
11569 3
13188 1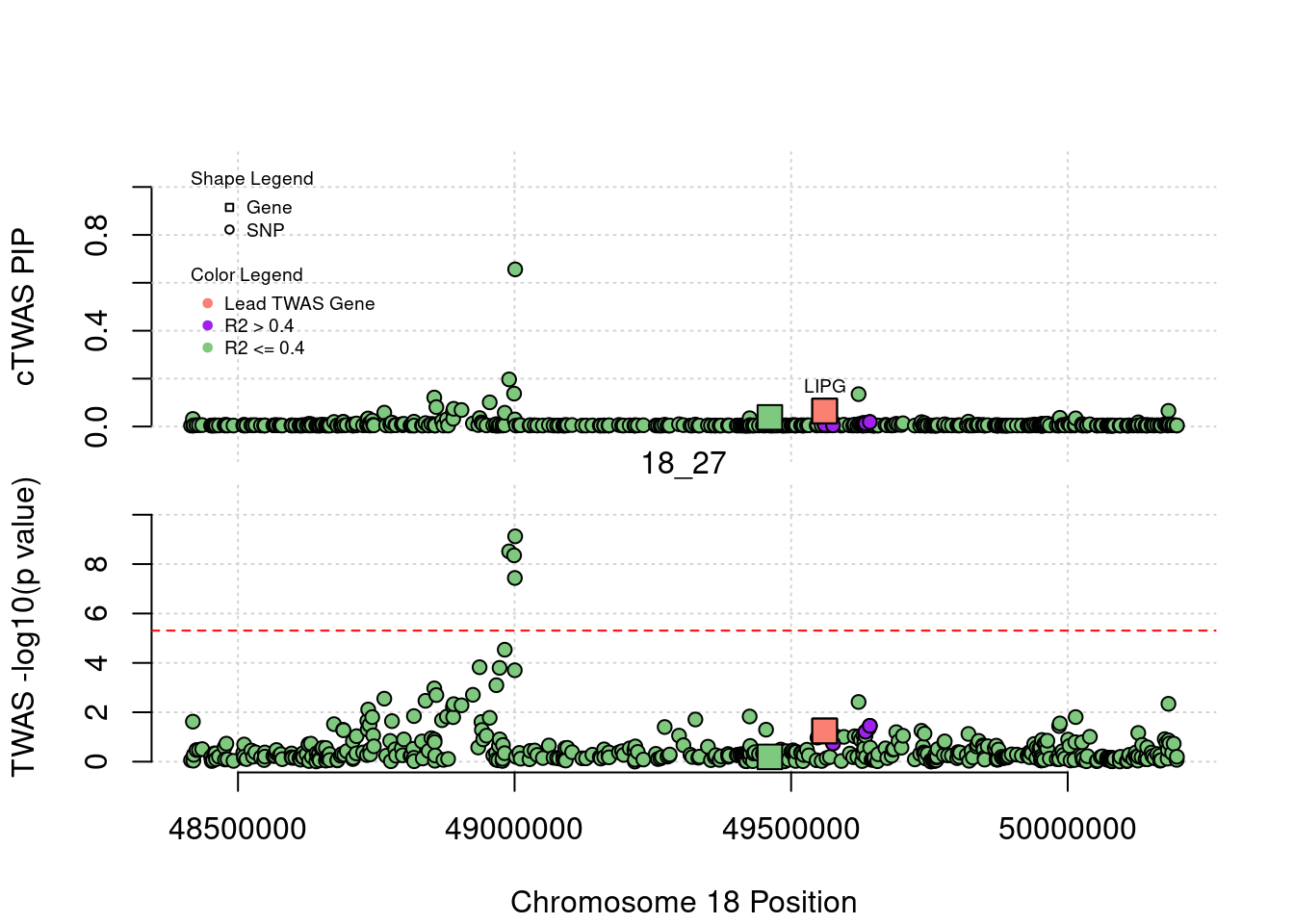
[1] "LIPG"
[1] "18_27"
genename region_tag susie_pip mu2 PVE z num_eqtl
1817 LIPG 18_27 0.06473438 10.897859 6.843631e-07 1.9012346 1
5621 DYM 18_27 0.03826757 4.803442 1.783175e-07 -0.4668737 2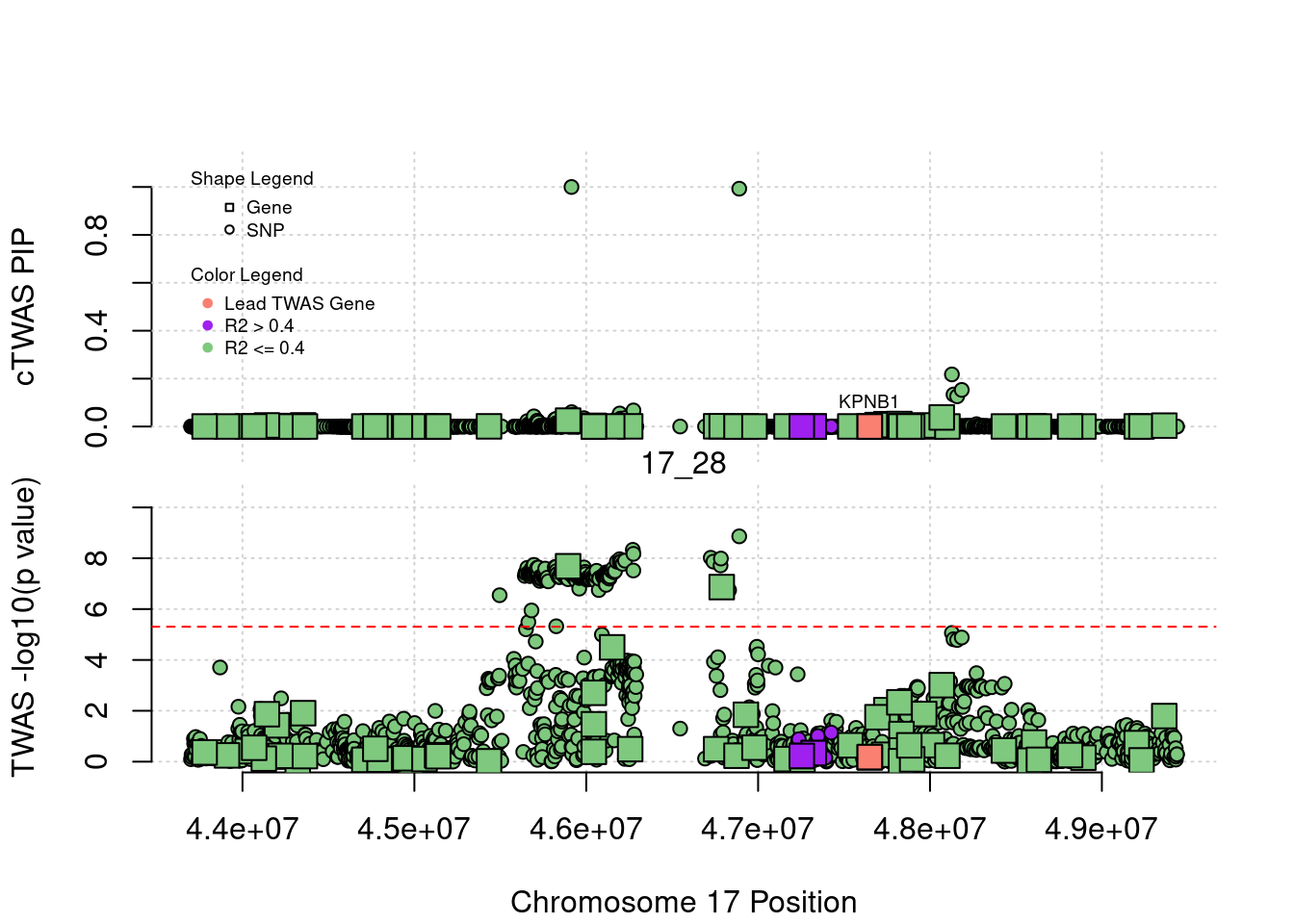
[1] "KPNB1"
[1] "17_28"
genename region_tag susie_pip mu2 PVE z
49 SLC4A1 17_28 0.0003180097 5.476102 1.689360e-09 0.48192771
231 SLC25A39 17_28 0.0002866459 4.529182 1.259436e-09 -0.05022464
340 GRN 17_28 0.0048166873 29.835259 1.394083e-07 -2.50000000
855 ADAM11 17_28 0.0003271419 5.727858 1.817770e-09 -0.54310345
1197 ATXN7L3 17_28 0.0020300106 21.984944 4.329464e-08 -2.08273381
1364 TMEM101 17_28 0.0004017452 7.523499 2.932115e-09 -0.86619718
2439 UBTF 17_28 0.0003263940 5.702325 1.805530e-09 0.53846154
2498 HDAC5 17_28 0.0002984882 4.891880 1.416490e-09 -0.30357143
2500 MPP2 17_28 0.0003317175 5.847920 1.881829e-09 -0.57075020
2502 DUSP3 17_28 0.0003767421 6.979751 2.550906e-09 0.77952756
3901 C17orf53 17_28 0.0044678567 29.074611 1.260154e-07 -2.46666667
4390 C1QL1 17_28 0.0002899284 6.379844 1.794367e-09 0.01151939
4391 HIGD1B 17_28 0.0002963348 4.829685 1.388392e-09 0.27586207
7214 LSM12 17_28 0.0004803592 9.177472 4.276610e-09 -1.07024793
7219 FAM171A2 17_28 0.0003229329 5.588266 1.750652e-09 0.51627907
7220 DBF4B 17_28 0.0002909955 4.671514 1.318725e-09 0.18918919
10186 KIF18B 17_28 0.0003010402 4.965591 1.450127e-09 -0.33322170
12610 CTC-296K1.4 17_28 0.0002920351 4.708543 1.333927e-09 -0.20888889
12615 CTC-296K1.3 17_28 0.0004542217 8.664653 3.817943e-09 1.01078096
48 CDC27 17_28 0.0005638306 9.313638 5.094228e-09 0.25974026
860 TBX21 17_28 0.0003305765 5.938589 1.904433e-09 0.62179487
862 NSF 17_28 0.0010635007 59.478721 6.136346e-08 0.98979592
2445 WNT3 17_28 0.0012204211 558.187743 6.608462e-07 -5.27058824
2451 KPNB1 17_28 0.0003313670 5.821022 1.871194e-09 0.43448276
2452 GOSR2 17_28 0.0003369155 14.101908 4.609028e-09 -2.44927536
2453 PNPO 17_28 0.0098829449 31.354986 3.006100e-07 2.59508717
3514 KANSL1 17_28 0.0003640200 21.509890 7.595805e-09 0.82274260
5563 NPEPPS 17_28 0.0004495803 10.909211 4.757854e-09 1.21000000
6976 WNT9B 17_28 0.0003090636 10.278593 3.081712e-09 0.54794521
7006 ARHGAP27 17_28 0.0007799120 82.455833 6.238460e-08 -0.03797468
8888 DCAKD 17_28 0.0002902251 28.608122 8.054428e-09 -0.26470588
9256 LRRC37A 17_28 0.0007051676 60.390985 4.131187e-08 0.98989899
9467 EFCAB13 17_28 0.0003327766 6.299482 2.033612e-09 -0.73719881
9549 RPRML 17_28 0.0003111989 6.081602 1.835974e-09 1.07613062
9691 ACBD4 17_28 0.0003375575 6.295973 2.061679e-09 0.58441558
10151 ARL17A 17_28 0.0006810977 537.906928 3.554078e-07 -4.15979988
10259 HEXIM1 17_28 0.0002995613 11.894480 3.456540e-09 -0.50724638
10265 MAPT 17_28 0.0246154818 498.175032 1.189599e-05 5.60645147
10901 MYL4 17_28 0.0002908602 6.579207 1.856386e-09 -0.21052632
11044 TBKBP1 17_28 0.0061761042 31.748763 1.902181e-07 2.36764706
11694 ARL17B 17_28 0.0028320351 185.449635 5.094893e-07 3.09554404
11902 LRRC37A2 17_28 0.0003019705 78.586006 2.302079e-08 -2.12048193
12370 ITGB3 17_28 0.0002968332 5.180641 1.491786e-09 -0.52597403
63 COPZ2 17_28 0.0005572602 10.503805 5.678257e-09 -1.21428571
86 OSBPL7 17_28 0.0105209292 24.039694 2.453542e-07 2.83041792
2455 CDK5RAP3 17_28 0.0044766936 13.795755 5.991192e-08 2.47418563
2456 CBX1 17_28 0.0003268446 4.764112 1.510545e-09 0.54589372
3515 HOXB5 17_28 0.0003394002 9.734487 3.205056e-09 0.59090909
3516 HOXB3 17_28 0.0006522950 10.398370 6.579907e-09 -1.35294118
5564 SKAP1 17_28 0.0004119036 5.218624 2.085268e-09 -0.90395750
5565 LRRC46 17_28 0.0011115832 9.434393 1.017341e-08 -1.74022390
5566 SCRN2 17_28 0.0004147273 6.569172 2.642918e-09 0.90923973
6986 MRPL10 17_28 0.0002867457 4.550373 1.265769e-09 0.05654812
6995 ATP5G1 17_28 0.0003066704 4.871136 1.449147e-09 -0.39173481
8090 GNGT2 17_28 0.0006284941 11.553020 7.043802e-09 -1.31942009
8104 SP2 17_28 0.0002936187 4.827525 1.375051e-09 0.23512748
8612 TTLL6 17_28 0.0003353892 5.400366 1.757044e-09 0.59420290
8981 PHOSPHO1 17_28 0.0002886628 4.594197 1.286503e-09 0.13432836
9804 HOXB4 17_28 0.0002880062 4.759400 1.329733e-09 0.10928962
10488 SP6 17_28 0.0005472424 6.256420 3.321361e-09 1.20472441
10980 ZNF652 17_28 0.0039011097 26.980080 1.021038e-07 2.40579710
12404 HOXB7 17_28 0.0002922864 6.285681 1.782261e-09 0.18888889
13137 RP5-890E16.5 17_28 0.0381096154 20.815031 7.695238e-07 -3.29629630
num_eqtl
49 1
231 2
340 1
855 1
1197 1
1364 1
2439 1
2498 1
2500 2
2502 1
3901 1
4390 2
4391 1
7214 1
7219 1
7220 1
10186 2
12610 1
12615 2
48 1
860 1
862 1
2445 1
2451 1
2452 1
2453 2
3514 2
5563 1
6976 1
7006 1
8888 1
9256 1
9467 2
9549 3
9691 1
10151 2
10259 1
10265 2
10901 1
11044 1
11694 2
11902 1
12370 1
63 1
86 2
2455 2
2456 1
3515 1
3516 1
5564 2
5565 2
5566 5
6986 2
6995 2
8090 2
8104 1
8612 1
8981 1
9804 1
10488 1
10980 1
12404 1
13137 1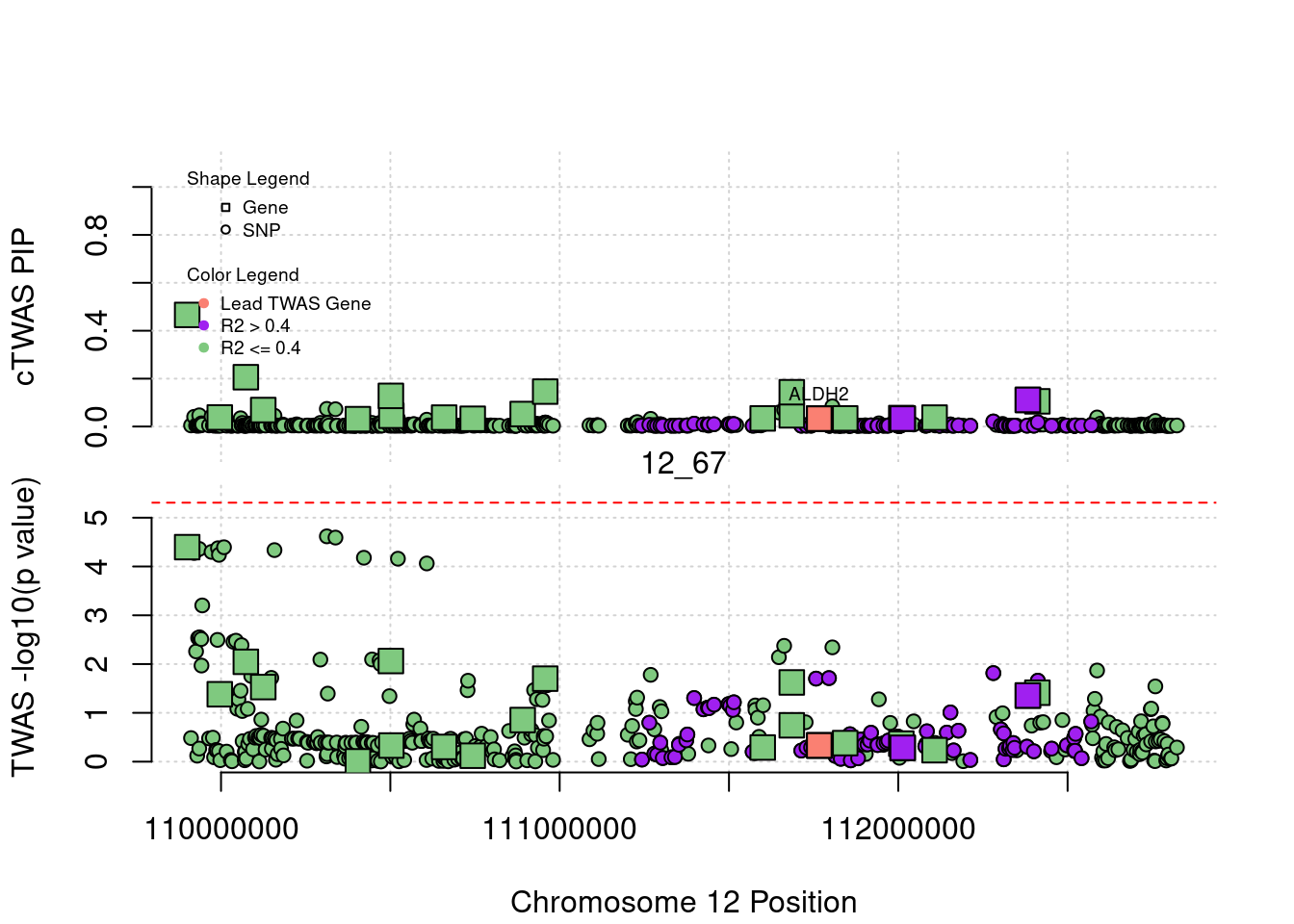
[1] "ALDH2"
[1] "12_67"
genename region_tag susie_pip mu2 PVE z
1280 BRAP 12_67 0.04675331 7.994322 3.625805e-07 1.34066358
1281 ERP29 12_67 0.03319527 5.119632 1.648638e-07 -0.64220183
2682 VPS29 12_67 0.04526175 7.644784 3.356657e-07 0.72667784
2689 ACAD10 12_67 0.14319178 17.884097 2.484251e-06 2.26027397
2690 ALDH2 12_67 0.03303767 5.097709 1.633785e-07 -0.72941176
2693 NAA25 12_67 0.03724561 6.593797 2.382435e-07 -0.54973822
3717 IFT81 12_67 0.06872120 12.430363 8.286765e-07 2.17500000
3719 HVCN1 12_67 0.03732208 6.442366 2.332500e-07 -0.67595819
5377 GIT2 12_67 0.03772658 9.022654 3.302115e-07 -2.03825370
5378 TCHP 12_67 0.46592142 19.210171 8.682691e-06 -4.10638298
6384 RAD9B 12_67 0.12795659 18.405551 2.284662e-06 2.62500000
8895 HECTD4 12_67 0.11125085 15.582348 1.681693e-06 -2.01275695
8900 CCDC63 12_67 0.05218572 9.901552 5.012626e-07 -1.47435897
9039 C12orf76 12_67 0.20590043 22.264369 4.447112e-06 2.61230769
9519 PTPN11 12_67 0.10518194 15.094079 1.540133e-06 2.06756757
10198 PPP1CC 12_67 0.03246320 4.964772 1.563511e-07 -0.31192661
10600 ANAPC7 12_67 0.03071825 4.555999 1.357658e-07 -0.01851852
10894 TMEM116 12_67 0.03538487 5.632015 1.933267e-07 0.79439252
11206 ATXN2 12_67 0.03390119 5.437734 1.788312e-07 -0.65322581
11819 MAPKAPK5-AS1 12_67 0.03398186 5.331891 1.757676e-07 0.81176471
12342 RP1-46F2.3 12_67 0.14583902 19.801867 2.801498e-06 -2.32835821
num_eqtl
1280 2
1281 1
2682 2
2689 1
2690 1
2693 1
3717 1
3719 1
5377 2
5378 1
6384 1
8895 2
8900 1
9039 1
9519 1
10198 1
10600 1
10894 1
11206 1
11819 1
12342 1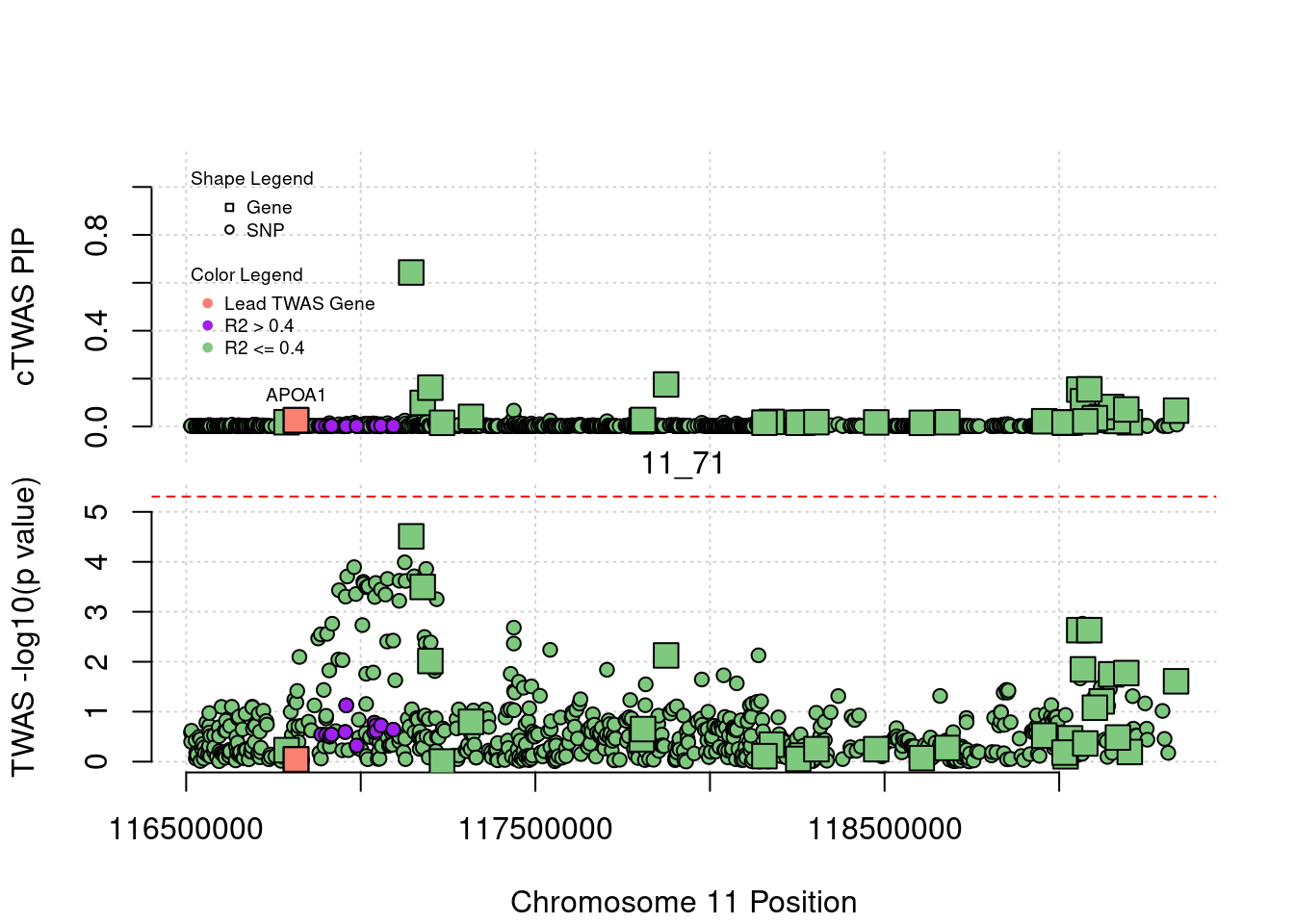
[1] "APOA1"
[1] "11_71"
genename region_tag susie_pip mu2 PVE z
2574 ZPR1 11_71 0.01790703 5.833847 1.013419e-07 -0.541501976
3348 APOA1 11_71 0.02628942 8.435404 2.151282e-07 0.119988826
5151 FXYD6 11_71 0.17556648 26.704647 4.548193e-06 -2.674833297
5152 FXYD2 11_71 0.02160276 7.609753 1.594741e-07 0.920315172
6308 SIDT2 11_71 0.09887605 15.572805 1.493717e-06 -3.600000000
6309 TAGLN 11_71 0.16221658 25.364693 3.991492e-06 2.587628866
8116 RNF214 11_71 0.01548425 4.529874 6.804353e-08 -0.008528785
8270 PAFAH1B2 11_71 0.64305971 21.059969 1.313770e-05 4.166666667
9300 DSCAML1 11_71 0.02745225 9.842213 2.621085e-07 1.218458904
10201 BACE1 11_71 0.04016866 12.905694 5.028971e-07 -1.406417112
268 PHLDB1 11_71 0.01559001 4.586187 6.935991e-08 -0.187022901
2613 DDX6 11_71 0.02202552 7.670877 1.639010e-07 -1.034883721
2614 CBL 11_71 0.01764533 5.737428 9.821040e-08 -0.467265741
3345 TREH 11_71 0.01789392 5.827467 1.011569e-07 -0.625000000
3346 IFT46 11_71 0.01712413 5.419494 9.002802e-08 -0.578805975
3350 RPS25 11_71 0.01571854 4.656502 7.100392e-08 -0.278667949
5145 SLC37A4 11_71 0.01688638 5.222010 8.554305e-08 -0.951456311
6288 HYOU1 11_71 0.15448100 24.965368 3.741308e-06 -3.044117647
6306 MPZL2 11_71 0.01619437 4.936535 7.755267e-08 0.313068811
6307 SCN2B 11_71 0.01925767 6.496184 1.213591e-07 -0.743421053
7128 MPZL3 11_71 0.01566375 4.634099 7.041603e-08 0.149253731
7137 VPS11 11_71 0.10560572 21.812486 2.234617e-06 -2.451773632
7138 NLRX1 11_71 0.02299510 8.109108 1.808917e-07 -0.977272727
8806 HINFP 11_71 0.04001481 12.911204 5.011848e-07 -1.865671642
8819 ABCG4 11_71 0.08152008 19.369046 1.531734e-06 -2.367578183
8823 C2CD2L 11_71 0.03477307 11.660357 3.933374e-07 -1.731343284
8942 RNF26 11_71 0.06652105 17.494079 1.128913e-06 -2.247474736
9299 SCN4B 11_71 0.01617218 4.927748 7.730852e-08 0.283582090
10410 H2AFX 11_71 0.15552293 25.032273 3.776636e-06 3.044117647
10624 TRAPPC4 11_71 0.01692983 5.340398 8.770750e-08 -0.443037975
11015 CD3E 11_71 0.01817585 5.991658 1.056458e-07 0.578735950
12106 CCDC153 11_71 0.07153757 18.076623 1.254475e-06 -2.389854562
12302 HMBS 11_71 0.02141686 7.510263 1.560347e-07 -0.784482759
num_eqtl
2574 1
3348 3
5151 2
5152 2
6308 1
6309 1
8116 1
8270 1
9300 3
10201 1
268 1
2613 1
2614 2
3345 1
3346 3
3350 2
5145 1
6288 1
6306 2
6307 1
7128 1
7137 2
7138 1
8806 1
8819 2
8823 1
8942 2
9299 1
10410 1
10624 1
11015 2
12106 3
12302 1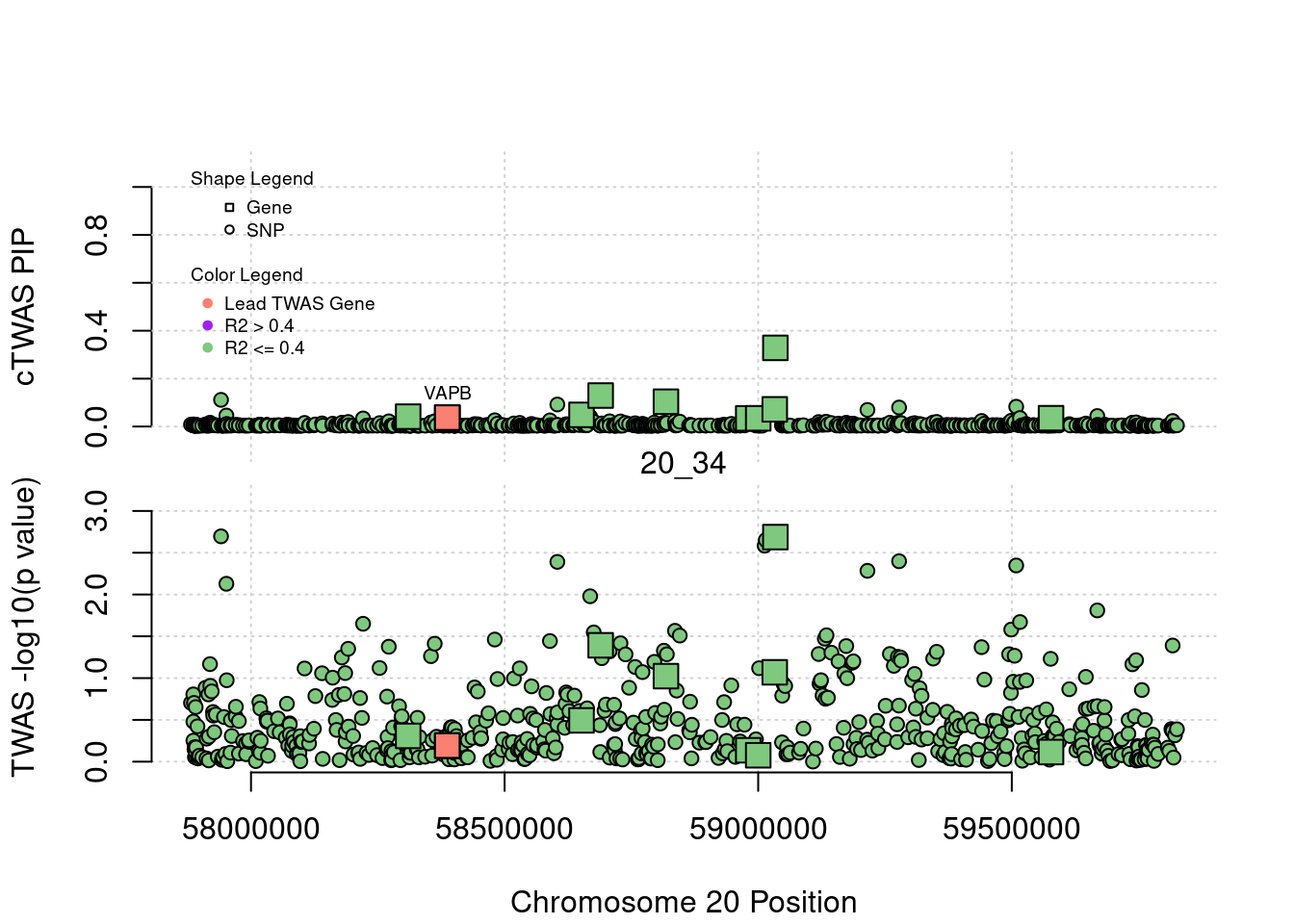
[1] "VAPB"
[1] "20_34"
genename region_tag susie_pip mu2 PVE z
1219 PHACTR3 20_34 0.03489479 4.798068 1.624192e-07 -0.2956861
1731 NELFCD 20_34 0.03400182 4.560485 1.504263e-07 -0.3443348
1732 CTSZ 20_34 0.03419707 4.612974 1.530313e-07 -0.2033651
1735 PRELID3B 20_34 0.32826632 26.301616 8.375663e-06 3.0820477
3796 VAPB 20_34 0.03727272 5.402612 1.953463e-07 0.4647532
3798 ATP5E 20_34 0.07184926 11.463582 7.990116e-07 1.7205882
3805 RAB22A 20_34 0.04134314 6.354345 2.548500e-07 -0.6880223
3811 STX16 20_34 0.04878657 7.878174 3.728519e-07 0.9925926
11491 NPEPL1 20_34 0.12933773 17.001918 2.133210e-06 -2.0485437
12650 RP4-806M20.4 20_34 0.10359351 14.893414 1.496708e-06 -1.6701031
num_eqtl
1219 2
1731 2
1732 3
1735 2
3796 2
3798 1
3805 1
3811 1
11491 1
12650 1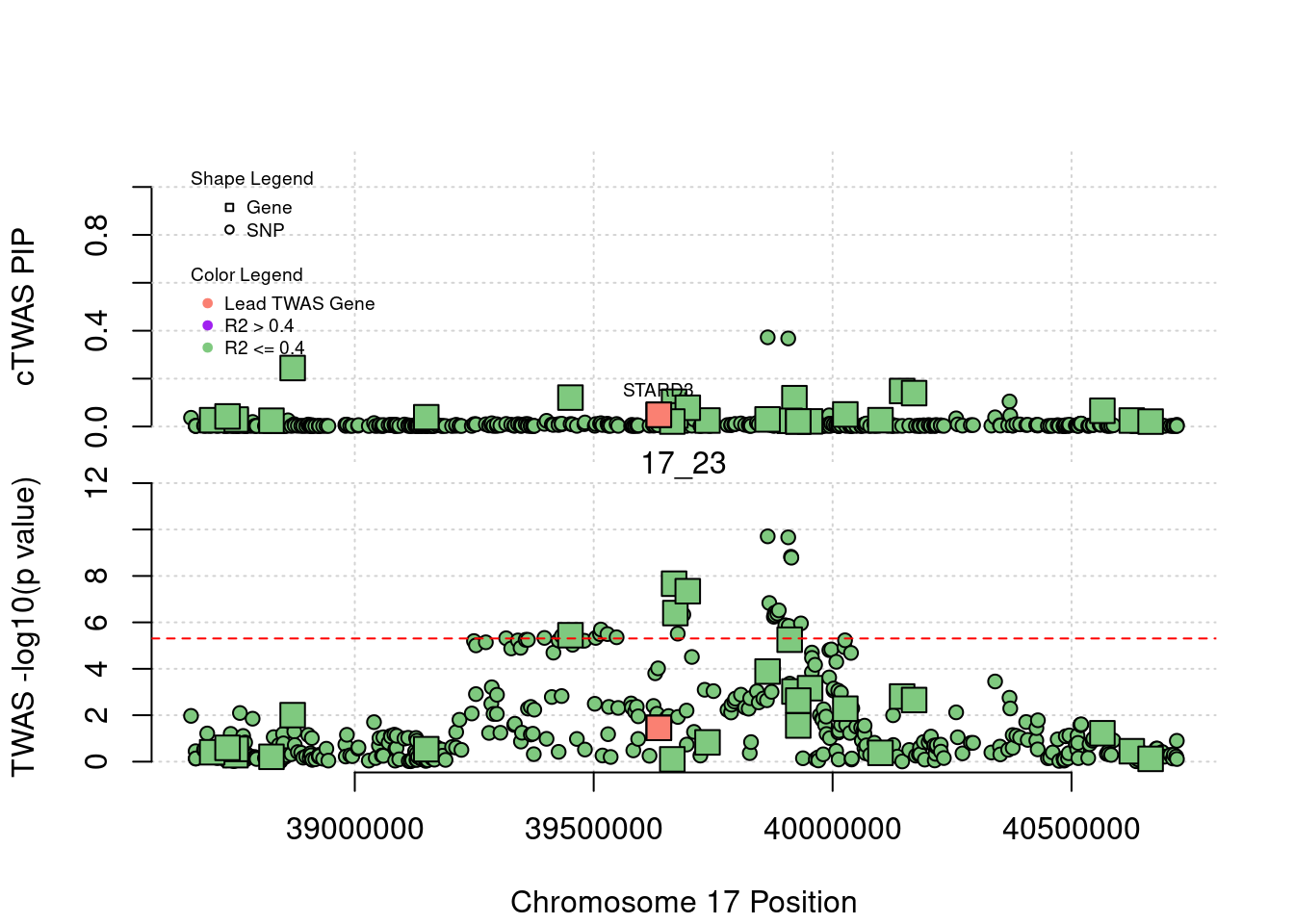
[1] "STARD3"
[1] "17_23"
genename region_tag susie_pip mu2 PVE z
22 LASP1 17_23 0.24428424 26.490940 6.277739e-06 2.5789474
156 MED24 17_23 0.05069764 15.124025 7.438161e-07 -2.7926629
853 SMARCE1 17_23 0.02519311 6.527192 1.595213e-07 -0.9227113
854 GSDMB 17_23 0.11843604 23.715838 2.724788e-06 -3.3053892
2440 PSMD3 17_23 0.02394550 18.058244 4.194786e-07 -4.5411022
2441 CASC3 17_23 0.14832067 24.210159 3.483451e-06 -3.1635688
2442 RAPGEFL1 17_23 0.13945876 23.328540 3.156049e-06 3.0590165
3941 MED1 17_23 0.12088620 28.011690 3.284933e-06 -4.6344778
4019 CCR7 17_23 0.06592876 15.577769 9.963011e-07 1.8811881
4020 NR1D1 17_23 0.02657843 6.725640 1.734097e-07 0.8127854
4444 STARD3 17_23 0.04900633 13.502687 6.419228e-07 -2.1062271
5630 ERBB2 17_23 0.10355184 31.617510 3.176113e-06 -5.5991906
5631 GRB7 17_23 0.02512553 7.082351 1.726248e-07 -1.4460432
5632 PNMT 17_23 0.04666788 23.785935 1.076834e-06 -5.0704225
7197 PLXDC1 17_23 0.03887163 9.912100 3.737738e-07 -1.0583333
7198 PGAP3 17_23 0.07840518 28.952979 2.202158e-06 -5.4680413
7199 IKZF3 17_23 0.02963216 16.164208 4.646524e-07 3.8235294
8234 GSDMA 17_23 0.02130786 12.074736 2.495904e-07 3.3941748
8780 ORMDL3 17_23 0.02134267 7.757592 1.606150e-07 -2.2000000
8995 TCAP 17_23 0.02080428 4.616384 9.316761e-08 0.2589928
11363 KRT222 17_23 0.02066725 4.574887 9.172200e-08 -0.3055556
12551 RP11-387H17.4 17_23 0.02117943 10.334739 2.123363e-07 3.0429362
12942 CWC25 17_23 0.02440978 5.869391 1.389848e-07 -0.4966938
13081 PCGF2 17_23 0.02476225 6.128785 1.472227e-07 0.6296296
13104 PSMB3 17_23 0.02917801 8.098124 2.292189e-07 1.0739073
13107 CTB-58E17.1 17_23 0.02645395 6.681254 1.714584e-07 -0.8539326
13108 CISD3 17_23 0.04229289 10.655726 4.371806e-07 -1.1408451
num_eqtl
22 1
156 2
853 2
854 1
2440 2
2441 1
2442 4
3941 2
4019 1
4020 1
4444 1
5630 3
5631 1
5632 1
7197 1
7198 2
7199 1
8234 2
8780 1
8995 1
11363 1
12551 2
12942 2
13081 1
13104 3
13107 1
13108 1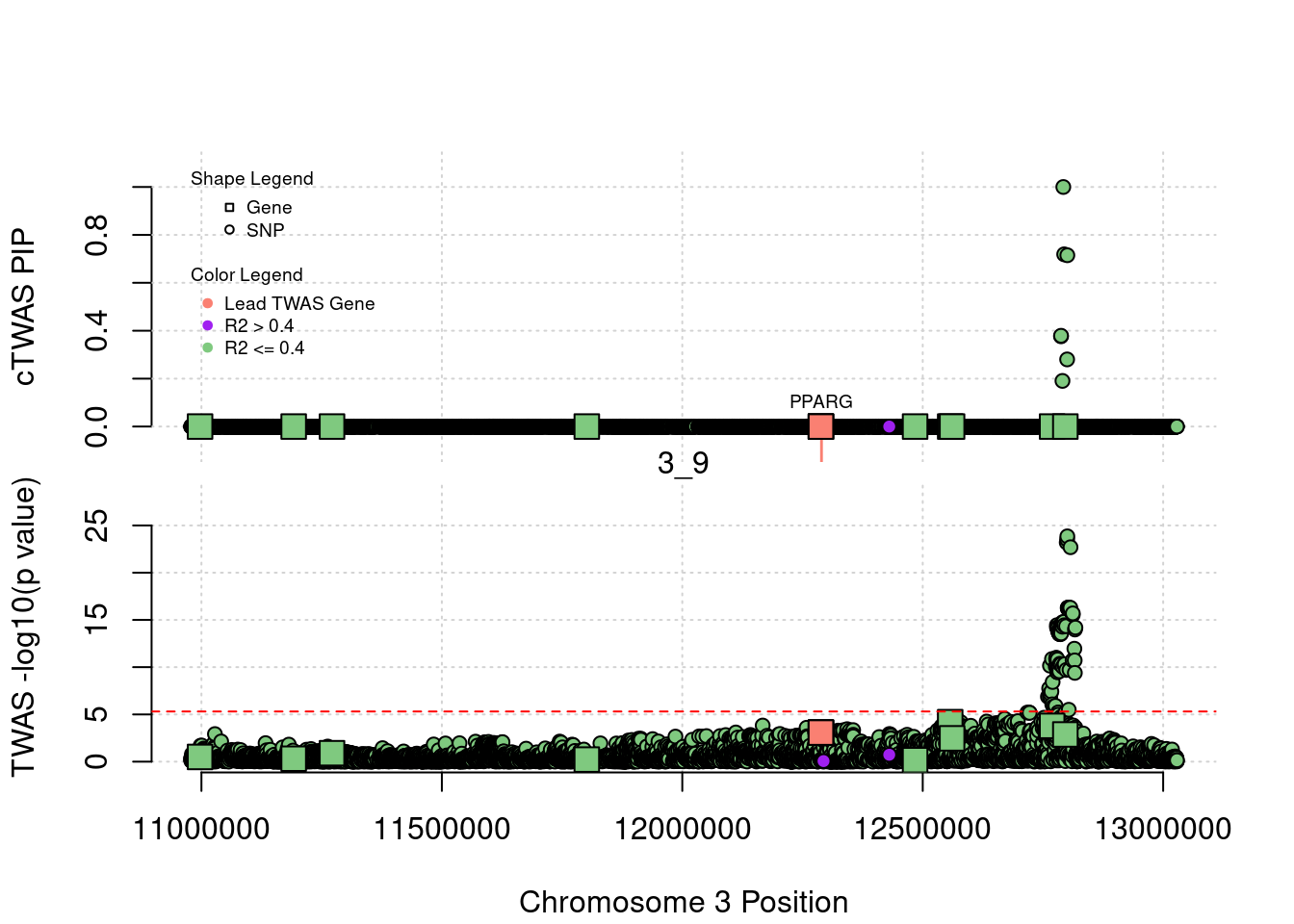
[1] "PPARG"
[1] "3_9"
genename region_tag susie_pip mu2 PVE z num_eqtl
924 MKRN2 3_9 0 29.475894 0 -3.9882864 2
1241 TMEM40 3_9 0 131.182002 0 -3.7679558 1
4476 PPARG 3_9 0 44.226334 0 -3.3398058 1
5912 TAMM41 3_9 0 9.115353 0 0.5181923 2
5933 CAND2 3_9 0 93.771766 0 -3.2111601 2
6666 TSEN2 3_9 0 165.262753 0 0.3926355 3
6833 SLC6A1 3_9 0 8.763910 0 -1.0000000 1
10620 HRH1 3_9 0 7.431693 0 -0.6944444 1
10763 ATG7 3_9 0 14.255822 0 -1.5204082 1
11600 MKRN2OS 3_9 0 326.284741 0 -2.9276210 2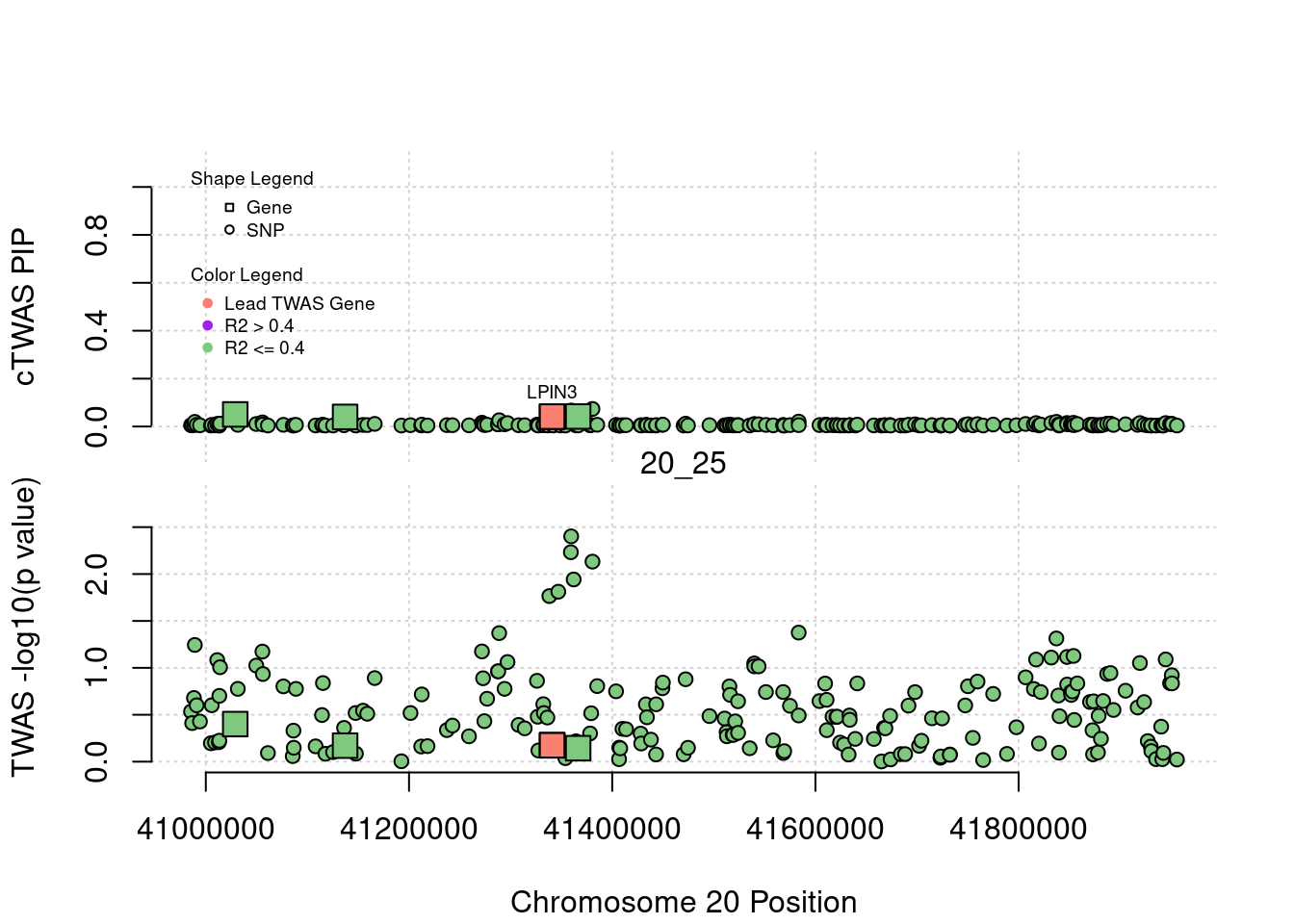
[1] "LPIN3"
[1] "20_25"
genename region_tag susie_pip mu2 PVE z num_eqtl
3799 PLCG1 20_25 0.04023364 5.053876 1.972533e-07 0.4185142 2
4553 LPIN3 20_25 0.04145891 5.329523 2.143467e-07 0.4270119 2
9925 EMILIN3 20_25 0.04214410 5.480196 2.240491e-07 -0.3592593 1
11032 TOP1 20_25 0.05057784 7.160215 3.513151e-07 0.8455882 1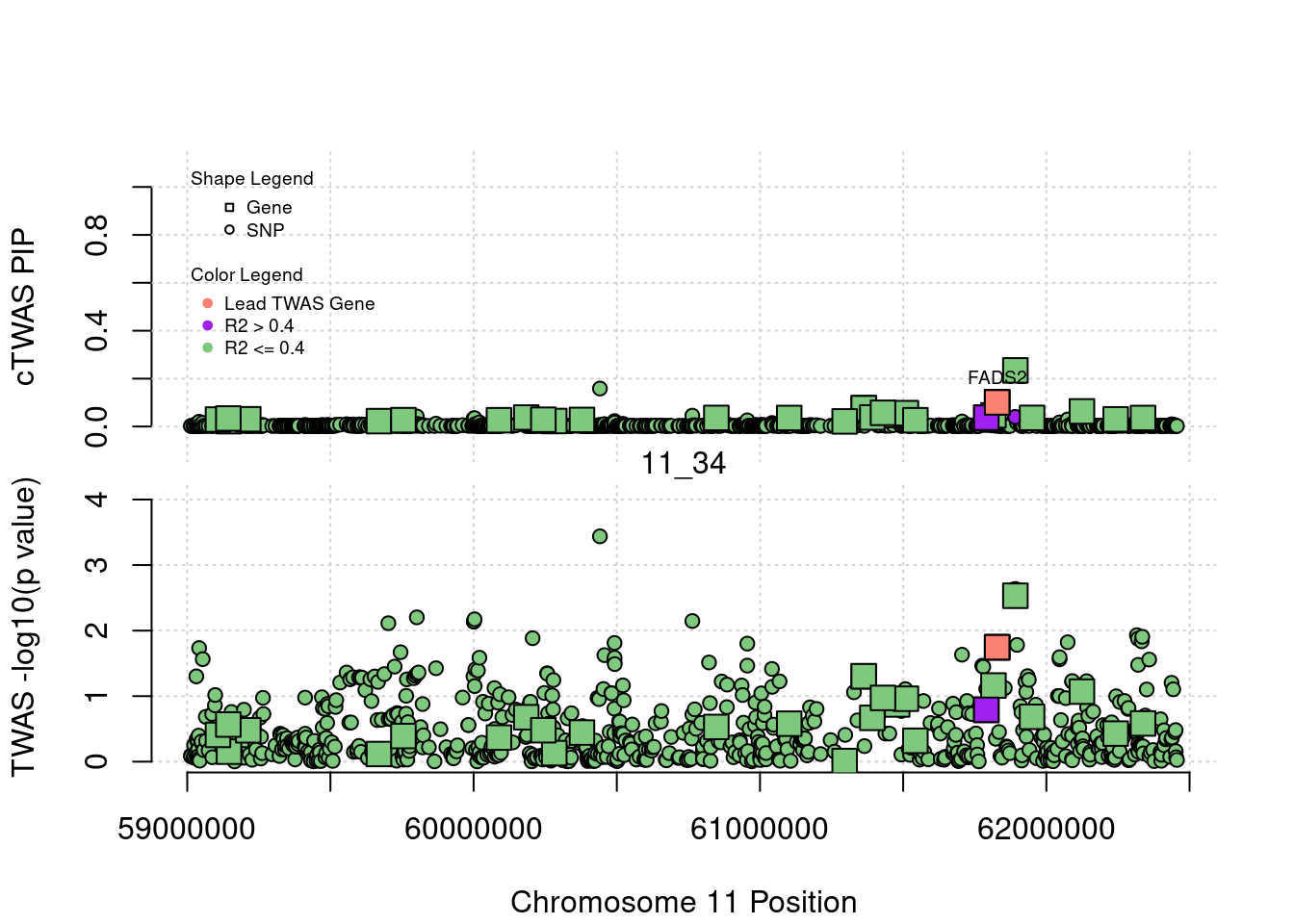
[1] "FADS2"
[1] "11_34"
genename region_tag susie_pip mu2 PVE z
2584 DTX4 11_34 0.02781763 6.861199 1.851529e-07 0.85897436
2593 MS4A6A 11_34 0.03760719 9.622151 3.510375e-07 1.25373134
2594 MS4A4A 11_34 0.02309081 5.160920 1.156051e-07 -0.32835821
2597 CCDC86 11_34 0.03597023 9.213902 3.215121e-07 1.04649029
2621 CD5 11_34 0.03568699 9.141412 3.164708e-07 1.11764706
3883 SCGB1D2 11_34 0.03004532 7.565569 2.205103e-07 -0.89156627
4751 FADS2 11_34 0.10100488 18.791985 1.841304e-06 2.36643571
4752 TMEM258 11_34 0.03697381 9.466299 3.395352e-07 1.39809594
6292 TMEM138 11_34 0.07669069 16.209207 1.205910e-06 1.96202532
6293 FADS1 11_34 0.04673113 11.618934 5.267239e-07 -1.81944444
6296 INCENP 11_34 0.06319853 14.409054 8.833908e-07 1.72072479
6299 MS4A2 11_34 0.02622115 6.321244 1.607921e-07 -0.79104478
7256 CYB561A3 11_34 0.07669069 16.209207 1.205910e-06 1.96202532
7257 PPP1R32 11_34 0.04776414 11.820495 5.477067e-07 1.49792139
7258 ASRGL1 11_34 0.03557232 9.111898 3.144354e-07 1.12328767
8045 FAM111A 11_34 0.02248651 4.919074 1.073040e-07 0.37903226
8061 PATL1 11_34 0.02241093 4.888373 1.062759e-07 -0.29903537
8063 STX3 11_34 0.02639309 6.380928 1.633746e-07 0.82994817
8072 MS4A14 11_34 0.02767618 6.814626 1.829611e-07 0.91549296
8249 VWCE 11_34 0.02151883 4.518039 9.431461e-08 0.02651515
8250 BEST1 11_34 0.03609758 9.246334 3.237860e-07 1.26126126
10279 TMEM216 11_34 0.03789266 9.691540 3.562529e-07 -1.24090909
10481 FAM111B 11_34 0.02911441 7.277705 2.055478e-07 -0.85853659
10784 MPEG1 11_34 0.03036725 7.663054 2.257448e-07 -0.96969697
11219 LRRC10B 11_34 0.05600778 13.290279 7.220926e-07 -1.60000000
11466 MS4A4E 11_34 0.02832931 7.027800 1.931372e-07 0.97058824
11539 FADS3 11_34 0.23438823 26.956236 6.129224e-06 -2.97402597
12060 AP001258.4 11_34 0.03358240 8.584346 2.796594e-07 1.10000000
12307 RP11-794G24.1 11_34 0.02633199 6.359770 1.624559e-07 -0.70680628
12313 RP11-286N22.8 11_34 0.05730574 13.502198 7.506077e-07 -1.61458333
num_eqtl
2584 1
2593 1
2594 1
2597 2
2621 1
3883 1
4751 2
4752 2
6292 1
6293 1
6296 2
6299 1
7256 1
7257 2
7258 1
8045 1
8061 1
8063 3
8072 1
8249 1
8250 1
10279 1
10481 1
10784 1
11219 1
11466 1
11539 1
12060 1
12307 1
12313 1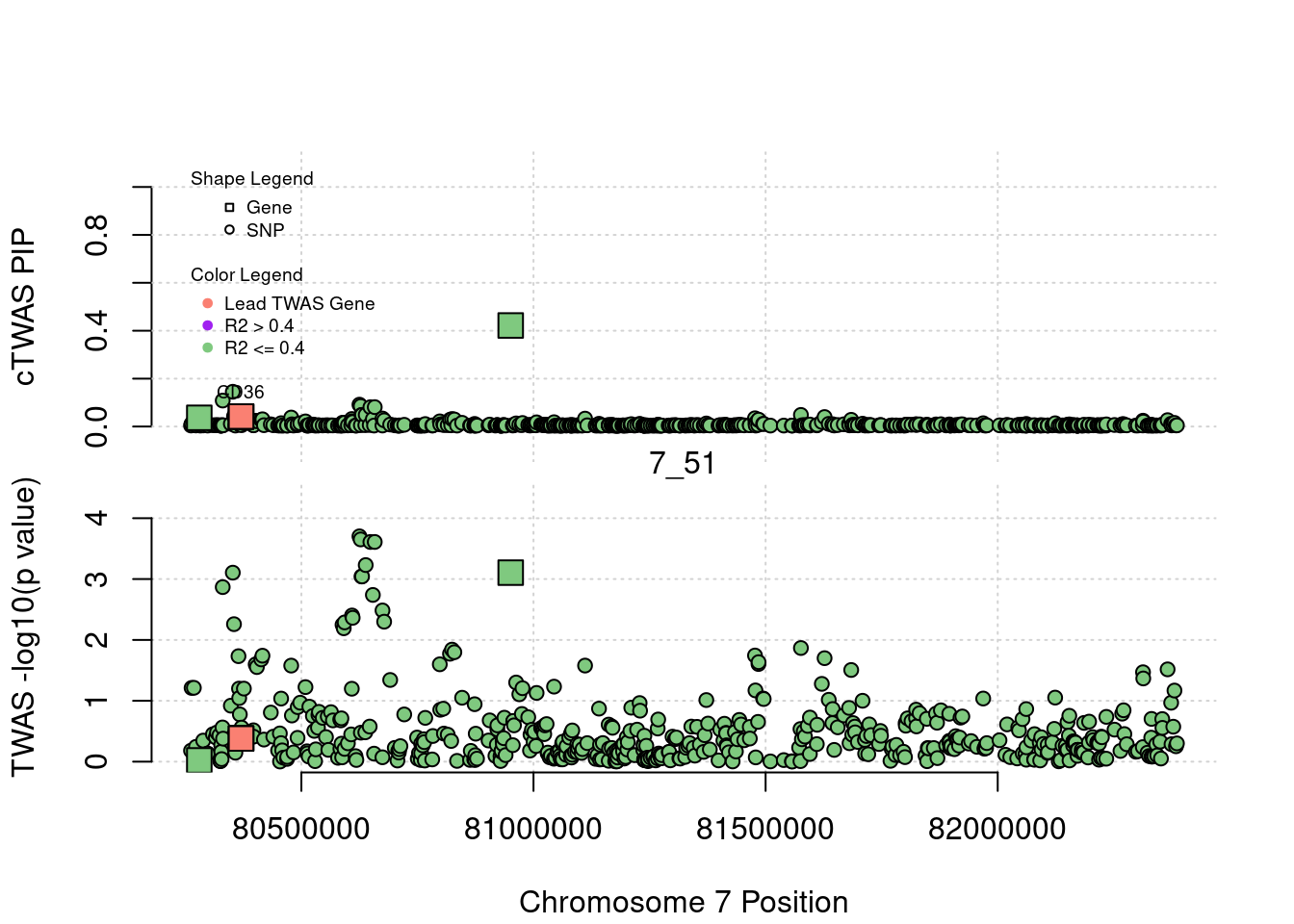
[1] "CD36"
[1] "7_51"
genename region_tag susie_pip mu2 PVE z num_eqtl
897 SEMA3C 7_51 0.42195791 28.413871 1.163081e-05 3.35820896 1
4116 GNAI1 7_51 0.03634843 4.524570 1.595414e-07 -0.05766061 2
4801 CD36 7_51 0.04178462 5.804239 2.352730e-07 0.81308411 1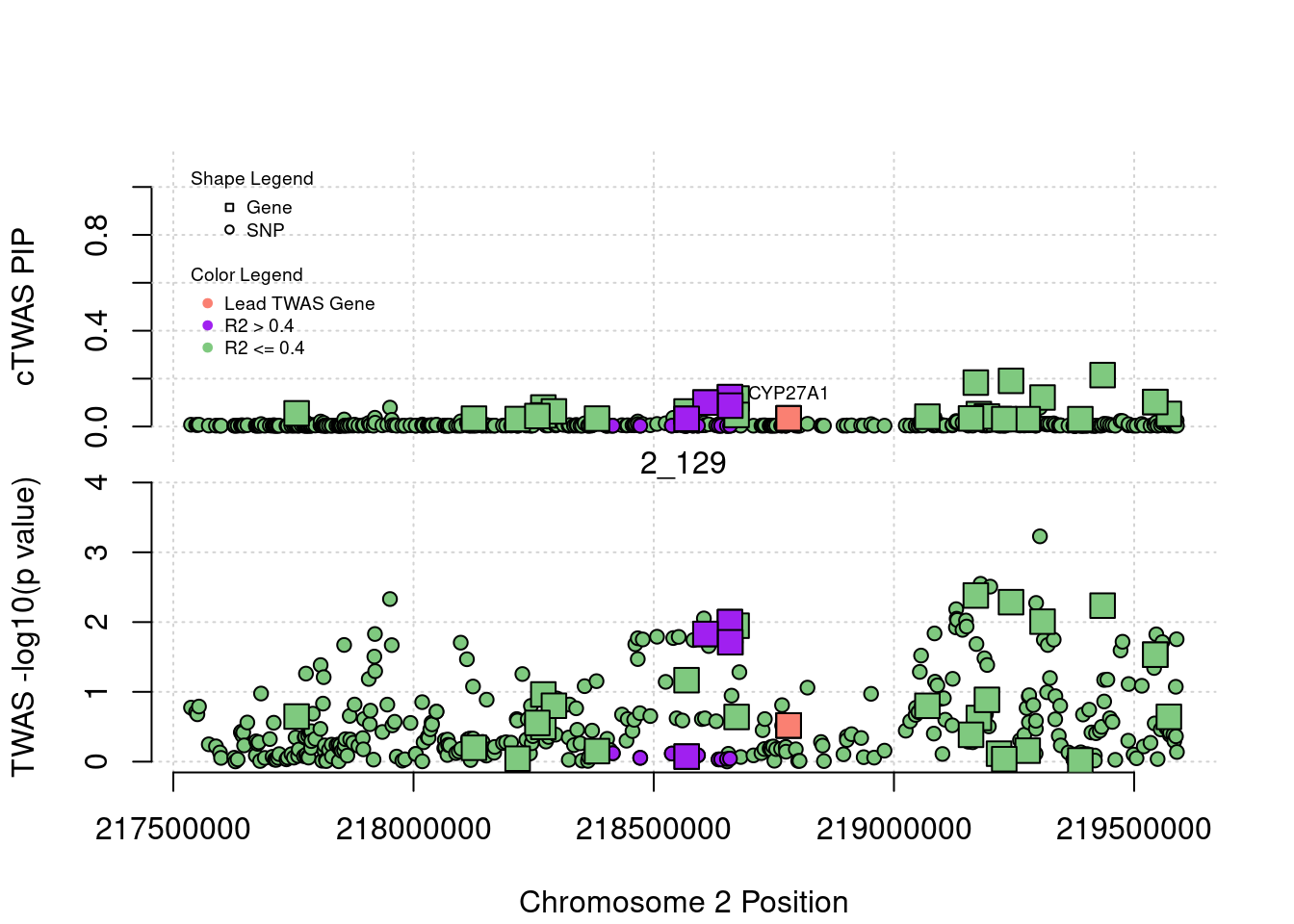
[1] "CYP27A1"
[1] "2_129"
genename region_tag susie_pip mu2 PVE z
261 SLC11A1 2_129 0.03319520 5.213021 1.678708e-07 -0.37762238
501 PTPRN 2_129 0.12121952 17.255893 2.029179e-06 2.58095238
815 SPEG 2_129 0.21477968 22.816469 4.753922e-06 -2.75857938
875 BCS1L 2_129 0.12268827 17.370641 2.067423e-06 2.57526882
3107 PLCD4 2_129 0.10007396 15.439848 1.498906e-06 2.43684211
3109 ZNF142 2_129 0.08722359 14.147743 1.197103e-06 2.33684211
3117 CNPPD1 2_129 0.05206533 9.350728 4.722854e-07 1.18372834
3119 ABCB6 2_129 0.03115380 4.631938 1.399859e-07 0.29464286
3780 CHPF 2_129 0.10233279 15.650444 1.553645e-06 -2.17910448
3781 DNPEP 2_129 0.03080248 4.528156 1.353061e-07 -0.05003297
3784 OBSL1 2_129 0.05265628 9.454937 4.829690e-07 -1.20588235
4101 TUBA4A 2_129 0.03077170 4.519008 1.348978e-07 0.39051095
4102 AAMP 2_129 0.07945239 13.274396 1.023133e-06 1.60294118
4103 PNKD 2_129 0.04656196 8.320530 3.758311e-07 1.00000000
4900 USP37 2_129 0.06293239 11.104810 6.779470e-07 -1.82472880
4905 TMBIM1 2_129 0.06330883 11.160103 6.853981e-07 1.41176471
4906 CYP27A1 2_129 0.03424827 5.499145 1.827024e-07 1.02997904
5916 CNOT9 2_129 0.03204614 4.890425 1.520312e-07 -0.19402985
6943 ZFAND2B 2_129 0.04218105 7.411254 3.032631e-07 -1.51428571
7463 ARPC2 2_129 0.03121591 4.650161 1.408168e-07 0.11676531
7467 RNF25 2_129 0.11709264 16.926435 1.922673e-06 -2.53409091
7468 STK36 2_129 0.04853678 8.703305 4.097940e-07 1.20289855
7477 GLB1L 2_129 0.19082480 21.644079 4.006677e-06 -2.79545455
9568 GPBAR1 2_129 0.04388210 7.774869 3.309717e-07 -1.08313614
9639 CXCR2 2_129 0.03308898 5.183663 1.663913e-07 0.47761194
10338 NHEJ1 2_129 0.03379454 5.376946 1.762757e-07 0.81975953
10447 TMEM198 2_129 0.10233279 15.650444 1.553645e-06 2.17910448
11041 ATG9A 2_129 0.03077614 4.520329 1.349568e-07 -0.09832389
11403 SLC23A3 2_129 0.18349763 21.259021 3.784288e-06 2.86602928
11749 DIRC3 2_129 0.05506282 9.867847 5.270980e-07 -1.21195652
12846 RP11-33O4.1 2_129 0.04131385 7.220305 2.893755e-07 1.41071429
num_eqtl
261 1
501 1
815 3
875 1
3107 1
3109 1
3117 2
3119 1
3780 1
3781 2
3784 1
4101 1
4102 1
4103 1
4900 2
4905 1
4906 2
5916 1
6943 1
7463 2
7467 1
7468 1
7477 1
9568 2
9639 1
10338 2
10447 1
11041 2
11403 2
11749 1
12846 1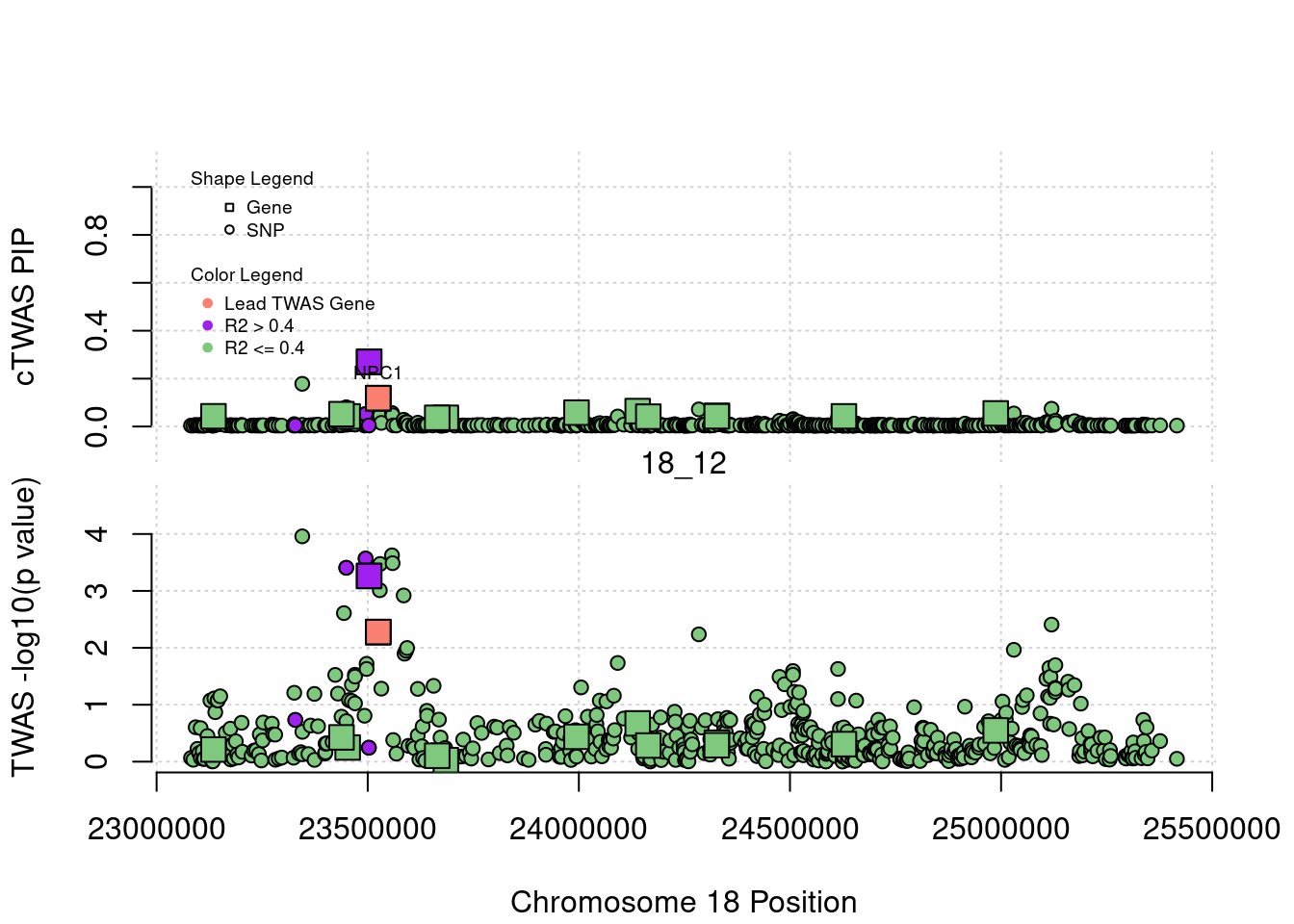
[1] "NPC1"
[1] "18_12"
genename region_tag susie_pip mu2 PVE z
490 LAMA3 18_12 0.03637321 4.547590 1.604624e-07 0.07352941
1823 RIOK3 18_12 0.04235391 5.945423 2.442793e-07 -0.57592109
4718 TMEM241 18_12 0.05164883 7.773134 3.894638e-07 0.88775510
4719 CABLES1 18_12 0.04290307 6.063854 2.523757e-07 0.50802139
5588 OSBPL1A 18_12 0.04483219 6.468596 2.813263e-07 -0.73134328
5590 C18orf8 18_12 0.27002235 23.626498 6.188843e-06 3.45722810
5592 NPC1 18_12 0.11844183 15.530864 1.784478e-06 2.78962603
6613 CABYR 18_12 0.06454667 9.836429 6.159164e-07 1.24637681
6614 ANKRD29 18_12 0.03634908 4.541529 1.601423e-07 0.27106054
8284 TTC39C 18_12 0.05818938 8.875187 5.009930e-07 -0.90476190
12545 LINC01894 18_12 0.05480973 8.321821 4.424727e-07 -1.07352941
12556 LINC01915 18_12 0.04270918 6.022276 2.495125e-07 -0.68750000
12559 RP11-799B12.4 18_12 0.04113012 5.675951 2.264691e-07 0.64285714
12923 RP11-621L6.3 18_12 0.04276926 6.035208 2.504000e-07 -0.65151127
num_eqtl
490 1
1823 2
4718 1
4719 1
5588 1
5590 2
5592 2
6613 1
6614 2
8284 1
12545 1
12556 1
12559 1
12923 2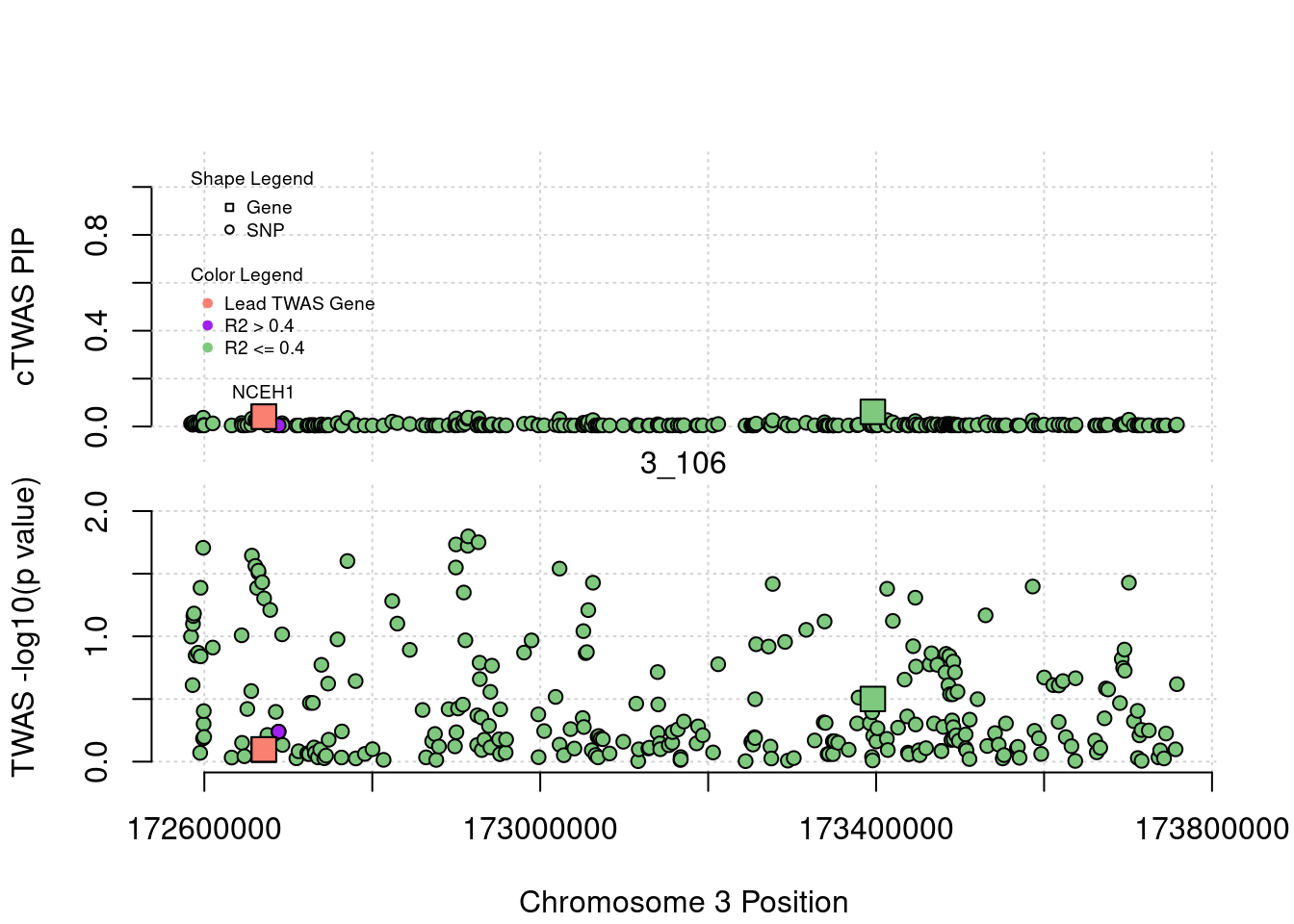
[1] "NCEH1"
[1] "3_106"
genename region_tag susie_pip mu2 PVE z num_eqtl
5959 NCEH1 3_106 0.04126151 4.747794 1.900410e-07 -0.2541279 2
8490 NLGN1 3_106 0.06127625 8.396673 4.991256e-07 1.0027548 1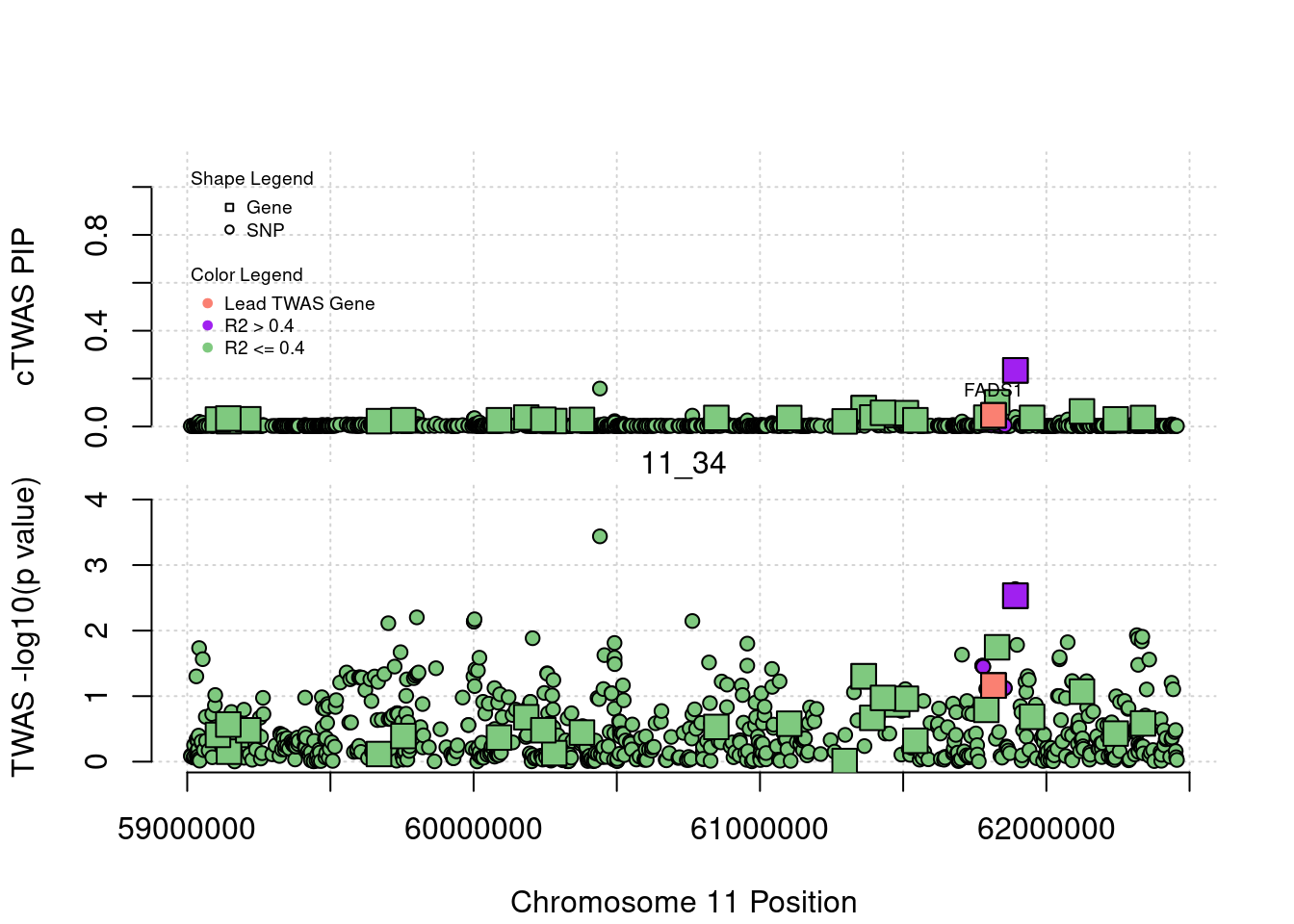
[1] "FADS1"
[1] "11_34"
genename region_tag susie_pip mu2 PVE z
2584 DTX4 11_34 0.02781763 6.861199 1.851529e-07 0.85897436
2593 MS4A6A 11_34 0.03760719 9.622151 3.510375e-07 1.25373134
2594 MS4A4A 11_34 0.02309081 5.160920 1.156051e-07 -0.32835821
2597 CCDC86 11_34 0.03597023 9.213902 3.215121e-07 1.04649029
2621 CD5 11_34 0.03568699 9.141412 3.164708e-07 1.11764706
3883 SCGB1D2 11_34 0.03004532 7.565569 2.205103e-07 -0.89156627
4751 FADS2 11_34 0.10100488 18.791985 1.841304e-06 2.36643571
4752 TMEM258 11_34 0.03697381 9.466299 3.395352e-07 1.39809594
6292 TMEM138 11_34 0.07669069 16.209207 1.205910e-06 1.96202532
6293 FADS1 11_34 0.04673113 11.618934 5.267239e-07 -1.81944444
6296 INCENP 11_34 0.06319853 14.409054 8.833908e-07 1.72072479
6299 MS4A2 11_34 0.02622115 6.321244 1.607921e-07 -0.79104478
7256 CYB561A3 11_34 0.07669069 16.209207 1.205910e-06 1.96202532
7257 PPP1R32 11_34 0.04776414 11.820495 5.477067e-07 1.49792139
7258 ASRGL1 11_34 0.03557232 9.111898 3.144354e-07 1.12328767
8045 FAM111A 11_34 0.02248651 4.919074 1.073040e-07 0.37903226
8061 PATL1 11_34 0.02241093 4.888373 1.062759e-07 -0.29903537
8063 STX3 11_34 0.02639309 6.380928 1.633746e-07 0.82994817
8072 MS4A14 11_34 0.02767618 6.814626 1.829611e-07 0.91549296
8249 VWCE 11_34 0.02151883 4.518039 9.431461e-08 0.02651515
8250 BEST1 11_34 0.03609758 9.246334 3.237860e-07 1.26126126
10279 TMEM216 11_34 0.03789266 9.691540 3.562529e-07 -1.24090909
10481 FAM111B 11_34 0.02911441 7.277705 2.055478e-07 -0.85853659
10784 MPEG1 11_34 0.03036725 7.663054 2.257448e-07 -0.96969697
11219 LRRC10B 11_34 0.05600778 13.290279 7.220926e-07 -1.60000000
11466 MS4A4E 11_34 0.02832931 7.027800 1.931372e-07 0.97058824
11539 FADS3 11_34 0.23438823 26.956236 6.129224e-06 -2.97402597
12060 AP001258.4 11_34 0.03358240 8.584346 2.796594e-07 1.10000000
12307 RP11-794G24.1 11_34 0.02633199 6.359770 1.624559e-07 -0.70680628
12313 RP11-286N22.8 11_34 0.05730574 13.502198 7.506077e-07 -1.61458333
num_eqtl
2584 1
2593 1
2594 1
2597 2
2621 1
3883 1
4751 2
4752 2
6292 1
6293 1
6296 2
6299 1
7256 1
7257 2
7258 1
8045 1
8061 1
8063 3
8072 1
8249 1
8250 1
10279 1
10481 1
10784 1
11219 1
11466 1
11539 1
12060 1
12307 1
12313 1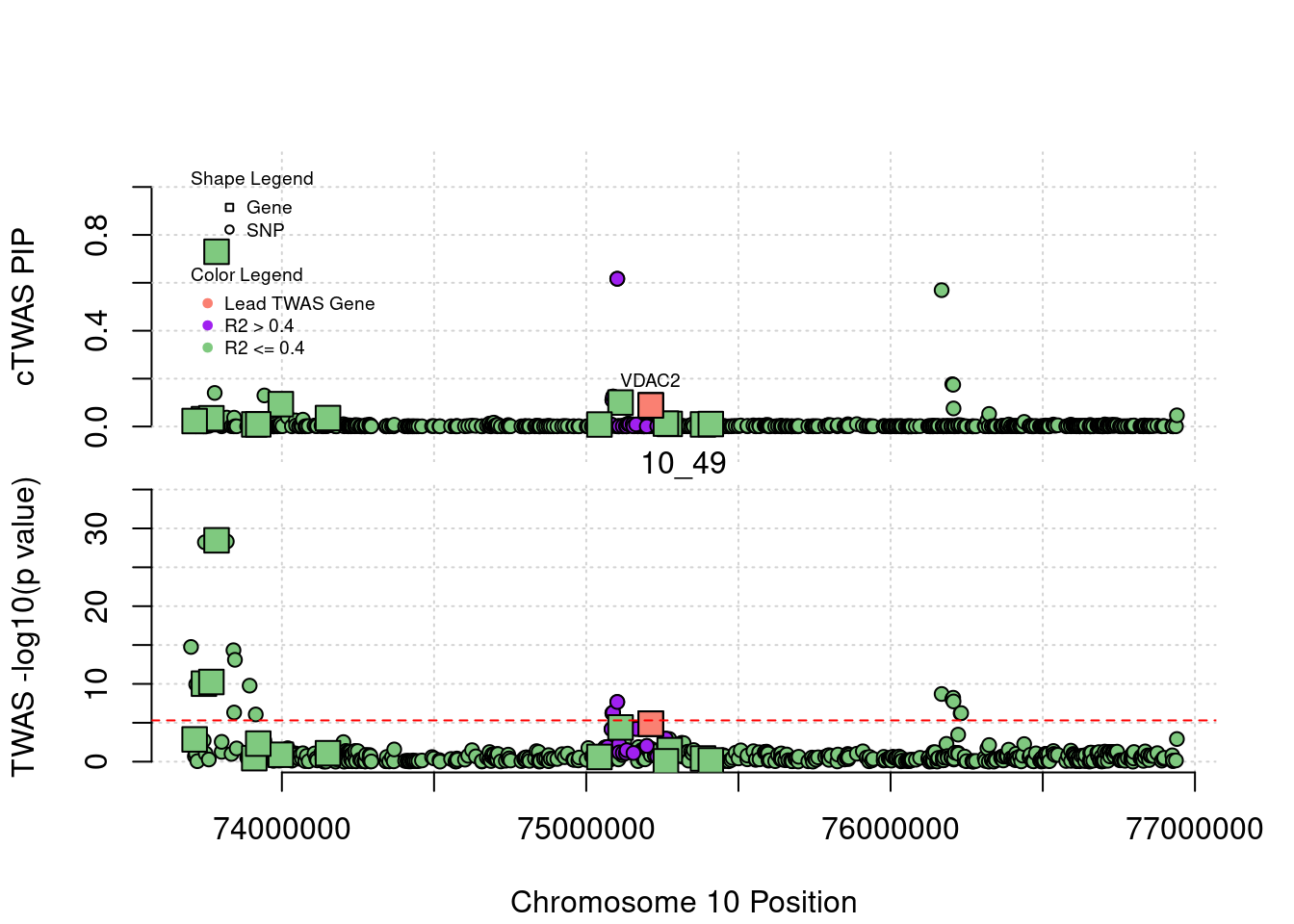
[1] "VDAC2"
[1] "10_49"
genename region_tag susie_pip mu2 PVE z
365 VCL 10_49 0.093489730 20.671759 1.874786e-06 1.5128205
1016 DUSP13 10_49 0.100284705 31.460222 3.060602e-06 4.1086957
3705 PLAU 10_49 0.008878220 5.750547 4.952739e-08 -0.9189189
6232 C10orf11 10_49 0.008899518 5.580033 4.817411e-08 -0.8573335
7858 VDAC2 10_49 0.088251394 21.153236 1.810960e-06 -4.3582090
9287 SEC24C 10_49 0.030788946 46.259307 1.381670e-06 6.4776119
10041 AP3M1 10_49 0.035279184 15.591795 5.336114e-07 -1.7388852
10442 DUPD1 10_49 0.008429365 5.258110 4.299668e-08 -1.1529412
10671 FUT11 10_49 0.034603540 47.907740 1.608187e-06 -6.5522388
11458 ZSWIM8 10_49 0.729519326 109.788618 7.769705e-05 -11.2164948
11550 C10orf55 10_49 0.009058826 11.033338 9.695925e-08 2.8208955
11619 ZNF503-AS1 10_49 0.010899216 10.034096 1.060923e-07 -2.1217391
12696 RP11-399K21.11 10_49 0.012183813 8.284501 9.791742e-08 -0.1303262
12792 RP11-574K11.29 10_49 0.022990638 19.165704 4.274509e-07 -3.2007683
12851 RP11-399K21.14 10_49 0.010355302 6.113393 6.141232e-08 0.3968750
num_eqtl
365 1
1016 1
3705 1
6232 2
7858 1
9287 1
10041 2
10442 1
10671 1
11458 1
11550 1
11619 1
12696 3
12792 2
12851 1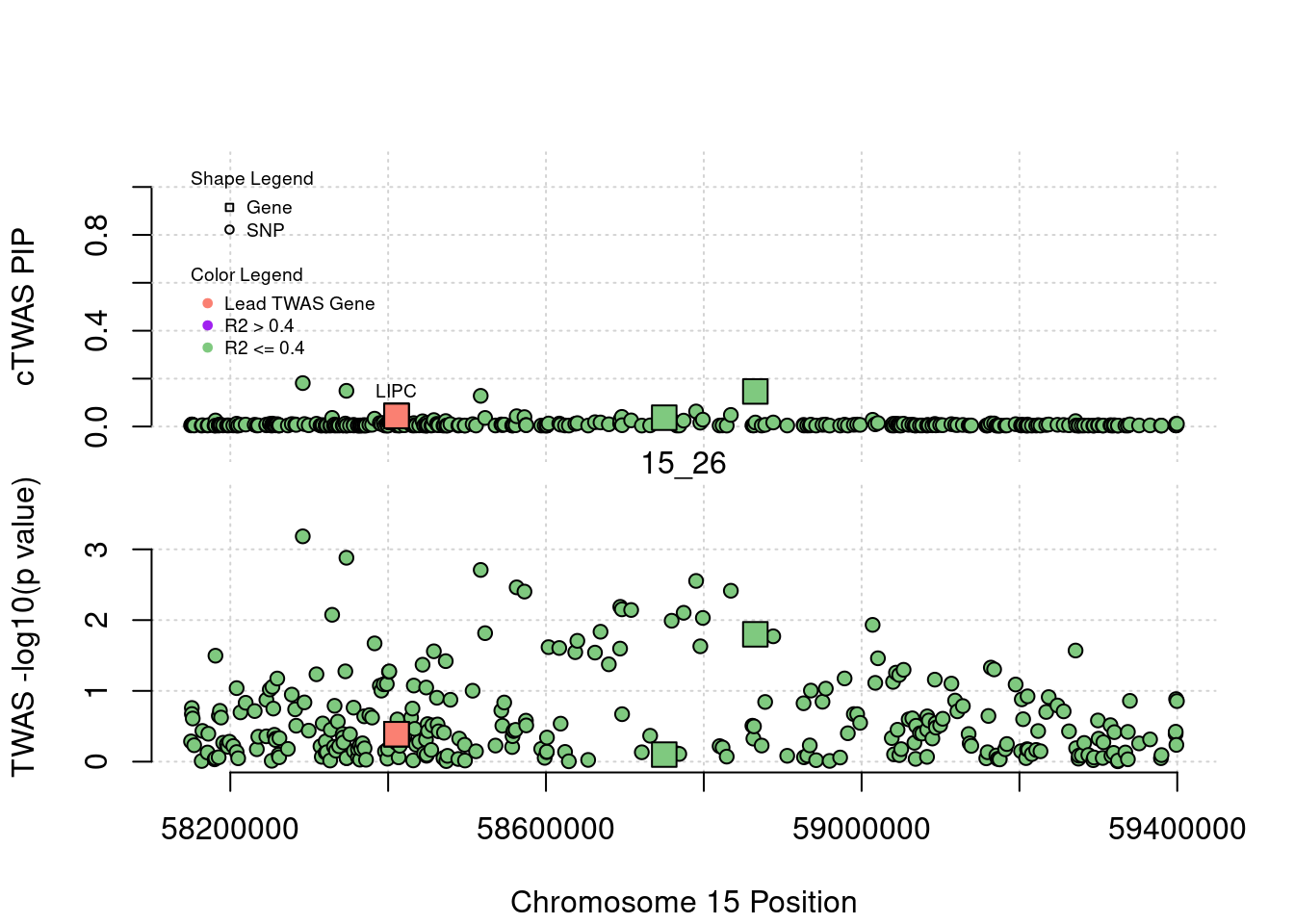
[1] "LIPC"
[1] "15_26"
genename region_tag susie_pip mu2 PVE z num_eqtl
5176 ADAM10 15_26 0.03703397 4.851525 1.742966e-07 0.2598425 1
6857 RNF111 15_26 0.14645672 17.702391 2.515079e-06 -2.4117647 1
7924 LIPC 15_26 0.04422266 6.481400 2.780508e-07 0.8250664 2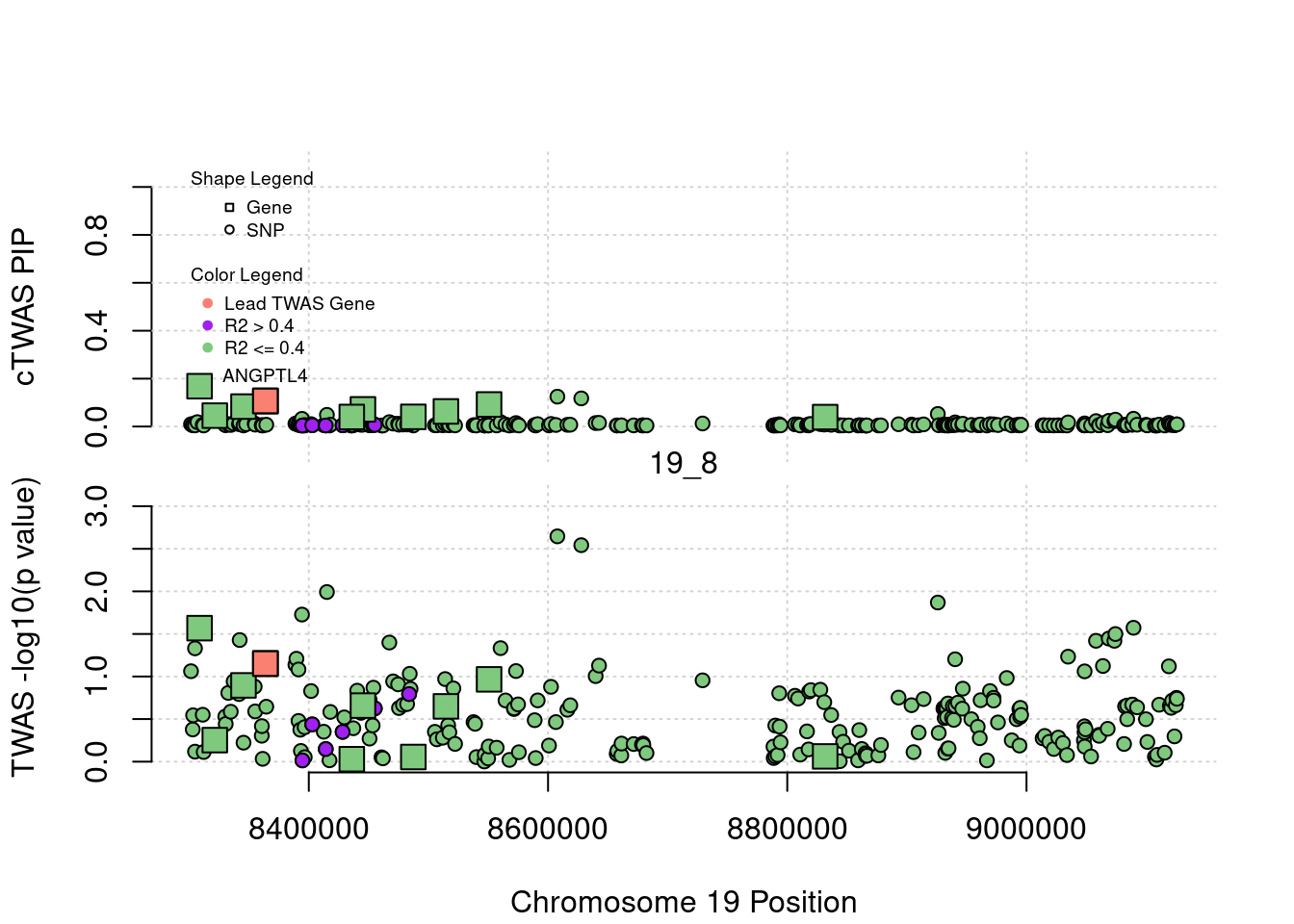
[1] "ANGPTL4"
[1] "19_8"
genename region_tag susie_pip mu2 PVE z
1483 HNRNPM 19_8 0.07173199 10.132429 7.050776e-07 1.22767857
1484 MARCH2 19_8 0.03980176 4.689135 1.810529e-07 0.08108108
4599 PRAM1 19_8 0.04039230 4.824423 1.890403e-07 -0.14864865
4601 ZNF414 19_8 0.06310548 8.941693 5.473905e-07 1.21505376
5673 MYO1F 19_8 0.09224704 12.484718 1.117227e-06 1.60696522
8213 ANGPTL4 19_8 0.10682901 13.868140 1.437202e-06 1.80882353
8214 CD320 19_8 0.16838445 18.229956 2.977817e-06 -2.21081081
8217 ZNF558 19_8 0.03938491 4.592445 1.754625e-07 -0.16666667
10274 KANK3 19_8 0.08336899 11.535792 9.329586e-07 -1.52564103
12631 NDUFA7 19_8 0.04559754 5.939216 2.627126e-07 0.58520936
num_eqtl
1483 1
1484 1
4599 1
4601 1
5673 2
8213 1
8214 1
8217 1
10274 1
12631 2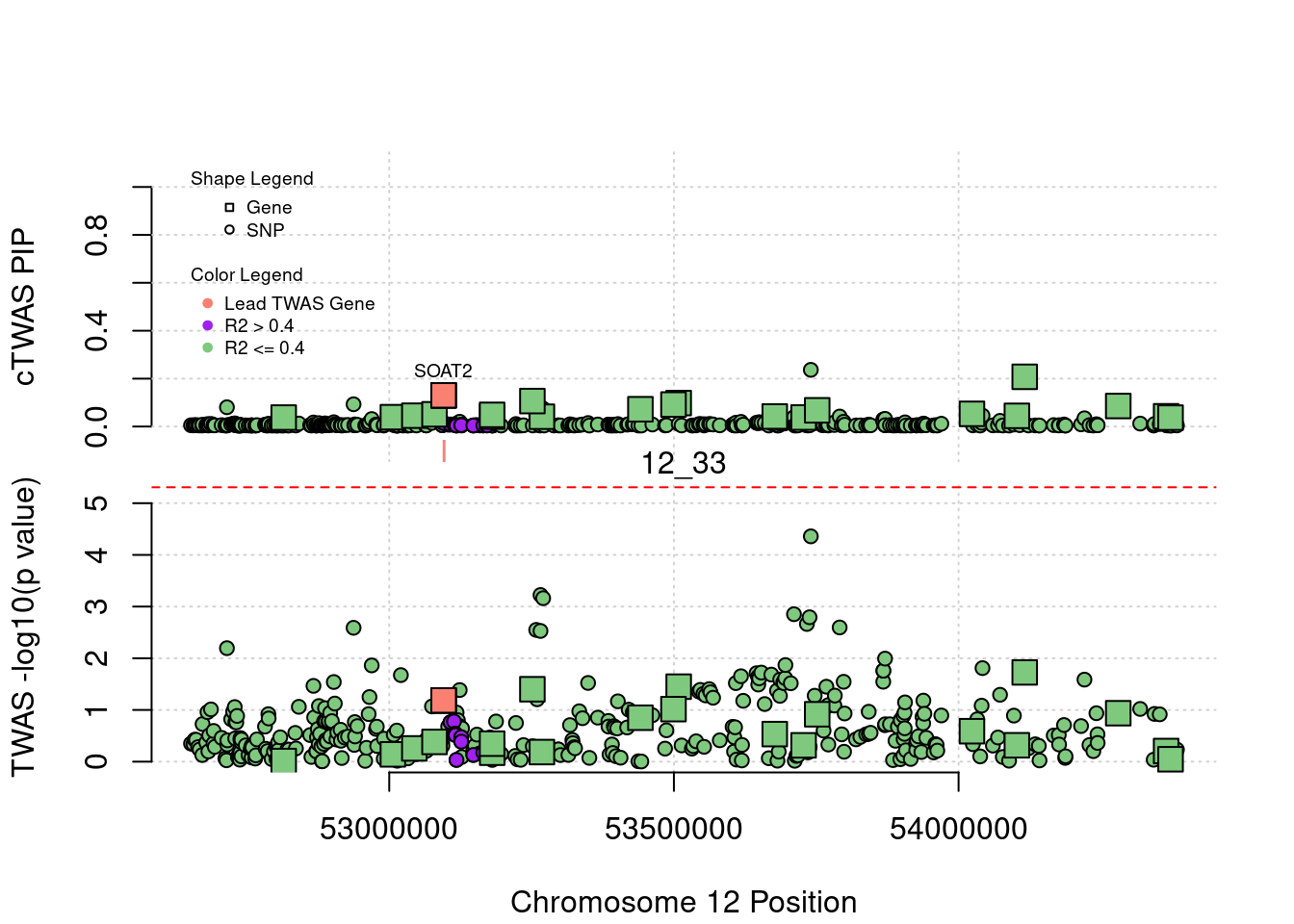
[1] "SOAT2"
[1] "12_33"
genename region_tag susie_pip mu2 PVE z
225 CALCOCO1 12_33 0.03778982 4.686097 1.717895e-07 0.70165746
585 EIF4B 12_33 0.03903239 4.983038 1.886817e-07 -0.35525461
1411 CBX5 12_33 0.08559634 12.255819 1.017672e-06 1.57352941
2670 TNS2 12_33 0.04537199 6.366685 2.802280e-07 0.59154930
3751 SMUG1 12_33 0.04475377 6.240417 2.709278e-07 0.70238095
4825 ATP5G2 12_33 0.04203984 5.665028 2.310327e-07 1.05090258
4843 ESPL1 12_33 0.04110618 5.458542 2.176678e-07 -0.44979920
5391 GPR84 12_33 0.04287162 5.845147 2.430948e-07 -0.49253731
5392 NPFF 12_33 0.09801321 13.528345 1.286292e-06 -2.10294118
5397 MAP3K12 12_33 0.09193530 12.926084 1.152815e-06 -1.66304348
5400 CSAD 12_33 0.04138287 5.520202 2.216083e-07 -0.42491301
5405 ZNF740 12_33 0.04876555 7.031210 3.326240e-07 0.75757576
7211 ZNF385A 12_33 0.03712370 4.522891 1.628838e-07 -0.12643678
8215 SPRYD3 12_33 0.05104420 7.452467 3.690260e-07 0.81437126
8216 SOAT2 12_33 0.13014201 16.218172 2.047528e-06 1.84210526
8583 KRT4 12_33 0.03744922 4.602988 1.672219e-07 -0.03296703
9784 MFSD5 12_33 0.10628486 14.293058 1.473693e-06 2.05639773
10799 HOXC6 12_33 0.05307684 7.813000 4.022845e-07 -1.13186813
11254 PRR13 12_33 0.07229305 10.678174 7.488658e-07 1.47058824
12161 RP11-834C11.4 12_33 0.20769029 20.766706 4.184025e-06 2.35007196
12433 CISTR 12_33 0.06779696 10.080773 6.630015e-07 -1.55036730
num_eqtl
225 1
585 2
1411 1
2670 1
3751 1
4825 2
4843 1
5391 1
5392 1
5397 1
5400 3
5405 1
7211 1
8215 1
8216 1
8583 1
9784 2
10799 1
11254 1
12161 2
12433 2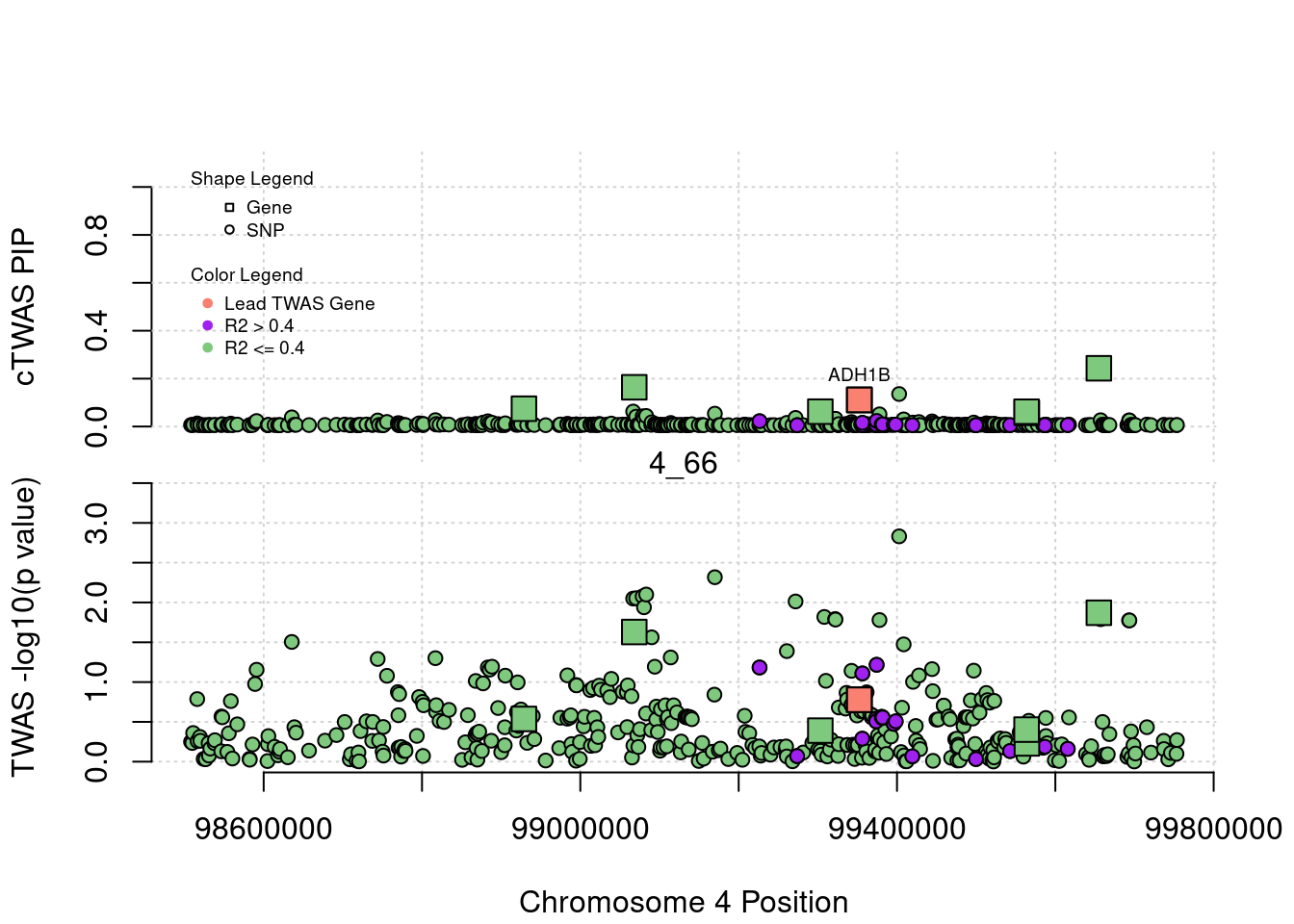
[1] "ADH1B"
[1] "4_66"
genename region_tag susie_pip mu2 PVE z
5323 MTTP 4_66 0.05713053 5.733633 3.177669e-07 0.5394737
5984 TRMT10A 4_66 0.06199325 6.490226 3.903145e-07 0.8490721
6388 EIF4E 4_66 0.07451430 8.200439 5.927712e-07 -1.0566038
10617 ADH1B 4_66 0.11117753 11.959347 1.289837e-06 -1.3819444
12092 ADH1C 4_66 0.06215161 6.513876 3.927374e-07 0.8287844
12107 RP11-766F14.2 4_66 0.24311774 19.587066 4.619516e-06 -2.4715447
12861 RP11-571L19.8 4_66 0.16383078 15.676799 2.491514e-06 -2.2652120
num_eqtl
5323 1
5984 2
6388 1
10617 1
12092 3
12107 1
12861 2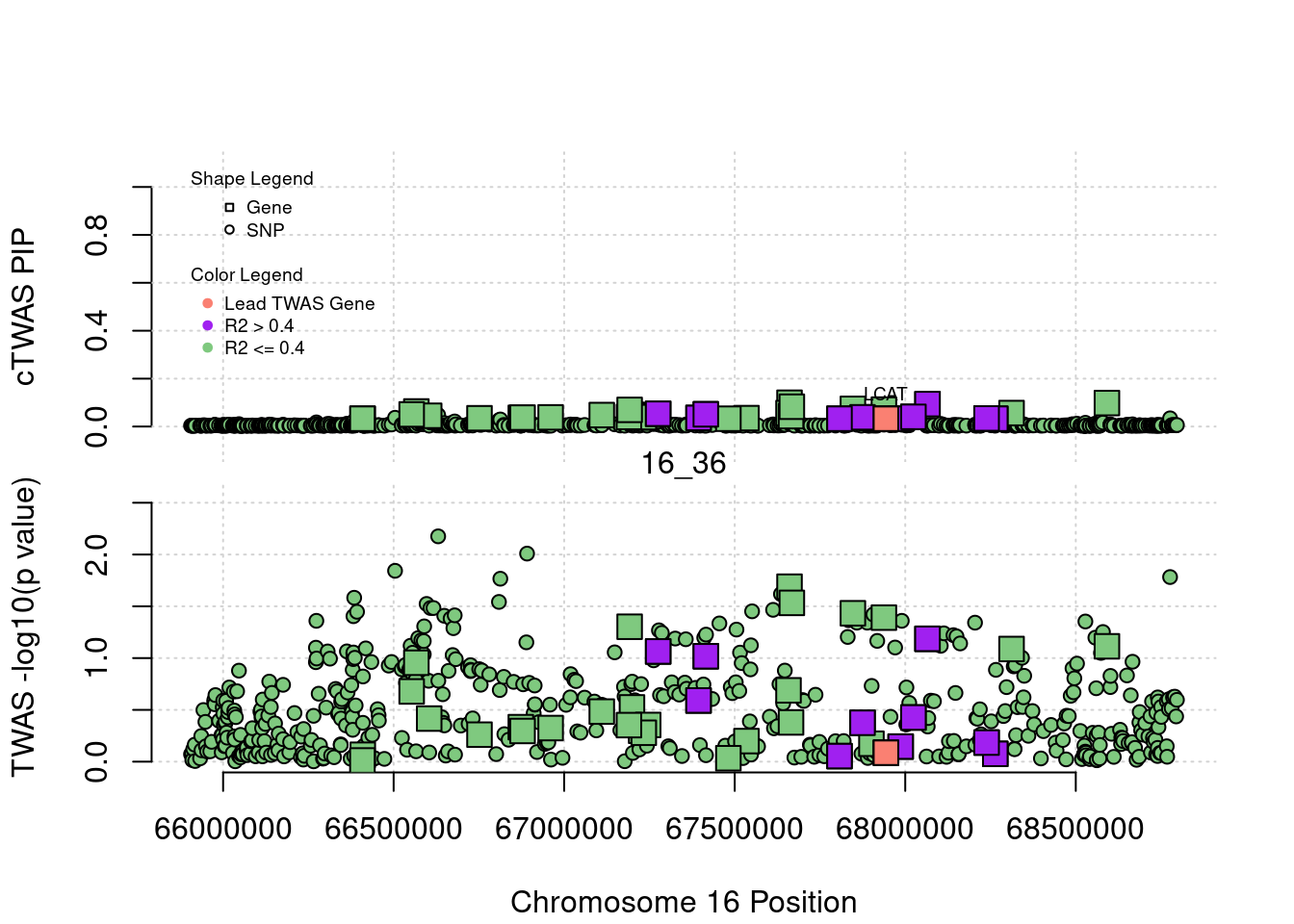
[1] "LCAT"
[1] "16_36"
genename region_tag susie_pip mu2 PVE z
385 EDC4 16_36 0.03864999 6.095171 2.285313e-07 0.80392157
388 FAM65A 16_36 0.03517201 5.230153 1.784523e-07 0.47712418
828 NFATC3 16_36 0.09364552 14.301824 1.299239e-06 1.84401694
1285 CMTM1 16_36 0.06293118 10.591698 6.466091e-07 -1.59405941
1855 ELMO3 16_36 0.04885492 8.250621 3.910258e-07 -1.03759398
1857 NUTF2 16_36 0.07385851 12.079624 8.654946e-07 2.08670520
1859 TSNAXIP1 16_36 0.03266295 4.552064 1.442362e-07 -0.14606742
1866 ACD 16_36 0.09943582 14.865698 1.433965e-06 2.31791908
1868 PARD6A 16_36 0.05634737 9.568173 5.230138e-07 -1.26797492
1881 SLC7A6OS 16_36 0.05629963 9.560069 5.221280e-07 -1.74074074
1882 SLC7A6 16_36 0.03260009 4.534436 1.434011e-07 0.20000000
1884 ESRP2 16_36 0.03381406 4.869319 1.597261e-07 0.44761905
3786 ENKD1 16_36 0.04331642 7.142583 3.001361e-07 0.80970301
3888 LRRC29 16_36 0.03781157 5.893867 2.161899e-07 0.66929134
3892 C16orf70 16_36 0.04785396 8.059797 3.741557e-07 -0.97706422
4527 PRMT7 16_36 0.03526055 5.253201 1.796898e-07 -0.42405017
4875 DYNC1LI2 16_36 0.03454736 5.065907 1.697784e-07 -0.60000000
4876 FHOD1 16_36 0.04326467 7.131586 2.993160e-07 0.76839164
4878 SLC9A5 16_36 0.03943120 6.278896 2.401783e-07 0.63377727
5524 CMTM3 16_36 0.04490788 7.474585 3.256267e-07 -0.87084871
7028 NAE1 16_36 0.03648713 5.566752 1.970389e-07 0.73170732
7035 TPPP3 16_36 0.03594180 5.428632 1.892782e-07 -1.13414634
7036 ZDHHC1 16_36 0.05104559 8.654980 4.285828e-07 -1.66463415
7037 ATP6V0D1 16_36 0.03262864 4.542446 1.437802e-07 0.09156734
7039 C16orf86 16_36 0.08265370 13.130075 1.052786e-06 2.17919075
8018 BEAN1 16_36 0.03260259 4.535136 1.434342e-07 -0.17833693
8117 DUS2 16_36 0.03280281 4.591212 1.460995e-07 -0.36470588
8868 CES3 16_36 0.03831276 6.014725 2.235474e-07 -0.71603194
8871 PDP2 16_36 0.03733128 5.776568 2.091959e-07 0.66544118
9492 EXOC3L1 16_36 0.03995691 6.400539 2.480954e-07 -0.76562500
9813 DDX28 16_36 0.04054143 6.533958 2.569720e-07 0.88775510
10534 PLEKHG4 16_36 0.05342158 9.075039 4.703007e-07 -1.71529720
11243 PSMB10 16_36 0.07059545 11.658849 7.984409e-07 2.04651163
11245 E2F4 16_36 0.06956550 11.522205 7.775708e-07 -1.96167427
11360 LCAT 16_36 0.03254254 4.518255 1.426371e-07 -0.22727273
11505 CKLF 16_36 0.05062503 8.578871 4.213140e-07 -1.25405405
12077 LINC00920 16_36 0.03256396 4.524282 1.429214e-07 -0.03193996
13014 RP11-615I2.6 16_36 0.09809835 14.738998 1.402620e-06 -1.76835994
num_eqtl
385 1
388 1
828 2
1285 1
1855 1
1857 1
1859 1
1866 1
1868 2
1881 1
1882 1
1884 1
3786 2
3888 1
3892 1
4527 3
4875 1
4876 2
4878 2
5524 1
7028 1
7035 1
7036 1
7037 2
7039 1
8018 2
8117 1
8868 2
8871 1
9492 1
9813 1
10534 2
11243 1
11245 2
11360 1
11505 1
12077 2
13014 2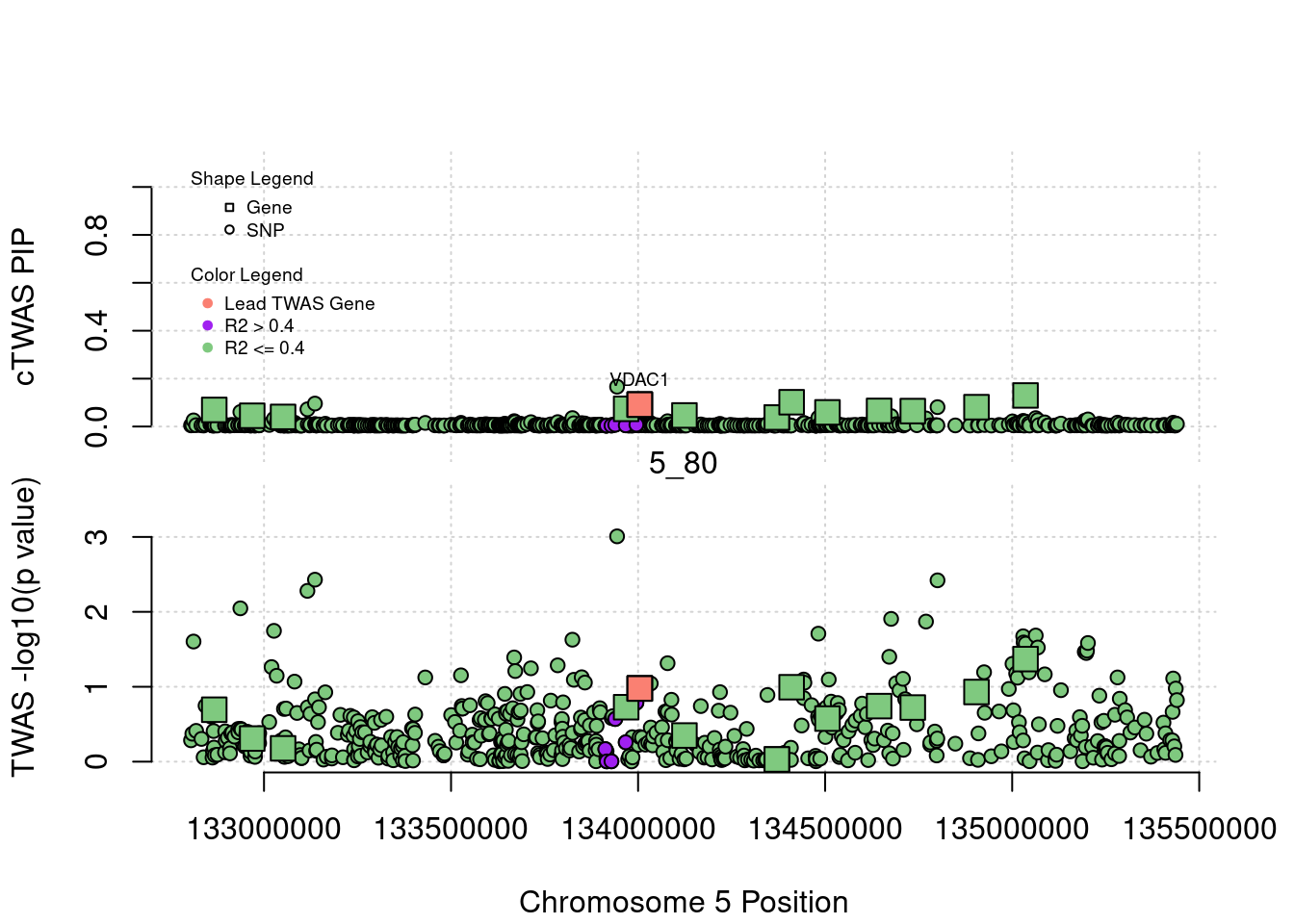
[1] "VDAC1"
[1] "5_80"
genename region_tag susie_pip mu2 PVE z
116 CDKL3 5_80 0.03761542 4.527426 1.652067e-07 -0.08130081
737 PITX1 5_80 0.12975728 16.073676 2.023286e-06 2.02631579
819 AFF4 5_80 0.04504579 6.184097 2.702346e-07 0.68807339
1055 TCF7 5_80 0.04643238 6.463251 2.911270e-07 -0.75974026
2928 C5orf15 5_80 0.07397067 10.775697 7.732419e-07 1.31781707
3415 UBE2B 5_80 0.03761542 4.527426 1.652067e-07 -0.08130081
4524 PCBD2 5_80 0.08078693 11.598468 9.089755e-07 -1.56504419
6523 SAR1B 5_80 0.06538367 9.627799 6.106701e-07 1.33644860
7688 GDF9 5_80 0.06939581 10.181266 6.854021e-07 1.27372263
7713 CAMLG 5_80 0.06450132 9.501662 5.945366e-07 1.31225633
8599 HSPA4 5_80 0.04010050 5.114777 1.989697e-07 0.43421053
11375 VDAC1 5_80 0.09151248 12.766560 1.133351e-06 1.61904762
11878 CDKN2AIPNL 5_80 0.10152738 13.744242 1.353675e-06 1.63768116
12174 LINC01843 5_80 0.05909288 8.689756 4.981420e-07 -1.11235955
num_eqtl
116 1
737 1
819 1
1055 1
2928 2
3415 1
4524 2
6523 1
7688 1
7713 2
8599 1
11375 1
11878 1
12174 1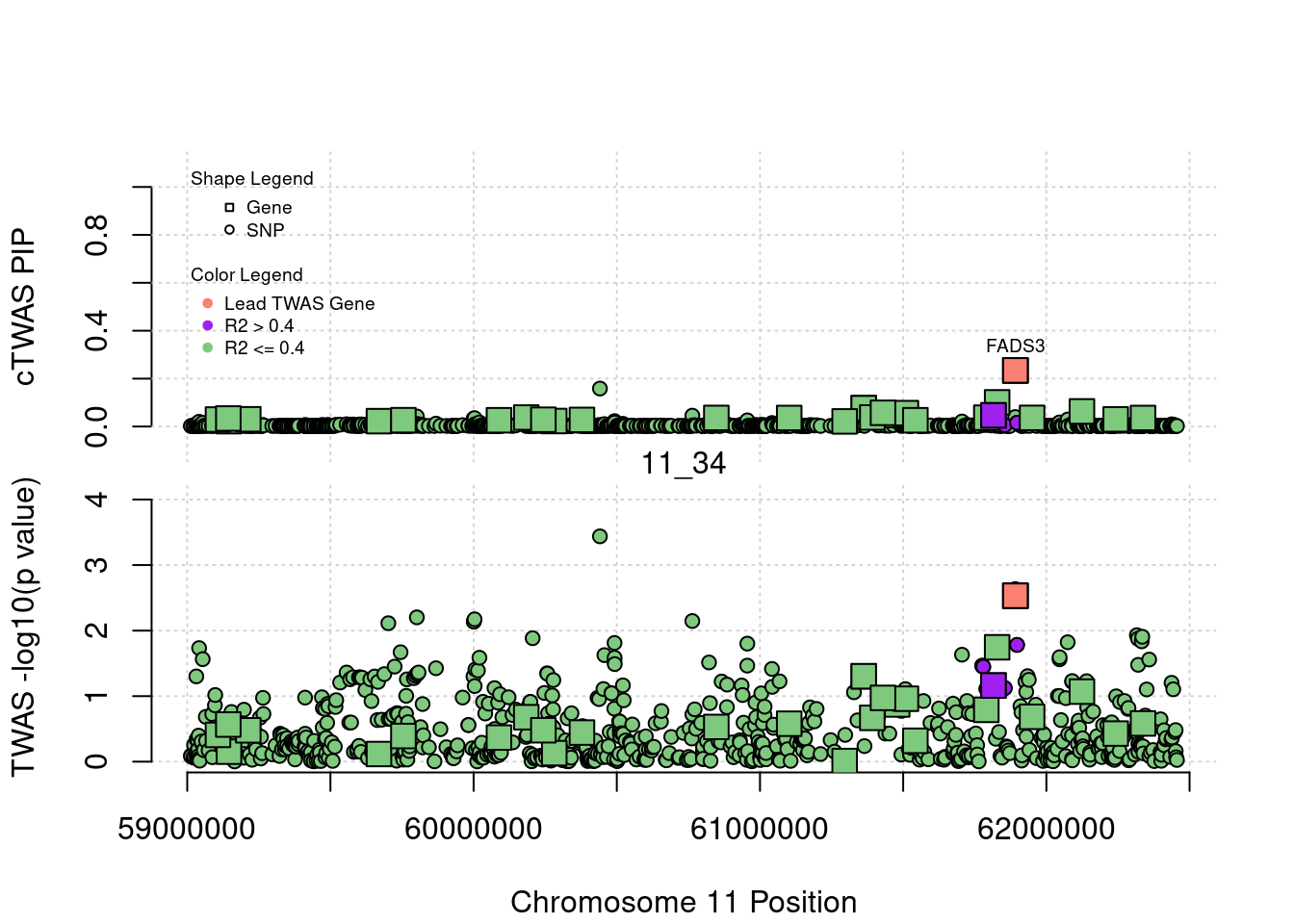
[1] "FADS3"
[1] "11_34"
genename region_tag susie_pip mu2 PVE z
2584 DTX4 11_34 0.02781763 6.861199 1.851529e-07 0.85897436
2593 MS4A6A 11_34 0.03760719 9.622151 3.510375e-07 1.25373134
2594 MS4A4A 11_34 0.02309081 5.160920 1.156051e-07 -0.32835821
2597 CCDC86 11_34 0.03597023 9.213902 3.215121e-07 1.04649029
2621 CD5 11_34 0.03568699 9.141412 3.164708e-07 1.11764706
3883 SCGB1D2 11_34 0.03004532 7.565569 2.205103e-07 -0.89156627
4751 FADS2 11_34 0.10100488 18.791985 1.841304e-06 2.36643571
4752 TMEM258 11_34 0.03697381 9.466299 3.395352e-07 1.39809594
6292 TMEM138 11_34 0.07669069 16.209207 1.205910e-06 1.96202532
6293 FADS1 11_34 0.04673113 11.618934 5.267239e-07 -1.81944444
6296 INCENP 11_34 0.06319853 14.409054 8.833908e-07 1.72072479
6299 MS4A2 11_34 0.02622115 6.321244 1.607921e-07 -0.79104478
7256 CYB561A3 11_34 0.07669069 16.209207 1.205910e-06 1.96202532
7257 PPP1R32 11_34 0.04776414 11.820495 5.477067e-07 1.49792139
7258 ASRGL1 11_34 0.03557232 9.111898 3.144354e-07 1.12328767
8045 FAM111A 11_34 0.02248651 4.919074 1.073040e-07 0.37903226
8061 PATL1 11_34 0.02241093 4.888373 1.062759e-07 -0.29903537
8063 STX3 11_34 0.02639309 6.380928 1.633746e-07 0.82994817
8072 MS4A14 11_34 0.02767618 6.814626 1.829611e-07 0.91549296
8249 VWCE 11_34 0.02151883 4.518039 9.431461e-08 0.02651515
8250 BEST1 11_34 0.03609758 9.246334 3.237860e-07 1.26126126
10279 TMEM216 11_34 0.03789266 9.691540 3.562529e-07 -1.24090909
10481 FAM111B 11_34 0.02911441 7.277705 2.055478e-07 -0.85853659
10784 MPEG1 11_34 0.03036725 7.663054 2.257448e-07 -0.96969697
11219 LRRC10B 11_34 0.05600778 13.290279 7.220926e-07 -1.60000000
11466 MS4A4E 11_34 0.02832931 7.027800 1.931372e-07 0.97058824
11539 FADS3 11_34 0.23438823 26.956236 6.129224e-06 -2.97402597
12060 AP001258.4 11_34 0.03358240 8.584346 2.796594e-07 1.10000000
12307 RP11-794G24.1 11_34 0.02633199 6.359770 1.624559e-07 -0.70680628
12313 RP11-286N22.8 11_34 0.05730574 13.502198 7.506077e-07 -1.61458333
num_eqtl
2584 1
2593 1
2594 1
2597 2
2621 1
3883 1
4751 2
4752 2
6292 1
6293 1
6296 2
6299 1
7256 1
7257 2
7258 1
8045 1
8061 1
8063 3
8072 1
8249 1
8250 1
10279 1
10481 1
10784 1
11219 1
11466 1
11539 1
12060 1
12307 1
12313 1
[1] "APOC2"
[1] "19_31"
genename region_tag susie_pip mu2 PVE z
123 TRAPPC6A 19_31 0.09915894 11.601044 1.115936e-06 -1.43410853
578 ZNF112 19_31 0.04768345 4.787592 2.214600e-07 0.15789474
842 PVR 19_31 0.04845305 4.935179 2.319714e-07 0.40965622
2052 CLPTM1 19_31 0.05877400 6.719066 3.830933e-07 0.77009814
2057 CKM 19_31 0.04813746 4.874945 2.276477e-07 0.36165100
2059 PPP1R13L 19_31 0.06371252 7.466884 4.615031e-07 0.94933333
3338 CD3EAP 19_31 0.05192595 5.573765 2.807653e-07 0.56301629
3949 FOSB 19_31 0.04665997 4.587703 2.076587e-07 0.11538462
3951 RTN2 19_31 0.04914093 5.065148 2.414604e-07 0.36842105
4280 NECTIN2 19_31 0.11432276 12.946862 1.435845e-06 1.65828323
4282 TOMM40 19_31 0.05203045 5.592327 2.822673e-07 -0.59268930
5668 GEMIN7 19_31 0.09449659 11.147586 1.021898e-06 -1.47151206
7051 ZNF233 19_31 0.22438507 19.499838 4.244586e-06 2.65656566
7052 ZNF235 19_31 0.13166512 14.291498 1.825404e-06 2.28712871
8611 ZNF296 19_31 0.04678271 4.611904 2.093033e-07 -0.19444444
10231 CEACAM19 19_31 0.15596201 15.922324 2.408994e-06 1.95939086
10300 BCAM 19_31 0.07444369 8.914531 6.437790e-07 -1.15789474
10487 BLOC1S3 19_31 0.04981758 5.191270 2.508804e-07 -0.41492537
11402 PPM1N 19_31 0.05741543 6.502596 3.621811e-07 -0.76470588
11503 IGSF23 19_31 0.04710462 4.675075 2.136301e-07 0.09745763
11829 APOC2 19_31 0.04744721 4.741844 2.182571e-07 -0.21225665
12617 ZNF285 19_31 0.05262760 5.697703 2.908867e-07 -0.63909774
13122 ZNF229 19_31 0.05013876 5.250555 2.553814e-07 -0.54634146
num_eqtl
123 1
578 1
842 2
2052 2
2057 2
2059 1
3338 2
3949 1
3951 1
4280 2
4282 1
5668 2
7051 1
7052 1
8611 1
10231 1
10300 1
10487 1
11402 1
11503 1
11829 2
12617 1
13122 1#run APOE locus again using full SNPs
# focus <- "APOE"
# region_tag <- ctwas_res$region_tag[which(ctwas_res$genename==focus)]
#
# locus_plot(region_tag, label="TWAS", rerun_ctwas = T)
#
# mtext(text=region_tag)
#
# print(focus)
# print(region_tag)
# print(ctwas_gene_res[ctwas_gene_res$region_tag==region_tag,report_cols,])Locus Plots - False positives
This section produces locus plots for all bystander genes with PIP>0.8 (false positives). The highlighted gene at each region is the false positive gene.
false_positives <- ctwas_gene_res$genename[ctwas_gene_res$genename %in% unrelated_genes & ctwas_gene_res$susie_pip>0.8]
for (i in 1:length(false_positives)){
focus <- false_positives[i]
region_tag <- ctwas_res$region_tag[which(ctwas_res$genename==focus)]
locus_plot3(region_tag, focus=focus)
mtext(text=region_tag)
print(focus)
print(region_tag)
print(ctwas_gene_res[ctwas_gene_res$region_tag==region_tag,report_cols,])
#genes at this locus that are in known annotations
ctwas_gene_res$genename[ctwas_gene_res$region_tag==region_tag][ctwas_gene_res$genename[ctwas_gene_res$region_tag==region_tag] %in% known_annotations]
}Genes with many eQTL
#distribution of number of eQTL for all imputed genes (after dropping ambiguous variants)
table(ctwas_gene_res$num_eqtl)
1 2 3 4 5 6
6701 2825 539 76 8 1 #all genes with 4+ eQTL
ctwas_gene_res[ctwas_gene_res$num_eqtl>3,] chrom id pos type region_tag1 region_tag2 cs_index
11112 1 ENSG00000204219.9 23381993 gene 1 16 0
531 1 ENSG00000057468.6 75779738 gene 1 47 0
3232 1 ENSG00000116791.13 74702183 gene 1 47 0
4675 1 ENSG00000134184.12 109673814 gene 1 67 0
12685 2 ENSG00000269973.1 9843557 gene 2 6 0
3049 2 ENSG00000115129.13 24064728 gene 2 14 0
3478 2 ENSG00000119771.14 23385014 gene 2 14 0
3160 2 ENSG00000116031.8 70823530 gene 2 47 0
5240 2 ENSG00000138376.10 214808273 gene 2 127 0
106 2 ENSG00000006607.13 241355498 gene 2 144 0
9645 2 ENSG00000180902.17 241734472 gene 2 144 0
5903 3 ENSG00000144455.13 4368850 gene 3 4 0
255 3 ENSG00000016391.10 53825497 gene 3 36 0
10512 3 ENSG00000189366.9 125937048 gene 3 78 0
12886 3 ENSG00000272970.1 184727389 gene 3 113 0
4063 3 ENSG00000127252.5 193241004 gene 3 118 0
10123 4 ENSG00000185619.18 705427 gene 4 2 0
9009 4 ENSG00000174137.12 1680955 gene 4 3 0
3830 4 ENSG00000124406.16 42619261 gene 4 34 0
10932 4 ENSG00000198515.13 48006903 gene 4 37 0
7510 4 ENSG00000163644.14 88284772 gene 4 59 0
6026 5 ENSG00000145779.7 119268542 gene 5 72 0
7759 5 ENSG00000164904.17 126538297 gene 5 77 0
6046 6 ENSG00000145949.9 2680498 gene 6 3 0
11722 6 ENSG00000230438.6 2876301 gene 6 3 0
11166 6 ENSG00000204516.9 31494471 gene 6 26 0
11126 6 ENSG00000204301.6 32205480 gene 6 26 0
12047 6 ENSG00000244731.7 31973120 gene 6 26 0
8639 6 ENSG00000170915.8 52356177 gene 6 39 0
6892 7 ENSG00000157927.16 4817947 gene 7 6 0
10553 7 ENSG00000196247.11 64665954 gene 7 43 0
24 7 ENSG00000002933.7 150788313 gene 7 93 0
233 7 ENSG00000013374.15 151341248 gene 7 94 0
6151 8 ENSG00000147576.15 66431109 gene 8 50 0
9587 8 ENSG00000180155.19 142777665 gene 8 93 0
2340 9 ENSG00000107099.15 214719 gene 9 1 0
5059 9 ENSG00000137074.18 33024705 gene 9 25 0
11846 9 ENSG00000235387.2 35865700 gene 9 27 0
5016 9 ENSG00000136816.15 129795323 gene 9 67 0
7973 10 ENSG00000166295.8 72178808 gene 10 48 0
4628 10 ENSG00000133661.15 79980164 gene 10 51 0
7970 10 ENSG00000166275.15 102846409 gene 10 66 0
6451 10 ENSG00000151893.14 118655864 gene 10 73 1
748 11 ENSG00000069696.6 627517 gene 11 1 0
9292 11 ENSG00000177042.14 688091 gene 11 1 0
10066 11 ENSG00000185201.16 307025 gene 11 1 0
8125 11 ENSG00000167311.13 3633446 gene 11 3 0
9633 11 ENSG00000180785.8 4643212 gene 11 3 0
6258 11 ENSG00000149089.12 34916384 gene 11 23 0
7918 11 ENSG00000166002.6 93498608 gene 11 53 0
5351 12 ENSG00000139197.10 7186351 gene 12 7 0
11940 12 ENSG00000240771.6 57605318 gene 12 36 0
10033 12 ENSG00000184967.6 132005752 gene 12 81 0
346 13 ENSG00000032742.17 20566173 gene 13 2 0
1836 13 ENSG00000102683.7 23180605 gene 13 4 0
4460 14 ENSG00000131966.13 58134632 gene 14 26 0
167 14 ENSG00000009830.11 77320520 gene 14 36 0
250 14 ENSG00000015133.18 91323427 gene 14 46 0
9780 14 ENSG00000182512.4 95533077 gene 14 49 0
11058 14 ENSG00000203485.12 104689664 gene 14 55 0
4192 15 ENSG00000128928.8 40361837 gene 15 14 0
1890 16 ENSG00000103148.15 112241 gene 16 1 0
1901 16 ENSG00000103199.13 4759007 gene 16 4 0
6591 16 ENSG00000153786.12 85009554 gene 16 49 0
5544 16 ENSG00000141013.16 89846600 gene 16 54 0
8194 17 ENSG00000167693.16 887726 gene 17 1 0
2442 17 ENSG00000108352.11 40170611 gene 17 23 0
5566 17 ENSG00000141295.13 47834936 gene 17 28 0
8119 17 ENSG00000167280.16 79074789 gene 17 44 0
8321 18 ENSG00000168461.12 9685412 gene 18 7 0
1814 18 ENSG00000101638.13 46714149 gene 18 26 0
1490 19 ENSG00000099817.11 1078821 gene 19 2 0
9732 19 ENSG00000182087.12 1000167 gene 19 2 0
2086 19 ENSG00000104980.7 7926838 gene 19 7 0
8210 19 ENSG00000167766.18 52618483 gene 19 36 0
8643 19 ENSG00000170949.17 53087254 gene 19 36 0
8644 19 ENSG00000170954.11 53093958 gene 19 36 0
11057 19 ENSG00000203326.11 53365182 gene 19 36 0
506 20 ENSG00000054793.13 51703540 gene 20 31 0
9888 22 ENSG00000183569.17 42553443 gene 22 18 0
11274 22 ENSG00000205593.11 50313740 gene 22 24 0
1961 15 ENSG00000103811.15 78937302 gene 15 37 0
1964 15 ENSG00000103876.11 80147571 gene 15 37 0
12376 15 ENSG00000259417.2 80252110 gene 15 37 0
130 16 ENSG00000007516.13 1333326 gene 16 2 0
susie_pip mu2 region_tag PVE genename
11112 0.2165531827 21.507177 1_16 4.518127e-06 TCEA3
531 0.0440342175 6.180003 1_47 2.639912e-07 MSH4
3232 0.0379537718 4.814549 1_47 1.772642e-07 CRYZ
4675 0.0244600436 8.346662 1_67 1.980526e-07 GSTM1
12685 0.0306431693 4.730411 2_6 1.406187e-07 RP11-95D17.1
3049 0.1280649183 13.581111 2_14 1.687236e-06 TP53I3
3478 0.0571029917 6.005463 2_14 3.326717e-07 KLHL29
3160 0.0238575187 4.673566 2_47 1.081643e-07 CD207
5240 0.0346456395 4.623896 2_127 1.554058e-07 BARD1
106 0.0369699941 4.535566 2_144 1.626640e-07 FARP2
9645 0.1213386828 15.600616 2_144 1.836333e-06 D2HGDH
5903 0.0244258772 6.426375 3_4 1.522743e-07 SUMF1
255 0.0507750627 9.180983 3_36 4.522203e-07 CHDH
10512 0.0519452702 5.571409 3_78 2.807511e-07 ALG1L
12886 0.0446447276 4.528488 3_113 1.961254e-07 RP11-329B9.4
4063 0.0461679442 4.572799 3_118 2.048014e-07 HRASLS
10123 0.0415752691 4.651024 4_2 1.875832e-07 PCGF3
9009 0.0546096961 5.324484 4_3 2.820705e-07 FAM53A
3830 0.1323480952 14.056297 4_34 1.804675e-06 ATP8A1
10932 0.1225852651 16.574520 4_37 1.971014e-06 CNGA1
7510 0.0596633378 5.060200 4_59 2.928772e-07 PPM1K
6026 0.1222296023 14.553489 5_72 1.725655e-06 TNFAIP8
7759 0.0574487010 4.600750 5_77 2.564007e-07 ALDH7A1
6046 0.0439488504 4.658363 6_3 1.986055e-07 MYLK4
11722 0.1198506687 14.017307 6_3 1.629729e-06 SERPINB9P1
11166 0.1149773520 30.739146 6_26 3.428582e-06 MICB
11126 0.0066330331 4.550173 6_26 2.927861e-08 NOTCH4
12047 0.0115304369 9.564307 6_26 1.069817e-07 C4A
8639 0.0471240512 4.532585 6_39 2.072044e-07 PAQR8
6892 0.0390673314 5.127604 7_6 1.943294e-07 RADIL
10553 0.0600366098 9.067138 7_43 5.280765e-07 ZNF107
24 0.0356333853 5.034035 7_93 1.740138e-07 TMEM176A
233 0.0519676493 5.037321 7_94 2.539470e-07 NUB1
6151 0.0782335708 13.908796 8_50 1.055585e-06 ADHFE1
9587 0.0490808165 6.017971 8_93 2.865314e-07 LYNX1
2340 0.0494136848 4.958825 9_1 2.377040e-07 DOCK8
5059 0.0479839679 5.063216 9_25 2.356856e-07 APTX
11846 0.0673676830 15.086440 9_27 9.859362e-07 SPAAR
5016 0.0471381096 5.007281 9_67 2.289731e-07 TOR1B
7973 0.0481151378 5.677193 10_48 2.649878e-07 ANAPC16
4628 0.0511950570 6.836265 10_51 3.395137e-07 SFTPD
7970 0.0006550480 7.207757 10_66 4.580192e-09 BORCS7
6451 0.7227588049 22.107581 10_73 1.550048e-05 CACUL1
748 0.0845027751 12.484376 11_1 1.023407e-06 DRD4
9292 0.0361365018 4.624666 11_1 1.621201e-07 TMEM80
10066 0.0357202865 4.518435 11_1 1.565717e-07 IFITM2
8125 0.0115413329 8.575870 11_3 9.601621e-08 ART5
9633 0.0083423383 6.216940 11_3 5.031238e-08 OR51E1
6258 0.0495298672 4.531703 11_23 2.177404e-07 APIP
7918 0.1087441019 15.854990 11_53 1.672562e-06 SMCO4
5351 0.0472982127 7.658971 12_7 3.514193e-07 PEX5
11940 0.0549328713 5.126961 12_36 2.732139e-07 ARHGEF25
10033 0.1145190870 13.470564 12_81 1.496491e-06 NOC4L
346 0.0383783120 4.579146 13_2 1.704829e-07 IFT88
1836 0.0434719760 4.837522 13_4 2.040059e-07 SGCG
4460 0.0561816376 4.610153 14_26 2.512582e-07 ACTR10
167 0.0220308723 4.532378 14_36 9.686529e-08 POMT2
250 0.0342051406 5.679435 14_46 1.884547e-07 CCDC88C
9780 0.0342395723 4.548660 14_49 1.510853e-07 GLRX5
11058 0.0580796189 6.034835 14_55 3.400162e-07 INF2
4192 0.0418828342 4.828395 15_14 1.961776e-07 IVD
1890 0.2017966776 23.219121 16_1 4.545380e-06 NPRL3
1901 0.0498766898 6.836741 16_4 3.307937e-07 ZNF500
6591 0.0998611432 12.028396 16_49 1.165238e-06 ZDHHC7
5544 0.0739153222 9.559528 16_54 6.854588e-07 GAS8
8194 0.0513564891 6.241564 17_1 3.109562e-07 NXN
2442 0.1394587619 23.328540 17_23 3.156049e-06 RAPGEFL1
5566 0.0004147273 6.569172 17_28 2.642918e-09 SCRN2
8119 0.1975915092 20.221028 17_44 3.875984e-06 ENGASE
8321 0.0189532463 5.083683 18_7 9.347006e-08 RAB31
1814 0.0292918992 4.519378 18_26 1.284212e-07 ST8SIA5
1490 0.3292345554 27.865676 19_2 8.899906e-06 POLR2E
9732 0.0303158041 5.041546 19_2 1.482666e-07 TMEM259
2086 0.0572222626 9.344200 19_7 5.187016e-07 TIMM44
8210 0.0148425117 4.832946 19_36 6.958726e-08 ZNF83
8643 0.0161796261 5.617267 19_36 8.816658e-08 ZNF160
8644 0.0209937643 7.988582 19_36 1.626936e-07 ZNF415
11057 0.0143593513 4.532138 19_36 6.313183e-08 ZNF525
506 0.0265247719 5.526450 20_31 1.422028e-07 ATP9A
9888 0.0433638436 4.567262 22_18 1.921295e-07 SERHL2
11274 0.0848633748 13.670071 22_24 1.125386e-06 DENND6B
1961 0.0187963047 5.781908 15_37 1.054275e-07 CTSH
1964 0.0162993022 4.773008 15_37 7.546952e-08 FAH
12376 0.8300210991 20.719695 15_37 1.668334e-05 LINC01314
130 0.0065793657 7.286811 16_2 4.650846e-08 BAIAP3
gene_type z num_eqtl
11112 protein_coding -2.440267951 4
531 protein_coding -0.620052310 4
3232 protein_coding 0.002855101 4
4675 protein_coding -0.973339711 4
12685 lincRNA 0.217340052 4
3049 protein_coding -1.976212808 4
3478 protein_coding -0.624890316 4
3160 protein_coding -0.102351022 4
5240 protein_coding 0.245540338 4
106 protein_coding 0.172114509 4
9645 protein_coding 1.891608686 4
5903 protein_coding 0.669021935 4
255 protein_coding -1.211958037 4
10512 protein_coding 0.608861176 4
12886 lincRNA 0.082489767 4
4063 protein_coding 0.086747178 4
10123 protein_coding -0.172329751 4
9009 protein_coding 0.674150321 4
3830 protein_coding 1.836540407 4
10932 protein_coding 2.302208079 4
7510 protein_coding 0.375710166 4
6026 protein_coding 1.766342942 4
7759 protein_coding 0.254481787 4
6046 protein_coding -0.229459393 4
11722 lincRNA -1.695032189 4
11166 protein_coding 2.916537842 4
11126 protein_coding -0.072751034 4
12047 protein_coding 1.316735788 4
8639 protein_coding -0.015891555 4
6892 protein_coding -0.413872489 4
10553 protein_coding 1.326350485 4
24 protein_coding -0.643253657 4
233 protein_coding 0.379208858 4
6151 protein_coding 1.998483498 4
9587 protein_coding 0.520495756 4
2340 protein_coding -0.356181735 4
5059 protein_coding -0.549963807 4
11846 protein_coding 1.675675285 5
5016 protein_coding 0.380445650 4
7973 protein_coding 0.399205152 4
4628 protein_coding -0.885721483 4
7970 protein_coding -0.526012182 4
6451 protein_coding 4.319545841 4
748 protein_coding -1.497374114 4
9292 protein_coding 0.049876581 4
10066 protein_coding -0.025005790 5
8125 protein_coding 0.507809839 4
9633 protein_coding -0.676154522 5
6258 protein_coding -0.027704193 4
7918 protein_coding -1.932558474 4
5351 protein_coding -0.902269046 4
11940 protein_coding 0.433006303 4
10033 protein_coding -1.805702949 6
346 protein_coding 0.131751846 4
1836 protein_coding 0.243117640 4
4460 protein_coding 0.119326035 4
167 protein_coding -0.374526500 4
250 protein_coding 0.609826113 4
9780 protein_coding 0.158475991 4
11058 protein_coding -0.476342392 4
4192 protein_coding 0.258688400 4
1890 protein_coding -2.449224925 4
1901 protein_coding -0.779958034 4
6591 protein_coding 1.538279013 4
5544 protein_coding -1.587793494 4
8194 protein_coding -0.655809230 4
2442 protein_coding 3.059016504 4
5566 protein_coding 0.909239725 5
8119 protein_coding -2.276500640 4
8321 protein_coding -0.427850673 5
1814 protein_coding -0.154330769 4
1490 protein_coding -3.150789744 5
9732 protein_coding -0.535091807 4
2086 protein_coding -1.221084055 4
8210 protein_coding 0.303018261 5
8643 protein_coding -0.511367254 4
8644 protein_coding -0.939733027 4
11057 protein_coding -0.062633062 4
506 protein_coding -0.509725048 4
9888 protein_coding 0.154400055 4
11274 protein_coding 2.245941958 4
1961 protein_coding 0.633053187 5
1964 protein_coding -0.142308981 4
12376 protein_coding -4.379164029 4
130 protein_coding 0.228653666 4#distribution of number of eQTL for genes with PIP>0.8
table(ctwas_gene_res$num_eqtl[ctwas_gene_res$susie_pip>0.8])/sum(ctwas_gene_res$susie_pip>0.8)
1 2 4
0.57894737 0.36842105 0.05263158 #genes with 2+ eQTL and PIP>0.8
ctwas_gene_res[ctwas_gene_res$num_eqtl>1 & ctwas_gene_res$susie_pip>0.8,] chrom id pos type region_tag1 region_tag2 cs_index
3169 1 ENSG00000116132.11 170662279 gene 1 84 1
1214 2 ENSG00000087338.4 69829233 gene 2 46 1
3147 2 ENSG00000115935.17 174683036 gene 2 105 1
9840 5 ENSG00000183072.9 173219180 gene 5 103 1
12133 8 ENSG00000249816.6 124849014 gene 8 82 1
9417 8 ENSG00000178209.14 143944186 gene 8 94 1
12376 15 ENSG00000259417.2 80252110 gene 15 37 0
6663 21 ENSG00000154721.14 25638800 gene 21 9 1
susie_pip mu2 region_tag PVE genename gene_type
3169 0.9999999 153.79916 1_84 1.491985e-04 PRRX1 protein_coding
1214 0.9944249 60.60721 2_46 5.846645e-05 GMCL1 protein_coding
3147 0.9993101 2124.21550 2_105 2.059251e-03 WIPF1 protein_coding
9840 0.9942183 61.83714 5_103 5.964054e-05 NKX2-5 protein_coding
12133 0.8180590 22.20129 8_82 1.761867e-05 LINC00964 lincRNA
9417 0.9666163 43.61991 8_94 4.090245e-05 PLEC protein_coding
12376 0.8300211 20.71969 15_37 1.668334e-05 LINC01314 protein_coding
6663 0.9633616 22.37976 21_9 2.091487e-05 JAM2 protein_coding
z num_eqtl
3169 14.848088 2
1214 -8.434995 2
3147 8.987351 2
9840 -9.616279 2
12133 4.554265 2
9417 3.093844 2
12376 -4.379164 4
6663 4.569598 2cTWAS genes in GO terms enriched for silver standard genes
#reload silver standard genes
known_annotations <- read_xlsx("data/summary_known_genes_annotations.xlsx", sheet="LDL")New names:
* `` -> ...4
* `` -> ...5known_annotations <- unique(known_annotations$`Gene Symbol`)
#GO enrichment analysis for silver standard genes
dbs <- c("GO_Biological_Process_2021", "GO_Cellular_Component_2021", "GO_Molecular_Function_2021")
genes <- known_annotations
GO_enrichment <- enrichr(genes, dbs)Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.for (db in dbs){
print(db)
df <- GO_enrichment[[db]]
df <- df[df$Adjusted.P.value<0.05,c("Term", "Overlap", "Adjusted.P.value", "Genes")]
plotEnrich(GO_enrichment[[db]])
print(df)
}[1] "GO_Biological_Process_2021"
Term
1 cholesterol transport (GO:0030301)
2 cholesterol homeostasis (GO:0042632)
3 sterol homeostasis (GO:0055092)
4 cholesterol efflux (GO:0033344)
5 sterol transport (GO:0015918)
6 cholesterol metabolic process (GO:0008203)
7 sterol metabolic process (GO:0016125)
8 triglyceride-rich lipoprotein particle remodeling (GO:0034370)
9 high-density lipoprotein particle remodeling (GO:0034375)
10 reverse cholesterol transport (GO:0043691)
11 secondary alcohol metabolic process (GO:1902652)
12 regulation of lipoprotein lipase activity (GO:0051004)
13 phospholipid transport (GO:0015914)
14 lipid transport (GO:0006869)
15 acylglycerol homeostasis (GO:0055090)
16 very-low-density lipoprotein particle remodeling (GO:0034372)
17 triglyceride homeostasis (GO:0070328)
18 triglyceride metabolic process (GO:0006641)
19 phospholipid efflux (GO:0033700)
20 chylomicron remodeling (GO:0034371)
21 regulation of cholesterol transport (GO:0032374)
22 chylomicron assembly (GO:0034378)
23 chylomicron remnant clearance (GO:0034382)
24 lipoprotein metabolic process (GO:0042157)
25 diterpenoid metabolic process (GO:0016101)
26 positive regulation of steroid metabolic process (GO:0045940)
27 retinoid metabolic process (GO:0001523)
28 lipid homeostasis (GO:0055088)
29 negative regulation of lipase activity (GO:0060192)
30 positive regulation of cholesterol esterification (GO:0010873)
31 intestinal cholesterol absorption (GO:0030299)
32 negative regulation of cholesterol transport (GO:0032375)
33 intestinal lipid absorption (GO:0098856)
34 acylglycerol metabolic process (GO:0006639)
35 regulation of cholesterol esterification (GO:0010872)
36 phospholipid homeostasis (GO:0055091)
37 very-low-density lipoprotein particle assembly (GO:0034379)
38 positive regulation of lipid metabolic process (GO:0045834)
39 high-density lipoprotein particle assembly (GO:0034380)
40 negative regulation of lipoprotein lipase activity (GO:0051005)
41 negative regulation of lipoprotein particle clearance (GO:0010985)
42 regulation of very-low-density lipoprotein particle remodeling (GO:0010901)
43 intracellular cholesterol transport (GO:0032367)
44 positive regulation of cholesterol transport (GO:0032376)
45 regulation of intestinal cholesterol absorption (GO:0030300)
46 positive regulation of lipoprotein lipase activity (GO:0051006)
47 acylglycerol catabolic process (GO:0046464)
48 positive regulation of lipid biosynthetic process (GO:0046889)
49 positive regulation of triglyceride metabolic process (GO:0090208)
50 positive regulation of triglyceride lipase activity (GO:0061365)
51 regulation of lipid catabolic process (GO:0050994)
52 steroid metabolic process (GO:0008202)
53 positive regulation of lipid catabolic process (GO:0050996)
54 triglyceride catabolic process (GO:0019433)
55 organophosphate ester transport (GO:0015748)
56 phosphatidylcholine metabolic process (GO:0046470)
57 regulation of triglyceride catabolic process (GO:0010896)
58 fatty acid metabolic process (GO:0006631)
59 regulation of fatty acid biosynthetic process (GO:0042304)
60 regulation of sterol transport (GO:0032371)
61 cellular response to low-density lipoprotein particle stimulus (GO:0071404)
62 low-density lipoprotein particle remodeling (GO:0034374)
63 regulation of macrophage derived foam cell differentiation (GO:0010743)
64 organic substance transport (GO:0071702)
65 regulation of cholesterol efflux (GO:0010874)
66 receptor-mediated endocytosis (GO:0006898)
67 secondary alcohol biosynthetic process (GO:1902653)
68 regulation of cholesterol storage (GO:0010885)
69 cholesterol import (GO:0070508)
70 sterol import (GO:0035376)
71 monocarboxylic acid biosynthetic process (GO:0072330)
72 cholesterol biosynthetic process (GO:0006695)
73 positive regulation of cellular metabolic process (GO:0031325)
74 sterol biosynthetic process (GO:0016126)
75 positive regulation of fatty acid metabolic process (GO:0045923)
76 regulation of receptor-mediated endocytosis (GO:0048259)
77 fatty acid biosynthetic process (GO:0006633)
78 regulation of Cdc42 protein signal transduction (GO:0032489)
79 positive regulation of triglyceride catabolic process (GO:0010898)
80 lipid biosynthetic process (GO:0008610)
81 positive regulation of cholesterol efflux (GO:0010875)
82 negative regulation of receptor-mediated endocytosis (GO:0048261)
83 lipoprotein transport (GO:0042953)
84 regulation of lipid metabolic process (GO:0019216)
85 monocarboxylic acid metabolic process (GO:0032787)
86 lipoprotein localization (GO:0044872)
87 lipid catabolic process (GO:0016042)
88 positive regulation of fatty acid biosynthetic process (GO:0045723)
89 intracellular sterol transport (GO:0032366)
90 steroid biosynthetic process (GO:0006694)
91 regulation of lipid biosynthetic process (GO:0046890)
92 regulation of lipase activity (GO:0060191)
93 positive regulation of cellular biosynthetic process (GO:0031328)
94 regulation of amyloid-beta clearance (GO:1900221)
95 phospholipid metabolic process (GO:0006644)
96 regulation of intestinal lipid absorption (GO:1904729)
97 positive regulation of protein catabolic process in the vacuole (GO:1904352)
98 positive regulation of biosynthetic process (GO:0009891)
99 regulation of cholesterol metabolic process (GO:0090181)
100 foam cell differentiation (GO:0090077)
101 positive regulation of cholesterol storage (GO:0010886)
102 macrophage derived foam cell differentiation (GO:0010742)
103 organic hydroxy compound biosynthetic process (GO:1901617)
104 regulation of steroid metabolic process (GO:0019218)
105 organonitrogen compound biosynthetic process (GO:1901566)
106 negative regulation of lipid metabolic process (GO:0045833)
107 regulation of lysosomal protein catabolic process (GO:1905165)
108 positive regulation of amyloid-beta clearance (GO:1900223)
109 cellular lipid catabolic process (GO:0044242)
110 chemical homeostasis (GO:0048878)
111 cholesterol catabolic process (GO:0006707)
112 sterol catabolic process (GO:0016127)
113 steroid hormone biosynthetic process (GO:0120178)
114 regulation of low-density lipoprotein particle clearance (GO:0010988)
115 bile acid metabolic process (GO:0008206)
116 phosphatidylcholine biosynthetic process (GO:0006656)
117 alcohol catabolic process (GO:0046164)
118 organophosphate catabolic process (GO:0046434)
119 regulation of phospholipase activity (GO:0010517)
120 positive regulation of lipid localization (GO:1905954)
121 positive regulation of phospholipid transport (GO:2001140)
122 glycerophospholipid metabolic process (GO:0006650)
123 organic hydroxy compound transport (GO:0015850)
124 negative regulation of macrophage derived foam cell differentiation (GO:0010745)
125 positive regulation of lipid transport (GO:0032370)
126 C21-steroid hormone biosynthetic process (GO:0006700)
127 membrane organization (GO:0061024)
128 positive regulation of endocytosis (GO:0045807)
129 positive regulation of multicellular organismal process (GO:0051240)
130 negative regulation of catabolic process (GO:0009895)
131 negative regulation of lipid catabolic process (GO:0050995)
132 carbohydrate derivative transport (GO:1901264)
133 positive regulation of macrophage derived foam cell differentiation (GO:0010744)
134 protein transport (GO:0015031)
135 fatty acid transport (GO:0015908)
136 positive regulation of lipid storage (GO:0010884)
137 C21-steroid hormone metabolic process (GO:0008207)
138 phospholipid catabolic process (GO:0009395)
139 negative regulation of endocytosis (GO:0045806)
140 regulation of primary metabolic process (GO:0080090)
141 negative regulation of multicellular organismal process (GO:0051241)
142 bile acid biosynthetic process (GO:0006699)
143 regulation of low-density lipoprotein particle receptor catabolic process (GO:0032803)
144 regulation of Rho protein signal transduction (GO:0035023)
145 regulation of small molecule metabolic process (GO:0062012)
146 positive regulation of cellular catabolic process (GO:0031331)
147 artery morphogenesis (GO:0048844)
148 negative regulation of cellular component organization (GO:0051129)
149 glycolipid transport (GO:0046836)
150 positive regulation of lipoprotein particle clearance (GO:0010986)
151 positive regulation of sterol transport (GO:0032373)
152 long-chain fatty acid transport (GO:0015909)
153 response to insulin (GO:0032868)
154 regulation of bile acid metabolic process (GO:1904251)
155 positive regulation of receptor catabolic process (GO:2000646)
156 positive regulation of transport (GO:0051050)
157 negative regulation of endothelial cell proliferation (GO:0001937)
158 negative regulation of endothelial cell migration (GO:0010596)
159 regulation of nitrogen compound metabolic process (GO:0051171)
160 negative regulation of amyloid-beta clearance (GO:1900222)
161 negative regulation of cellular metabolic process (GO:0031324)
162 glycerophospholipid biosynthetic process (GO:0046474)
163 negative regulation of production of molecular mediator of immune response (GO:0002701)
164 unsaturated fatty acid biosynthetic process (GO:0006636)
165 anion transport (GO:0006820)
166 positive regulation of receptor-mediated endocytosis (GO:0048260)
167 negative regulation of cholesterol storage (GO:0010887)
168 regulation of bile acid biosynthetic process (GO:0070857)
169 peptidyl-amino acid modification (GO:0018193)
170 low-density lipoprotein particle receptor catabolic process (GO:0032802)
171 low-density lipoprotein receptor particle metabolic process (GO:0032799)
172 protein oxidation (GO:0018158)
173 positive regulation by host of viral process (GO:0044794)
174 positive regulation of triglyceride biosynthetic process (GO:0010867)
175 receptor internalization (GO:0031623)
176 response to glucose (GO:0009749)
177 positive regulation of cellular component organization (GO:0051130)
178 negative regulation of fatty acid biosynthetic process (GO:0045717)
179 negative regulation of hemostasis (GO:1900047)
180 peptidyl-methionine modification (GO:0018206)
181 ethanol oxidation (GO:0006069)
182 negative regulation of amyloid fibril formation (GO:1905907)
183 negative regulation of protein metabolic process (GO:0051248)
184 unsaturated fatty acid metabolic process (GO:0033559)
185 alpha-linolenic acid metabolic process (GO:0036109)
186 platelet degranulation (GO:0002576)
187 negative regulation of metabolic process (GO:0009892)
188 negative regulation of cell activation (GO:0050866)
189 negative regulation of coagulation (GO:0050819)
190 cGMP-mediated signaling (GO:0019934)
191 intestinal absorption (GO:0050892)
192 receptor metabolic process (GO:0043112)
193 regulation of phagocytosis (GO:0050764)
194 regulation of amyloid fibril formation (GO:1905906)
195 regulation of sequestering of triglyceride (GO:0010889)
196 regulation of triglyceride biosynthetic process (GO:0010866)
197 post-translational protein modification (GO:0043687)
198 regulation of endocytosis (GO:0030100)
199 amyloid fibril formation (GO:1990000)
200 positive regulation of small molecule metabolic process (GO:0062013)
201 cellular response to nutrient levels (GO:0031669)
202 negative regulation of cytokine production involved in immune response (GO:0002719)
203 negative regulation of fatty acid metabolic process (GO:0045922)
204 regulation of cholesterol biosynthetic process (GO:0045540)
205 regulation of steroid biosynthetic process (GO:0050810)
206 positive regulation of catabolic process (GO:0009896)
207 amyloid precursor protein metabolic process (GO:0042982)
208 nitric oxide mediated signal transduction (GO:0007263)
209 positive regulation of nitric-oxide synthase activity (GO:0051000)
210 ethanol metabolic process (GO:0006067)
211 positive regulation of cellular protein catabolic process (GO:1903364)
212 response to fatty acid (GO:0070542)
213 long-term memory (GO:0007616)
214 negative regulation of lipid storage (GO:0010888)
215 linoleic acid metabolic process (GO:0043651)
216 negative regulation of lipid biosynthetic process (GO:0051055)
217 regulation of lipid storage (GO:0010883)
218 regulation of interleukin-1 beta production (GO:0032651)
219 long-chain fatty acid metabolic process (GO:0001676)
220 regulation of cytokine production involved in immune response (GO:0002718)
221 cellular protein metabolic process (GO:0044267)
222 negative regulation of defense response (GO:0031348)
223 transport across blood-brain barrier (GO:0150104)
224 receptor catabolic process (GO:0032801)
225 response to hexose (GO:0009746)
226 regulated exocytosis (GO:0045055)
227 regulation of endothelial cell migration (GO:0010594)
228 negative regulation of protein transport (GO:0051224)
229 positive regulation of binding (GO:0051099)
230 regulation of blood coagulation (GO:0030193)
231 positive regulation of monooxygenase activity (GO:0032770)
232 negative regulation of wound healing (GO:0061045)
233 negative regulation of macromolecule metabolic process (GO:0010605)
234 long-chain fatty acid biosynthetic process (GO:0042759)
235 regulation of developmental growth (GO:0048638)
236 regulation of cell death (GO:0010941)
237 regulation of angiogenesis (GO:0045765)
238 regulation of inflammatory response (GO:0050727)
239 apoptotic cell clearance (GO:0043277)
240 cellular response to peptide hormone stimulus (GO:0071375)
241 negative regulation of blood vessel endothelial cell migration (GO:0043537)
242 phosphate-containing compound metabolic process (GO:0006796)
243 negative regulation of epithelial cell migration (GO:0010633)
244 cellular response to amyloid-beta (GO:1904646)
245 cyclic-nucleotide-mediated signaling (GO:0019935)
246 regulation of receptor internalization (GO:0002090)
247 response to lipid (GO:0033993)
248 regulation of vascular associated smooth muscle cell proliferation (GO:1904705)
249 regulation of protein-containing complex assembly (GO:0043254)
250 ion transport (GO:0006811)
251 negative regulation of response to external stimulus (GO:0032102)
252 regulation of protein binding (GO:0043393)
253 regulation of cellular component biogenesis (GO:0044087)
254 negative regulation of protein secretion (GO:0050709)
255 negative regulation of secretion by cell (GO:1903531)
256 regulation of nitric-oxide synthase activity (GO:0050999)
257 negative regulation of blood coagulation (GO:0030195)
258 cellular response to organic substance (GO:0071310)
259 cellular response to insulin stimulus (GO:0032869)
260 response to amyloid-beta (GO:1904645)
261 establishment of protein localization to extracellular region (GO:0035592)
262 regulation of cellular metabolic process (GO:0031323)
263 regulation of protein metabolic process (GO:0051246)
264 positive regulation of cell differentiation (GO:0045597)
265 negative regulation of cell projection organization (GO:0031345)
266 positive regulation of fat cell differentiation (GO:0045600)
267 negative regulation of BMP signaling pathway (GO:0030514)
268 negative regulation of cellular biosynthetic process (GO:0031327)
269 positive regulation of phagocytosis (GO:0050766)
Overlap Adjusted.P.value
1 28/51 3.291336e-55
2 29/71 1.134840e-52
3 29/72 1.264418e-52
4 16/24 1.003687e-32
5 15/21 2.203564e-31
6 20/77 5.273224e-31
7 19/70 7.882518e-30
8 12/13 1.520527e-27
9 13/18 2.514128e-27
10 12/17 5.731565e-25
11 15/49 2.711706e-24
12 12/21 2.245426e-23
13 15/59 5.665269e-23
14 17/109 2.748742e-22
15 12/25 3.145271e-22
16 9/9 2.283165e-21
17 12/31 7.414146e-21
18 13/55 1.935295e-19
19 9/12 4.196729e-19
20 8/9 5.374944e-18
21 10/25 1.639871e-17
22 8/10 2.436689e-17
23 7/7 1.679428e-16
24 7/9 5.763376e-15
25 11/64 8.362646e-15
26 7/13 2.508790e-13
27 11/92 5.133855e-13
28 10/64 5.133855e-13
29 6/9 3.296018e-12
30 6/9 3.296018e-12
31 6/9 3.296018e-12
32 6/11 1.693836e-11
33 6/11 1.693836e-11
34 8/41 3.013332e-11
35 6/12 3.013332e-11
36 6/12 3.013332e-11
37 6/12 3.013332e-11
38 7/25 4.654994e-11
39 6/13 5.294950e-11
40 5/6 5.459029e-11
41 5/6 5.459029e-11
42 5/6 5.459029e-11
43 6/15 1.393181e-10
44 7/33 3.496187e-10
45 5/8 4.627510e-10
46 5/8 4.627510e-10
47 7/35 5.017364e-10
48 7/35 5.017364e-10
49 6/19 6.556580e-10
50 5/9 9.553575e-10
51 6/21 1.253116e-09
52 9/104 1.493946e-09
53 6/22 1.653549e-09
54 6/23 2.189811e-09
55 6/25 3.733511e-09
56 8/77 3.733511e-09
57 5/12 5.225810e-09
58 9/124 6.552660e-09
59 6/29 9.279729e-09
60 4/5 9.798195e-09
61 5/14 1.207993e-08
62 5/14 1.207993e-08
63 6/31 1.339781e-08
64 9/136 1.355933e-08
65 6/33 1.942867e-08
66 9/143 2.054367e-08
67 6/34 2.282600e-08
68 5/16 2.390304e-08
69 4/6 2.513038e-08
70 4/6 2.513038e-08
71 7/63 2.556641e-08
72 6/35 2.556641e-08
73 8/105 3.517095e-08
74 6/38 4.196695e-08
75 5/18 4.228478e-08
76 6/39 4.816188e-08
77 7/71 5.609765e-08
78 4/8 1.033779e-07
79 4/8 1.033779e-07
80 7/80 1.258618e-07
81 5/23 1.517283e-07
82 5/26 2.906610e-07
83 4/10 2.936601e-07
84 7/92 3.199662e-07
85 8/143 3.471498e-07
86 4/11 4.442138e-07
87 5/29 4.906437e-07
88 4/13 9.252034e-07
89 4/13 9.252034e-07
90 6/65 9.598111e-07
91 5/35 1.249797e-06
92 4/14 1.249797e-06
93 8/180 1.884951e-06
94 4/16 2.212485e-06
95 6/76 2.336232e-06
96 3/5 3.664395e-06
97 3/5 3.664395e-06
98 5/44 3.827136e-06
99 4/21 6.819051e-06
100 3/6 6.952354e-06
101 3/6 6.952354e-06
102 3/6 6.952354e-06
103 5/50 6.991423e-06
104 4/23 9.554179e-06
105 7/158 1.040987e-05
106 4/24 1.121951e-05
107 3/7 1.146235e-05
108 3/7 1.146235e-05
109 4/27 1.788032e-05
110 5/65 2.452122e-05
111 3/9 2.639634e-05
112 3/9 2.639634e-05
113 4/31 3.060279e-05
114 3/10 3.695601e-05
115 4/33 3.856854e-05
116 4/33 3.856854e-05
117 3/11 4.775659e-05
118 3/11 4.775659e-05
119 3/11 4.775659e-05
120 3/11 4.775659e-05
121 3/11 4.775659e-05
122 5/80 6.182763e-05
123 4/40 7.973389e-05
124 3/13 7.973389e-05
125 3/13 7.973389e-05
126 3/15 1.252218e-04
127 7/242 1.420233e-04
128 4/48 1.598563e-04
129 8/345 1.704405e-04
130 4/49 1.709436e-04
131 3/18 2.111817e-04
132 3/18 2.111817e-04
133 3/18 2.111817e-04
134 8/369 2.646441e-04
135 3/20 2.892290e-04
136 3/21 3.341258e-04
137 3/24 4.974036e-04
138 3/24 4.974036e-04
139 3/25 5.597802e-04
140 5/130 5.616142e-04
141 6/214 6.166972e-04
142 3/27 6.934199e-04
143 2/5 7.406583e-04
144 4/73 7.449454e-04
145 3/28 7.586859e-04
146 5/141 7.898802e-04
147 3/30 9.228897e-04
148 4/80 1.034287e-03
149 2/6 1.049782e-03
150 2/6 1.049782e-03
151 2/6 1.049782e-03
152 3/32 1.085012e-03
153 4/84 1.208000e-03
154 2/7 1.428577e-03
155 2/7 1.428577e-03
156 4/91 1.611630e-03
157 3/37 1.625395e-03
158 3/38 1.749224e-03
159 2/8 1.841132e-03
160 2/8 1.841132e-03
161 3/39 1.855101e-03
162 5/177 2.046657e-03
163 2/9 2.304288e-03
164 2/9 2.304288e-03
165 3/43 2.420344e-03
166 3/44 2.575438e-03
167 2/10 2.756295e-03
168 2/10 2.756295e-03
169 2/10 2.756295e-03
170 2/10 2.756295e-03
171 2/10 2.756295e-03
172 2/11 3.303347e-03
173 2/11 3.303347e-03
174 2/11 3.303347e-03
175 3/49 3.337798e-03
176 3/49 3.337798e-03
177 4/114 3.341630e-03
178 2/12 3.781332e-03
179 2/12 3.781332e-03
180 2/12 3.781332e-03
181 2/12 3.781332e-03
182 2/12 3.781332e-03
183 3/52 3.822266e-03
184 3/54 4.245657e-03
185 2/13 4.386588e-03
186 4/125 4.489058e-03
187 3/56 4.645735e-03
188 2/14 4.945884e-03
189 2/14 4.945884e-03
190 2/14 4.945884e-03
191 2/14 4.945884e-03
192 3/58 4.985945e-03
193 3/58 4.985945e-03
194 2/15 5.548828e-03
195 2/15 5.548828e-03
196 2/15 5.548828e-03
197 6/345 5.596173e-03
198 3/61 5.626994e-03
199 3/63 6.147256e-03
200 2/16 6.200855e-03
201 3/66 6.840974e-03
202 2/17 6.840974e-03
203 2/17 6.840974e-03
204 2/17 6.840974e-03
205 2/17 6.840974e-03
206 3/67 7.093595e-03
207 2/18 7.532008e-03
208 2/18 7.532008e-03
209 2/18 7.532008e-03
210 2/19 8.241667e-03
211 2/19 8.241667e-03
212 2/19 8.241667e-03
213 2/19 8.241667e-03
214 2/20 9.094348e-03
215 2/21 9.982653e-03
216 2/22 1.085553e-02
217 2/22 1.085553e-02
218 3/83 1.230478e-02
219 3/83 1.230478e-02
220 2/24 1.273659e-02
221 6/417 1.291939e-02
222 3/85 1.298477e-02
223 3/86 1.336097e-02
224 2/25 1.350640e-02
225 2/25 1.350640e-02
226 4/180 1.399465e-02
227 3/89 1.440735e-02
228 2/26 1.440735e-02
229 3/90 1.479001e-02
230 2/27 1.539039e-02
231 2/28 1.646589e-02
232 2/29 1.757027e-02
233 4/194 1.771224e-02
234 2/30 1.862299e-02
235 2/31 1.977865e-02
236 3/102 2.036757e-02
237 4/203 2.042828e-02
238 4/206 2.141587e-02
239 2/33 2.198466e-02
240 3/106 2.228428e-02
241 2/34 2.311351e-02
242 4/212 2.328380e-02
243 2/35 2.415927e-02
244 2/35 2.415927e-02
245 2/36 2.531623e-02
246 2/36 2.531623e-02
247 3/114 2.646649e-02
248 2/37 2.648825e-02
249 3/116 2.742785e-02
250 3/116 2.742785e-02
251 3/118 2.842391e-02
252 3/118 2.842391e-02
253 2/39 2.842391e-02
254 2/39 2.842391e-02
255 2/39 2.842391e-02
256 2/39 2.842391e-02
257 2/40 2.973753e-02
258 3/123 3.119488e-02
259 3/129 3.533580e-02
260 2/44 3.533580e-02
261 2/46 3.834204e-02
262 2/47 3.980515e-02
263 2/48 4.128649e-02
264 4/258 4.183273e-02
265 2/49 4.262417e-02
266 2/51 4.583569e-02
267 2/52 4.720898e-02
268 2/52 4.720898e-02
269 2/53 4.877011e-02
Genes
1 SCARB1;CETP;LCAT;LIPC;NPC1L1;LIPG;CD36;APOE;LDLRAP1;APOB;LDLR;ABCA1;ABCG8;STARD3;ABCG5;OSBPL5;APOA2;APOA1;APOC3;APOA4;APOA5;NPC1;SOAT1;STAR;NPC2;SOAT2;APOC2;APOC1
2 SCARB1;CETP;MTTP;PCSK9;LPL;LCAT;ABCB11;CYP7A1;LIPC;LIPG;APOE;LDLRAP1;APOB;LDLR;ABCA1;ABCG8;ABCG5;EPHX2;APOA2;APOA1;APOC3;APOA4;APOA5;SOAT1;NPC1;NPC2;SOAT2;APOC2;ANGPTL3
3 SCARB1;CETP;MTTP;PCSK9;LPL;LCAT;ABCB11;CYP7A1;LIPC;LIPG;APOE;LDLRAP1;APOB;LDLR;ABCA1;ABCG8;ABCG5;EPHX2;APOA2;APOA1;APOC3;APOA4;APOA5;SOAT1;NPC1;NPC2;SOAT2;APOC2;ANGPTL3
4 ABCA1;ABCG8;SCARB1;ABCG5;APOA2;APOA1;APOC3;APOA4;APOA5;NPC1;SOAT1;NPC2;SOAT2;APOC2;APOC1;APOE
5 ABCG8;CETP;STARD3;ABCG5;OSBPL5;APOA2;APOA1;LCAT;NPC1;NPC1L1;NPC2;CD36;APOB;LDLRAP1;LDLR
6 ABCA1;STARD3;CETP;OSBPL5;APOA2;APOA1;LCAT;APOA4;HMGCR;APOA5;CYP7A1;CYP27A1;SOAT1;SOAT2;NPC1L1;ANGPTL3;APOE;DHCR7;LDLRAP1;APOB
7 ABCA1;STARD3;CETP;OSBPL5;APOA2;APOA1;LCAT;APOA4;HMGCR;LIPA;CYP7A1;CYP27A1;SOAT1;SOAT2;ANGPTL3;APOE;DHCR7;LDLRAP1;APOB
8 CETP;LIPC;APOC2;APOA2;APOA1;APOC3;LCAT;LPL;APOA4;APOE;APOB;APOA5
9 CETP;SCARB1;APOA2;APOA1;APOC3;LCAT;APOA4;LIPC;APOC2;APOC1;LIPG;APOE;PLTP
10 ABCA1;CETP;SCARB1;LIPC;APOC2;LIPG;APOA2;APOA1;APOC3;LCAT;APOA4;APOE
11 ABCA1;STARD3;CETP;OSBPL5;APOA2;APOA1;LCAT;APOA4;CYP27A1;SOAT1;SOAT2;ANGPTL3;APOE;LDLRAP1;APOB
12 LIPC;SORT1;APOC2;APOH;APOC1;ANGPTL3;APOA1;APOC3;LPL;APOA4;ANGPTL4;APOA5
13 ABCA1;SCARB1;OSBPL5;MTTP;APOA2;APOA1;APOC3;APOA4;APOA5;NPC2;APOC2;APOC1;APOE;LDLR;PLTP
14 ABCA1;SCARB1;ABCG8;CETP;ABCG5;OSBPL5;MTTP;APOA1;APOA4;ABCB11;APOA5;NPC2;NPC1L1;CD36;APOE;LDLR;PLTP
15 CETP;SCARB1;LIPC;APOC2;ANGPTL3;LPL;APOA1;APOC3;APOA4;APOE;ANGPTL4;APOA5
16 CETP;LIPC;APOC2;APOA1;LCAT;LPL;APOA4;APOE;APOA5
17 CETP;SCARB1;LIPC;APOC2;ANGPTL3;LPL;APOA1;APOC3;APOA4;APOE;ANGPTL4;APOA5
18 CETP;APOA2;LPL;APOC3;APOA5;LIPC;LIPI;APOH;LIPG;APOC1;APOE;APOB;LPIN3
19 ABCA1;APOC2;APOC1;APOA2;APOA1;APOC3;APOA4;APOE;APOA5
20 APOC2;APOA2;APOA1;APOC3;LPL;APOA4;APOE;APOB
21 CETP;LRP1;APOC2;LIPG;APOC1;APOA2;TSPO;APOA1;APOA4;APOA5
22 APOC2;MTTP;APOA2;APOA1;APOC3;APOA4;APOE;APOB
23 LIPC;APOC2;APOC1;APOC3;APOE;APOB;LDLR
24 NPC1L1;MTTP;APOA2;APOA1;APOA4;APOE;APOA5
25 LRP1;ADH1B;APOC2;APOA2;APOA1;LPL;APOC3;APOA4;LRP2;APOE;APOB
26 APOC1;APOA2;APOA1;APOA4;APOE;LDLRAP1;APOA5
27 LRP1;ADH1B;APOC2;APOA2;APOA1;LPL;APOC3;APOA4;LRP2;APOE;APOB
28 ABCA1;CETP;LIPG;ANGPTL3;APOA1;APOA4;PPARG;APOE;ABCB11;APOA5
29 SORT1;APOC1;ANGPTL3;APOA2;APOC3;ANGPTL4
30 APOC1;APOA2;APOA1;APOA4;APOE;APOA5
31 ABCG8;ABCG5;NPC1L1;SOAT2;CD36;LDLR
32 ABCG8;ABCG5;APOC2;APOC1;APOA2;APOC3
33 ABCG8;ABCG5;NPC1L1;SOAT2;CD36;LDLR
34 CETP;APOH;APOC1;APOA2;LPL;APOC3;APOE;APOA5
35 APOC1;APOA2;APOA1;APOA4;APOE;APOA5
36 ABCA1;CETP;LIPG;ANGPTL3;APOA1;ABCB11
37 SOAT1;SOAT2;APOC1;MTTP;APOC3;APOB
38 APOA2;ANGPTL3;APOA1;APOA4;PPARG;APOE;APOA5
39 ABCA1;APOA2;APOA1;APOA4;APOE;APOA5
40 SORT1;APOC1;ANGPTL3;APOC3;ANGPTL4
41 LRPAP1;APOC2;APOC1;APOC3;PCSK9
42 APOC2;APOA2;APOA1;APOC3;APOA5
43 ABCA1;NPC1;STAR;NPC2;LDLRAP1;LDLR
44 CETP;LRP1;LIPG;APOA1;PPARG;APOE;PLTP
45 ABCG8;ABCG5;APOA1;APOA4;APOA5
46 APOC2;APOH;APOA1;APOA4;APOA5
47 LIPC;LIPI;LIPG;APOA2;LPL;APOC3;APOA5
48 SCARB1;APOC2;APOA1;APOA4;APOE;LDLR;APOA5
49 SCARB1;APOC2;APOA1;APOA4;APOA5;LDLR
50 APOC2;APOH;APOA1;APOA4;APOA5
51 APOC1;APOA2;ANGPTL3;APOC3;ABCB11;APOA5
52 CYP27A1;STARD3;NPC1;STAR;TSPO;LRP2;ABCB11;LIPA;CYP7A1
53 APOC2;APOA2;ANGPTL3;APOA1;APOA4;APOA5
54 LIPC;LIPI;LIPG;APOC3;LPL;APOA5
55 SCARB1;OSBPL5;NPC2;MTTP;LDLR;PLTP
56 CETP;LIPC;APOA2;APOA1;LCAT;APOA4;APOA5;LPIN3
57 APOC2;APOA1;APOC3;APOA4;APOA5
58 LIPC;LIPI;LIPG;ANGPTL3;LPL;PPARG;CD36;ABCB11;LPIN3
59 APOC2;APOC1;APOA1;APOC3;APOA4;APOA5
60 LRP1;APOC1;TSPO;APOA4
61 ABCA1;LPL;PPARG;CD36;LDLR
62 CETP;LIPC;APOA2;APOB;LPA
63 ABCA1;CETP;LPL;PPARG;CD36;APOB
64 ABCA1;ABCG8;CETP;ABCG5;APOA1;APOA4;LRP2;APOA5;PLTP
65 CETP;LRP1;APOA1;PPARG;APOE;PLTP
66 SCARB1;LRP1;APOA1;CD36;LRP2;APOE;LDLRAP1;APOB;LDLR
67 NPC1L1;APOA1;APOA4;HMGCR;DHCR7;APOA5
68 ABCA1;SCARB1;LPL;PPARG;APOB
69 SCARB1;APOA1;CD36;LDLR
70 SCARB1;APOA1;CD36;LDLR
71 CYP27A1;LIPC;LIPI;LIPG;LPL;ABCB11;CYP7A1
72 NPC1L1;APOA1;APOA4;HMGCR;DHCR7;APOA5
73 APOC1;APOA2;PCSK9;APOA1;APOA4;PPARG;APOE;APOA5
74 NPC1L1;APOA1;APOA4;HMGCR;DHCR7;APOA5
75 APOC2;APOA1;APOA4;PPARG;APOA5
76 LRPAP1;APOC2;APOC1;APOC3;LDLRAP1;APOA5
77 FADS3;LIPC;LIPI;EPHX2;LIPG;LPL;FADS1
78 ABCA1;APOA1;APOC3;APOE
79 APOC2;APOA1;APOA4;APOA5
80 LIPC;STAR;LIPI;LIPG;LPL;HMGCR;FADS1
81 LRP1;APOA1;PPARG;APOE;PLTP
82 LRPAP1;APOC2;APOC1;PCSK9;APOC3
83 LRP1;PPARG;CD36;APOB
84 NPC2;APOC2;APOC1;APOC3;PPARG;HMGCR;DHCR7
85 NPC1;ADH1B;ANGPTL3;LPL;PPARG;VDAC1;CD36;ABCB11
86 LRP1;PPARG;CD36;APOB
87 LIPC;LIPI;LIPG;LPL;APOA4
88 APOC2;APOA1;APOA4;APOA5
89 ABCA1;NPC1;STAR;NPC2
90 CYP27A1;STAR;HMGCR;DHCR7;ABCB11;CYP7A1
91 STAR;APOA1;APOA4;APOE;APOA5
92 LIPC;APOA2;ANGPTL3;LPL
93 SCARB1;STAR;APOC2;APOA1;APOA4;CD36;APOA5;LDLR
94 LRPAP1;LRP1;HMGCR;APOE
95 LIPG;APOA2;ANGPTL3;LPL;LCAT;FADS1
96 APOA1;APOA4;APOA5
97 LRP1;LRP2;LDLR
98 APOA1;APOA4;APOE;CD36;APOA5
99 EPHX2;APOE;LDLRAP1;KPNB1
100 SOAT1;SOAT2;PPARG
101 SCARB1;LPL;APOB
102 SOAT1;SOAT2;PPARG
103 CYP27A1;HMGCR;DHCR7;ABCB11;CYP7A1
104 STAR;EPHX2;APOE;ABCB11
105 VAPA;VAPB;APOA2;APOA1;LCAT;APOE;LPIN3
106 APOC2;APOC1;APOA2;APOC3
107 LRP1;LRP2;LDLR
108 LRPAP1;LRP1;APOE
109 LIPG;APOA2;ANGPTL3;LPIN3
110 CETP;ANGPTL3;APOA4;PPARG;ABCB11
111 CYP27A1;APOE;CYP7A1
112 CYP27A1;APOE;CYP7A1
113 STARD3;STAR;TSPO;DHCR7
114 APOC3;PCSK9;LDLRAP1
115 CYP27A1;NPC1;ABCB11;CYP7A1
116 APOA2;LCAT;APOA1;LPIN3
117 CYP27A1;APOE;CYP7A1
118 LIPG;ANGPTL3;APOA2
119 LRP1;APOC2;ANGPTL3
120 LRP1;LPL;APOB
121 CETP;APOA1;APOE
122 CETP;APOA1;LCAT;APOA4;APOA5
123 ABCG8;ABCG5;NPC2;ABCB11
124 ABCA1;CETP;PPARG
125 CETP;LRP1;APOE
126 STARD3;STAR;TSPO
127 NPC1;VAPA;VAPB;LRP2;LDLRAP1;APOB;LDLR
128 LRP1;APOE;LDLRAP1;APOA5
129 GHR;ABCA1;LRPAP1;LRP1;APOC2;CD36;APOE;APOA5
130 APOC1;APOA2;APOC3;HMGCR
131 APOC1;APOA2;APOC3
132 SCARB1;NPC2;PLTP
133 LPL;CD36;APOB
134 ABCA1;LRP1;MTTP;PPARG;CD36;LRP2;APOE;APOB
135 PPARG;APOE;CD36
136 SCARB1;LPL;APOB
137 STARD3;STAR;TSPO
138 LIPG;APOA2;ANGPTL3
139 APOC2;APOC1;APOC3
140 PPARG;HMGCR;APOE;DHCR7;LDLR
141 LRPAP1;APOA2;APOA1;APOC3;APOA4;HMGCR
142 CYP27A1;ABCB11;CYP7A1
143 PCSK9;APOE
144 ABCA1;APOA1;APOC3;APOE
145 EPHX2;APOE;ABCB11
146 APOC2;APOA1;APOA4;APOE;APOA5
147 LRP1;ANGPTL3;LRP2
148 APOA2;APOA1;APOC3;APOA4
149 NPC2;PLTP
150 LIPG;LDLRAP1
151 CETP;LIPG
152 PPARG;APOE;CD36
153 SORT1;PCSK9;PPARG;LPIN3
154 ABCB11;CYP7A1
155 PCSK9;APOE
156 LRP1;APOA2;APOA1;APOE
157 APOH;PPARG;APOE
158 APOH;PPARG;APOE
159 APOE;LDLR
160 LRPAP1;HMGCR
161 LRPAP1;PCSK9;APOE
162 LIPI;APOA2;APOA1;LCAT;LPIN3
163 APOA2;APOA1
164 FADS3;FADS1
165 TSPO;VDAC2;VDAC1
166 PCSK9;LDLRAP1;APOA5
167 ABCA1;PPARG
168 STAR;CYP7A1
169 APOA2;APOA1
170 MYLIP;PCSK9
171 MYLIP;PCSK9
172 APOA2;APOA1
173 VAPA;APOE
174 SCARB1;LDLR
175 LRP1;CD36;LDLRAP1
176 APOA2;LPL;CYP7A1
177 LRP1;APOC2;APOE;APOA5
178 APOC1;APOC3
179 APOH;APOE
180 APOA2;APOA1
181 ALDH2;ADH1B
182 APOE;LDLR
183 HMGCR;APOE;LDLR
184 FADS3;FADS2;FADS1
185 FADS2;FADS1
186 ITIH4;APOH;APOA1;CD36
187 APOC2;APOC1;APOC3
188 APOE;LDLR
189 APOH;APOE
190 APOE;CD36
191 NPC1L1;CD36
192 LRP1;CD36;LDLRAP1
193 SCARB1;APOA2;APOA1
194 APOE;LDLR
195 LPL;PPARG
196 SCARB1;LDLR
197 APOA2;PCSK9;APOA1;APOE;APOB;APOA5
198 LRPAP1;LRP1;APOE
199 APOA1;APOA4;CD36
200 PPARG;LDLRAP1
201 PCSK9;LPL;FADS1
202 APOA2;APOA1
203 APOC1;APOC3
204 APOE;KPNB1
205 STAR;CYP7A1
206 APOA2;ANGPTL3;APOA5
207 APOE;LDLRAP1
208 APOE;CD36
209 SCARB1;APOE
210 ALDH2;ADH1B
211 PCSK9;APOE
212 LPL;CD36
213 APOE;LDLR
214 ABCA1;PPARG
215 FADS2;FADS1
216 APOC1;APOC3
217 LPL;APOB
218 APOA1;LPL;CD36
219 FADS2;EPHX2;FADS1
220 APOA2;APOA1
221 APOA2;PCSK9;APOA1;APOE;APOB;APOA5
222 APOA1;PPARG;APOE
223 LRP1;CD36;LRP2
224 MYLIP;PCSK9
225 APOA2;LPL
226 ITIH4;APOH;APOA1;CD36
227 SCARB1;APOH;APOE
228 HMGCR;APOE
229 LRP1;PPARG;APOE
230 APOH;APOE
231 SCARB1;APOE
232 APOH;APOE
233 LRPAP1;PCSK9;APOE;LDLR
234 EPHX2;FADS1
235 GHR;APOE
236 LRPAP1;LRP1;CD36
237 APOH;ANGPTL3;PPARG;ANGPTL4
238 APOA1;LPL;PPARG;APOE
239 SCARB1;LRP1
240 PCSK9;PPARG;LPIN3
241 PPARG;APOE
242 EPHX2;ANGPTL3;LPL;LCAT
243 APOH;APOE
244 LRP1;CD36
245 APOE;CD36
246 LRPAP1;PCSK9
247 APOA4;PPARG;CD36
248 PPARG;LDLRAP1
249 ABCA1;CD36;APOE
250 TSPO;VDAC2;VDAC1
251 APOA1;PPARG;APOE
252 LRPAP1;LRP1;LDLRAP1
253 APOE;CD36
254 HMGCR;APOE
255 HMGCR;APOE
256 SCARB1;APOE
257 APOH;APOE
258 GHR;LRP2;LDLRAP1
259 PCSK9;PPARG;LPIN3
260 LRP1;CD36
261 ABCA1;MTTP
262 NPC2;ABCB11
263 APOE;LDLR
264 LPL;PPARG;CD36;APOB
265 MYLIP;APOE
266 LPL;PPARG
267 PPARG;LRP2
268 APOC1;APOC3
269 APOA2;APOA1
[1] "GO_Cellular_Component_2021"
Term Overlap
1 high-density lipoprotein particle (GO:0034364) 12/19
2 chylomicron (GO:0042627) 10/10
3 triglyceride-rich plasma lipoprotein particle (GO:0034385) 10/15
4 very-low-density lipoprotein particle (GO:0034361) 10/15
5 early endosome (GO:0005769) 13/266
6 low-density lipoprotein particle (GO:0034362) 4/7
7 spherical high-density lipoprotein particle (GO:0034366) 4/8
8 endoplasmic reticulum lumen (GO:0005788) 10/285
9 endocytic vesicle membrane (GO:0030666) 8/158
10 endoplasmic reticulum membrane (GO:0005789) 14/712
11 lysosome (GO:0005764) 11/477
12 lytic vacuole (GO:0000323) 8/219
13 endocytic vesicle (GO:0030139) 7/189
14 clathrin-coated endocytic vesicle membrane (GO:0030669) 5/69
15 clathrin-coated endocytic vesicle (GO:0045334) 5/85
16 clathrin-coated vesicle membrane (GO:0030665) 5/90
17 lysosomal membrane (GO:0005765) 8/330
18 intracellular organelle lumen (GO:0070013) 12/848
19 collagen-containing extracellular matrix (GO:0062023) 8/380
20 endocytic vesicle lumen (GO:0071682) 3/21
21 organelle outer membrane (GO:0031968) 5/142
22 ATP-binding cassette (ABC) transporter complex (GO:0043190) 2/6
23 lytic vacuole membrane (GO:0098852) 6/267
24 endosome membrane (GO:0010008) 6/325
25 mitochondrial outer membrane (GO:0005741) 4/126
26 platelet dense granule lumen (GO:0031089) 2/14
27 vesicle (GO:0031982) 5/226
28 endolysosome membrane (GO:0036020) 2/17
29 basolateral plasma membrane (GO:0016323) 4/151
30 cytoplasmic vesicle membrane (GO:0030659) 6/380
31 platelet dense granule (GO:0042827) 2/21
32 lysosomal lumen (GO:0043202) 3/86
33 endolysosome (GO:0036019) 2/25
34 secretory granule lumen (GO:0034774) 5/316
35 brush border membrane (GO:0031526) 2/37
36 mitochondrial envelope (GO:0005740) 3/127
37 extracellular membrane-bounded organelle (GO:0065010) 2/56
38 extracellular vesicle (GO:1903561) 2/59
39 vacuolar lumen (GO:0005775) 3/161
40 caveola (GO:0005901) 2/60
Adjusted.P.value
1 5.261200e-24
2 6.209923e-24
3 9.203512e-21
4 9.203512e-21
5 1.246888e-10
6 7.731648e-08
7 1.321996e-07
8 6.153361e-07
9 8.075627e-07
10 1.200431e-06
11 6.211359e-06
12 7.353950e-06
13 2.938912e-05
14 2.938912e-05
15 7.671871e-05
16 9.509306e-05
17 1.065748e-04
18 1.698308e-04
19 2.574496e-04
20 2.574496e-04
21 6.432532e-04
22 8.164449e-04
23 1.420908e-03
24 3.827987e-03
25 3.898444e-03
26 4.116966e-03
27 4.199032e-03
28 5.675277e-03
29 6.551600e-03
30 6.795770e-03
31 7.845057e-03
32 1.043474e-02
33 1.043474e-02
34 1.423039e-02
35 2.126720e-02
36 2.763580e-02
37 4.460396e-02
38 4.700422e-02
39 4.700422e-02
40 4.700422e-02
Genes
1 CETP;APOC2;APOH;APOC1;APOA2;APOA1;APOC3;LCAT;APOA4;APOE;APOA5;PLTP
2 APOC2;APOH;APOC1;APOA2;APOA1;APOC3;APOA4;APOE;APOB;APOA5
3 APOC2;APOH;APOC1;APOA2;APOA1;APOC3;APOA4;APOE;APOB;APOA5
4 APOC2;APOH;APOC1;APOA2;APOA1;APOC3;APOA4;APOE;APOB;APOA5
5 LRP1;SORT1;APOA2;PCSK9;APOA1;APOC3;APOA4;APOC2;LIPG;APOE;LDLRAP1;APOB;LDLR
6 APOC2;APOE;APOB;APOA5
7 APOC2;APOA2;APOA1;APOC3
8 LRPAP1;LIPC;MTTP;APOA2;PCSK9;APOA1;APOA4;APOE;APOB;APOA5
9 SCARB1;LRP1;CD36;LRP2;APOE;LDLRAP1;APOB;LDLR
10 ABCA1;STARD3;HMGCR;CYP7A1;FADS2;NCEH1;SOAT1;VAPA;SOAT2;VAPB;DHCR7;APOB;FADS1;LPIN3
11 SCARB1;STARD3;NPC1;LRP1;NPC2;SORT1;PCSK9;LRP2;APOB;LIPA;LDLR
12 SCARB1;NPC1;NPC2;SORT1;PCSK9;LRP2;LIPA;LDLR
13 ABCA1;SCARB1;LRP1;APOA1;CD36;APOE;APOB
14 LRP2;APOE;LDLRAP1;APOB;LDLR
15 LRP2;APOE;LDLRAP1;APOB;LDLR
16 LRP2;APOE;LDLRAP1;APOB;LDLR
17 SCARB1;STARD3;NPC1;LRP1;VAPA;PCSK9;LRP2;LDLR
18 CYP27A1;LIPC;ALDH2;MTTP;APOA2;PCSK9;APOA1;APOA4;APOE;APOB;APOA5;KPNB1
19 ITIH4;APOH;ANGPTL3;APOA1;APOC3;APOA4;ANGPTL4;APOE
20 APOA1;APOE;APOB
21 VDAC3;TSPO;VDAC2;VDAC1;DHCR7
22 ABCG8;ABCG5
23 SCARB1;STARD3;NPC1;LRP1;PCSK9;LRP2
24 STARD3;SORT1;PCSK9;ABCB11;APOB;LDLR
25 VDAC3;TSPO;VDAC2;VDAC1
26 ITIH4;APOH
27 ABCA1;CETP;VAPA;APOA1;APOE
28 PCSK9;LDLR
29 LRP1;MTTP;ABCB11;LDLR
30 SCARB1;LRP1;SORT1;CD36;APOB;LDLR
31 ITIH4;APOH
32 NPC2;APOB;LIPA
33 PCSK9;LDLR
34 ITIH4;NPC2;APOH;APOA1;KPNB1
35 LRP2;CD36
36 STAR;VDAC2;VDAC1
37 APOA1;APOE
38 APOA1;APOE
39 NPC2;APOB;LIPA
40 SCARB1;CD36
[1] "GO_Molecular_Function_2021"
Term
1 cholesterol binding (GO:0015485)
2 sterol binding (GO:0032934)
3 cholesterol transfer activity (GO:0120020)
4 sterol transfer activity (GO:0120015)
5 lipoprotein particle receptor binding (GO:0070325)
6 phosphatidylcholine-sterol O-acyltransferase activator activity (GO:0060228)
7 lipoprotein particle binding (GO:0071813)
8 low-density lipoprotein particle binding (GO:0030169)
9 lipase inhibitor activity (GO:0055102)
10 low-density lipoprotein particle receptor binding (GO:0050750)
11 lipoprotein lipase activity (GO:0004465)
12 amyloid-beta binding (GO:0001540)
13 triglyceride lipase activity (GO:0004806)
14 lipase activity (GO:0016298)
15 lipase binding (GO:0035473)
16 apolipoprotein A-I binding (GO:0034186)
17 apolipoprotein receptor binding (GO:0034190)
18 phospholipase A1 activity (GO:0008970)
19 lipase activator activity (GO:0060229)
20 carboxylic ester hydrolase activity (GO:0052689)
21 voltage-gated anion channel activity (GO:0008308)
22 voltage-gated ion channel activity (GO:0005244)
23 phosphatidylcholine transporter activity (GO:0008525)
24 protein heterodimerization activity (GO:0046982)
25 phosphatidylcholine transfer activity (GO:0120019)
26 phospholipase activity (GO:0004620)
27 O-acyltransferase activity (GO:0008374)
28 high-density lipoprotein particle binding (GO:0008035)
29 ceramide transfer activity (GO:0120017)
30 clathrin heavy chain binding (GO:0032050)
31 phospholipase inhibitor activity (GO:0004859)
32 protein homodimerization activity (GO:0042803)
33 anion channel activity (GO:0005253)
34 phospholipid transfer activity (GO:0120014)
35 oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen, NAD(P)H as one donor, and incorporation of one atom of oxygen (GO:0016709)
36 steroid hydroxylase activity (GO:0008395)
37 NADP binding (GO:0050661)
38 peptidase inhibitor activity (GO:0030414)
39 endopeptidase regulator activity (GO:0061135)
Overlap Adjusted.P.value
1 17/50 2.288174e-28
2 17/60 4.390322e-27
3 11/18 5.744987e-22
4 11/19 1.020647e-21
5 10/28 4.677379e-17
6 6/6 3.484605e-14
7 8/24 2.055320e-13
8 6/17 3.140441e-10
9 5/10 1.805253e-09
10 6/23 2.016360e-09
11 4/5 9.113232e-09
12 7/80 1.430772e-07
13 5/23 1.612041e-07
14 6/49 1.859889e-07
15 3/5 3.788104e-06
16 3/5 3.788104e-06
17 3/6 7.112969e-06
18 3/10 3.991032e-05
19 3/12 6.897596e-05
20 5/96 1.501407e-04
21 3/16 1.501407e-04
22 3/16 1.501407e-04
23 3/18 2.082322e-04
24 6/188 3.016202e-04
25 2/5 7.035280e-04
26 4/73 7.035280e-04
27 3/30 8.567836e-04
28 2/6 9.653527e-04
29 2/8 1.732107e-03
30 2/9 2.147965e-03
31 2/10 2.592555e-03
32 8/636 7.345938e-03
33 3/68 7.879809e-03
34 2/22 1.181407e-02
35 2/36 2.870121e-02
36 2/36 2.870121e-02
37 2/36 2.870121e-02
38 2/40 3.429432e-02
39 2/46 4.375411e-02
Genes
1 ABCA1;STARD3;CETP;OSBPL5;APOA2;APOA1;APOC3;APOA4;APOA5;NPC1;SOAT1;STAR;NPC2;SOAT2;TSPO;VDAC2;VDAC1
2 ABCA1;STARD3;CETP;OSBPL5;APOA2;APOA1;APOC3;APOA4;APOA5;NPC1;SOAT1;STAR;NPC2;SOAT2;TSPO;VDAC2;VDAC1
3 ABCA1;ABCG8;CETP;ABCG5;NPC2;MTTP;APOA2;APOA1;APOA4;APOB;PLTP
4 ABCA1;ABCG8;CETP;ABCG5;NPC2;MTTP;APOA2;APOA1;APOA4;APOB;PLTP
5 LRPAP1;LRP1;APOA2;PCSK9;APOA1;APOC3;APOE;APOB;LDLRAP1;APOA5
6 APOC1;APOA2;APOA1;APOA4;APOE;APOA5
7 SCARB1;LIPC;APOA2;LPL;PCSK9;CD36;LDLR;PLTP
8 SCARB1;LIPC;PCSK9;CD36;LDLR;PLTP
9 APOC2;APOC1;ANGPTL3;APOA2;APOC3
10 LRPAP1;PCSK9;APOE;APOB;LDLRAP1;APOA5
11 LIPC;LIPI;LIPG;LPL
12 LRPAP1;LRP1;APOA1;CD36;APOE;LDLRAP1;LDLR
13 LIPC;LIPI;LIPG;LCAT;LPL
14 LIPC;LIPI;LIPG;LCAT;LPL;LIPA
15 LRPAP1;APOB;APOA5
16 ABCA1;SCARB1;LCAT
17 APOA2;APOA1;PCSK9
18 LIPC;LIPG;LPL
19 APOC2;APOH;APOA5
20 LIPC;LIPG;LPL;LCAT;LIPA
21 VDAC3;VDAC2;VDAC1
22 VDAC3;VDAC2;VDAC1
23 ABCA1;MTTP;PLTP
24 ABCG8;ABCG5;VAPA;VAPB;MTTP;APOA2
25 MTTP;PLTP
26 LIPC;LIPI;LIPG;LPL
27 SOAT1;SOAT2;LCAT
28 APOA2;PLTP
29 MTTP;PLTP
30 LRP1;LDLR
31 APOC1;ANGPTL3
32 GHR;STARD3;VAPB;EPHX2;APOA2;LPL;APOA4;APOE
33 VDAC3;VDAC2;VDAC1
34 MTTP;PLTP
35 CYP27A1;CYP7A1
36 CYP27A1;CYP7A1
37 HMGCR;DHCR7
38 ITIH4;LPA
39 ITIH4;LPAGO_known_annotations <- do.call(rbind, GO_enrichment)
GO_known_annotations <- GO_known_annotations[GO_known_annotations$Adjusted.P.value<0.05,]
#GO enrichment analysis for cTWAS genes
genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
GO_enrichment <- enrichr(genes, dbs)Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.GO_ctwas_genes <- do.call(rbind, GO_enrichment)
#optionally subset to only significant GO terms
#GO_ctwas_genes <- GO_ctwas_genes[GO_ctwas_genes$Adjusted.P.value<0.05,]
#identify cTWAS genes in silver standard enriched GO terms
GO_ctwas_genes <- GO_ctwas_genes[GO_ctwas_genes$Term %in% GO_known_annotations$Term,]
overlap_genes <- lapply(GO_ctwas_genes$Genes, function(x){unlist(strsplit(x, ";"))})
overlap_genes <- -sort(-table(unlist(overlap_genes)))
#ctwas genes in silver standard enriched GO terms, not already in silver standard
overlap_genes[!(names(overlap_genes) %in% known_annotations)]
PGP PSMB7 GNB4 PCCB WASHC2C MSRB1 NKX2-5
4 4 3 2 2 1 1 save(overlap_genes, file=paste0(results_dir, "/overlap_genes.Rd"))
load(paste0(results_dir, "/overlap_genes.Rd"))
overlap_genes <- overlap_genes[!(names(overlap_genes) %in% known_annotations)]
overlap_genes
PGP PSMB7 GNB4 PCCB WASHC2C MSRB1 NKX2-5
4 4 3 2 2 1 1 overlap_genes <- names(overlap_genes)
#ctwas_gene_res[ctwas_gene_res$genename %in% overlap_genes, report_cols,]Results for Paper
out_table <- ctwas_gene_res
report_cols <- report_cols[!(report_cols %in% c("mu2", "PVE"))]
report_cols <- c(report_cols,"silver","GO_overlap_silver", "bystander")
#reload silver standard genes
known_annotations <- read_xlsx("data/summary_known_genes_annotations.xlsx", sheet="LDL")New names:
* `` -> ...4
* `` -> ...5known_annotations <- unique(known_annotations$`Gene Symbol`)
out_table$silver <- F
out_table$silver[out_table$genename %in% known_annotations] <- T
library(biomaRt)
library(GenomicRanges)Loading required package: stats4Loading required package: BiocGenericsLoading required package: parallel
Attaching package: 'BiocGenerics'The following objects are masked from 'package:parallel':
clusterApply, clusterApplyLB, clusterCall, clusterEvalQ,
clusterExport, clusterMap, parApply, parCapply, parLapply,
parLapplyLB, parRapply, parSapply, parSapplyLBThe following objects are masked from 'package:dplyr':
combine, intersect, setdiff, unionThe following objects are masked from 'package:stats':
IQR, mad, sd, var, xtabsThe following objects are masked from 'package:base':
anyDuplicated, append, as.data.frame, basename, cbind, colnames,
dirname, do.call, duplicated, eval, evalq, Filter, Find, get, grep,
grepl, intersect, is.unsorted, lapply, Map, mapply, match, mget,
order, paste, pmax, pmax.int, pmin, pmin.int, Position, rank,
rbind, Reduce, rownames, sapply, setdiff, sort, table, tapply,
union, unique, unsplit, which, which.max, which.minLoading required package: S4Vectors
Attaching package: 'S4Vectors'The following objects are masked from 'package:dplyr':
first, renameThe following object is masked from 'package:tidyr':
expandThe following object is masked from 'package:base':
expand.gridLoading required package: IRanges
Attaching package: 'IRanges'The following objects are masked from 'package:dplyr':
collapse, desc, sliceThe following object is masked from 'package:purrr':
reduceLoading required package: GenomeInfoDbensembl <- useEnsembl(biomart="ENSEMBL_MART_ENSEMBL", dataset="hsapiens_gene_ensembl")
G_list <- getBM(filters= "chromosome_name", attributes= c("hgnc_symbol","chromosome_name","start_position","end_position","gene_biotype"), values=1:22, mart=ensembl)
G_list <- G_list[G_list$hgnc_symbol!="",]
G_list <- G_list[G_list$gene_biotype %in% c("protein_coding","lncRNA"),]
G_list$start <- G_list$start_position
G_list$end <- G_list$end_position
G_list_granges <- makeGRangesFromDataFrame(G_list, keep.extra.columns=T)
known_annotations_positions <- G_list[G_list$hgnc_symbol %in% known_annotations,]
half_window <- 1000000
known_annotations_positions$start <- known_annotations_positions$start_position - half_window
known_annotations_positions$end <- known_annotations_positions$end_position + half_window
known_annotations_positions$start[known_annotations_positions$start<1] <- 1
known_annotations_granges <- makeGRangesFromDataFrame(known_annotations_positions, keep.extra.columns=T)
bystanders_extended <- findOverlaps(known_annotations_granges,G_list_granges)
bystanders_extended <- unique(subjectHits(bystanders_extended))
bystanders_extended <- G_list$hgnc_symbol[bystanders_extended]
bystanders_extended <- unique(bystanders_extended[!(bystanders_extended %in% known_annotations)])
save(bystanders_extended, file=paste0(results_dir, "/bystanders_extended.Rd"))
load(paste0(results_dir, "/bystanders_extended.Rd"))
#add extended bystanders list to output
out_table$bystander <- F
out_table$bystander[out_table$genename %in% bystanders_extended] <- T
#reload GO overlaps with silver standard
load(paste0(results_dir, "/overlap_genes.Rd"))
out_table$GO_overlap_silver <- NA
out_table$GO_overlap_silver[out_table$susie_pip>0.8] <- 0
for (i in names(overlap_genes)){
out_table$GO_overlap_silver[out_table$genename==i] <- overlap_genes[i]
}cTWAS identifies high-confidence liver genes associated with LDL cholesterol
full.gene.pip.summary <- data.frame(gene_name = ctwas_gene_res$genename,
gene_pip = ctwas_gene_res$susie_pip,
gene_id = ctwas_gene_res$id,
chr = as.integer(ctwas_gene_res$chrom),
start = ctwas_gene_res$pos / 1e3,
is_highlight = F, stringsAsFactors = F) %>% as_tibble()
full.gene.pip.summary$is_highlight <- full.gene.pip.summary$gene_pip > 0.80
don <- full.gene.pip.summary %>%
# Compute chromosome size
group_by(chr) %>%
summarise(chr_len=max(start)) %>%
# Calculate cumulative position of each chromosome
mutate(tot=cumsum(chr_len)-chr_len) %>%
dplyr::select(-chr_len) %>%
# Add this info to the initial dataset
left_join(full.gene.pip.summary, ., by=c("chr"="chr")) %>%
# Add a cumulative position of each SNP
arrange(chr, start) %>%
mutate( BPcum=start+tot)
axisdf <- don %>% group_by(chr) %>% summarize(center=( max(BPcum) + min(BPcum) ) / 2 )
x_axis_labels <- axisdf$chr
x_axis_labels[seq(1,21,2)] <- ""
ggplot(don, aes(x=BPcum, y=gene_pip)) +
# Show all points
ggrastr::geom_point_rast(aes(color=as.factor(chr)), size=2) +
scale_color_manual(values = rep(c("grey", "skyblue"), 22 )) +
# custom X axis:
# scale_x_continuous(label = axisdf$chr,
# breaks= axisdf$center,
# guide = guide_axis(n.dodge = 2)) +
scale_x_continuous(label = x_axis_labels,
breaks = axisdf$center) +
scale_y_continuous(expand = c(0, 0), limits = c(0,1.25), breaks=(1:5)*0.2, minor_breaks=(1:10)*0.1) + # remove space between plot area and x axis
# Add highlighted points
ggrastr::geom_point_rast(data=subset(don, is_highlight==T), color="orange", size=2) +
# Add label using ggrepel to avoid overlapping
ggrepel::geom_label_repel(data=subset(don, is_highlight==T),
aes(label=gene_name),
size=4,
min.segment.length = 0,
label.size = NA,
fill = alpha(c("white"),0)) +
# Custom the theme:
theme_bw() +
theme(
text = element_text(size = 14),
legend.position="none",
panel.border = element_blank(),
panel.grid.major.x = element_blank(),
panel.grid.minor.x = element_blank()
) +
xlab("Chromosome") +
ylab("cTWAS PIP")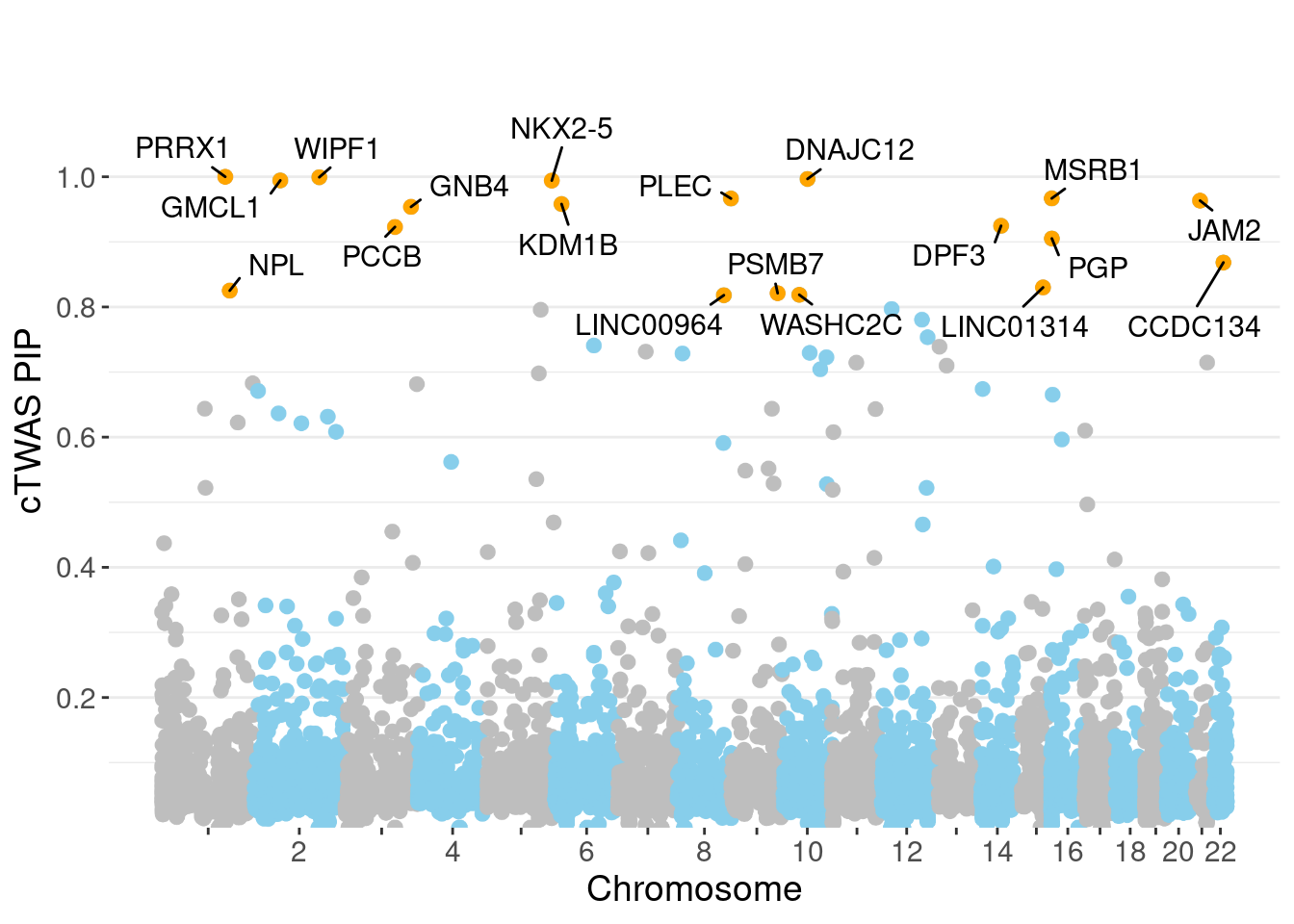
#number of SNPs at PIP>0.8 threshold
sum(out_table$susie_pip>0.8)[1] 19#number of SNPs at PIP>0.5 threshold
sum(out_table$susie_pip>0.5)[1] 60#genes with PIP>0.8
head(out_table[order(-out_table$susie_pip),report_cols], sum(out_table$susie_pip>0.8)) genename region_tag susie_pip z num_eqtl silver
3169 PRRX1 1_84 0.9999999 14.848088 2 FALSE
3147 WIPF1 2_105 0.9993101 8.987351 2 FALSE
2431 DNAJC12 10_44 0.9967458 -5.328244 1 FALSE
1214 GMCL1 2_46 0.9944249 -8.434995 2 FALSE
9840 NKX2-5 5_103 0.9942183 -9.616279 2 FALSE
10979 MSRB1 16_2 0.9667967 5.929412 1 FALSE
9417 PLEC 8_94 0.9666163 3.093844 2 FALSE
6663 JAM2 21_9 0.9633616 4.569598 2 FALSE
7794 KDM1B 6_14 0.9581444 -9.000000 1 FALSE
2987 GNB4 3_110 0.9537748 -5.869048 1 FALSE
11279 DPF3 14_34 0.9246749 6.100000 1 FALSE
2960 PCCB 3_84 0.9229236 -5.382353 1 FALSE
9960 PGP 16_2 0.9051693 5.943820 1 FALSE
1551 CCDC134 22_17 0.8683170 -5.180556 1 FALSE
12376 LINC01314 15_37 0.8300211 -4.379164 4 FALSE
4891 NPL 1_90 0.8251030 -3.825175 1 FALSE
5042 PSMB7 9_64 0.8211676 -4.820896 1 FALSE
8847 WASHC2C 10_31 0.8186904 -3.972152 1 FALSE
12133 LINC00964 8_82 0.8180590 4.554265 2 FALSE
GO_overlap_silver bystander
3169 0 FALSE
3147 0 FALSE
2431 0 FALSE
1214 0 FALSE
9840 1 FALSE
10979 1 FALSE
9417 0 FALSE
6663 0 FALSE
7794 0 FALSE
2987 3 FALSE
11279 0 FALSE
2960 2 FALSE
9960 4 FALSE
1551 0 FALSE
12376 0 FALSE
4891 0 FALSE
5042 4 FALSE
8847 2 FALSE
12133 0 FALSEhead(out_table[order(-out_table$susie_pip),report_cols[-(7:8)]], sum(out_table$susie_pip>0.8)) genename region_tag susie_pip z num_eqtl silver
3169 PRRX1 1_84 0.9999999 14.848088 2 FALSE
3147 WIPF1 2_105 0.9993101 8.987351 2 FALSE
2431 DNAJC12 10_44 0.9967458 -5.328244 1 FALSE
1214 GMCL1 2_46 0.9944249 -8.434995 2 FALSE
9840 NKX2-5 5_103 0.9942183 -9.616279 2 FALSE
10979 MSRB1 16_2 0.9667967 5.929412 1 FALSE
9417 PLEC 8_94 0.9666163 3.093844 2 FALSE
6663 JAM2 21_9 0.9633616 4.569598 2 FALSE
7794 KDM1B 6_14 0.9581444 -9.000000 1 FALSE
2987 GNB4 3_110 0.9537748 -5.869048 1 FALSE
11279 DPF3 14_34 0.9246749 6.100000 1 FALSE
2960 PCCB 3_84 0.9229236 -5.382353 1 FALSE
9960 PGP 16_2 0.9051693 5.943820 1 FALSE
1551 CCDC134 22_17 0.8683170 -5.180556 1 FALSE
12376 LINC01314 15_37 0.8300211 -4.379164 4 FALSE
4891 NPL 1_90 0.8251030 -3.825175 1 FALSE
5042 PSMB7 9_64 0.8211676 -4.820896 1 FALSE
8847 WASHC2C 10_31 0.8186904 -3.972152 1 FALSE
12133 LINC00964 8_82 0.8180590 4.554265 2 FALSEhead(out_table[order(-out_table$susie_pip),report_cols[c(1,7:8)]], sum(out_table$susie_pip>0.8)) genename GO_overlap_silver bystander
3169 PRRX1 0 FALSE
3147 WIPF1 0 FALSE
2431 DNAJC12 0 FALSE
1214 GMCL1 0 FALSE
9840 NKX2-5 1 FALSE
10979 MSRB1 1 FALSE
9417 PLEC 0 FALSE
6663 JAM2 0 FALSE
7794 KDM1B 0 FALSE
2987 GNB4 3 FALSE
11279 DPF3 0 FALSE
2960 PCCB 2 FALSE
9960 PGP 4 FALSE
1551 CCDC134 0 FALSE
12376 LINC01314 0 FALSE
4891 NPL 0 FALSE
5042 PSMB7 4 FALSE
8847 WASHC2C 2 FALSE
12133 LINC00964 0 FALSEcTWAS avoids false positives when multiple genes are in a region
TNKS is a silver standard (assumed true positive gene) that is correctly detected. The bystander gene RP11-115J16.2 is significant using TWAS but has low PIP using cTWAS.
#TNKS gene
locus_plot4("8_12", label="cTWAS")
out_table[out_table$region_tag=="8_12",report_cols[-(7:8)]]
out_table[out_table$region_tag=="8_12",report_cols[c(1,7:8)]]FADS1 is a silver standard gene (assumed true positive gene) that is correctly detected. There are 5 significant TWAS genes at this locus, including FADS2, another silver standard gene. FADS2 is not detected due to its high LD with FADS1. The remaining 3 bystander genes at this locus have low PIP using cTWAS.
#FADS1 gene
locus_plot3("11_34", focus="FADS1")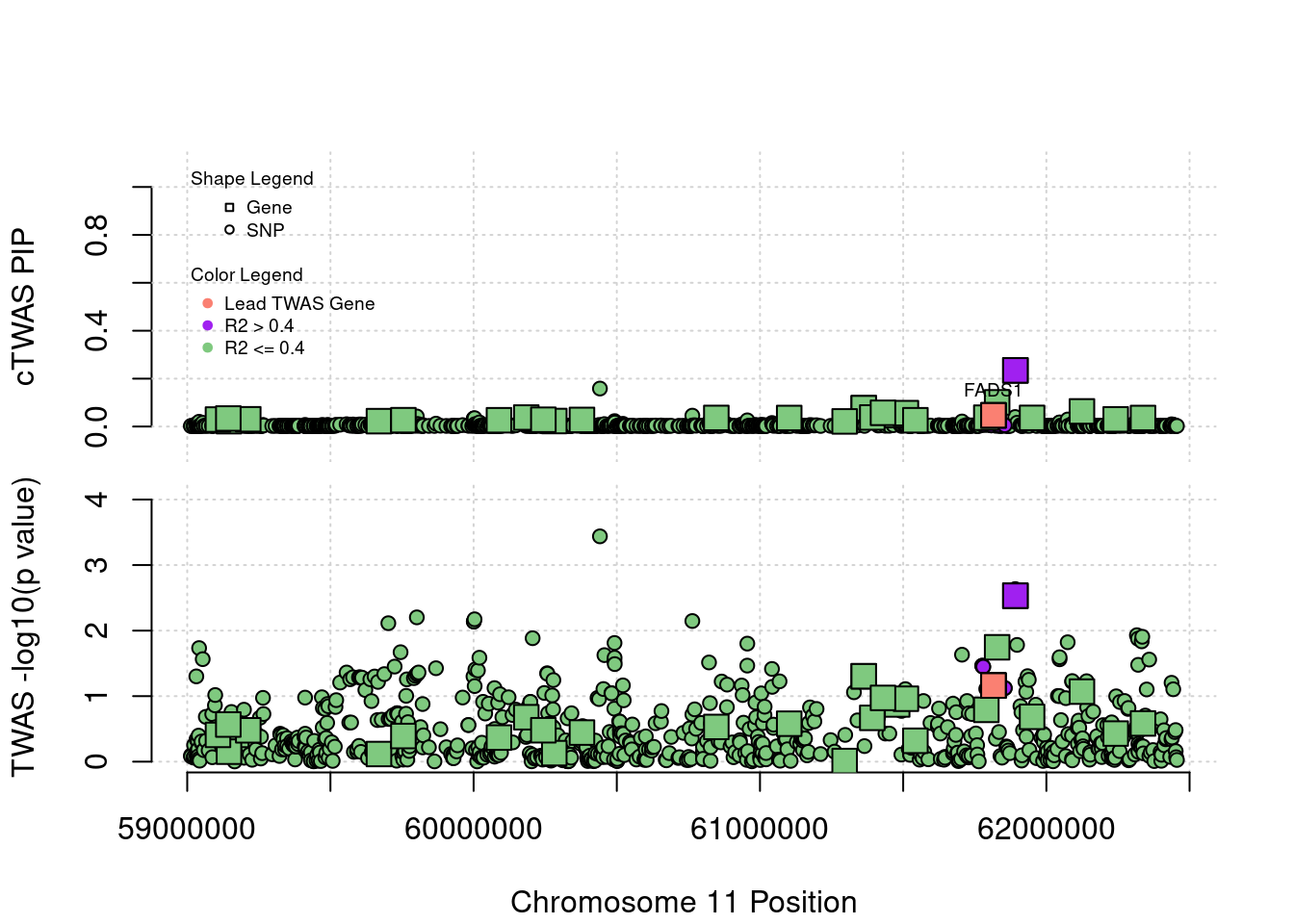
out_table[out_table$region_tag=="11_34",report_cols[-(7:8)]] genename region_tag susie_pip z num_eqtl silver
2584 DTX4 11_34 0.02781763 0.85897436 1 FALSE
2593 MS4A6A 11_34 0.03760719 1.25373134 1 FALSE
2594 MS4A4A 11_34 0.02309081 -0.32835821 1 FALSE
2597 CCDC86 11_34 0.03597023 1.04649029 2 FALSE
2621 CD5 11_34 0.03568699 1.11764706 1 FALSE
3883 SCGB1D2 11_34 0.03004532 -0.89156627 1 FALSE
4751 FADS2 11_34 0.10100488 2.36643571 2 TRUE
4752 TMEM258 11_34 0.03697381 1.39809594 2 FALSE
6292 TMEM138 11_34 0.07669069 1.96202532 1 FALSE
6293 FADS1 11_34 0.04673113 -1.81944444 1 TRUE
6296 INCENP 11_34 0.06319853 1.72072479 2 FALSE
6299 MS4A2 11_34 0.02622115 -0.79104478 1 FALSE
7256 CYB561A3 11_34 0.07669069 1.96202532 1 FALSE
7257 PPP1R32 11_34 0.04776414 1.49792139 2 FALSE
7258 ASRGL1 11_34 0.03557232 1.12328767 1 FALSE
8045 FAM111A 11_34 0.02248651 0.37903226 1 FALSE
8061 PATL1 11_34 0.02241093 -0.29903537 1 FALSE
8063 STX3 11_34 0.02639309 0.82994817 3 FALSE
8072 MS4A14 11_34 0.02767618 0.91549296 1 FALSE
8249 VWCE 11_34 0.02151883 0.02651515 1 FALSE
8250 BEST1 11_34 0.03609758 1.26126126 1 FALSE
10279 TMEM216 11_34 0.03789266 -1.24090909 1 FALSE
10481 FAM111B 11_34 0.02911441 -0.85853659 1 FALSE
10784 MPEG1 11_34 0.03036725 -0.96969697 1 FALSE
11219 LRRC10B 11_34 0.05600778 -1.60000000 1 FALSE
11466 MS4A4E 11_34 0.02832931 0.97058824 1 FALSE
11539 FADS3 11_34 0.23438823 -2.97402597 1 TRUE
12060 AP001258.4 11_34 0.03358240 1.10000000 1 FALSE
12307 RP11-794G24.1 11_34 0.02633199 -0.70680628 1 FALSE
12313 RP11-286N22.8 11_34 0.05730574 -1.61458333 1 FALSEout_table[out_table$region_tag=="11_34",report_cols[c(1,7:8)]] genename GO_overlap_silver bystander
2584 DTX4 NA FALSE
2593 MS4A6A NA FALSE
2594 MS4A4A NA FALSE
2597 CCDC86 NA TRUE
2621 CD5 NA TRUE
3883 SCGB1D2 NA TRUE
4751 FADS2 NA FALSE
4752 TMEM258 NA TRUE
6292 TMEM138 NA TRUE
6293 FADS1 NA FALSE
6296 INCENP NA TRUE
6299 MS4A2 NA FALSE
7256 CYB561A3 NA TRUE
7257 PPP1R32 NA TRUE
7258 ASRGL1 NA TRUE
8045 FAM111A NA FALSE
8061 PATL1 NA FALSE
8063 STX3 NA FALSE
8072 MS4A14 NA FALSE
8249 VWCE NA TRUE
8250 BEST1 NA TRUE
10279 TMEM216 NA TRUE
10481 FAM111B NA FALSE
10784 MPEG1 NA FALSE
11219 LRRC10B NA TRUE
11466 MS4A4E NA FALSE
11539 FADS3 NA FALSE
12060 AP001258.4 NA FALSE
12307 RP11-794G24.1 NA FALSE
12313 RP11-286N22.8 NA FALSE#number of significant TWAS genes at this locus
sum(abs(out_table$z[out_table$region_tag=="11_34"])>sig_thresh)[1] 0cTWAS avoids false positives when SNPs in a region are (considerably) more significant
POLK is a gene that is significant using TWAS but not detected using TWAS. cTWAS places a high posterior probability on SNPs are this locus. OpenTargets suggets that the causal gene at this locus is HMGCR (note: different GWAS, similar population), which is not imputed in our dataset. cTWAS selected the variants at this locus because the causal gene is not imputed. Note that MR-JTI claims POLK is causal using their method, and their paper includes a discussion of its potential relevance to LDL.
locus_plot("5_45", label="TWAS")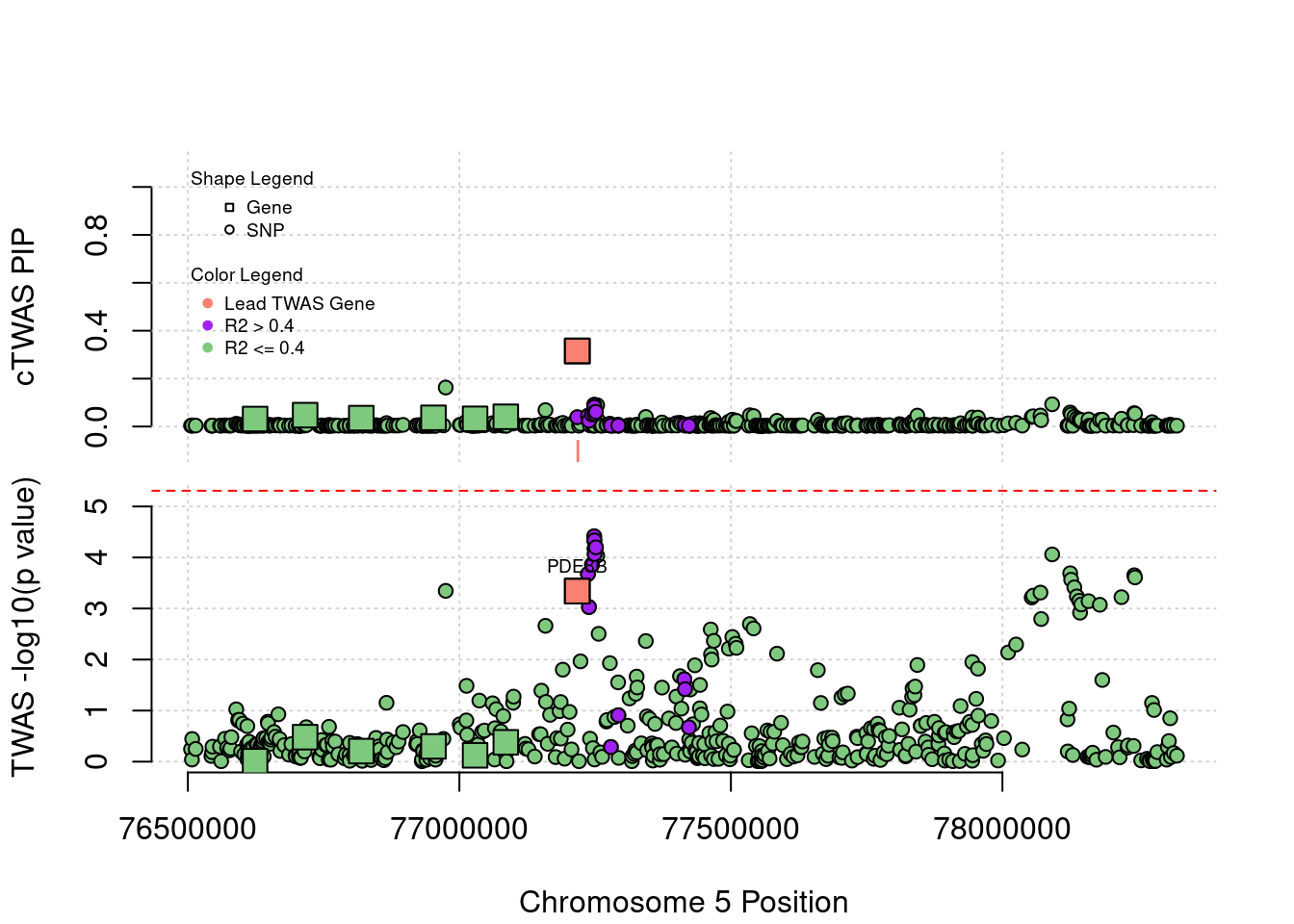
#locus_plot("5_45", label="TWAS", rerun_ctwas = T)
out_table[out_table$region_tag=="5_45",report_cols[-(7:8)]] genename region_tag susie_pip z num_eqtl silver
2891 PDE8B 5_45 0.31566331 -3.50495050 1 FALSE
4561 ZBED3 5_45 0.04067004 -0.81037007 2 FALSE
6015 CRHBP 5_45 0.03635826 0.67080227 2 FALSE
7654 F2RL2 5_45 0.03134154 0.02696569 2 FALSE
7660 F2RL1 5_45 0.03500783 -0.49283464 2 FALSE
7661 AGGF1 5_45 0.03208742 -0.31506849 1 FALSE
9671 F2R 5_45 0.04764434 0.97134670 1 FALSEout_table[out_table$region_tag=="5_45",report_cols[c(1,7:8)]] genename GO_overlap_silver bystander
2891 PDE8B NA FALSE
4561 ZBED3 NA FALSE
6015 CRHBP NA FALSE
7654 F2RL2 NA FALSE
7660 F2RL1 NA FALSE
7661 AGGF1 NA FALSE
9671 F2R NA FALSEcTWAS is more precise than TWAS in distinguishing silver standard and bystander genes
load(paste0(results_dir, "/known_annotations.Rd"))
load(paste0(results_dir, "/bystanders.Rd"))
#remove genes without imputed expression from bystander list
unrelated_genes <- unrelated_genes[unrelated_genes %in% ctwas_gene_res$genename]
#subset results to genes in known annotations or bystanders
ctwas_gene_res_subset <- ctwas_gene_res[ctwas_gene_res$genename %in% c(known_annotations, unrelated_genes),]
#assign ctwas and TWAS genes
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>0.8]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>sig_thresh]
#sensitivity / recall
sensitivity <- rep(NA,2)
names(sensitivity) <- c("ctwas", "TWAS")
sensitivity["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
sensitivity["TWAS"] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
sensitivityctwas TWAS
0 0 #specificity / (1 - False Positive Rate)
specificity <- rep(NA,2)
names(specificity) <- c("ctwas", "TWAS")
specificity["ctwas"] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
specificity["TWAS"] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
specificity ctwas TWAS
1.0000000 0.9886878 #precision / PPV / (1 - False Discovery Rate)
precision <- rep(NA,2)
names(precision) <- c("ctwas", "TWAS")
precision["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
precision["TWAS"] <- sum(twas_genes %in% known_annotations)/length(twas_genes)
precisionctwas TWAS
NaN 0 #store sensitivity and specificity calculations for plots
sensitivity_plot <- sensitivity
specificity_plot <- specificity
#precision / PPV by PIP threshold
pip_range <- c(0.5, 0.8, 1)
precision_range <- rep(NA, length(pip_range))
number_detected <- rep(NA, length(pip_range))
for (i in 1:length(pip_range)){
pip_upper <- pip_range[i]
if (i==1){
pip_lower <- 0
} else {
pip_lower <- pip_range[i-1]
}
#assign ctwas genes using PIP threshold
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>=pip_lower]
number_detected[i] <- length(ctwas_genes)
precision_range[i] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
}
names(precision_range) <- paste0(">= ", c(0, pip_range[-length(pip_range)]))
precision_range <- precision_range*100
precision_range <- c(precision_range, precision["TWAS"]*100)
names(precision_range)[4] <- "TWAS Bonferroni"
number_detected <- c(number_detected, length(twas_genes))
barplot(precision_range, ylim=c(0,100), main="Precision for Distinguishing Silver Standard and Bystander Genes", xlab="PIP Threshold for Detection", ylab="% of Detected Genes in Silver Standard")
abline(h=20, lty=2)
abline(h=40, lty=2)
abline(h=60, lty=2)
abline(h=80, lty=2)
xx <- barplot(precision_range, add=T, col=c(rep("darkgrey",3), "white"))
text(x = xx, y = rep(0, length(number_detected)), label = paste0(number_detected, " detected"), pos = 3, cex=0.8)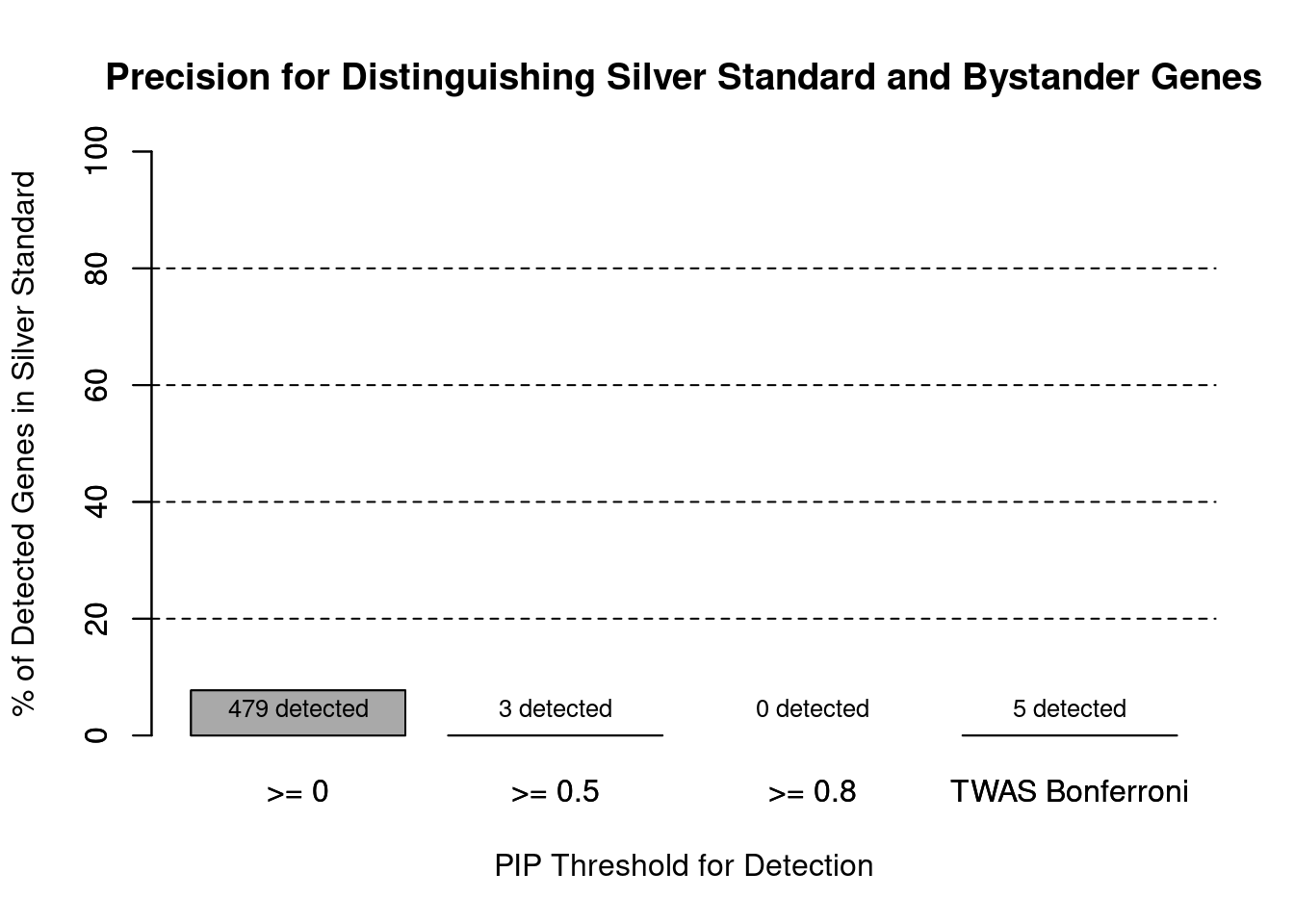
#text(x = xx, y = precision_range, label = paste0(round(precision_range,1), "%"), pos = 3, cex=0.8, offset = 1.5)
#false discovery rate by PIP threshold
barplot(100-precision_range, ylim=c(0,100), main="False Discovery Rate for Distinguishing Silver Standard and Bystander Genes", xlab="PIP Threshold for Detection", ylab="% Bystanders in Detected Genes")
abline(h=20, lty=2)
abline(h=40, lty=2)
abline(h=60, lty=2)
abline(h=80, lty=2)
xx <- barplot(100-precision_range, add=T, col=c(rep("darkgrey",3), "white"))
text(x = xx, y = rep(0, length(number_detected)), label = paste0(number_detected, " detected"), pos = 3, cex=0.8)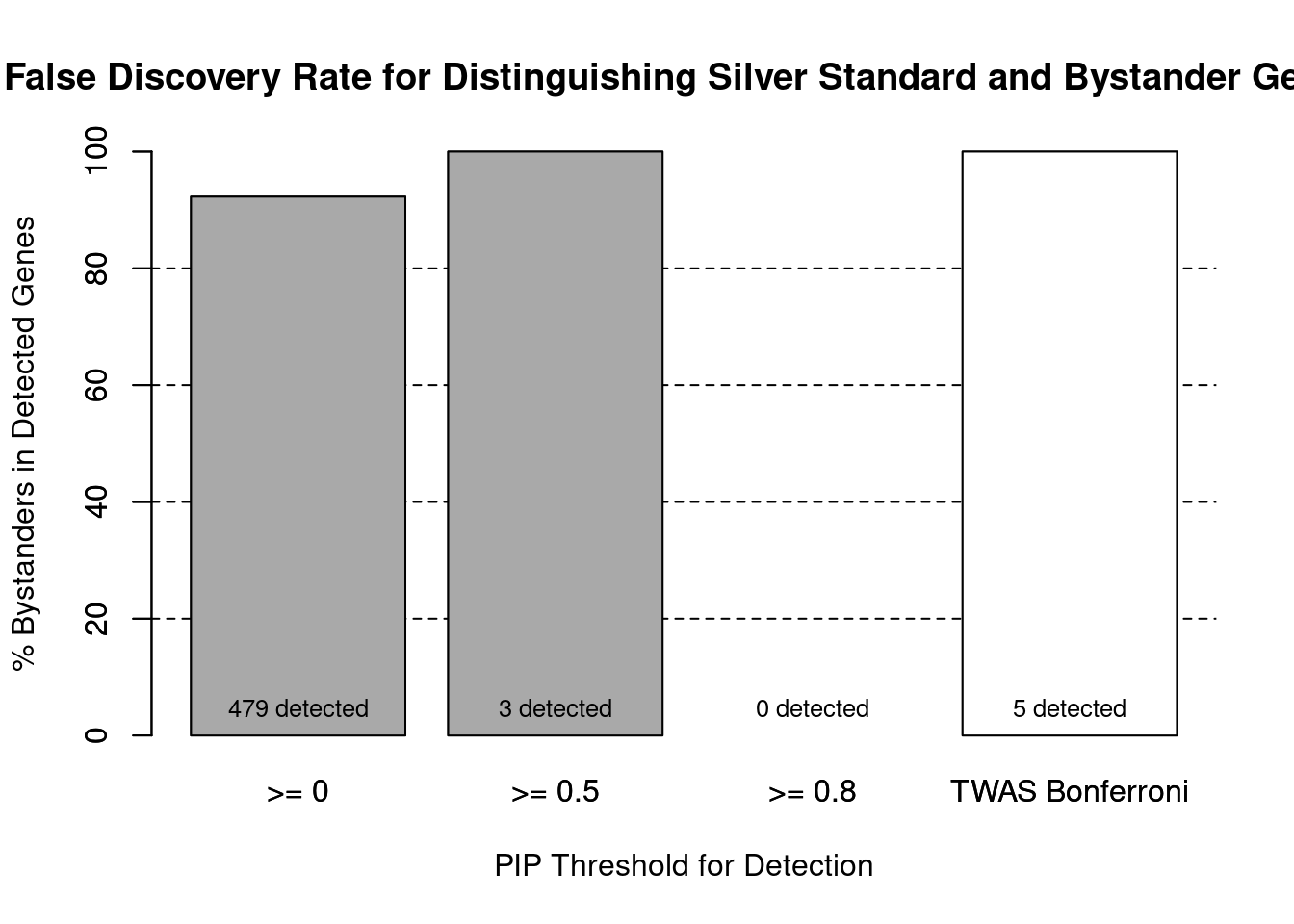
#text(x = xx, y = precision_range, label = paste0(round(precision_range,1), "%"), pos = 3, cex=0.8, offset = 1.5)Undetected silver standard genes have low TWAS z-scores or stronger signal from nearby variants
For all 69 silver standard genes, sequentially bin each gene using the following criteria: 1) gene not imputed; 2) gene detected by cTWAS at PIP>0.8; 3) gene insignificant by TWAS; 4) gene nearby a detected silver standard gene; 5) gene nearby a detected bystander gene; 6) gene nearby a detected SNP; 7) inconclusive.
#reload silver standard genes
known_annotations <- read_xlsx("data/summary_known_genes_annotations.xlsx", sheet="LDL")New names:
* `` -> ...4
* `` -> ...5known_annotations <- unique(known_annotations$`Gene Symbol`)
#categorize silver standard genes by case
silver_standard_case <- c()
uncertain_regions <- matrix(NA, 0, 2)
for (i in 1:length(known_annotations)){
current_gene <- known_annotations[i]
if (current_gene %in% ctwas_gene_res$genename) {
if (ctwas_gene_res$susie_pip[ctwas_gene_res$genename == current_gene] > 0.8){
silver_standard_case <- c(silver_standard_case, "Detected (PIP > 0.8)")
} else {
if (abs(ctwas_gene_res$z[ctwas_gene_res$genename == current_gene]) < sig_thresh){
silver_standard_case <- c(silver_standard_case, "Insignificant z-score")
} else {
current_region <- ctwas_gene_res$region_tag[ctwas_gene_res$genename == current_gene]
current_gene_res <- ctwas_gene_res[ctwas_gene_res$region_tag==current_region,]
current_snp_res <- ctwas_snp_res[ctwas_snp_res$region_tag==current_region,]
if (any(current_gene_res$susie_pip>0.8)){
if (any(current_gene_res$genename[current_gene_res$susie_pip>0.8] %in% known_annotations)){
silver_standard_case <- c(silver_standard_case, "Nearby Silver Standard Gene")
} else {
silver_standard_case <- c(silver_standard_case, "Nearby Bystander Gene")
}
} else {
#if (any(current_snp_res$susie_pip>0.8)){
if (sum(current_snp_res$susie_pip)>0.8){
silver_standard_case <- c(silver_standard_case, "Nearby SNP(s)")
} else {
silver_standard_case <- c(silver_standard_case, "Inconclusive")
uncertain_regions <- rbind(uncertain_regions, c(current_gene, ctwas_gene_res$region_tag[ctwas_gene_res$genename == current_gene]))
print(c(current_gene, ctwas_gene_res$region_tag[ctwas_gene_res$genename == current_gene]))
}
}
}
}
} else {
silver_standard_case <- c(silver_standard_case, "Not Imputed")
}
}
names(silver_standard_case) <- known_annotations
#table of outcomes for silver standard genes
-sort(-table(silver_standard_case))silver_standard_case
Insignificant z-score Not Imputed
37 32 #show inconclusive genes
silver_standard_case[silver_standard_case=="Inconclusive"]named character(0)# for (i in 1:nrow(uncertain_regions)){
# locus_plot3(uncertain_regions[i,2], focus=uncertain_regions[i,1])
# }
#pie chart of outcomes for silver standard genes
df <- data.frame(-sort(-table(silver_standard_case)))
names(df) <- c("Outcome", "Frequency")
#df <- df[df$Outcome!="Not Imputed",] #exclude genes not imputed
df$Outcome <- droplevels(df$Outcome) #exclude genes not imputed
bp<- ggplot(df, aes(x=Outcome, y=Frequency, fill=Outcome)) + geom_bar(width = 1, stat = "identity", position=position_dodge()) +
theme(axis.text.x = element_text(angle = 45, vjust = 1, hjust=1)) + theme(legend.position = "none")
bp
pie <- ggplot(df, aes(x="", y=Frequency, fill=Outcome)) + geom_bar(width = 1, stat = "identity")
pie <- pie + coord_polar("y", start=0) + theme_minimal() + theme(axis.title.y=element_blank())
pie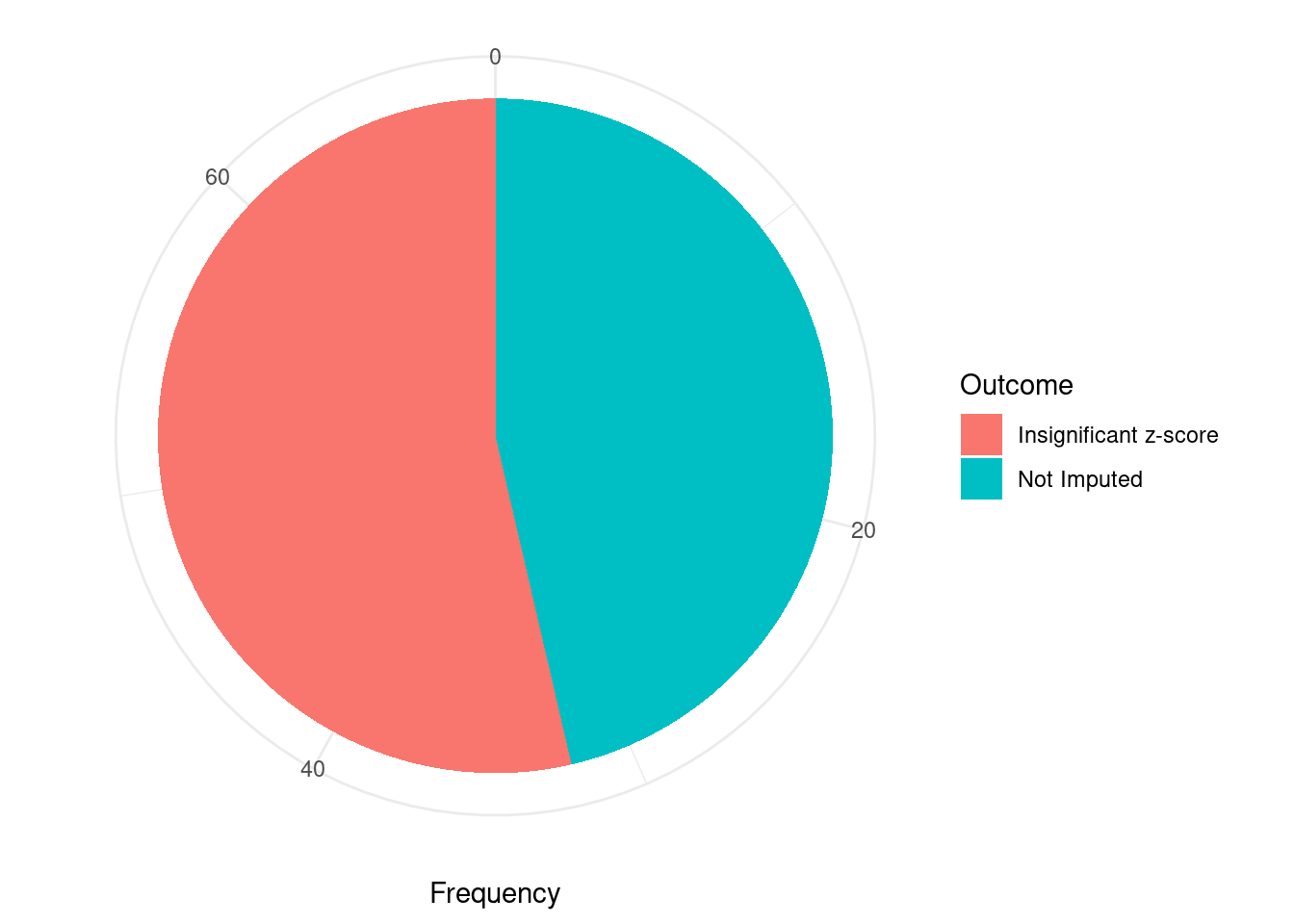
#locus_plot3(focus="KPNB1", region_tag="17_27")
#locus_plot3(focus="LPIN3", region_tag="20_25")
#locus_plot3(focus="LIPC", region_tag="15_26")cTWAS detects some genes that are not significant using a stringent TWAS threshold
TTC39B is a member of the Dyslipidaemia term in the disease_GLAD4U. This gene was not included in our silver standard. This gene is not significant using TWAS but is detected by cTWAS.
#locus_plot3(focus="TTC39B", region_tag="9_13")
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] parallel stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] GenomicRanges_1.36.1 GenomeInfoDb_1.20.0 IRanges_2.18.1
[4] S4Vectors_0.22.1 BiocGenerics_0.30.0 biomaRt_2.40.1
[7] ctwas_0.1.31 forcats_0.5.1 stringr_1.4.0
[10] dplyr_1.0.7 purrr_0.3.4 readr_2.1.1
[13] tidyr_1.1.4 tidyverse_1.3.1 tibble_3.1.6
[16] readxl_1.3.1 WebGestaltR_0.4.4 disgenet2r_0.99.2
[19] enrichR_3.0 cowplot_1.0.0 ggplot2_3.3.5
[22] workflowr_1.6.2
loaded via a namespace (and not attached):
[1] ggbeeswarm_0.6.0 colorspace_2.0-2 rjson_0.2.20
[4] ellipsis_0.3.2 rprojroot_2.0.2 XVector_0.24.0
[7] fs_1.5.2 rstudioapi_0.13 farver_2.1.0
[10] ggrepel_0.8.1 bit64_4.0.5 AnnotationDbi_1.46.0
[13] fansi_0.5.0 lubridate_1.8.0 xml2_1.3.3
[16] codetools_0.2-16 logging_0.10-108 doParallel_1.0.16
[19] cachem_1.0.6 knitr_1.36 jsonlite_1.7.2
[22] apcluster_1.4.8 Cairo_1.5-12.2 broom_0.7.10
[25] dbplyr_2.1.1 compiler_3.6.1 httr_1.4.2
[28] backports_1.4.1 assertthat_0.2.1 Matrix_1.2-18
[31] fastmap_1.1.0 cli_3.1.0 later_0.8.0
[34] prettyunits_1.1.1 htmltools_0.5.2 tools_3.6.1
[37] igraph_1.2.10 GenomeInfoDbData_1.2.1 gtable_0.3.0
[40] glue_1.5.1 reshape2_1.4.4 doRNG_1.8.2
[43] Rcpp_1.0.7 Biobase_2.44.0 cellranger_1.1.0
[46] jquerylib_0.1.4 vctrs_0.3.8 svglite_1.2.2
[49] iterators_1.0.13 xfun_0.29 rvest_1.0.2
[52] lifecycle_1.0.1 rngtools_1.5.2 XML_3.99-0.3
[55] zlibbioc_1.30.0 scales_1.1.1 vroom_1.5.7
[58] hms_1.1.1 promises_1.0.1 yaml_2.2.1
[61] curl_4.3.2 memoise_2.0.1 ggrastr_1.0.1
[64] gdtools_0.1.9 stringi_1.7.6 RSQLite_2.2.8
[67] highr_0.9 foreach_1.5.1 rlang_0.4.12
[70] pkgconfig_2.0.3 bitops_1.0-7 evaluate_0.14
[73] lattice_0.20-38 labeling_0.4.2 bit_4.0.4
[76] tidyselect_1.1.1 plyr_1.8.6 magrittr_2.0.1
[79] R6_2.5.1 generics_0.1.1 DBI_1.1.1
[82] pgenlibr_0.3.1 pillar_1.6.4 haven_2.4.3
[85] whisker_0.3-2 withr_2.4.3 RCurl_1.98-1.5
[88] modelr_0.1.8 crayon_1.4.2 utf8_1.2.2
[91] tzdb_0.2.0 rmarkdown_2.11 progress_1.2.2
[94] grid_3.6.1 data.table_1.14.2 blob_1.2.2
[97] git2r_0.26.1 reprex_2.0.1 digest_0.6.29
[100] httpuv_1.5.1 munsell_0.5.0 beeswarm_0.2.3
[103] vipor_0.4.5