Autism - Brain Hippocampus
sheng Qian
2021-2-6
Last updated: 2022-02-27
Checks: 6 1
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 3dd5b4c. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .ipynb_checkpoints/
Ignored: data/AF/
Untracked files:
Untracked: Rplot.png
Untracked: analysis/.ipynb_checkpoints/
Untracked: analysis/Autism_Brain_Amygdala.Rmd
Untracked: analysis/Autism_Brain_Anterior_cingulate_cortex_BA24.Rmd
Untracked: analysis/Autism_Brain_Caudate_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Cerebellar_Hemisphere.Rmd
Untracked: analysis/Autism_Brain_Cerebellum.Rmd
Untracked: analysis/Autism_Brain_Cortex.Rmd
Untracked: analysis/Autism_Brain_Frontal_Cortex_BA9.Rmd
Untracked: analysis/Autism_Brain_Hippocampus.Rmd
Untracked: analysis/Autism_Brain_Hypothalamus.Rmd
Untracked: analysis/Autism_Brain_Nucleus_accumbens_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Putamen_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Spinal_cord_cervical_c-1.Rmd
Untracked: analysis/Autism_Brain_Substantia_nigra.Rmd
Untracked: analysis/Glucose_Adipose_Subcutaneous.Rmd
Untracked: analysis/Glucose_Adipose_Visceral_Omentum.Rmd
Untracked: analysis/Splicing_Test.Rmd
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: data/.ipynb_checkpoints/
Untracked: data/Autism/
Untracked: data/BMI/
Untracked: data/BMI_S/
Untracked: data/Glucose/
Untracked: data/LDL_S/
Untracked: data/SCZ/
Untracked: data/T2D/
Untracked: data/TEST/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Unstaged changes:
Modified: analysis/BMI_Brain_Amygdala_S.Rmd
Modified: analysis/BMI_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/BMI_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/BMI_Brain_Cerebellum_S.Rmd
Modified: analysis/BMI_Brain_Cortex.Rmd
Modified: analysis/BMI_Brain_Cortex_S.Rmd
Modified: analysis/BMI_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/BMI_Brain_Hippocampus_S.Rmd
Modified: analysis/BMI_Brain_Hypothalamus_S.Rmd
Modified: analysis/BMI_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/BMI_Brain_Substantia_nigra_S.Rmd
Modified: analysis/LDL_Liver_S.Rmd
Modified: analysis/index.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_Brain_Hippocampus.Rmd) and HTML (docs/SCZ_Brain_Hippocampus.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 3dd5b4c | sq-96 | 2022-02-27 | update |
Weight QC
#number of imputed weights
nrow(qclist_all)[1] 11027#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1084 780 648 424 542 552 509 409 421 444 660 622 226 379 378 525
17 18 19 20 21 22
658 168 860 330 120 288 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 8830#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8008Check convergence of parameters
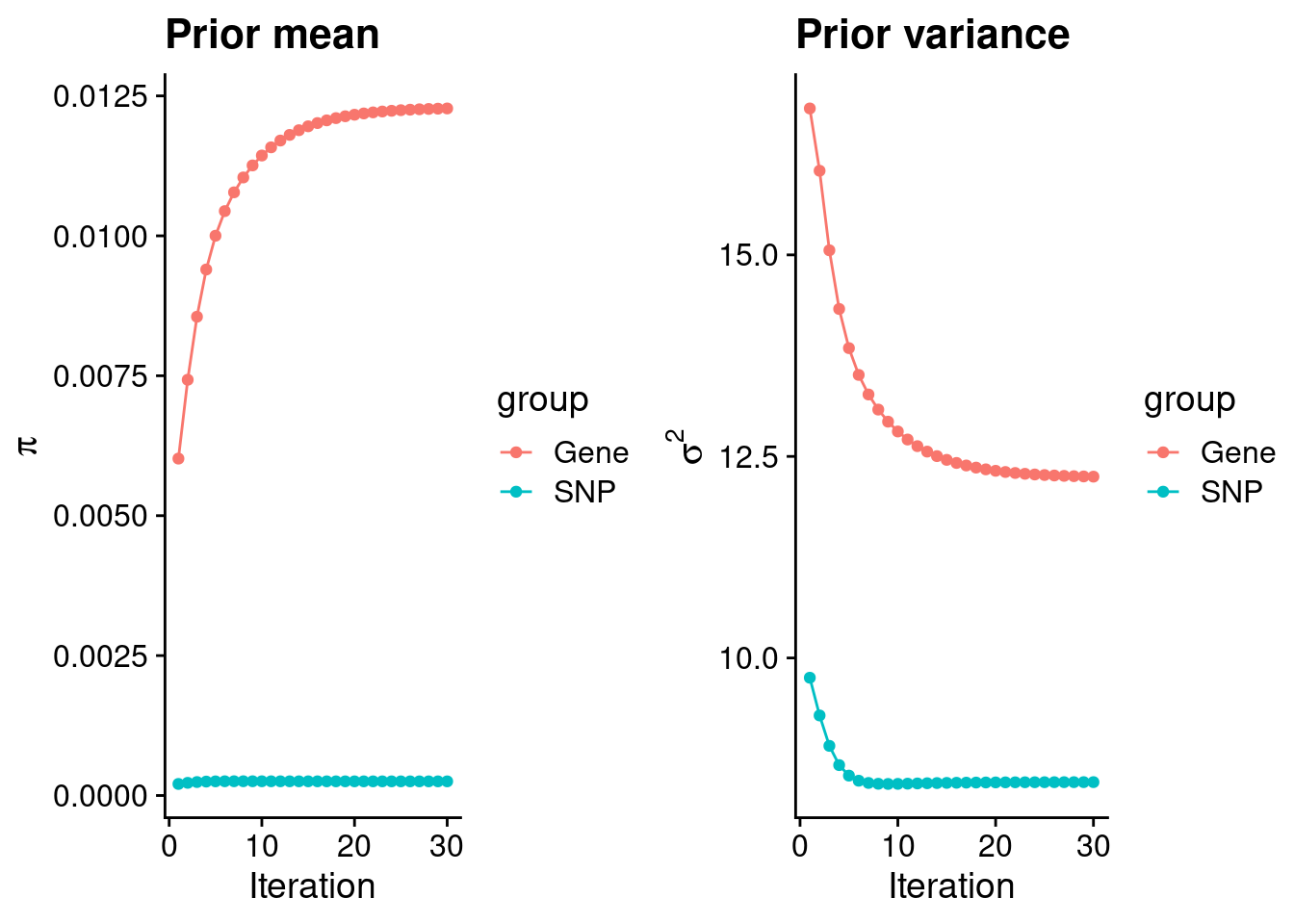
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0122760 0.0002528 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
12.25 8.46 #report sample size
print(sample_size)[1] 82315#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 11027 7573890#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.02014 0.19680 #compare sum(PIP*mu2/sample_size) with above PVE calculation
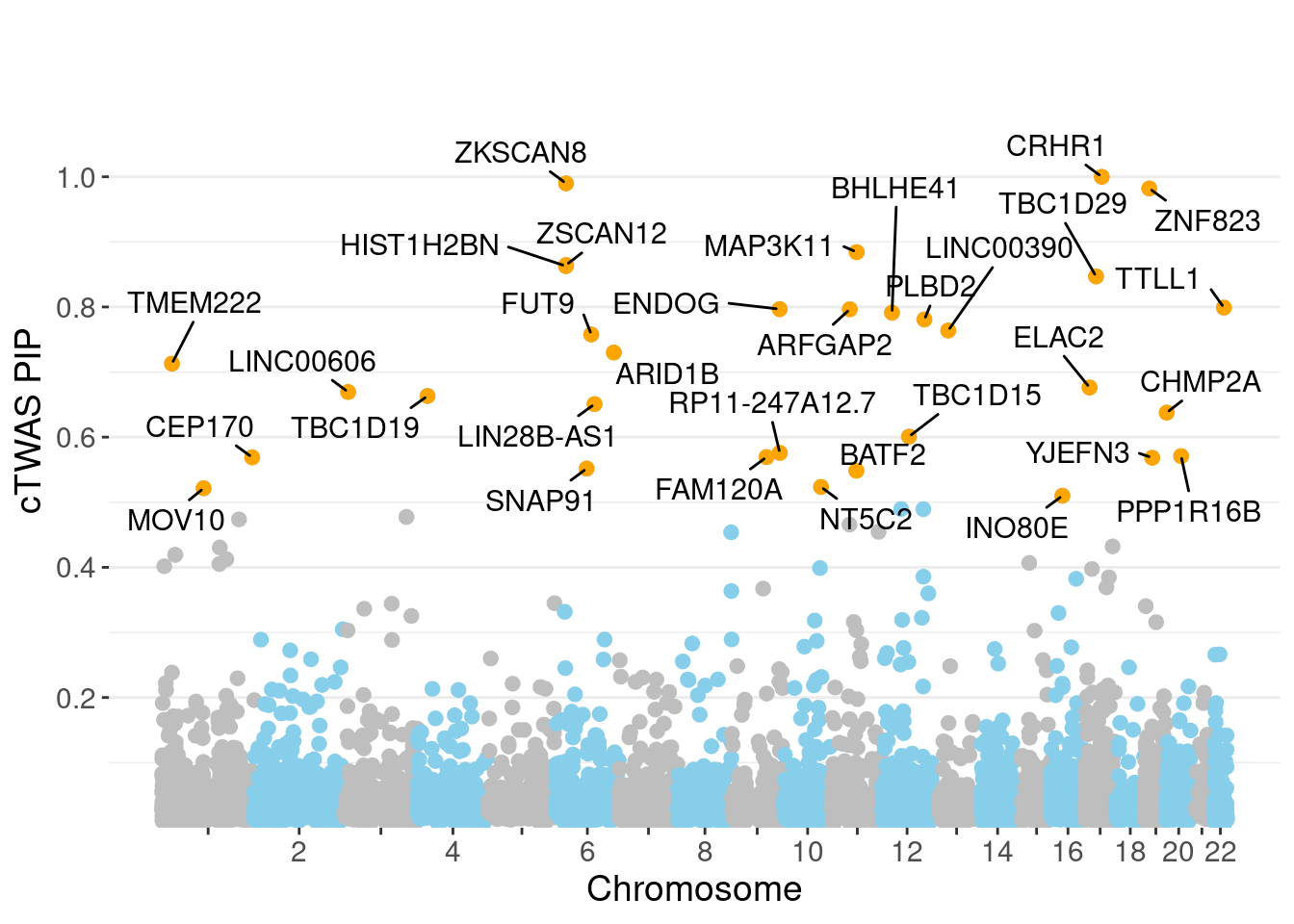
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.1542 1.5603Genes with highest PIPs
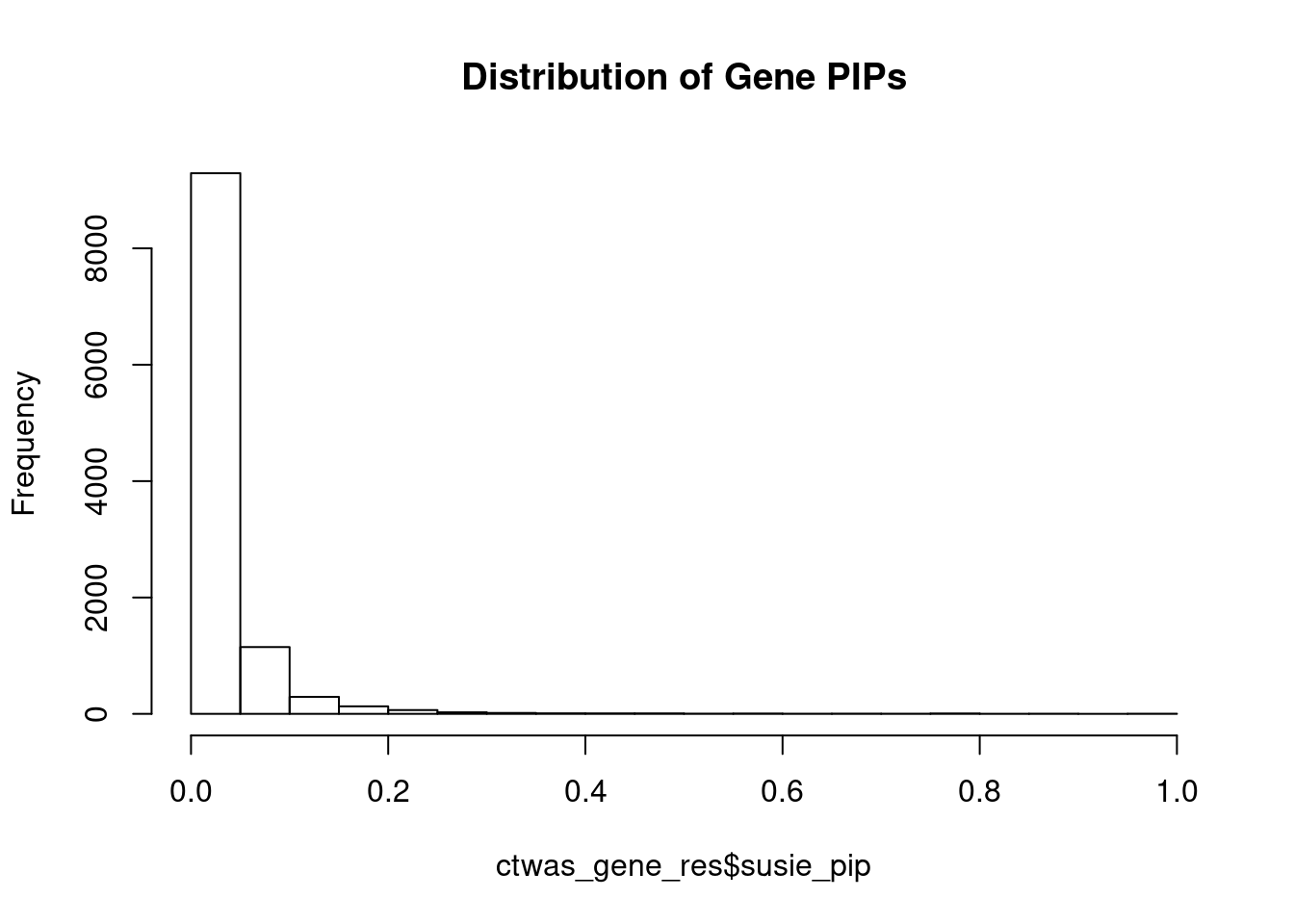
genename region_tag susie_pip mu2 PVE z num_eqtl
3391 CRHR1 17_27 1.0000 4090.65 0.0496950 3.362 1
10755 ZKSCAN8 6_22 0.9899 1791.68 0.0215453 5.406 2
10687 ZNF823 19_10 0.9820 30.13 0.0003594 5.455 1
8759 MAP3K11 11_36 0.8843 23.83 0.0002560 -4.544 1
6779 ZSCAN12 6_22 0.8645 846.93 0.0088944 10.940 1
11759 HIST1H2BN 6_21 0.8628 95.15 0.0009973 10.773 1
12742 TBC1D29 17_18 0.8469 22.50 0.0002315 4.578 2
1516 TTLL1 22_18 0.7990 23.82 0.0002313 -4.667 2
7929 ENDOG 9_66 0.7967 23.37 0.0002262 4.806 2
6084 ARFGAP2 11_29 0.7965 25.00 0.0002419 4.740 1
3607 BHLHE41 12_18 0.7912 22.15 0.0002129 -4.024 1
6202 PLBD2 12_68 0.7808 20.44 0.0001938 3.986 1
11532 LINC00390 13_17 0.7638 20.77 0.0001927 -4.166 1
8652 FUT9 6_65 0.7578 30.23 0.0002783 5.427 1
436 ARID1B 6_102 0.7300 21.88 0.0001940 -3.907 1
10075 TMEM222 1_19 0.7129 23.45 0.0002031 3.902 1
104 ELAC2 17_11 0.6763 23.15 0.0001902 4.227 1
11534 LINC00606 3_8 0.6693 23.42 0.0001904 -3.964 1
2469 TBC1D19 4_22 0.6633 21.39 0.0001723 4.146 1
10925 LIN28B-AS1 6_70 0.6508 23.57 0.0001863 -4.651 1Genes with largest effect sizes
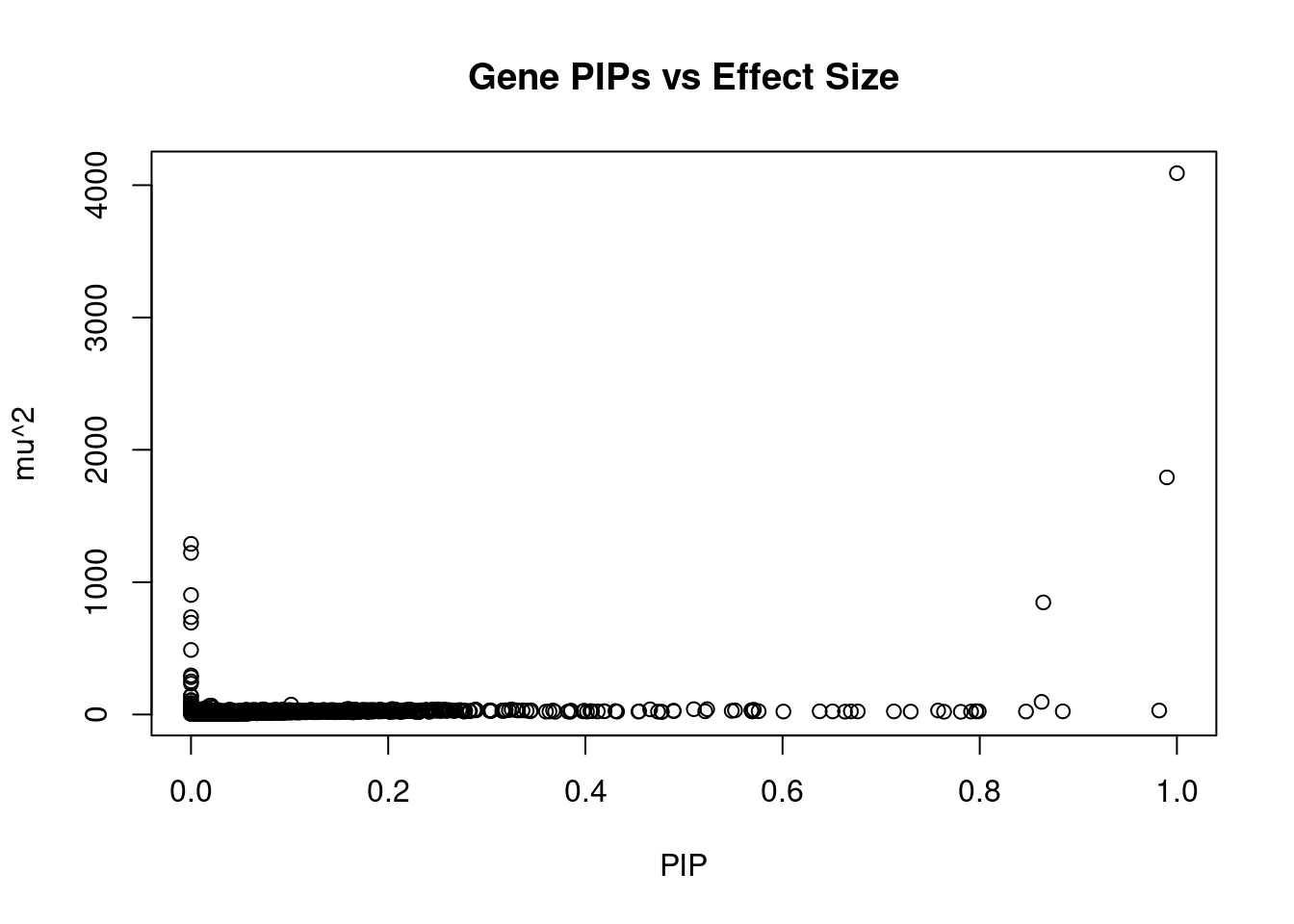
genename region_tag susie_pip mu2 PVE z num_eqtl
3391 CRHR1 17_27 1.000e+00 4090.65 4.970e-02 3.3623 1
10755 ZKSCAN8 6_22 9.899e-01 1791.68 2.155e-02 5.4057 2
10581 ZNF165 6_22 0.000e+00 1289.20 0.000e+00 4.3541 2
10546 ZSCAN26 6_22 7.559e-12 1221.35 1.121e-13 8.3004 2
10362 ZKSCAN3 6_22 0.000e+00 903.12 0.000e+00 6.5137 1
6779 ZSCAN12 6_22 8.645e-01 846.93 8.894e-03 10.9401 1
6833 ARHGAP27 17_27 0.000e+00 735.33 0.000e+00 0.6410 2
11810 ZSCAN31 6_22 0.000e+00 693.84 0.000e+00 -3.0550 2
10383 HLA-DRB1 6_27 0.000e+00 487.58 0.000e+00 4.3158 1
10219 ZSCAN23 6_22 0.000e+00 295.33 0.000e+00 -6.9089 1
12006 HLA-DMB 6_27 0.000e+00 281.88 0.000e+00 -8.0544 1
10782 HLA-DRB5 6_27 0.000e+00 250.39 0.000e+00 2.9680 1
9350 HLA-DQB1 6_27 0.000e+00 232.89 0.000e+00 1.0000 1
10106 HEXIM1 17_27 0.000e+00 142.54 0.000e+00 -3.3281 1
4828 NMT1 17_27 0.000e+00 138.82 0.000e+00 2.4473 2
9374 RPRML 17_27 0.000e+00 108.90 0.000e+00 0.4344 2
4944 PGBD1 6_22 0.000e+00 104.14 0.000e+00 1.6011 1
11759 HIST1H2BN 6_21 8.628e-01 95.15 9.973e-04 10.7729 1
10966 COL11A2 6_27 0.000e+00 86.40 0.000e+00 4.3742 2
2357 GOSR2 17_27 0.000e+00 78.76 0.000e+00 -2.5096 1Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_eqtl
3391 CRHR1 17_27 1.0000 4090.65 0.0496950 3.362 1
10755 ZKSCAN8 6_22 0.9899 1791.68 0.0215453 5.406 2
6779 ZSCAN12 6_22 0.8645 846.93 0.0088944 10.940 1
11759 HIST1H2BN 6_21 0.8628 95.15 0.0009973 10.773 1
10687 ZNF823 19_10 0.9820 30.13 0.0003594 5.455 1
8652 FUT9 6_65 0.7578 30.23 0.0002783 5.427 1
8759 MAP3K11 11_36 0.8843 23.83 0.0002560 -4.544 1
910 NT5C2 10_66 0.5235 40.16 0.0002554 -7.489 2
8275 INO80E 16_24 0.5100 40.11 0.0002485 6.350 1
1736 PPP1R16B 20_23 0.5709 35.72 0.0002477 6.091 1
6084 ARFGAP2 11_29 0.7965 25.00 0.0002419 4.740 1
12742 TBC1D29 17_18 0.8469 22.50 0.0002315 4.578 2
1516 TTLL1 22_18 0.7990 23.82 0.0002313 -4.667 2
7929 ENDOG 9_66 0.7967 23.37 0.0002262 4.806 2
12176 YJEFN3 19_15 0.5684 32.08 0.0002215 -5.736 1
2535 MDK 11_28 0.4657 39.04 0.0002209 -6.357 1
3607 BHLHE41 12_18 0.7912 22.15 0.0002129 -4.024 1
626 SNAP91 6_57 0.5517 30.94 0.0002074 5.814 1
10075 TMEM222 1_19 0.7129 23.45 0.0002031 3.902 1
436 ARID1B 6_102 0.7300 21.88 0.0001940 -3.907 1Genes with largest z scores
genename region_tag susie_pip mu2 PVE z num_eqtl
6779 ZSCAN12 6_22 8.645e-01 846.93 8.894e-03 10.940 1
11759 HIST1H2BN 6_21 8.628e-01 95.15 9.973e-04 10.773 1
12628 CTA-14H9.5 6_20 2.067e-02 67.63 1.698e-05 9.082 1
13065 RP1-86C11.7 6_21 1.016e-01 74.47 9.190e-05 -9.033 1
10071 BTN3A2 6_20 1.858e-02 65.31 1.474e-05 8.998 2
10546 ZSCAN26 6_22 7.559e-12 1221.35 1.121e-13 8.300 2
12006 HLA-DMB 6_27 0.000e+00 281.88 0.000e+00 -8.054 1
9448 HIST1H2BC 6_20 2.125e-02 52.71 1.361e-05 -8.028 1
2719 TRIM38 6_20 1.646e-02 47.14 9.423e-06 -7.700 2
6064 CNNM2 10_66 8.581e-02 37.30 3.889e-05 -7.691 1
910 NT5C2 10_66 5.235e-01 40.16 2.554e-04 -7.489 2
10219 ZSCAN23 6_22 0.000e+00 295.33 0.000e+00 -6.909 1
10362 ZKSCAN3 6_22 0.000e+00 903.12 0.000e+00 6.514 1
6186 ABCB9 12_75 8.133e-03 39.30 3.883e-06 6.404 1
2535 MDK 11_28 4.657e-01 39.04 2.209e-04 -6.357 1
8275 INO80E 16_24 5.100e-01 40.11 2.485e-04 6.350 1
2981 KCNJ13 2_137 2.463e-01 35.54 1.064e-04 6.333 1
12375 APOPT1 14_54 3.889e-02 35.96 1.699e-05 -6.260 2
10290 DPYD 1_60 1.148e-02 36.66 5.112e-06 -6.222 1
6146 TAOK2 16_24 2.213e-01 37.86 1.018e-04 6.189 1Comparing z scores and PIPs
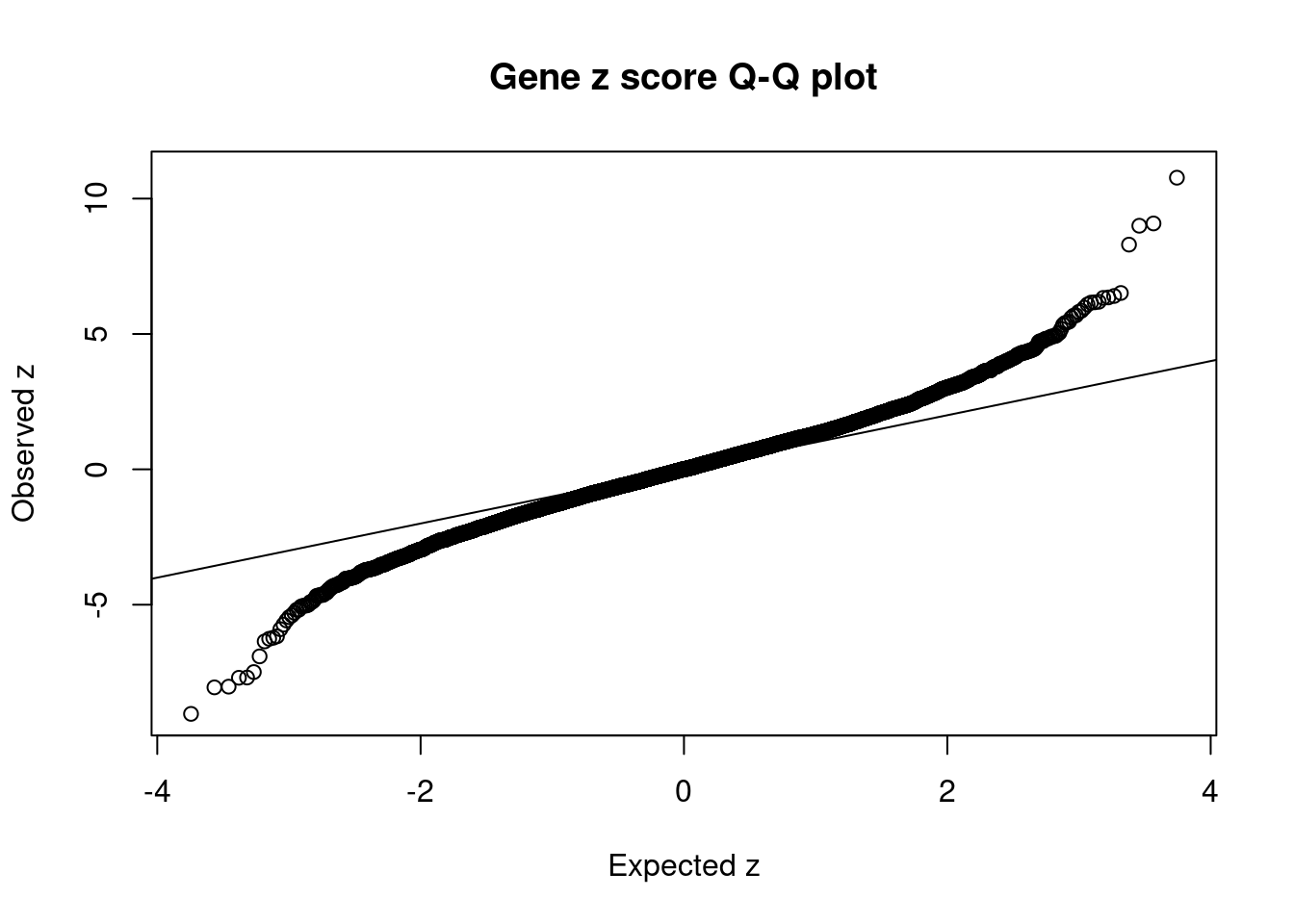
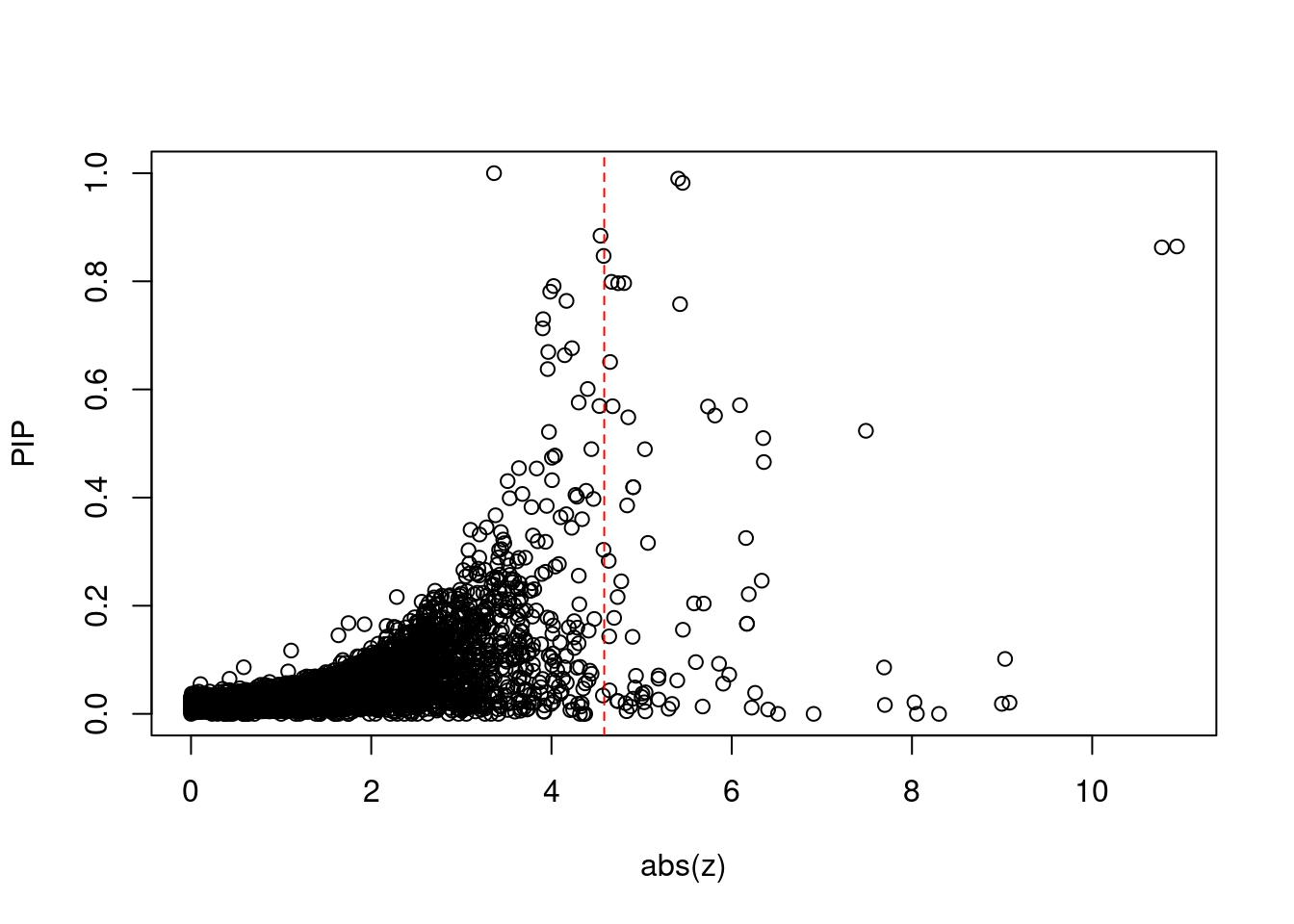
[1] 0.006711 genename region_tag susie_pip mu2 PVE z num_eqtl
6779 ZSCAN12 6_22 8.645e-01 846.93 8.894e-03 10.940 1
11759 HIST1H2BN 6_21 8.628e-01 95.15 9.973e-04 10.773 1
12628 CTA-14H9.5 6_20 2.067e-02 67.63 1.698e-05 9.082 1
13065 RP1-86C11.7 6_21 1.016e-01 74.47 9.190e-05 -9.033 1
10071 BTN3A2 6_20 1.858e-02 65.31 1.474e-05 8.998 2
10546 ZSCAN26 6_22 7.559e-12 1221.35 1.121e-13 8.300 2
12006 HLA-DMB 6_27 0.000e+00 281.88 0.000e+00 -8.054 1
9448 HIST1H2BC 6_20 2.125e-02 52.71 1.361e-05 -8.028 1
2719 TRIM38 6_20 1.646e-02 47.14 9.423e-06 -7.700 2
6064 CNNM2 10_66 8.581e-02 37.30 3.889e-05 -7.691 1
910 NT5C2 10_66 5.235e-01 40.16 2.554e-04 -7.489 2
10219 ZSCAN23 6_22 0.000e+00 295.33 0.000e+00 -6.909 1
10362 ZKSCAN3 6_22 0.000e+00 903.12 0.000e+00 6.514 1
6186 ABCB9 12_75 8.133e-03 39.30 3.883e-06 6.404 1
2535 MDK 11_28 4.657e-01 39.04 2.209e-04 -6.357 1
8275 INO80E 16_24 5.100e-01 40.11 2.485e-04 6.350 1
2981 KCNJ13 2_137 2.463e-01 35.54 1.064e-04 6.333 1
12375 APOPT1 14_54 3.889e-02 35.96 1.699e-05 -6.260 2
10290 DPYD 1_60 1.148e-02 36.66 5.112e-06 -6.222 1
6146 TAOK2 16_24 2.213e-01 37.86 1.018e-04 6.189 1GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 32Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"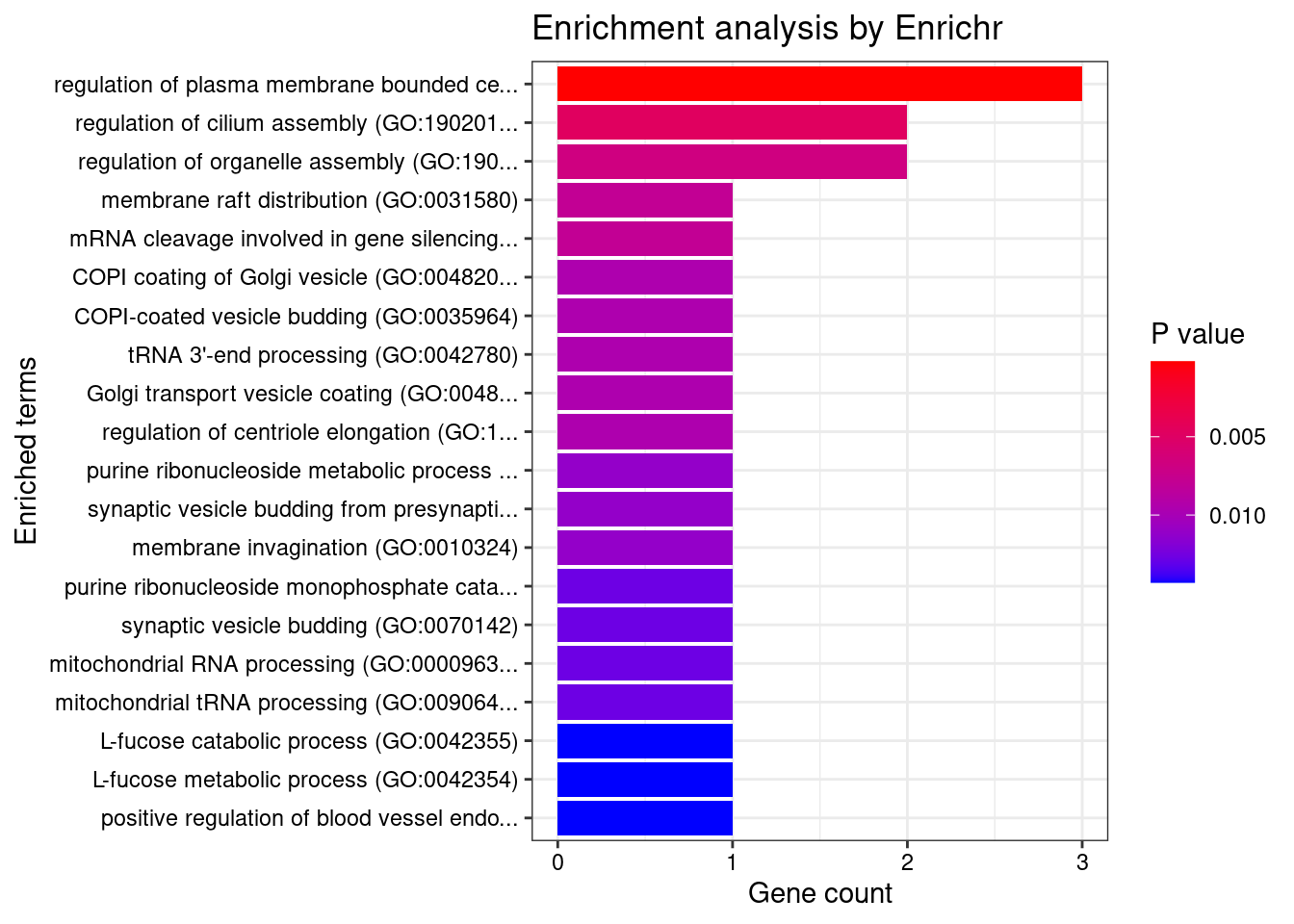
Term
1 regulation of plasma membrane bounded cell projection assembly (GO:0120032)
Overlap Adjusted.P.value Genes
1 3/70 0.04393 PPP1R16B;TBC1D19;TBC1D15
[1] "GO_Cellular_Component_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio BgRatio
72 PROSTATE CANCER, HEREDITARY, 2 0.02144 1/8 1/9703
74 COMBINED OXIDATIVE PHOSPHORYLATION DEFICIENCY 17 0.02144 1/8 1/9703
76 SPASTIC PARAPLEGIA 45, AUTOSOMAL RECESSIVE 0.02144 1/8 1/9703
24 Pain, Postoperative 0.02572 1/8 2/9703
70 CHROMOSOME 6q24-q25 DELETION SYNDROME 0.02572 1/8 2/9703
51 Long Sleeper Syndrome 0.03744 1/8 7/9703
52 Short Sleeper Syndrome 0.03744 1/8 7/9703
53 Sleep-Related Neurogenic Tachypnea 0.03744 1/8 7/9703
54 Subwakefullness Syndrome 0.03744 1/8 7/9703
55 Sleep Disorders 0.03744 1/8 7/9703WebGestalt enrichment analysis for genes with PIP>0.5
Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 41#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 23#significance threshold for TWAS
print(sig_thresh)[1] 4.585#number of ctwas genes
length(ctwas_genes)[1] 7#number of TWAS genes
length(twas_genes)[1] 74#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_eqtl
8759 MAP3K11 11_36 0.8843 23.83 0.0002560 -4.544 1
12742 TBC1D29 17_18 0.8469 22.50 0.0002315 4.578 2
3391 CRHR1 17_27 1.0000 4090.65 0.0496950 3.362 1#sensitivity / recall
print(sensitivity)ctwas TWAS
0 0 #specificity
print(specificity) ctwas TWAS
0.9994 0.9933 #precision / PPV
print(precision)ctwas TWAS
0 0 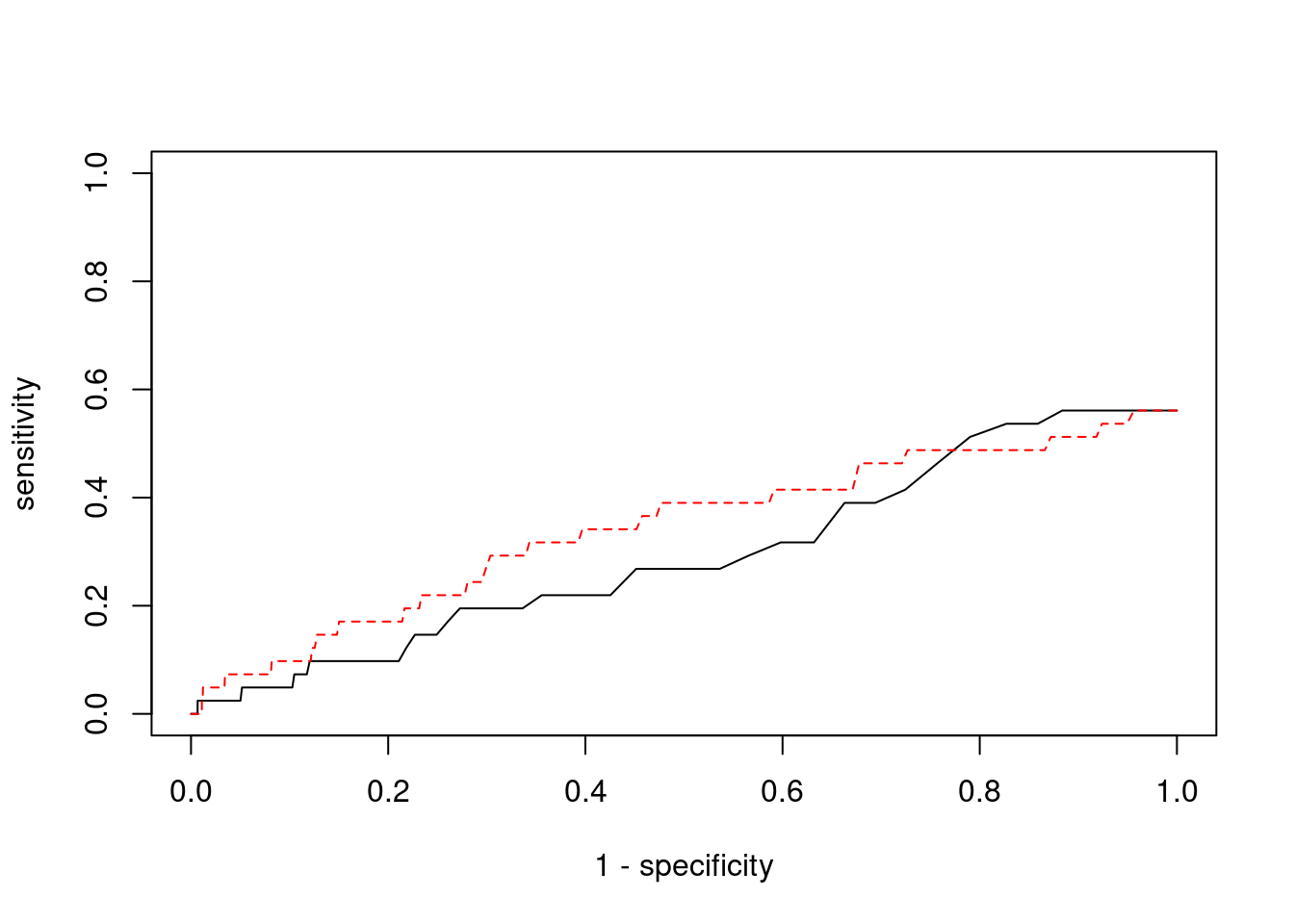
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.3.1 forcats_0.5.1 stringr_1.4.0 dplyr_1.0.7
[5] purrr_0.3.4 readr_2.1.1 tidyr_1.1.4 tidyverse_1.3.1
[9] tibble_3.1.6 WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0
[13] cowplot_1.0.0 ggplot2_3.3.5 workflowr_1.6.2
loaded via a namespace (and not attached):
[1] fs_1.5.2 lubridate_1.8.0 bit64_4.0.5 doParallel_1.0.17
[5] httr_1.4.2 rprojroot_2.0.2 tools_3.6.1 backports_1.4.1
[9] doRNG_1.8.2 utf8_1.2.2 R6_2.5.1 vipor_0.4.5
[13] DBI_1.1.2 colorspace_2.0-2 withr_2.4.3 ggrastr_1.0.1
[17] tidyselect_1.1.1 bit_4.0.4 curl_4.3.2 compiler_3.6.1
[21] git2r_0.26.1 rvest_1.0.2 cli_3.1.0 Cairo_1.5-12.2
[25] xml2_1.3.3 labeling_0.4.2 scales_1.1.1 apcluster_1.4.8
[29] digest_0.6.29 rmarkdown_2.11 svglite_1.2.2 pkgconfig_2.0.3
[33] htmltools_0.5.2 dbplyr_2.1.1 fastmap_1.1.0 highr_0.9
[37] rlang_1.0.1 rstudioapi_0.13 RSQLite_2.2.8 jquerylib_0.1.4
[41] farver_2.1.0 generics_0.1.1 jsonlite_1.7.2 vroom_1.5.7
[45] magrittr_2.0.2 Matrix_1.2-18 ggbeeswarm_0.6.0 Rcpp_1.0.8
[49] munsell_0.5.0 fansi_1.0.2 gdtools_0.1.9 lifecycle_1.0.1
[53] stringi_1.7.6 whisker_0.3-2 yaml_2.2.1 plyr_1.8.6
[57] grid_3.6.1 blob_1.2.2 ggrepel_0.9.1 parallel_3.6.1
[61] promises_1.0.1 crayon_1.5.0 lattice_0.20-38 haven_2.4.3
[65] hms_1.1.1 knitr_1.36 pillar_1.6.4 igraph_1.2.10
[69] rjson_0.2.20 rngtools_1.5.2 reshape2_1.4.4 codetools_0.2-16
[73] reprex_2.0.1 glue_1.6.2 evaluate_0.14 data.table_1.14.2
[77] modelr_0.1.8 vctrs_0.3.8 tzdb_0.2.0 httpuv_1.5.1
[81] foreach_1.5.2 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] cachem_1.0.6 xfun_0.29 broom_0.7.10 later_0.8.0
[89] iterators_1.0.14 beeswarm_0.2.3 memoise_2.0.1 ellipsis_0.3.2