BMI - Brain Cerebellar Hemisphere
sheng Qian
2021-2-6
Last updated: 2022-02-21
Checks: 6 1
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version bbf6737. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .ipynb_checkpoints/
Untracked files:
Untracked: Rplot.png
Untracked: analysis/Glucose_Adipose_Subcutaneous.Rmd
Untracked: analysis/Glucose_Adipose_Visceral_Omentum.Rmd
Untracked: analysis/Splicing_Test.Rmd
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: data/.ipynb_checkpoints/
Untracked: data/AF/
Untracked: data/BMI/
Untracked: data/BMI_S/
Untracked: data/Glucose/
Untracked: data/LDL_S/
Untracked: data/T2D/
Untracked: data/TEST/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/BMI_Brain_Cerebellar_Hemisphere.Rmd) and HTML (docs/BMI_Brain_Cerebellar_Hemisphere.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | bbf6737 | sq-96 | 2022-02-21 | update |
| html | 91f38fa | sq-96 | 2022-02-13 | Build site. |
| Rmd | eb13ecf | sq-96 | 2022-02-13 | update |
| html | e6bc169 | sq-96 | 2022-02-13 | Build site. |
| Rmd | 87fee8b | sq-96 | 2022-02-13 | update |
Weight QC
#number of imputed weights
nrow(qclist_all)[1] 11315#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1087 770 652 425 535 625 556 423 440 443 698 615 209 381 372 538
17 18 19 20 21 22
709 170 904 333 134 296 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 8732#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.7717Check convergence of parameters

| Version | Author | Date |
|---|---|---|
| e6bc169 | sq-96 | 2022-02-13 |
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0097195 0.0002858 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
17.70 17.92 #report sample size
print(sample_size)[1] 336107#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 11315 7535010#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.005791 0.114815 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.06977 17.00375Genes with highest PIPs
genename region_tag susie_pip mu2 PVE z num_eqtl
11541 NDUFS3 11_29 0.9962 1179.68 3.497e-03 -11.094 2
3395 CCND2 12_4 0.9680 28.45 8.194e-05 -5.094 2
978 PIK3C3 18_23 0.9528 51.82 1.469e-04 6.896 2
7720 ZNF12 7_10 0.9349 27.64 7.689e-05 5.106 2
4962 DCAF7 17_37 0.8835 28.48 7.487e-05 5.437 1
7905 CASP7 10_71 0.8757 24.36 6.346e-05 4.584 1
9464 ZBTB41 1_98 0.8621 1788.23 4.587e-03 4.618 1
8843 LAMB2 3_34 0.8204 138.48 3.380e-04 -7.471 1
518 KCNH2 7_93 0.7943 40.89 9.662e-05 6.352 2
8913 EFEMP2 11_36 0.7872 56.09 1.314e-04 -8.201 1
1242 XRN2 20_15 0.7859 23.48 5.491e-05 -4.449 3
7481 SERPINI1 3_103 0.7809 21.25 4.937e-05 -3.916 2
4684 YWHAQ 2_6 0.7693 25.68 5.878e-05 4.911 1
3471 YIPF4 2_20 0.7572 628.63 1.416e-03 2.868 4
1398 CBX5 12_33 0.7455 25.07 5.560e-05 -4.691 1
8350 TAP1 6_27 0.7394 29.03 6.387e-05 5.285 1
3479 SLF2 10_64 0.7338 30.52 6.662e-05 4.780 2
8202 NCKAP5L 12_31 0.7242 49.53 1.067e-04 -8.217 1
8279 NLRC3 16_3 0.7209 33.49 7.182e-05 5.243 1
4586 CSNK1G2 19_2 0.7170 31.70 6.763e-05 -5.549 2Genes with largest effect sizes

genename region_tag susie_pip mu2 PVE z num_eqtl
7785 CCDC171 9_13 0.000e+00 53826 0.000e+00 7.951 1
7784 PSIP1 9_13 0.000e+00 45986 0.000e+00 8.364 1
6221 CNNM2 10_66 1.346e-03 36526 1.463e-04 -5.132 1
6469 ARL14EP 11_21 0.000e+00 28862 0.000e+00 6.331 2
5419 MFAP1 15_16 0.000e+00 24545 0.000e+00 4.303 1
12479 RP11-757G1.6 11_38 1.092e-05 24452 7.943e-07 4.319 2
8035 LEO1 15_21 8.436e-04 23922 6.004e-05 4.647 1
13446 LINC02019 3_35 1.410e-07 23283 9.769e-09 -4.344 2
3007 CISH 3_35 0.000e+00 22928 0.000e+00 -4.823 1
11730 CKMT1A 15_16 0.000e+00 21983 0.000e+00 4.130 1
10888 MRPL21 11_38 0.000e+00 21546 0.000e+00 3.982 2
3006 HEMK1 3_35 0.000e+00 19749 0.000e+00 -4.682 1
1065 CCNT2 2_80 1.380e-04 19271 7.913e-06 3.713 1
3139 PLCL1 2_117 0.000e+00 19186 0.000e+00 -5.642 1
5423 LYSMD2 15_21 0.000e+00 18805 0.000e+00 -5.232 1
8114 MAP1A 15_16 0.000e+00 17091 0.000e+00 3.818 2
1452 MAST3 19_14 0.000e+00 16327 0.000e+00 -2.208 1
9538 NSUN3 3_59 0.000e+00 16205 0.000e+00 4.755 1
8409 ADAL 15_16 0.000e+00 15308 0.000e+00 -2.861 1
130 CACNA2D2 3_35 0.000e+00 14672 0.000e+00 -4.014 1Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_eqtl
2642 PTPMT1 11_29 0.456199 14326.39 1.945e-02 -3.623 2
9530 ERBB4 2_125 0.703049 5989.36 1.253e-02 -7.023 1
286 CPS1 2_124 0.364179 4800.48 5.201e-03 -3.562 1
9464 ZBTB41 1_98 0.862082 1788.23 4.587e-03 4.618 1
3081 LANCL1 2_124 0.316378 4817.32 4.535e-03 -3.535 1
11541 NDUFS3 11_29 0.996248 1179.68 3.497e-03 -11.094 2
3471 YIPF4 2_20 0.757159 628.63 1.416e-03 2.868 4
8843 LAMB2 3_34 0.820428 138.48 3.380e-04 -7.471 1
11726 VPS52 6_28 0.631264 126.68 2.379e-04 1.603 1
978 PIK3C3 18_23 0.952810 51.82 1.469e-04 6.896 2
6221 CNNM2 10_66 0.001346 36525.78 1.463e-04 -5.132 1
11281 RNF5 6_26 0.246996 181.55 1.334e-04 6.337 2
8913 EFEMP2 11_36 0.787153 56.09 1.314e-04 -8.201 1
1460 STX1B 16_24 0.512675 80.29 1.225e-04 -10.209 1
7606 MFSD8 4_84 0.005705 7091.90 1.204e-04 2.512 1
8202 NCKAP5L 12_31 0.724170 49.53 1.067e-04 -8.217 1
518 KCNH2 7_93 0.794299 40.89 9.662e-05 6.352 2
13683 DHRS11 17_22 0.480896 61.80 8.842e-05 -8.128 1
3395 CCND2 12_4 0.968047 28.45 8.194e-05 -5.094 2
7263 TAL1 1_29 0.563761 47.91 8.036e-05 -6.866 1Genes with largest z scores
genename region_tag susie_pip mu2 PVE z num_eqtl
41 RBM6 3_35 1.197e-03 934.02 3.327e-06 12.536 1
33 RBM5 3_35 6.485e-04 978.25 1.888e-06 12.473 1
7609 MST1R 3_35 3.362e-10 248.31 2.484e-13 -11.521 3
9166 KCTD13 16_24 1.073e-01 109.75 3.504e-05 -11.491 1
11541 NDUFS3 11_29 9.962e-01 1179.68 3.497e-03 -11.094 2
8510 INO80E 16_24 2.414e-02 98.53 7.075e-06 11.077 1
7604 RNF123 3_35 1.410e-11 847.57 3.555e-14 -10.957 1
12511 RP11-1348G14.4 16_23 2.267e-01 91.80 6.192e-05 10.676 1
10122 APOBR 16_23 1.382e-01 93.79 3.855e-05 -10.540 1
9282 NUPR1 16_23 1.382e-01 93.79 3.855e-05 -10.540 1
12037 NPIPB7 16_23 1.036e-01 90.83 2.801e-05 10.510 1
6310 DOC2A 16_24 3.833e-02 87.50 9.978e-06 -10.320 2
10802 C6orf106 6_28 4.122e-05 118.83 1.457e-08 -10.264 1
1460 STX1B 16_24 5.127e-01 80.29 1.225e-04 -10.209 1
8172 ZNF646 16_24 5.766e-02 75.22 1.290e-05 -10.000 1
8171 ZNF668 16_24 5.766e-02 75.22 1.290e-05 10.000 1
2889 COL4A3BP 5_44 3.736e-02 69.80 7.759e-06 9.828 1
484 PRSS8 16_24 1.768e-02 71.39 3.754e-06 9.765 1
649 UHRF1BP1 6_28 1.067e-07 88.10 2.797e-11 -9.654 2
1937 BCKDK 16_24 1.390e-02 68.03 2.813e-06 -9.638 1Comparing z scores and PIPs
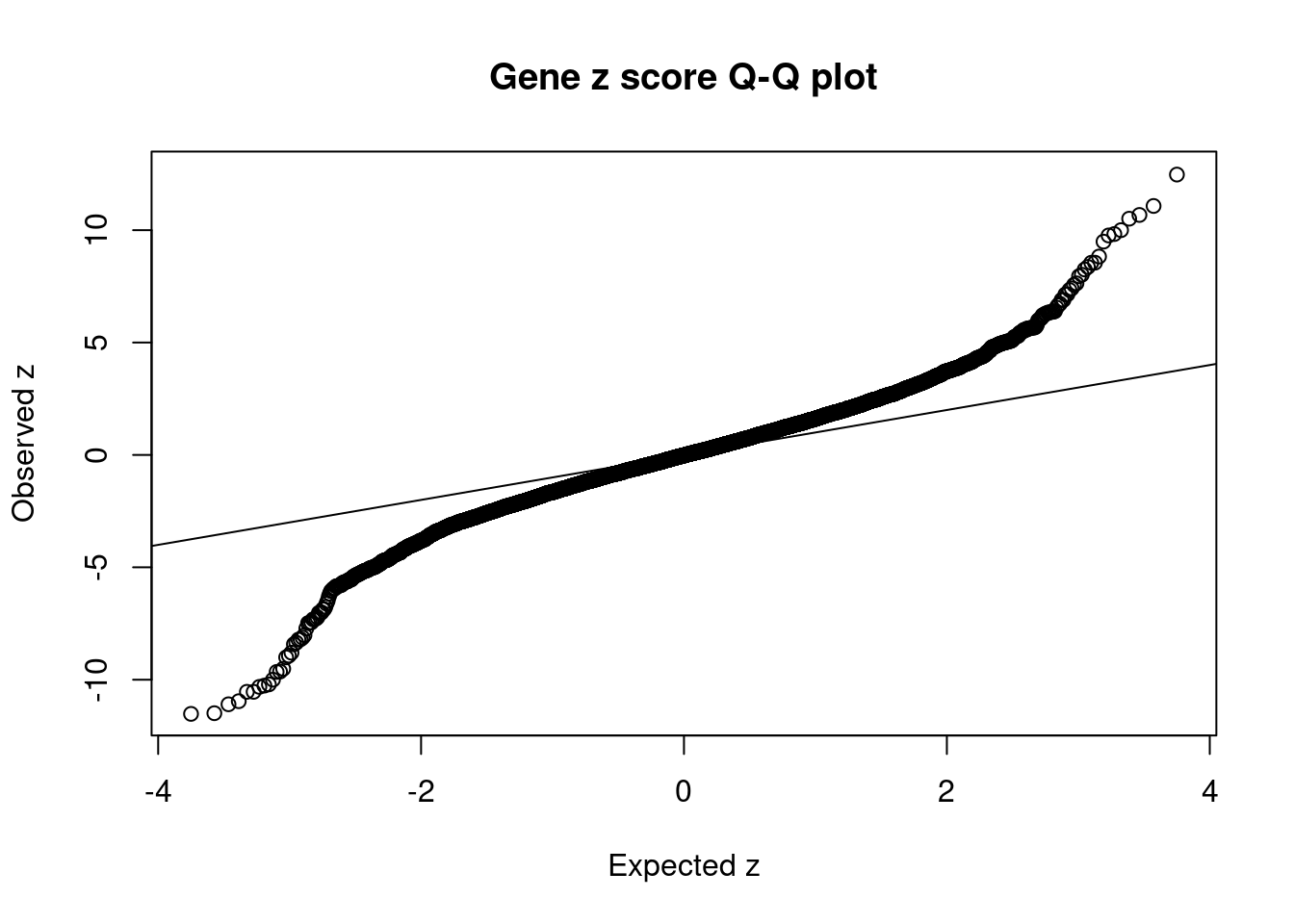
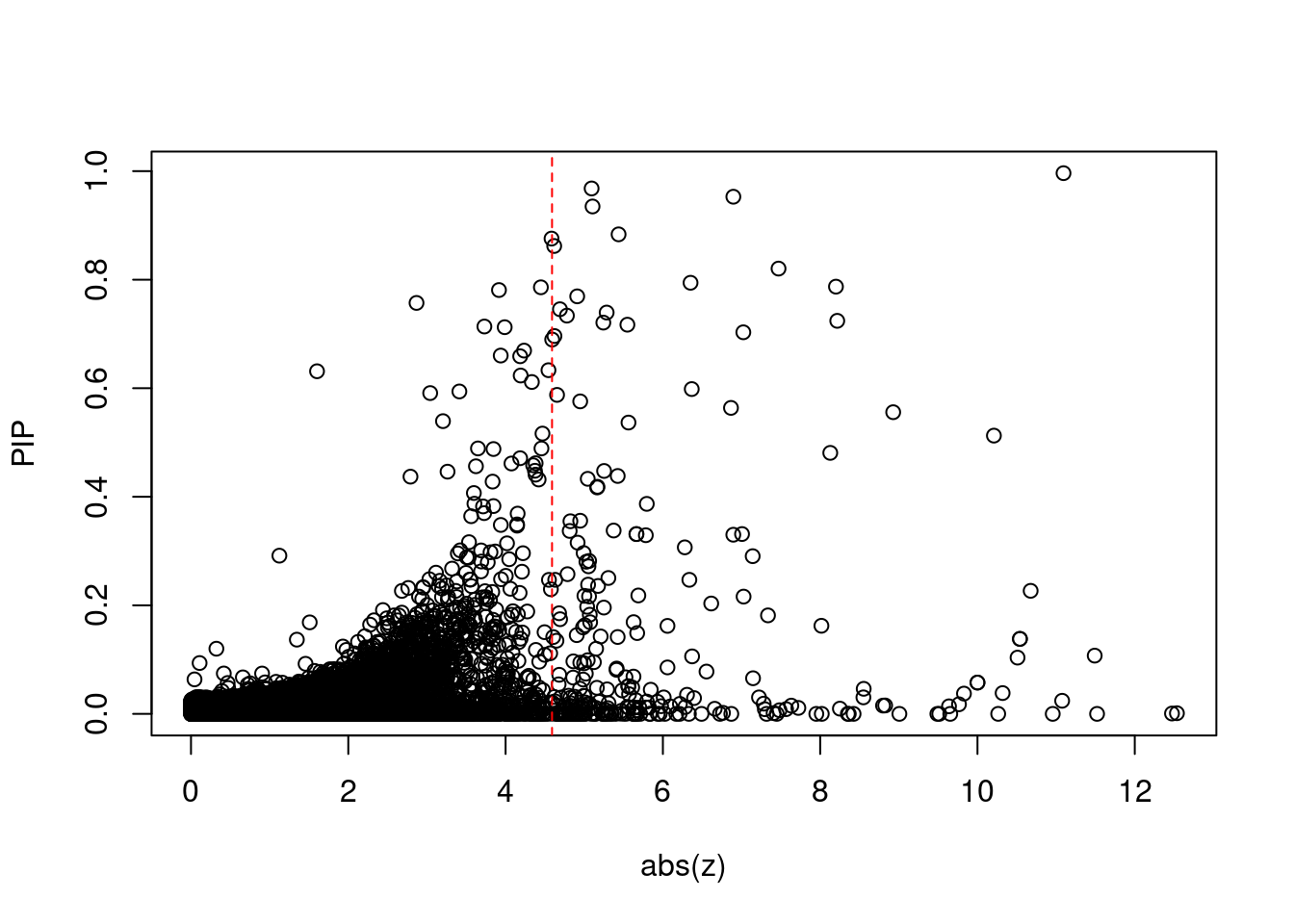
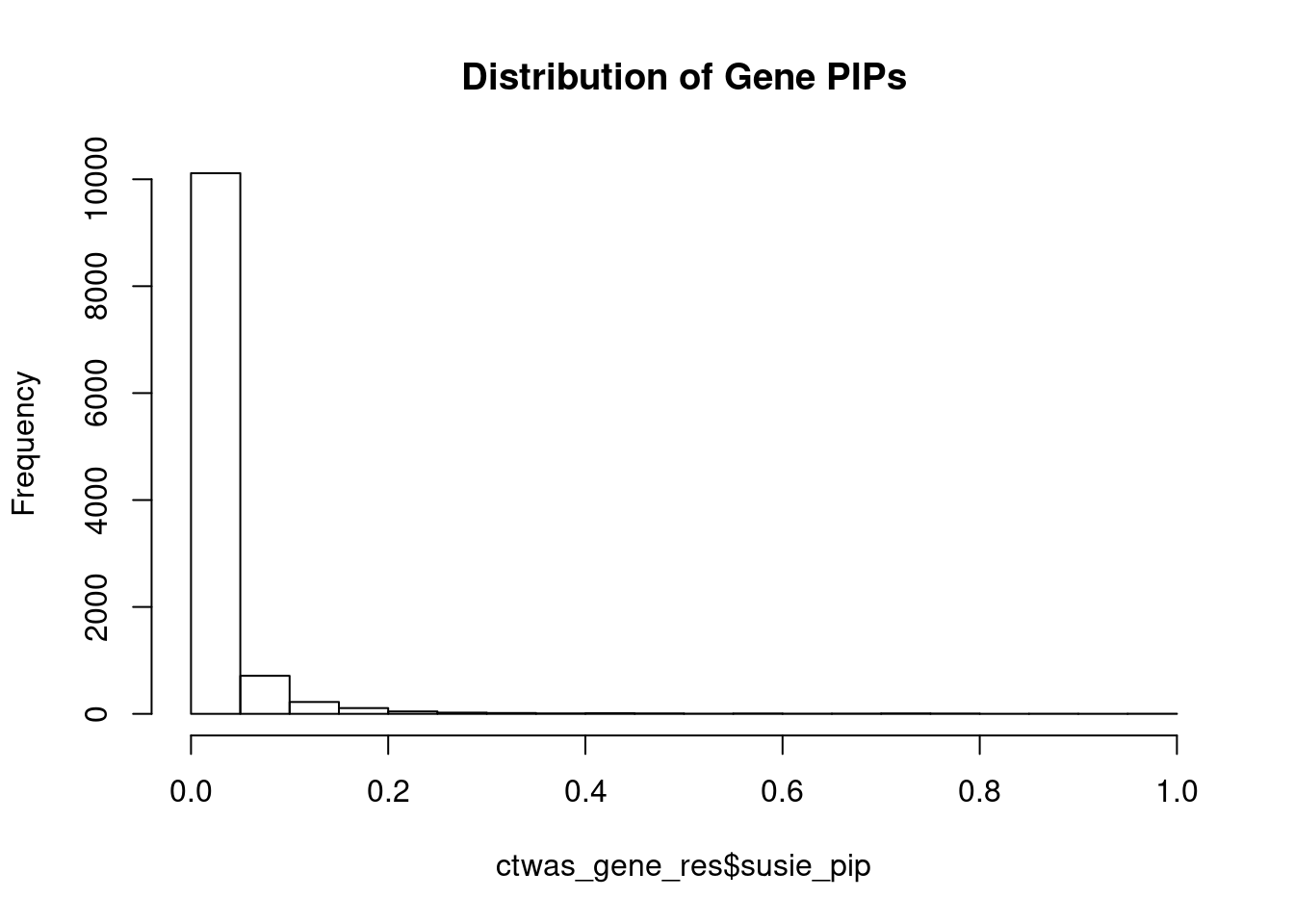
[1] 0.02307 genename region_tag susie_pip mu2 PVE z num_eqtl
41 RBM6 3_35 1.197e-03 934.02 3.327e-06 12.536 1
33 RBM5 3_35 6.485e-04 978.25 1.888e-06 12.473 1
7609 MST1R 3_35 3.362e-10 248.31 2.484e-13 -11.521 3
9166 KCTD13 16_24 1.073e-01 109.75 3.504e-05 -11.491 1
11541 NDUFS3 11_29 9.962e-01 1179.68 3.497e-03 -11.094 2
8510 INO80E 16_24 2.414e-02 98.53 7.075e-06 11.077 1
7604 RNF123 3_35 1.410e-11 847.57 3.555e-14 -10.957 1
12511 RP11-1348G14.4 16_23 2.267e-01 91.80 6.192e-05 10.676 1
10122 APOBR 16_23 1.382e-01 93.79 3.855e-05 -10.540 1
9282 NUPR1 16_23 1.382e-01 93.79 3.855e-05 -10.540 1
12037 NPIPB7 16_23 1.036e-01 90.83 2.801e-05 10.510 1
6310 DOC2A 16_24 3.833e-02 87.50 9.978e-06 -10.320 2
10802 C6orf106 6_28 4.122e-05 118.83 1.457e-08 -10.264 1
1460 STX1B 16_24 5.127e-01 80.29 1.225e-04 -10.209 1
8172 ZNF646 16_24 5.766e-02 75.22 1.290e-05 -10.000 1
8171 ZNF668 16_24 5.766e-02 75.22 1.290e-05 10.000 1
2889 COL4A3BP 5_44 3.736e-02 69.80 7.759e-06 9.828 1
484 PRSS8 16_24 1.768e-02 71.39 3.754e-06 9.765 1
649 UHRF1BP1 6_28 1.067e-07 88.10 2.797e-11 -9.654 2
1937 BCKDK 16_24 1.390e-02 68.03 2.813e-06 -9.638 1GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 43Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"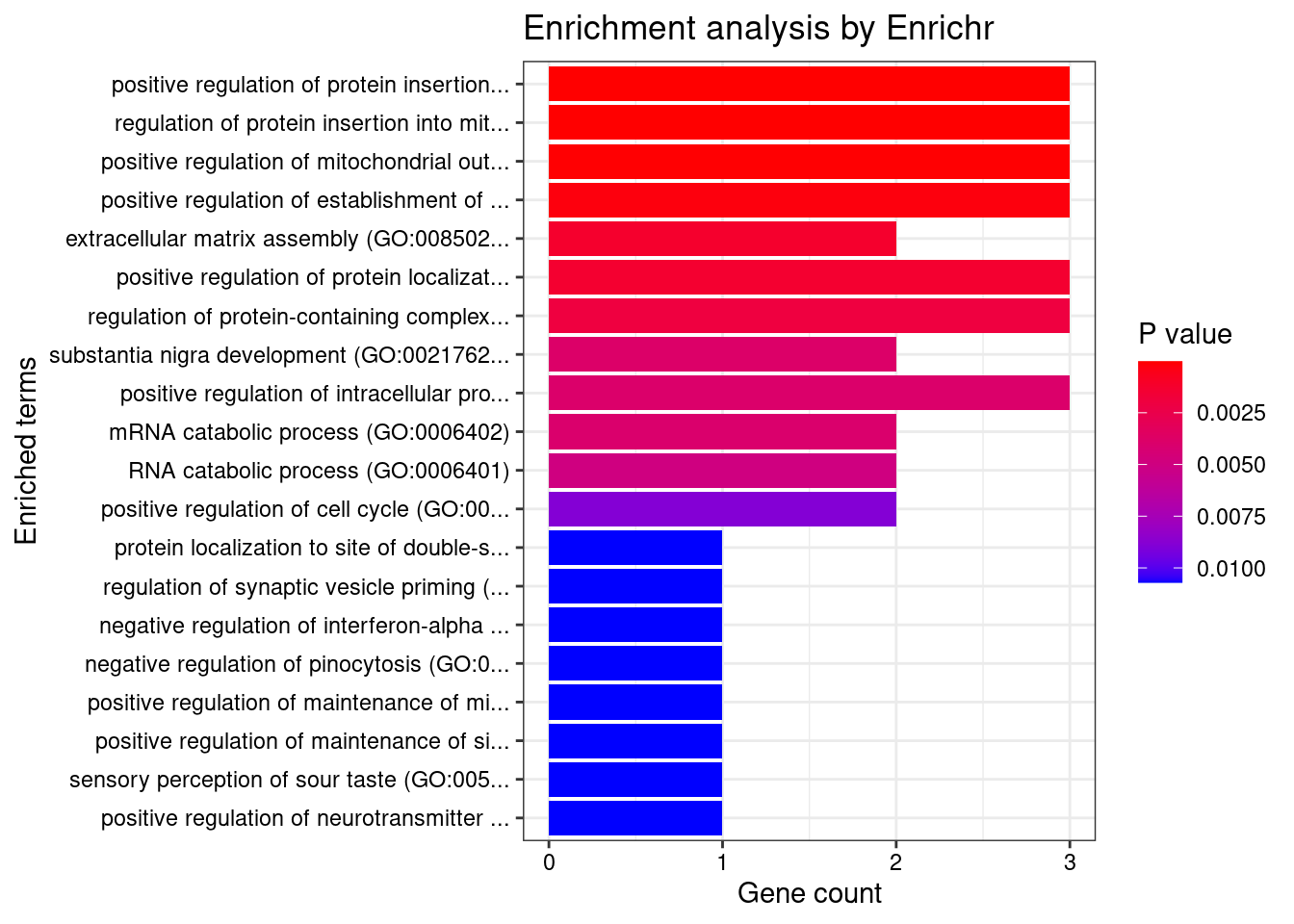
Term
1 positive regulation of protein insertion into mitochondrial membrane involved in apoptotic signaling pathway (GO:1900740)
2 regulation of protein insertion into mitochondrial membrane involved in apoptotic signaling pathway (GO:1900739)
3 positive regulation of mitochondrial outer membrane permeabilization involved in apoptotic signaling pathway (GO:1901030)
4 positive regulation of establishment of protein localization to mitochondrion (GO:1903749)
Overlap Adjusted.P.value Genes
1 3/26 0.004615 YWHAQ;YWHAB;YWHAZ
2 3/26 0.004615 YWHAQ;YWHAB;YWHAZ
3 3/34 0.006997 YWHAQ;YWHAB;YWHAZ
4 3/56 0.023522 YWHAQ;YWHAB;YWHAZ
[1] "GO_Cellular_Component_2021"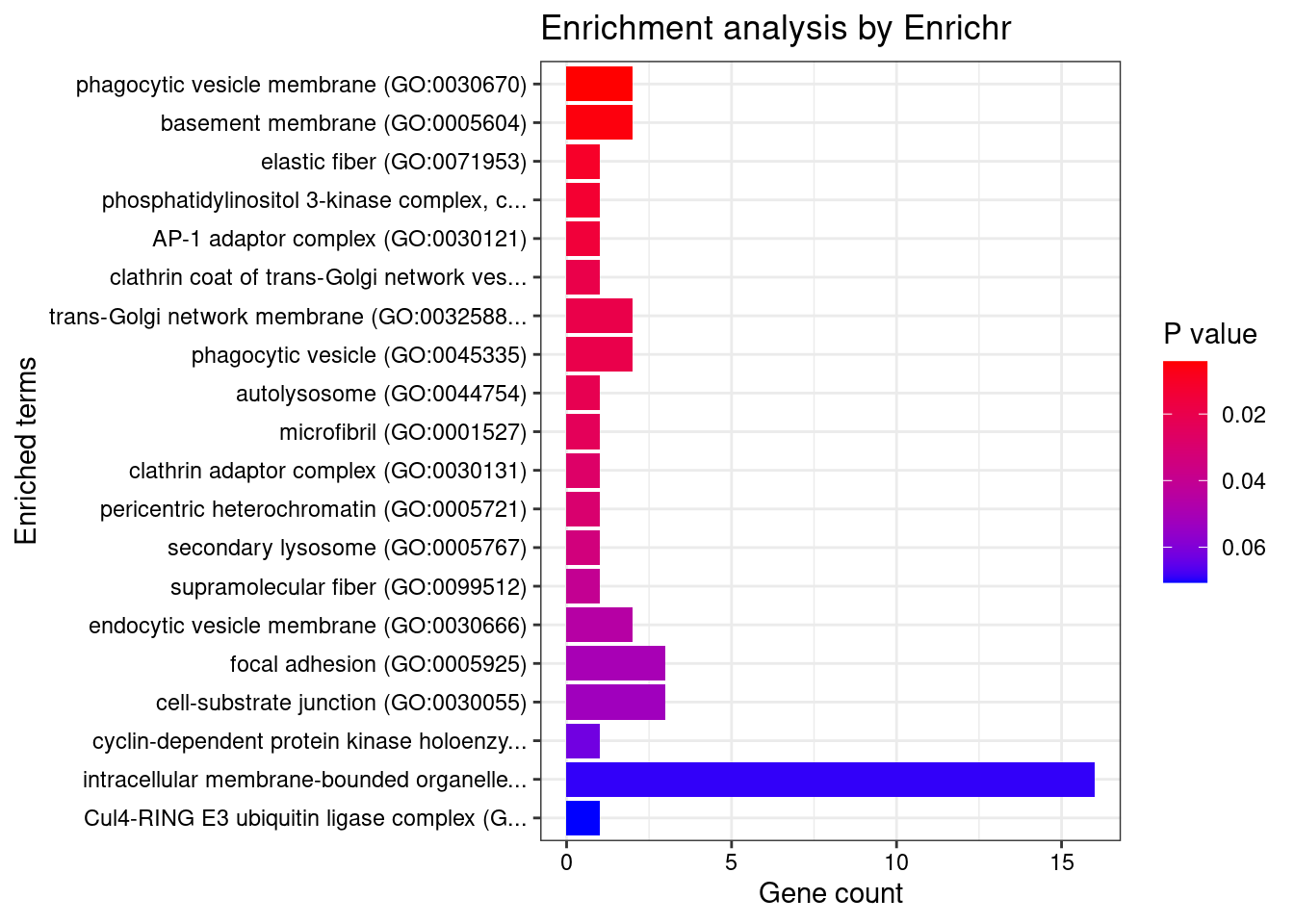
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description
79 Diffuse mesangial sclerosis (disorder)
117 ERYTHROKERATODERMIA VARIABILIS 3 (disorder)
118 Pierson syndrome
119 ATAXIA, SENSORY, AUTOSOMAL DOMINANT
128 Familial encephalopathy with neuroserpin inclusion bodies
143 NEPHROTIC SYNDROME, TYPE 5, WITH OR WITHOUT OCULAR ABNORMALITIES
144 CUTIS LAXA, AUTOSOMAL RECESSIVE, TYPE IB
146 Familial mesangial sclerosis
147 Nephrotic Syndrome, Congenital, With Ocular Abnormalities And Congenital Myasthenic Syndrome
150 AMYOTROPHIC LATERAL SCLEROSIS 19
FDR Ratio BgRatio
79 0.02608 1/22 1/9703
117 0.02608 1/22 1/9703
118 0.02608 1/22 1/9703
119 0.02608 1/22 1/9703
128 0.02608 1/22 1/9703
143 0.02608 1/22 1/9703
144 0.02608 1/22 1/9703
146 0.02608 1/22 1/9703
147 0.02608 1/22 1/9703
150 0.02608 1/22 1/9703WebGestalt enrichment analysis for genes with PIP>0.5
Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Warning: ggrepel: 3 unlabeled data points (too many overlaps). Consider
increasing max.overlaps
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 41#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 25#significance threshold for TWAS
print(sig_thresh)[1] 4.591#number of ctwas genes
length(ctwas_genes)[1] 8#number of TWAS genes
length(twas_genes)[1] 261#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_eqtl
7905 CASP7 10_71 0.8757 24.36 6.346e-05 4.584 1#sensitivity / recall
print(sensitivity) ctwas TWAS
0.00000 0.07317 #specificity
print(specificity) ctwas TWAS
0.9993 0.9771 #precision / PPV
print(precision) ctwas TWAS
0.00000 0.01149 
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.3.1 forcats_0.5.1 stringr_1.4.0 dplyr_1.0.7
[5] purrr_0.3.4 readr_2.1.1 tidyr_1.1.4 tidyverse_1.3.1
[9] tibble_3.1.6 WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0
[13] cowplot_1.0.0 ggplot2_3.3.5 workflowr_1.6.2
loaded via a namespace (and not attached):
[1] fs_1.5.2 lubridate_1.8.0 bit64_4.0.5 doParallel_1.0.16
[5] httr_1.4.2 rprojroot_2.0.2 tools_3.6.1 backports_1.4.1
[9] doRNG_1.8.2 utf8_1.2.2 R6_2.5.1 vipor_0.4.5
[13] DBI_1.1.1 colorspace_2.0-2 withr_2.4.3 ggrastr_1.0.1
[17] tidyselect_1.1.1 bit_4.0.4 curl_4.3.2 compiler_3.6.1
[21] git2r_0.26.1 cli_3.1.0 rvest_1.0.2 Cairo_1.5-12.2
[25] xml2_1.3.3 labeling_0.4.2 scales_1.1.1 apcluster_1.4.8
[29] digest_0.6.29 rmarkdown_2.11 svglite_1.2.2 pkgconfig_2.0.3
[33] htmltools_0.5.2 dbplyr_2.1.1 fastmap_1.1.0 highr_0.9
[37] rlang_0.4.12 rstudioapi_0.13 RSQLite_2.2.8 jquerylib_0.1.4
[41] farver_2.1.0 generics_0.1.1 jsonlite_1.7.2 vroom_1.5.7
[45] magrittr_2.0.1 Matrix_1.2-18 ggbeeswarm_0.6.0 Rcpp_1.0.7
[49] munsell_0.5.0 fansi_0.5.0 gdtools_0.1.9 lifecycle_1.0.1
[53] stringi_1.7.6 whisker_0.3-2 yaml_2.2.1 plyr_1.8.6
[57] grid_3.6.1 blob_1.2.2 ggrepel_0.9.1 parallel_3.6.1
[61] promises_1.0.1 crayon_1.4.2 lattice_0.20-38 haven_2.4.3
[65] hms_1.1.1 knitr_1.36 pillar_1.6.4 igraph_1.2.10
[69] rjson_0.2.20 rngtools_1.5.2 reshape2_1.4.4 codetools_0.2-16
[73] reprex_2.0.1 glue_1.5.1 evaluate_0.14 data.table_1.14.2
[77] modelr_0.1.8 vctrs_0.3.8 tzdb_0.2.0 httpuv_1.5.1
[81] foreach_1.5.1 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] cachem_1.0.6 xfun_0.29 broom_0.7.10 later_0.8.0
[89] iterators_1.0.13 beeswarm_0.2.3 memoise_2.0.1 ellipsis_0.3.2