Autism - Brain Caudate basal ganglia
sheng Qian
2021-2-6
Last updated: 2022-02-27
Checks: 6 1
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 3dd5b4c. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .ipynb_checkpoints/
Ignored: data/AF/
Untracked files:
Untracked: Rplot.png
Untracked: analysis/.ipynb_checkpoints/
Untracked: analysis/Autism_Brain_Amygdala.Rmd
Untracked: analysis/Autism_Brain_Anterior_cingulate_cortex_BA24.Rmd
Untracked: analysis/Autism_Brain_Caudate_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Cerebellar_Hemisphere.Rmd
Untracked: analysis/Autism_Brain_Cerebellum.Rmd
Untracked: analysis/Autism_Brain_Cortex.Rmd
Untracked: analysis/Autism_Brain_Frontal_Cortex_BA9.Rmd
Untracked: analysis/Autism_Brain_Hippocampus.Rmd
Untracked: analysis/Autism_Brain_Hypothalamus.Rmd
Untracked: analysis/Autism_Brain_Nucleus_accumbens_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Putamen_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Spinal_cord_cervical_c-1.Rmd
Untracked: analysis/Autism_Brain_Substantia_nigra.Rmd
Untracked: analysis/Glucose_Adipose_Subcutaneous.Rmd
Untracked: analysis/Glucose_Adipose_Visceral_Omentum.Rmd
Untracked: analysis/Splicing_Test.Rmd
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: data/.ipynb_checkpoints/
Untracked: data/Autism/
Untracked: data/BMI/
Untracked: data/BMI_S/
Untracked: data/Glucose/
Untracked: data/LDL_S/
Untracked: data/SCZ/
Untracked: data/T2D/
Untracked: data/TEST/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Unstaged changes:
Modified: analysis/BMI_Brain_Amygdala_S.Rmd
Modified: analysis/BMI_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/BMI_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/BMI_Brain_Cerebellum_S.Rmd
Modified: analysis/BMI_Brain_Cortex.Rmd
Modified: analysis/BMI_Brain_Cortex_S.Rmd
Modified: analysis/BMI_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/BMI_Brain_Hippocampus_S.Rmd
Modified: analysis/BMI_Brain_Hypothalamus_S.Rmd
Modified: analysis/BMI_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/BMI_Brain_Substantia_nigra_S.Rmd
Modified: analysis/LDL_Liver_S.Rmd
Modified: analysis/index.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_Brain_Caudate_basal_ganglia.Rmd) and HTML (docs/SCZ_Brain_Caudate_basal_ganglia.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 3dd5b4c | sq-96 | 2022-02-27 | update |
Weight QC
#number of imputed weights
nrow(qclist_all)[1] 11590#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1115 851 695 452 579 584 548 433 433 450 693 657 241 393 396 529
17 18 19 20 21 22
713 185 887 340 130 286 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 9058#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.7815Check convergence of parameters
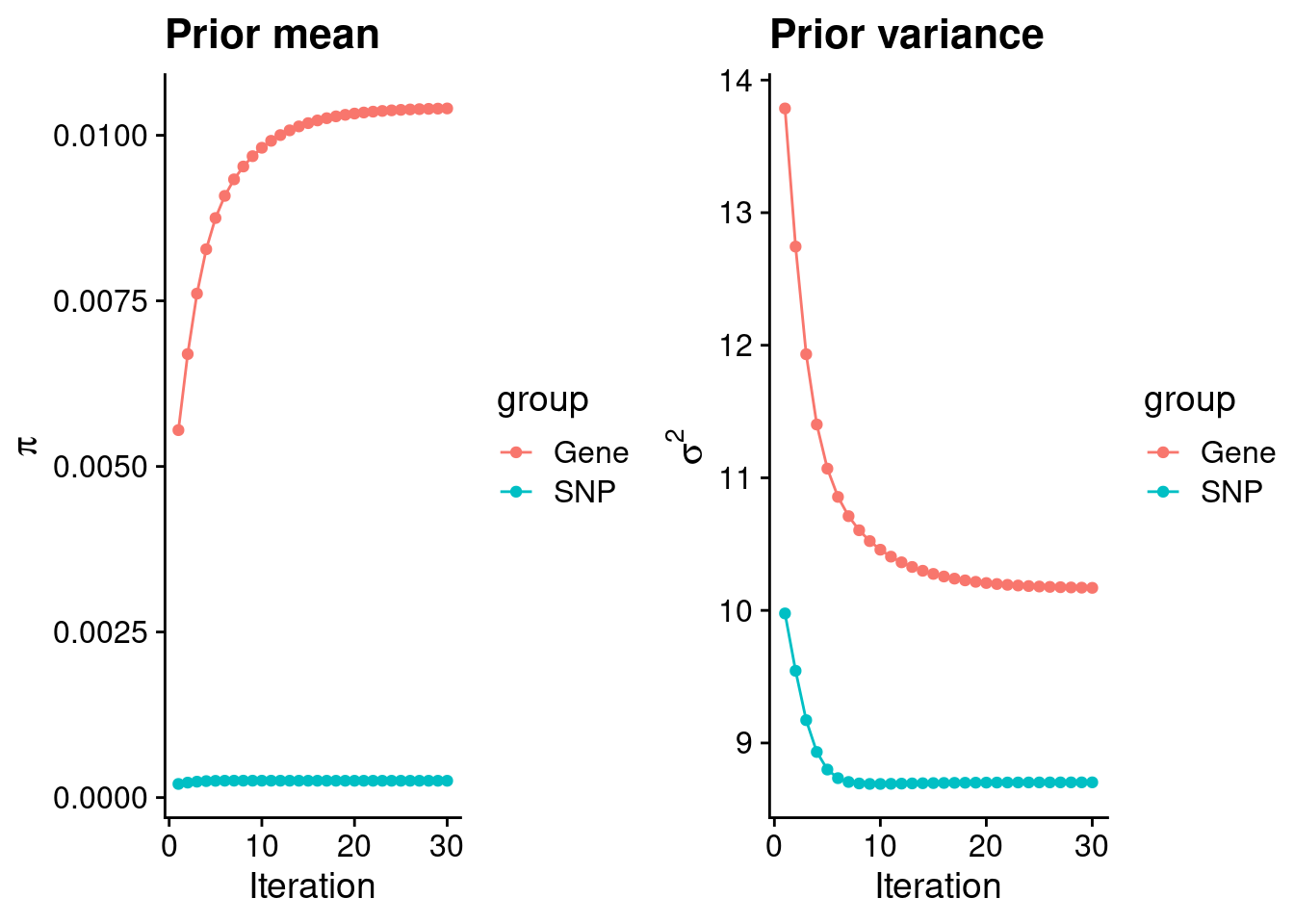
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0104054 0.0002537 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
10.170 8.703 #report sample size
print(sample_size)[1] 82315#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 11590 7573890#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.0149 0.2031 #compare sum(PIP*mu2/sample_size) with above PVE calculation
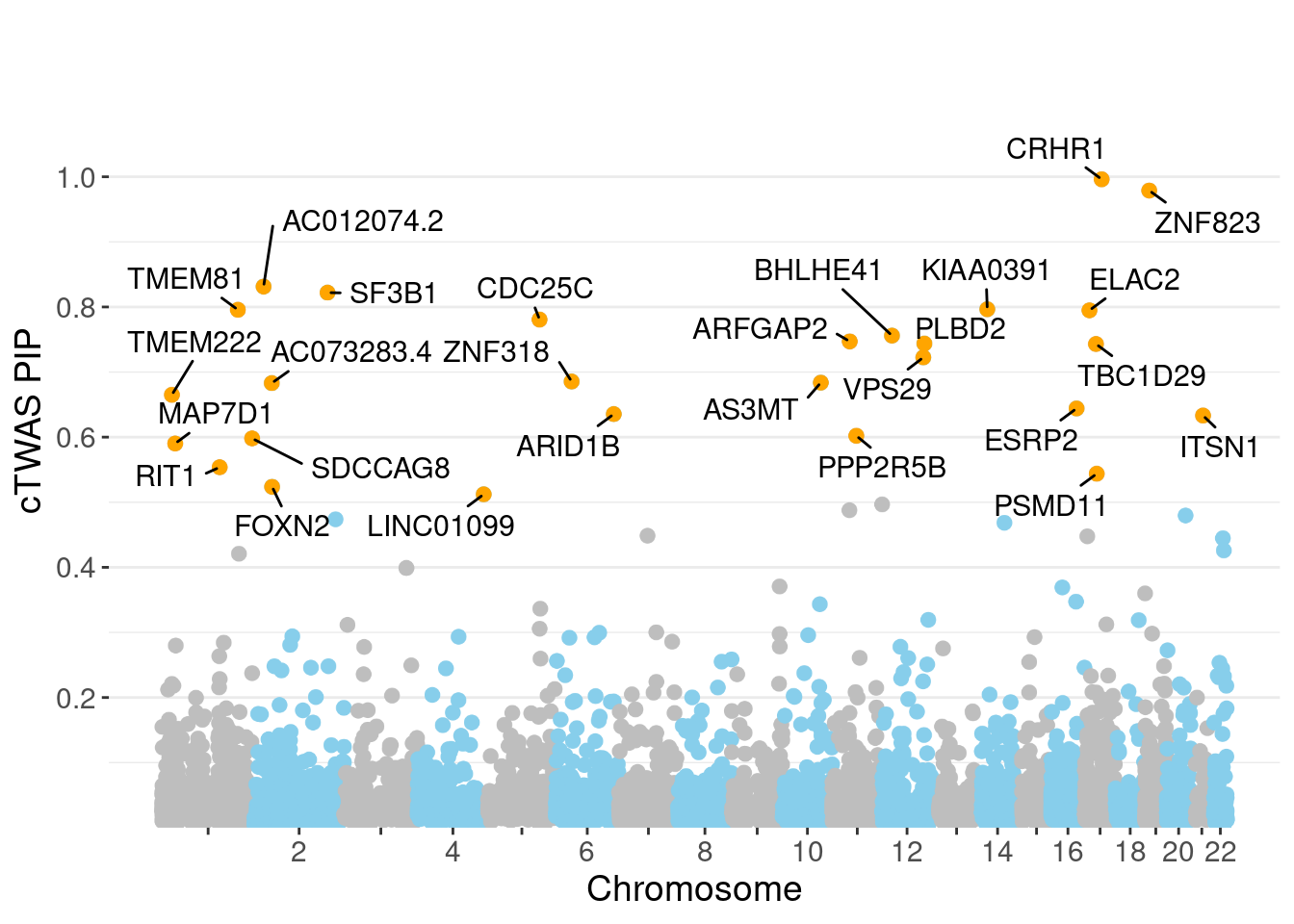
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.1133 1.4480Genes with highest PIPs
genename region_tag susie_pip mu2 PVE z num_eqtl
3517 CRHR1 17_27 0.9961 3564.35 0.0431343 3.362 1
11135 ZNF823 19_10 0.9789 29.83 0.0003547 5.485 2
12311 AC012074.2 2_16 0.8313 23.83 0.0002407 4.623 1
3091 SF3B1 2_117 0.8220 43.62 0.0004356 6.725 1
1678 KIAA0391 14_9 0.7965 25.21 0.0002440 -5.164 1
9254 TMEM81 1_104 0.7956 26.02 0.0002515 4.938 1
110 ELAC2 17_11 0.7948 21.79 0.0002104 4.509 1
7042 CDC25C 5_82 0.7806 25.25 0.0002395 -5.591 1
3750 BHLHE41 12_18 0.7560 22.76 0.0002091 -4.024 1
6310 ARFGAP2 11_29 0.7470 24.29 0.0002204 4.740 1
6443 PLBD2 12_68 0.7438 20.62 0.0001864 3.986 1
13314 TBC1D29 17_18 0.7431 23.05 0.0002081 4.407 1
2684 VPS29 12_67 0.7225 25.07 0.0002201 -4.965 2
8894 ZNF318 6_33 0.6854 24.43 0.0002034 -4.832 1
11792 AS3MT 10_66 0.6837 38.64 0.0003209 6.812 2
12298 AC073283.4 2_30 0.6830 22.95 0.0001904 -3.812 4
10494 TMEM222 1_19 0.6650 24.78 0.0002002 3.902 1
1879 ESRP2 16_36 0.6441 27.28 0.0002134 5.203 2
461 ARID1B 6_102 0.6354 24.30 0.0001876 -3.907 1
11616 ITSN1 21_14 0.6332 24.19 0.0001861 3.954 2Genes with largest effect sizes
genename region_tag susie_pip mu2 PVE z num_eqtl
3517 CRHR1 17_27 9.961e-01 3564.35 4.313e-02 3.3623 1
12229 HLA-DQB2 6_26 7.161e-14 841.86 7.324e-16 -4.1487 1
10939 HLA-DQA1 6_26 1.436e-13 799.37 1.394e-15 3.4460 1
12390 HLA-DQA2 6_26 1.401e-13 516.30 8.788e-16 -3.5679 1
12115 ARL17B 17_27 0.000e+00 374.03 0.000e+00 -3.0672 1
11731 CLIC1 6_26 9.395e-13 368.28 4.203e-15 8.8118 2
7119 ARHGAP27 17_27 0.000e+00 340.94 0.000e+00 0.3401 1
11469 MSH5 6_26 3.538e-13 318.93 1.371e-15 7.6794 2
11464 HSPA1L 6_26 3.716e-13 230.85 1.042e-15 7.1259 1
9922 ACBD4 17_27 0.000e+00 208.81 0.000e+00 1.3121 1
4990 NMT1 17_27 0.000e+00 184.54 0.000e+00 2.4459 1
12582 C4A 6_26 1.657e-12 156.41 3.148e-15 5.2909 1
10283 FMNL1 17_27 0.000e+00 141.39 0.000e+00 -0.6638 1
10529 HEXIM1 17_27 0.000e+00 130.59 0.000e+00 -3.3358 1
1319 PUS7 7_65 0.000e+00 108.05 0.000e+00 0.6328 2
2439 GOSR2 17_27 0.000e+00 71.67 0.000e+00 -2.5096 1
4823 RINT1 7_65 0.000e+00 68.20 0.000e+00 1.1750 1
5119 PGBD1 6_22 1.352e-02 67.44 1.107e-05 -8.4933 1
10491 BTN3A2 6_20 1.634e-02 64.75 1.285e-05 8.9338 2
9090 DCAKD 17_27 0.000e+00 55.42 0.000e+00 -2.1069 2Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_eqtl
3517 CRHR1 17_27 0.9961 3564.35 0.0431343 3.362 1
3091 SF3B1 2_117 0.8220 43.62 0.0004356 6.725 1
11135 ZNF823 19_10 0.9789 29.83 0.0003547 5.485 2
11792 AS3MT 10_66 0.6837 38.64 0.0003209 6.812 2
9254 TMEM81 1_104 0.7956 26.02 0.0002515 4.938 1
1678 KIAA0391 14_9 0.7965 25.21 0.0002440 -5.164 1
12311 AC012074.2 2_16 0.8313 23.83 0.0002407 4.623 1
7042 CDC25C 5_82 0.7806 25.25 0.0002395 -5.591 1
2630 MDK 11_28 0.4877 38.14 0.0002260 -6.357 1
6310 ARFGAP2 11_29 0.7470 24.29 0.0002204 4.740 1
2684 VPS29 12_67 0.7225 25.07 0.0002201 -4.965 2
1879 ESRP2 16_36 0.6441 27.28 0.0002134 5.203 2
110 ELAC2 17_11 0.7948 21.79 0.0002104 4.509 1
3750 BHLHE41 12_18 0.7560 22.76 0.0002091 -4.024 1
13314 TBC1D29 17_18 0.7431 23.05 0.0002081 4.407 1
8894 ZNF318 6_33 0.6854 24.43 0.0002034 -4.832 1
10494 TMEM222 1_19 0.6650 24.78 0.0002002 3.902 1
12298 AC073283.4 2_30 0.6830 22.95 0.0001904 -3.812 4
506 SDCCAG8 1_128 0.5981 25.90 0.0001882 -4.982 1
461 ARID1B 6_102 0.6354 24.30 0.0001876 -3.907 1Genes with largest z scores
genename region_tag susie_pip mu2 PVE z num_eqtl
10491 BTN3A2 6_20 1.634e-02 64.75 1.285e-05 8.934 2
11731 CLIC1 6_26 9.395e-13 368.28 4.203e-15 8.812 2
5119 PGBD1 6_22 1.352e-02 67.44 1.107e-05 -8.493 1
6289 CNNM2 10_66 1.509e-01 46.14 8.461e-05 -8.118 2
11469 MSH5 6_26 3.538e-13 318.93 1.371e-15 7.679 2
13068 RP11-490G2.2 1_60 1.266e-02 48.12 7.404e-06 7.322 1
7064 ZSCAN12 6_22 1.413e-02 34.93 5.996e-06 7.214 1
9628 C2orf69 2_118 2.481e-01 40.18 1.211e-04 7.151 2
11464 HSPA1L 6_26 3.716e-13 230.85 1.042e-15 7.126 1
11792 AS3MT 10_66 6.837e-01 38.64 3.209e-04 6.812 2
1226 PPP1R13B 14_54 1.408e-01 46.70 7.990e-05 6.798 3
10643 ZSCAN23 6_22 8.404e-02 46.85 4.784e-05 -6.793 1
7500 TYW5 2_118 3.373e-02 36.46 1.494e-05 -6.774 2
3091 SF3B1 2_117 8.220e-01 43.62 4.356e-04 6.725 1
6279 CYP17A1 10_66 5.195e-03 27.80 1.754e-06 -6.720 1
9851 HIST1H2BC 6_20 1.561e-02 39.53 7.498e-06 -6.675 2
10986 ZSCAN26 6_22 1.420e-02 37.06 6.395e-06 6.584 3
4024 XRCC3 14_54 7.584e-02 42.09 3.877e-05 6.524 2
9986 ARL6IP4 12_75 8.382e-03 39.48 4.020e-06 6.491 1
6424 ABCB9 12_75 6.686e-03 38.21 3.104e-06 6.404 1Comparing z scores and PIPs
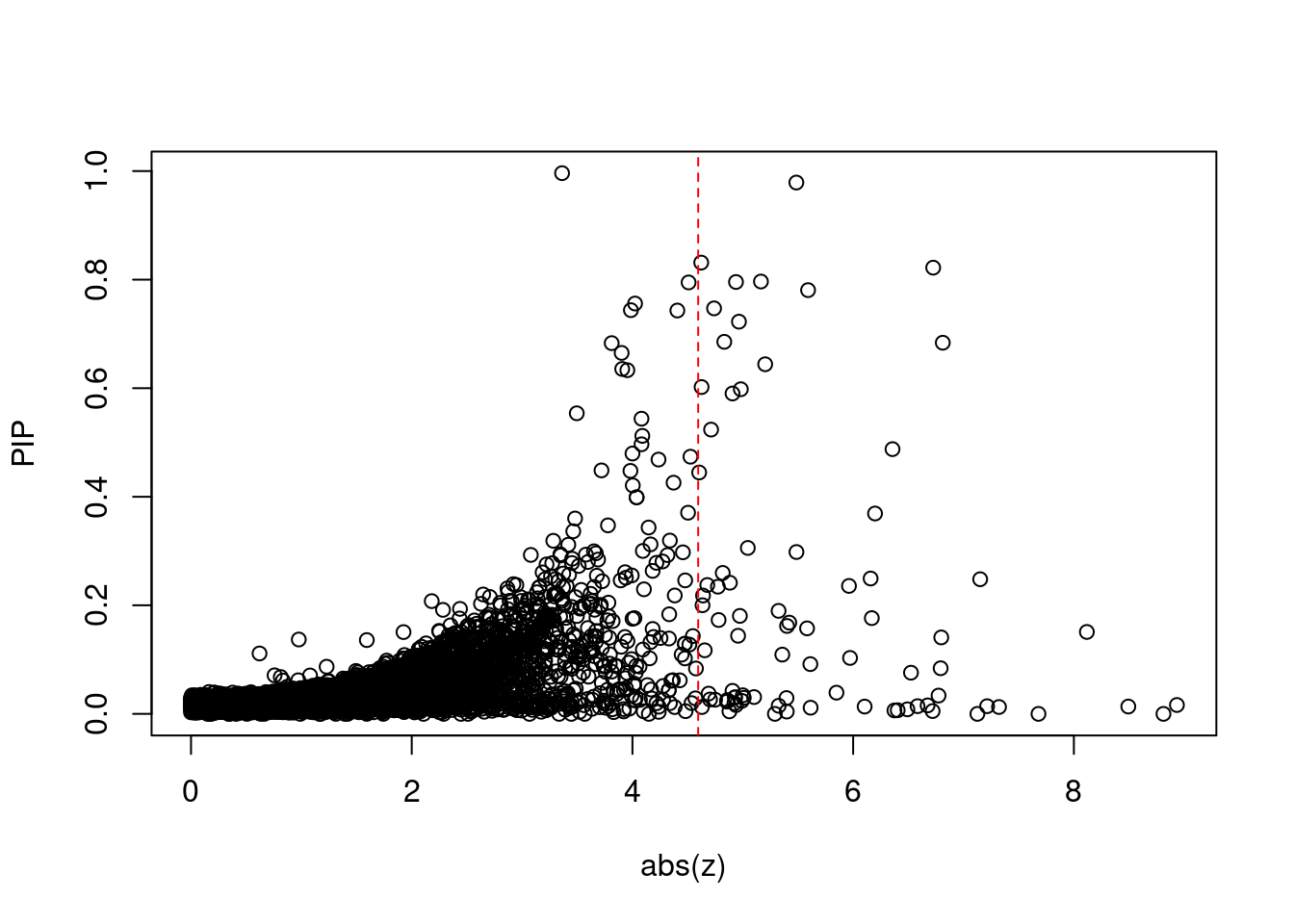
[1] 0.007075 genename region_tag susie_pip mu2 PVE z num_eqtl
10491 BTN3A2 6_20 1.634e-02 64.75 1.285e-05 8.934 2
11731 CLIC1 6_26 9.395e-13 368.28 4.203e-15 8.812 2
5119 PGBD1 6_22 1.352e-02 67.44 1.107e-05 -8.493 1
6289 CNNM2 10_66 1.509e-01 46.14 8.461e-05 -8.118 2
11469 MSH5 6_26 3.538e-13 318.93 1.371e-15 7.679 2
13068 RP11-490G2.2 1_60 1.266e-02 48.12 7.404e-06 7.322 1
7064 ZSCAN12 6_22 1.413e-02 34.93 5.996e-06 7.214 1
9628 C2orf69 2_118 2.481e-01 40.18 1.211e-04 7.151 2
11464 HSPA1L 6_26 3.716e-13 230.85 1.042e-15 7.126 1
11792 AS3MT 10_66 6.837e-01 38.64 3.209e-04 6.812 2
1226 PPP1R13B 14_54 1.408e-01 46.70 7.990e-05 6.798 3
10643 ZSCAN23 6_22 8.404e-02 46.85 4.784e-05 -6.793 1
7500 TYW5 2_118 3.373e-02 36.46 1.494e-05 -6.774 2
3091 SF3B1 2_117 8.220e-01 43.62 4.356e-04 6.725 1
6279 CYP17A1 10_66 5.195e-03 27.80 1.754e-06 -6.720 1
9851 HIST1H2BC 6_20 1.561e-02 39.53 7.498e-06 -6.675 2
10986 ZSCAN26 6_22 1.420e-02 37.06 6.395e-06 6.584 3
4024 XRCC3 14_54 7.584e-02 42.09 3.877e-05 6.524 2
9986 ARL6IP4 12_75 8.382e-03 39.48 4.020e-06 6.491 1
6424 ABCB9 12_75 6.686e-03 38.21 3.104e-06 6.404 1GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 27Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Cellular_Component_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio BgRatio
27 Neoplasms, Glandular and Epithelial 0.02897 1/13 2/9703
32 Pain, Postoperative 0.02897 1/13 2/9703
46 Glandular Neoplasms 0.02897 1/13 2/9703
88 Refractory anemia with ringed sideroblasts 0.02897 1/13 2/9703
93 Epithelioma 0.02897 1/13 2/9703
106 CHROMOSOME 6q24-q25 DELETION SYNDROME 0.02897 1/13 2/9703
107 SENIOR-LOKEN SYNDROME 7 0.02897 1/13 1/9703
111 PROSTATE CANCER, HEREDITARY, 2 0.02897 1/13 1/9703
113 NOONAN SYNDROME 8 0.02897 1/13 1/9703
114 COMBINED OXIDATIVE PHOSPHORYLATION DEFICIENCY 17 0.02897 1/13 1/9703WebGestalt enrichment analysis for genes with PIP>0.5
Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 41#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 23#significance threshold for TWAS
print(sig_thresh)[1] 4.596#number of ctwas genes
length(ctwas_genes)[1] 4#number of TWAS genes
length(twas_genes)[1] 82#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_eqtl
3517 CRHR1 17_27 0.9961 3564 0.04313 3.362 1#sensitivity / recall
print(sensitivity) ctwas TWAS
0.00000 0.02439 #specificity
print(specificity) ctwas TWAS
0.9997 0.9930 #precision / PPV
print(precision) ctwas TWAS
0.0000 0.0122 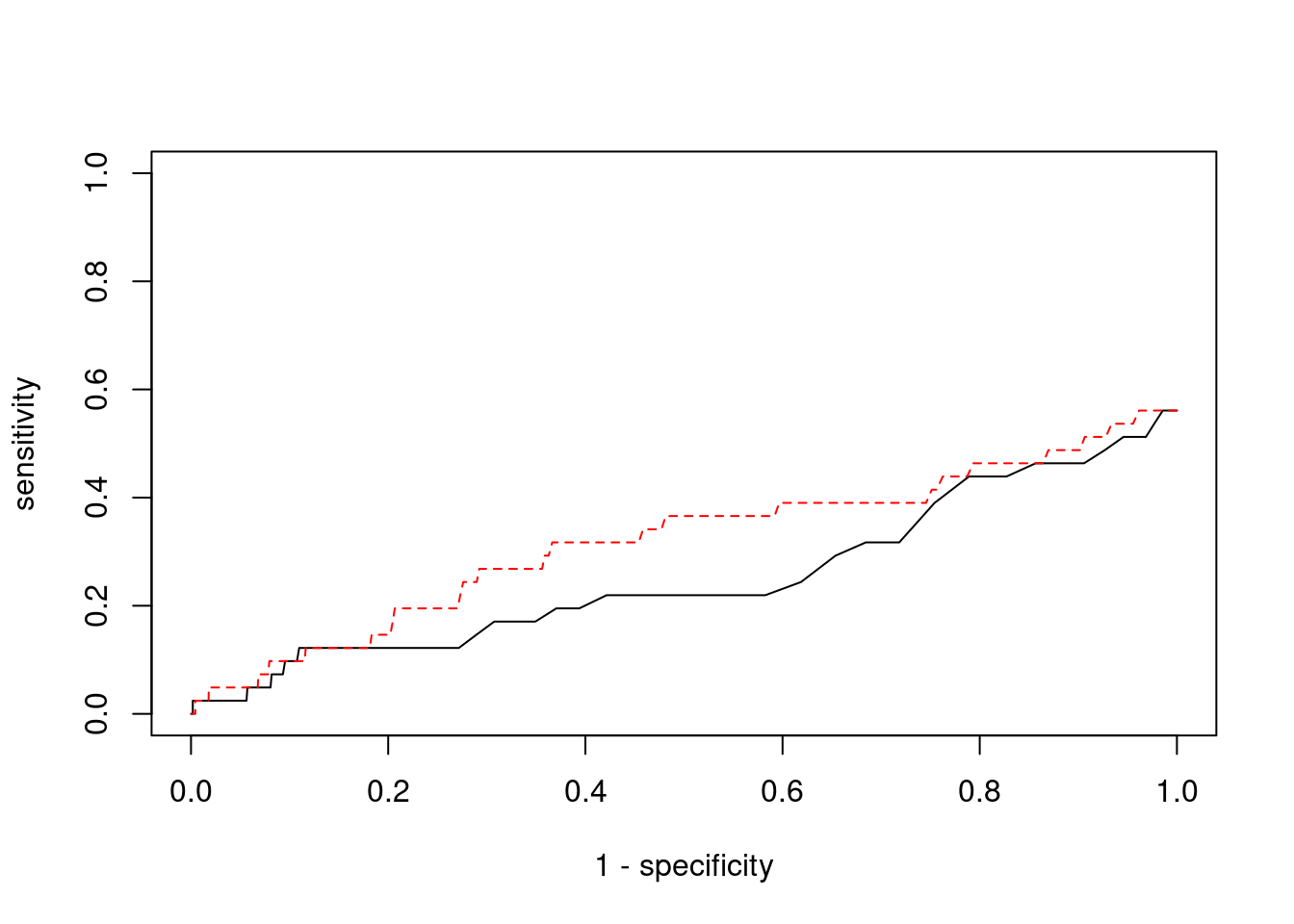
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.3.1 forcats_0.5.1 stringr_1.4.0 dplyr_1.0.7
[5] purrr_0.3.4 readr_2.1.1 tidyr_1.1.4 tidyverse_1.3.1
[9] tibble_3.1.6 WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0
[13] cowplot_1.0.0 ggplot2_3.3.5 workflowr_1.6.2
loaded via a namespace (and not attached):
[1] fs_1.5.2 lubridate_1.8.0 bit64_4.0.5 doParallel_1.0.17
[5] httr_1.4.2 rprojroot_2.0.2 tools_3.6.1 backports_1.4.1
[9] doRNG_1.8.2 utf8_1.2.2 R6_2.5.1 vipor_0.4.5
[13] DBI_1.1.2 colorspace_2.0-2 withr_2.4.3 ggrastr_1.0.1
[17] tidyselect_1.1.1 bit_4.0.4 curl_4.3.2 compiler_3.6.1
[21] git2r_0.26.1 rvest_1.0.2 cli_3.1.0 Cairo_1.5-12.2
[25] xml2_1.3.3 labeling_0.4.2 scales_1.1.1 apcluster_1.4.8
[29] digest_0.6.29 rmarkdown_2.11 svglite_1.2.2 pkgconfig_2.0.3
[33] htmltools_0.5.2 dbplyr_2.1.1 fastmap_1.1.0 highr_0.9
[37] rlang_1.0.1 rstudioapi_0.13 RSQLite_2.2.8 jquerylib_0.1.4
[41] farver_2.1.0 generics_0.1.1 jsonlite_1.7.2 vroom_1.5.7
[45] magrittr_2.0.2 Matrix_1.2-18 ggbeeswarm_0.6.0 Rcpp_1.0.8
[49] munsell_0.5.0 fansi_1.0.2 gdtools_0.1.9 lifecycle_1.0.1
[53] stringi_1.7.6 whisker_0.3-2 yaml_2.2.1 plyr_1.8.6
[57] grid_3.6.1 blob_1.2.2 ggrepel_0.9.1 parallel_3.6.1
[61] promises_1.0.1 crayon_1.5.0 lattice_0.20-38 haven_2.4.3
[65] hms_1.1.1 knitr_1.36 pillar_1.6.4 igraph_1.2.10
[69] rjson_0.2.20 rngtools_1.5.2 reshape2_1.4.4 codetools_0.2-16
[73] reprex_2.0.1 glue_1.6.2 evaluate_0.14 data.table_1.14.2
[77] modelr_0.1.8 vctrs_0.3.8 tzdb_0.2.0 httpuv_1.5.1
[81] foreach_1.5.2 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] cachem_1.0.6 xfun_0.29 broom_0.7.10 later_0.8.0
[89] iterators_1.0.14 beeswarm_0.2.3 memoise_2.0.1 ellipsis_0.3.2