T2D - Liver
sheng Qian
2021-2-6
Last updated: 2022-02-13
Checks: 6 1
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 87fee8b. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .ipynb_checkpoints/
Untracked files:
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/BMI_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: data/.ipynb_checkpoints/
Untracked: data/AF/
Untracked: data/BMI/
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/T2D_Liver.Rmd) and HTML (docs/T2D_Liver.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 87fee8b | sq-96 | 2022-02-13 | update |
Introduction
Weight QC
qclist_all <- list()
qc_files <- paste0(results_dir, "/", list.files(results_dir, pattern="exprqc.Rd"))
for (i in 1:length(qc_files)){
load(qc_files[i])
chr <- unlist(strsplit(rev(unlist(strsplit(qc_files[i], "_")))[1], "[.]"))[1]
qclist_all[[chr]] <- cbind(do.call(rbind, lapply(qclist,unlist)), as.numeric(substring(chr,4)))
}
qclist_all <- data.frame(do.call(rbind, qclist_all))
colnames(qclist_all)[ncol(qclist_all)] <- "chr"
rm(qclist, wgtlist, z_gene_chr)
#number of imputed weights
nrow(qclist_all)[1] 10290#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1015 728 627 403 457 577 509 392 388 394 607 572 190 353 340 483
17 18 19 20 21 22
629 151 808 290 108 269 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 8215#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.7983479Load ctwas results
Check convergence of parameters
library(ggplot2)
library(cowplot)
********************************************************Note: As of version 1.0.0, cowplot does not change the default ggplot2 theme anymore. To recover the previous behavior, execute:
theme_set(theme_cowplot())********************************************************load(paste0(results_dir, "/", analysis_id, "_ctwas.s2.susieIrssres.Rd"))
df <- data.frame(niter = rep(1:ncol(group_prior_rec), 2),
value = c(group_prior_rec[1,], group_prior_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_rec)))
df$group <- as.factor(df$group)
df$value[df$group=="SNP"] <- df$value[df$group=="SNP"]*thin #adjust parameter to account for thin argument
p_pi <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(pi)) +
ggtitle("Prior mean") +
theme_cowplot()
df <- data.frame(niter = rep(1:ncol(group_prior_var_rec), 2),
value = c(group_prior_var_rec[1,], group_prior_var_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_var_rec)))
df$group <- as.factor(df$group)
p_sigma2 <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(sigma^2)) +
ggtitle("Prior variance") +
theme_cowplot()
plot_grid(p_pi, p_sigma2)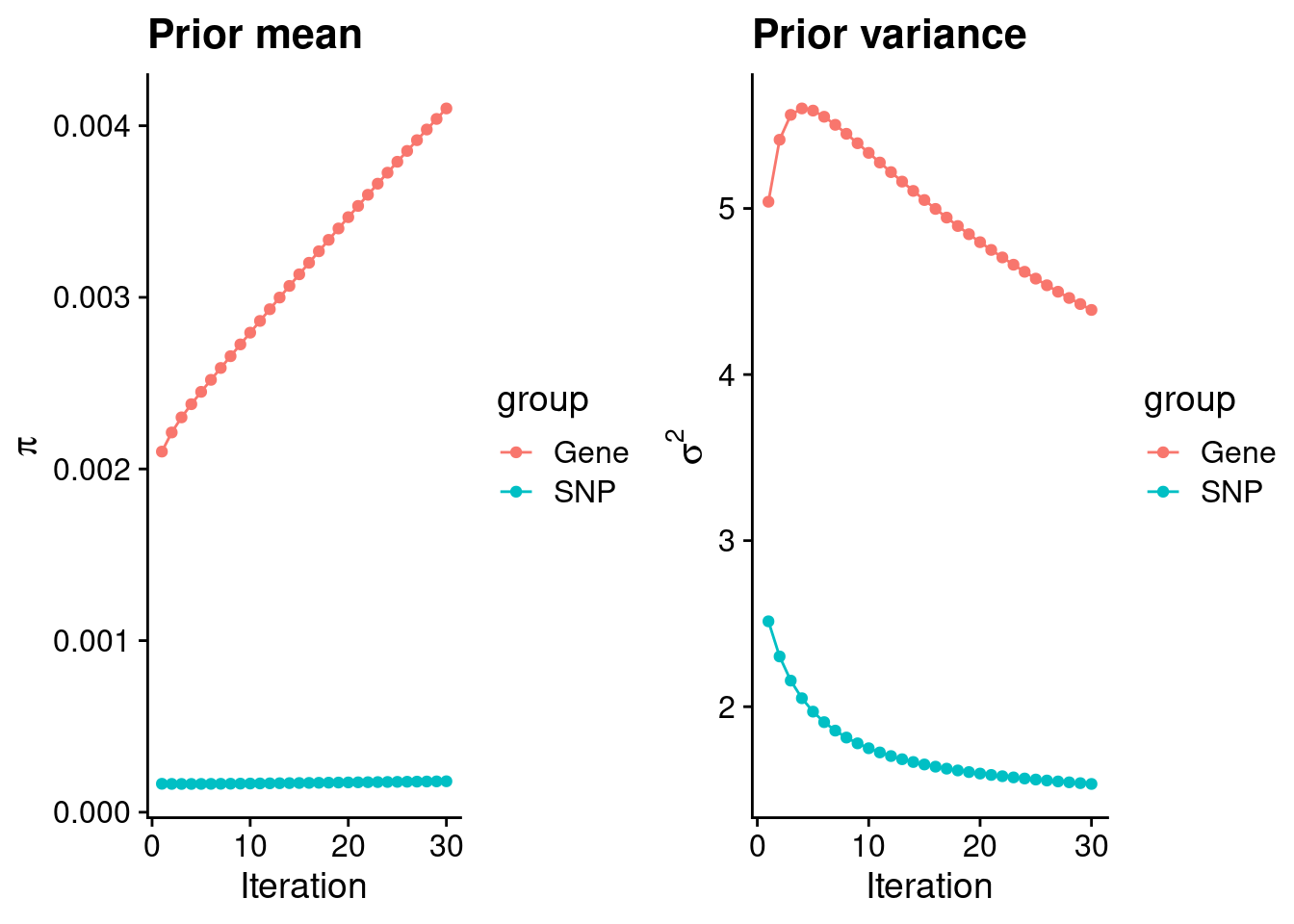
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0041007085 0.0001802713 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
4.388896 1.534856 #report sample size
print(sample_size)[1] 337159#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 10290 7535010#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.0005492813 0.0061836269 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.004834584 0.111688709Genes with highest PIPs
#distribution of PIPs
hist(ctwas_gene_res$susie_pip, xlim=c(0,1), main="Distribution of Gene PIPs")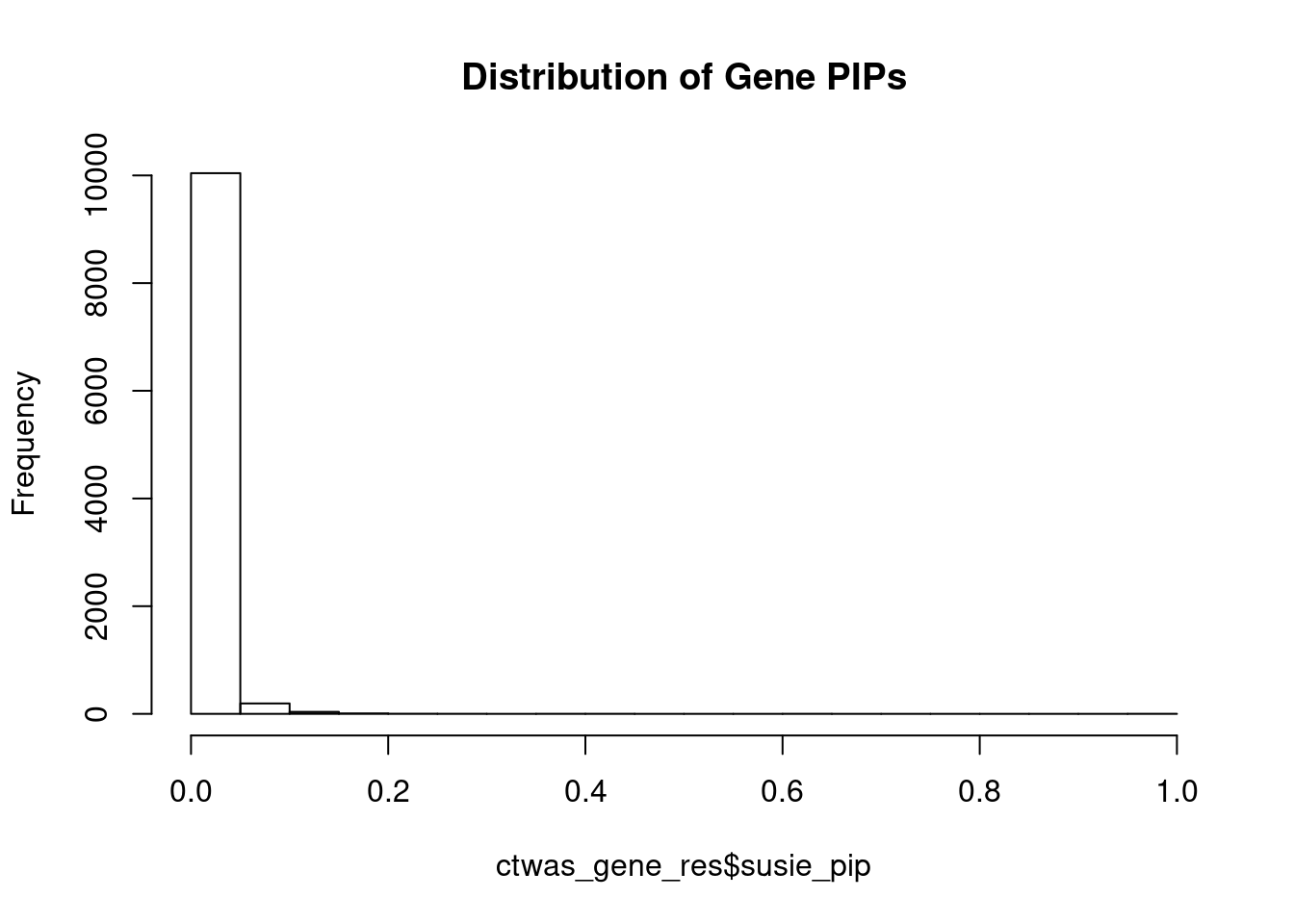
#genes with PIP>0.8 or 20 highest PIPs
head(ctwas_gene_res[order(-ctwas_gene_res$susie_pip),report_cols], max(sum(ctwas_gene_res$susie_pip>0.8), 20)) genename region_tag susie_pip mu2 PVE z
3212 CCND2 12_4 0.9959793 27.97577 8.264138e-05 5.657050
2240 SEC23IP 10_74 0.6381754 61.38049 1.161811e-04 -3.610724
12661 LINC01126 2_27 0.4142141 28.18774 3.462982e-05 4.620415
10283 MCMBP 10_74 0.3685673 60.32290 6.594232e-05 3.522402
703 GUCY2C 12_12 0.2541535 35.09246 2.645301e-05 3.878767
6307 NUS1 6_78 0.2350043 32.14897 2.240826e-05 3.716370
12541 RP6-65G23.5 14_33 0.2244479 30.24036 2.013111e-05 3.369949
5911 CIZ1 9_66 0.2067132 30.11029 1.846071e-05 -3.513905
6558 AP3S2 15_41 0.1990701 30.35974 1.792543e-05 -3.745658
7641 NDEL1 17_8 0.1907755 28.90161 1.635346e-05 -3.136833
10118 RABL6 9_74 0.1894969 30.14016 1.693998e-05 3.491221
2577 GNPTAB 12_61 0.1868989 29.62473 1.642201e-05 3.600816
4089 UBAC1 9_72 0.1742949 28.55651 1.476233e-05 3.438703
9318 LIPF 10_56 0.1709291 29.37002 1.488969e-05 -2.991710
12431 RP11-535A5.1 18_11 0.1656953 28.08816 1.380381e-05 -2.997627
12123 UPK3BL 7_63 0.1607226 28.40836 1.354217e-05 3.134982
1624 TPD52L2 20_38 0.1531083 28.20869 1.280993e-05 -3.090905
1483 RPL3 22_16 0.1466952 27.86306 1.212300e-05 3.284537
4539 ISCA1 9_44 0.1449422 27.76422 1.193563e-05 3.269765
3541 ARHGAP9 12_36 0.1430809 26.51095 1.125051e-05 2.925673
num_eqtl
3212 1
2240 1
12661 1
10283 1
703 1
6307 1
12541 1
5911 2
6558 1
7641 1
10118 1
2577 1
4089 1
9318 1
12431 1
12123 1
1624 1
1483 1
4539 1
3541 1Genes with largest effect sizes
#plot PIP vs effect size
plot(ctwas_gene_res$susie_pip, ctwas_gene_res$mu2, xlab="PIP", ylab="mu^2", main="Gene PIPs vs Effect Size")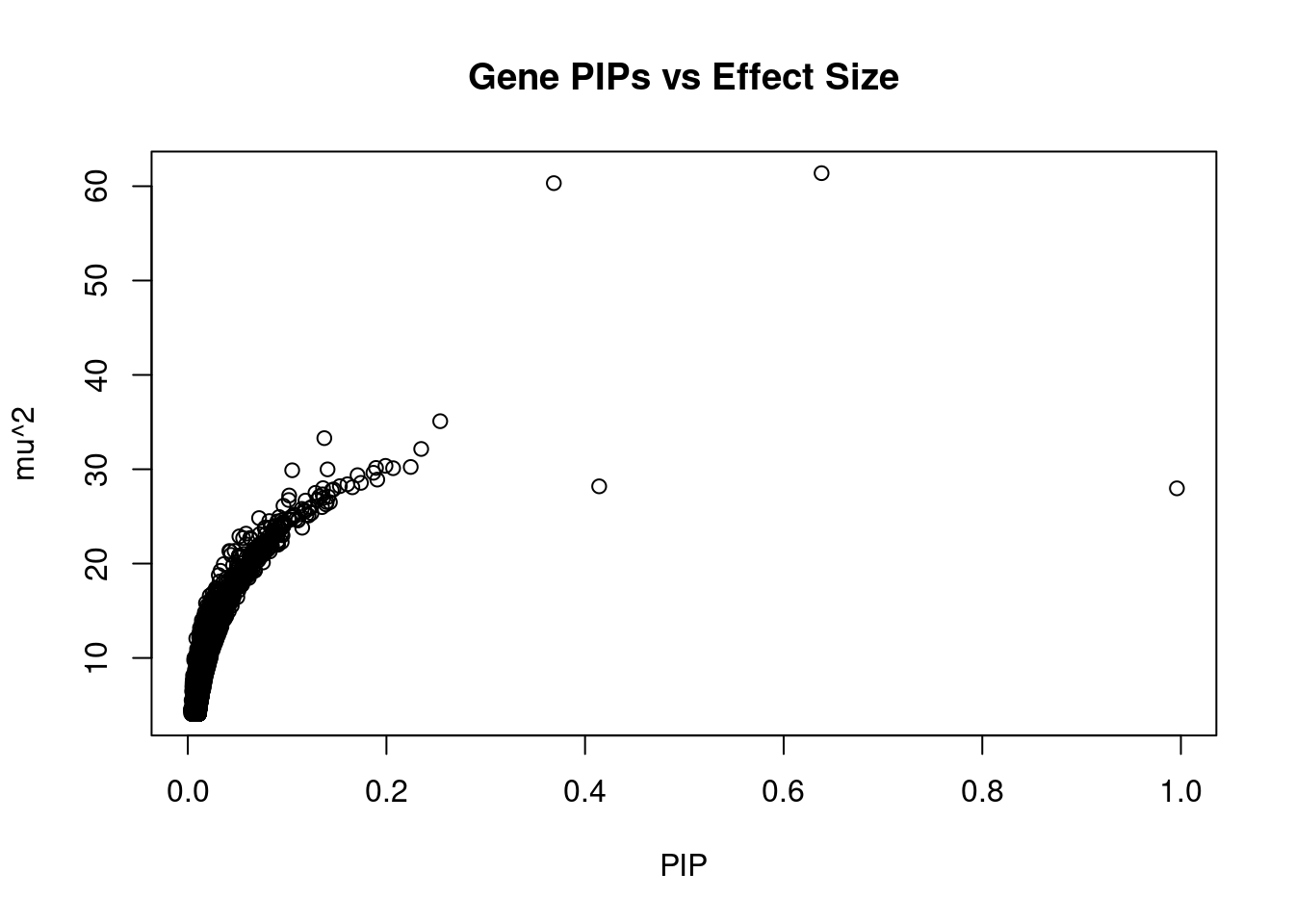
#genes with 20 largest effect sizes
head(ctwas_gene_res[order(-ctwas_gene_res$mu2),report_cols],20) genename region_tag susie_pip mu2 PVE z
2240 SEC23IP 10_74 0.6381754 61.38049 1.161811e-04 -3.610724
10283 MCMBP 10_74 0.3685673 60.32290 6.594232e-05 3.522402
703 GUCY2C 12_12 0.2541535 35.09246 2.645301e-05 3.878767
8349 GPHN 14_32 0.1374525 33.29520 1.357374e-05 -3.426575
6307 NUS1 6_78 0.2350043 32.14897 2.240826e-05 3.716370
6558 AP3S2 15_41 0.1990701 30.35974 1.792543e-05 -3.745658
12541 RP6-65G23.5 14_33 0.2244479 30.24036 2.013111e-05 3.369949
10118 RABL6 9_74 0.1894969 30.14016 1.693998e-05 3.491221
5911 CIZ1 9_66 0.2067132 30.11029 1.846071e-05 -3.513905
5073 ETNK1 12_16 0.1407134 29.98608 1.251470e-05 3.169725
7288 AGGF1 5_45 0.1050999 29.89108 9.317706e-06 -3.154473
2577 GNPTAB 12_61 0.1868989 29.62473 1.642201e-05 3.600816
9318 LIPF 10_56 0.1709291 29.37002 1.488969e-05 -2.991710
7641 NDEL1 17_8 0.1907755 28.90161 1.635346e-05 -3.136833
4089 UBAC1 9_72 0.1742949 28.55651 1.476233e-05 3.438703
12123 UPK3BL 7_63 0.1607226 28.40836 1.354217e-05 3.134982
1624 TPD52L2 20_38 0.1531083 28.20869 1.280993e-05 -3.090905
12661 LINC01126 2_27 0.4142141 28.18774 3.462982e-05 4.620415
12431 RP11-535A5.1 18_11 0.1656953 28.08816 1.380381e-05 -2.997627
1460 PPP6R2 22_24 0.1361427 27.99436 1.130395e-05 -3.283527
num_eqtl
2240 1
10283 1
703 1
8349 2
6307 1
6558 1
12541 1
10118 1
5911 2
5073 1
7288 2
2577 1
9318 1
7641 1
4089 1
12123 1
1624 1
12661 1
12431 1
1460 1Genes with highest PVE
#genes with 20 highest pve
head(ctwas_gene_res[order(-ctwas_gene_res$PVE),report_cols],20) genename region_tag susie_pip mu2 PVE z
2240 SEC23IP 10_74 0.6381754 61.38049 1.161811e-04 -3.610724
3212 CCND2 12_4 0.9959793 27.97577 8.264138e-05 5.657050
10283 MCMBP 10_74 0.3685673 60.32290 6.594232e-05 3.522402
12661 LINC01126 2_27 0.4142141 28.18774 3.462982e-05 4.620415
703 GUCY2C 12_12 0.2541535 35.09246 2.645301e-05 3.878767
6307 NUS1 6_78 0.2350043 32.14897 2.240826e-05 3.716370
12541 RP6-65G23.5 14_33 0.2244479 30.24036 2.013111e-05 3.369949
5911 CIZ1 9_66 0.2067132 30.11029 1.846071e-05 -3.513905
6558 AP3S2 15_41 0.1990701 30.35974 1.792543e-05 -3.745658
10118 RABL6 9_74 0.1894969 30.14016 1.693998e-05 3.491221
2577 GNPTAB 12_61 0.1868989 29.62473 1.642201e-05 3.600816
7641 NDEL1 17_8 0.1907755 28.90161 1.635346e-05 -3.136833
9318 LIPF 10_56 0.1709291 29.37002 1.488969e-05 -2.991710
4089 UBAC1 9_72 0.1742949 28.55651 1.476233e-05 3.438703
12431 RP11-535A5.1 18_11 0.1656953 28.08816 1.380381e-05 -2.997627
8349 GPHN 14_32 0.1374525 33.29520 1.357374e-05 -3.426575
12123 UPK3BL 7_63 0.1607226 28.40836 1.354217e-05 3.134982
1624 TPD52L2 20_38 0.1531083 28.20869 1.280993e-05 -3.090905
5073 ETNK1 12_16 0.1407134 29.98608 1.251470e-05 3.169725
1483 RPL3 22_16 0.1466952 27.86306 1.212300e-05 3.284537
num_eqtl
2240 1
3212 1
10283 1
12661 1
703 1
6307 1
12541 1
5911 2
6558 1
10118 1
2577 1
7641 1
9318 1
4089 1
12431 1
8349 2
12123 1
1624 1
5073 1
1483 1Genes with largest z scores
#genes with 20 largest z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
3212 CCND2 12_4 0.99597929 27.97577 8.264138e-05 5.657050
12661 LINC01126 2_27 0.41421408 28.18774 3.462982e-05 4.620415
703 GUCY2C 12_12 0.25415347 35.09246 2.645301e-05 3.878767
6558 AP3S2 15_41 0.19907014 30.35974 1.792543e-05 -3.745658
6307 NUS1 6_78 0.23500434 32.14897 2.240826e-05 3.716370
2240 SEC23IP 10_74 0.63817542 61.38049 1.161811e-04 -3.610724
2577 GNPTAB 12_61 0.18689890 29.62473 1.642201e-05 3.600816
10283 MCMBP 10_74 0.36856732 60.32290 6.594232e-05 3.522402
1505 RBX1 22_17 0.13906601 26.28028 1.083967e-05 -3.521311
5911 CIZ1 9_66 0.20671321 30.11029 1.846071e-05 -3.513905
10118 RABL6 9_74 0.18949686 30.14016 1.693998e-05 3.491221
4089 UBAC1 9_72 0.17429489 28.55651 1.476233e-05 3.438703
10840 PPP1CB 2_17 0.08612930 23.42382 5.983755e-06 3.433773
7172 SPDYA 2_17 0.08537626 23.34966 5.912661e-06 -3.429510
8349 GPHN 14_32 0.13745250 33.29520 1.357374e-05 -3.426575
5040 CNOT6L 4_52 0.13254813 27.11893 1.066133e-05 3.423769
12541 RP6-65G23.5 14_33 0.22444790 30.24036 2.013111e-05 3.369949
1483 RPL3 22_16 0.14669520 27.86306 1.212300e-05 3.284537
1460 PPP6R2 22_24 0.13614270 27.99436 1.130395e-05 -3.283527
2417 GLRB 4_101 0.13567594 27.33830 1.100119e-05 3.269896
num_eqtl
3212 1
12661 1
703 1
6558 1
6307 1
2240 1
2577 1
10283 1
1505 1
5911 2
10118 1
4089 1
10840 3
7172 2
8349 2
5040 1
12541 1
1483 1
1460 1
2417 1Comparing z scores and PIPs
#set nominal signifiance threshold for z scores
alpha <- 0.05
#bonferroni adjusted threshold for z scores
sig_thresh <- qnorm(1-(alpha/nrow(ctwas_gene_res)/2), lower=T)
#Q-Q plot for z scores
obs_z <- ctwas_gene_res$z[order(ctwas_gene_res$z)]
exp_z <- qnorm((1:nrow(ctwas_gene_res))/nrow(ctwas_gene_res))
plot(exp_z, obs_z, xlab="Expected z", ylab="Observed z", main="Gene z score Q-Q plot")
abline(a=0,b=1)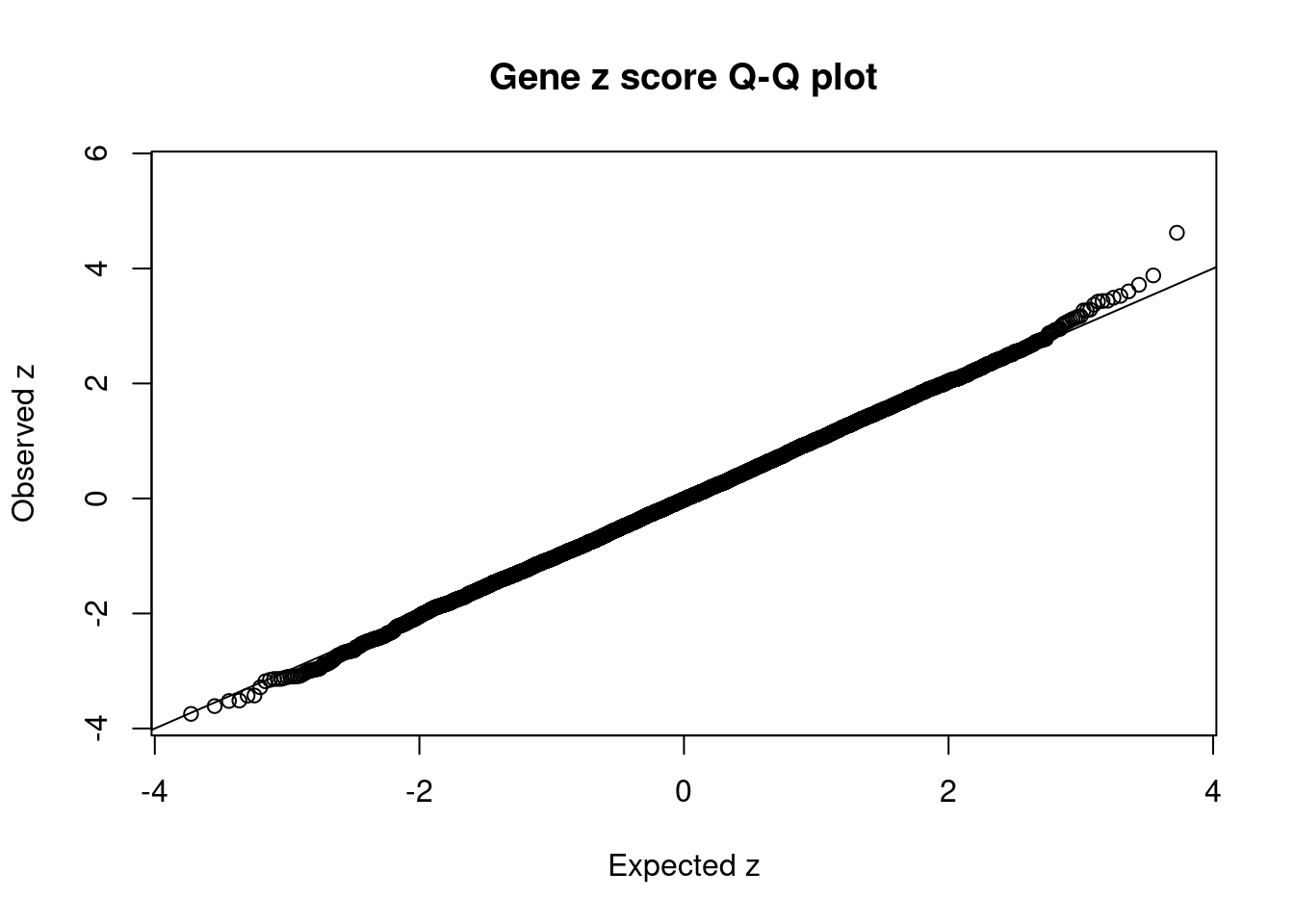
#plot z score vs PIP
plot(abs(ctwas_gene_res$z), ctwas_gene_res$susie_pip, xlab="abs(z)", ylab="PIP")
abline(v=sig_thresh, col="red", lty=2)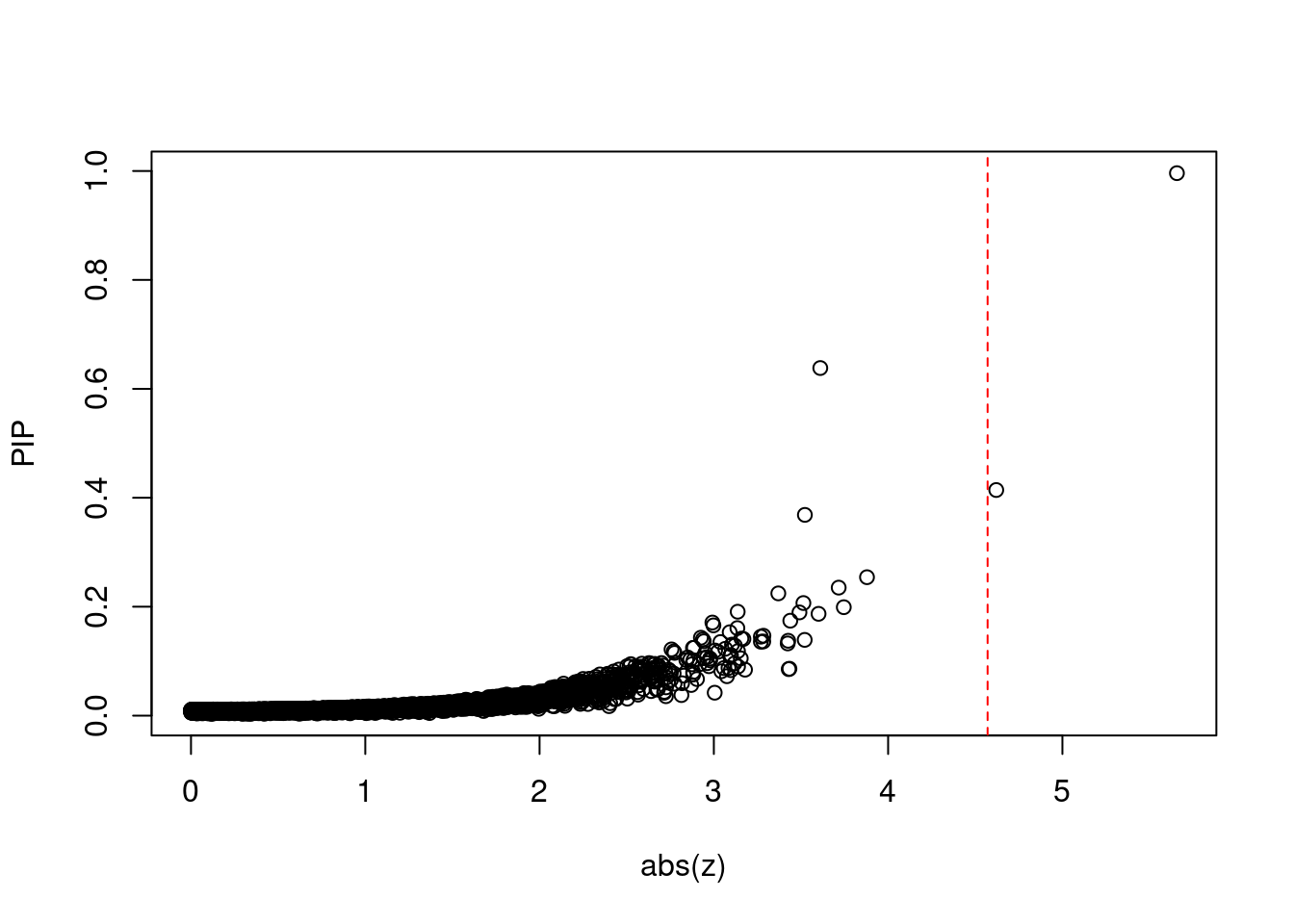
#proportion of significant z scores
mean(abs(ctwas_gene_res$z) > sig_thresh)[1] 0.0001943635#genes with most significant z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
3212 CCND2 12_4 0.99597929 27.97577 8.264138e-05 5.657050
12661 LINC01126 2_27 0.41421408 28.18774 3.462982e-05 4.620415
703 GUCY2C 12_12 0.25415347 35.09246 2.645301e-05 3.878767
6558 AP3S2 15_41 0.19907014 30.35974 1.792543e-05 -3.745658
6307 NUS1 6_78 0.23500434 32.14897 2.240826e-05 3.716370
2240 SEC23IP 10_74 0.63817542 61.38049 1.161811e-04 -3.610724
2577 GNPTAB 12_61 0.18689890 29.62473 1.642201e-05 3.600816
10283 MCMBP 10_74 0.36856732 60.32290 6.594232e-05 3.522402
1505 RBX1 22_17 0.13906601 26.28028 1.083967e-05 -3.521311
5911 CIZ1 9_66 0.20671321 30.11029 1.846071e-05 -3.513905
10118 RABL6 9_74 0.18949686 30.14016 1.693998e-05 3.491221
4089 UBAC1 9_72 0.17429489 28.55651 1.476233e-05 3.438703
10840 PPP1CB 2_17 0.08612930 23.42382 5.983755e-06 3.433773
7172 SPDYA 2_17 0.08537626 23.34966 5.912661e-06 -3.429510
8349 GPHN 14_32 0.13745250 33.29520 1.357374e-05 -3.426575
5040 CNOT6L 4_52 0.13254813 27.11893 1.066133e-05 3.423769
12541 RP6-65G23.5 14_33 0.22444790 30.24036 2.013111e-05 3.369949
1483 RPL3 22_16 0.14669520 27.86306 1.212300e-05 3.284537
1460 PPP6R2 22_24 0.13614270 27.99436 1.130395e-05 -3.283527
2417 GLRB 4_101 0.13567594 27.33830 1.100119e-05 3.269896
num_eqtl
3212 1
12661 1
703 1
6558 1
6307 1
2240 1
2577 1
10283 1
1505 1
5911 2
10118 1
4089 1
10840 3
7172 2
8349 2
5040 1
12541 1
1483 1
1460 1
2417 1Sensitivity, specificity and precision for silver standard genes
library("readxl")
known_annotations <- read_xlsx("data/summary_known_genes_annotations.xlsx", sheet="T2D")
known_annotations <- unique(known_annotations$`Gene Symbol`)
unrelated_genes <- ctwas_gene_res$genename[!(ctwas_gene_res$genename %in% known_annotations)]
#number of genes in known annotations
print(length(known_annotations))[1] 72#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 33#assign ctwas, TWAS, and bystander genes
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>sig_thresh]
novel_genes <- ctwas_genes[!(ctwas_genes %in% twas_genes)]
#significance threshold for TWAS
print(sig_thresh)[1] 4.570782#number of ctwas genes
length(ctwas_genes)[1] 1#number of TWAS genes
length(twas_genes)[1] 2#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols][1] genename region_tag susie_pip mu2 PVE z num_eqtl
<0 rows> (or 0-length row.names)#sensitivity / recall
sensitivity <- rep(NA,2)
names(sensitivity) <- c("ctwas", "TWAS")
sensitivity["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
sensitivity["TWAS"] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
sensitivityctwas TWAS
0 0 #specificity
specificity <- rep(NA,2)
names(specificity) <- c("ctwas", "TWAS")
specificity["ctwas"] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
specificity["TWAS"] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
specificity ctwas TWAS
0.9999025 0.9998050 #precision / PPV
precision <- rep(NA,2)
names(precision) <- c("ctwas", "TWAS")
precision["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
precision["TWAS"] <- sum(twas_genes %in% known_annotations)/length(twas_genes)
precisionctwas TWAS
0 0 #ROC curves
pip_range <- (0:1000)/1000
sensitivity <- rep(NA, length(pip_range))
specificity <- rep(NA, length(pip_range))
for (index in 1:length(pip_range)){
pip <- pip_range[index]
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>=pip]
sensitivity[index] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
}
plot(1-specificity, sensitivity, type="l", xlim=c(0,1), ylim=c(0,1))
sig_thresh_range <- seq(from=0, to=max(abs(ctwas_gene_res$z)), length.out=length(pip_range))
for (index in 1:length(sig_thresh_range)){
sig_thresh_plot <- sig_thresh_range[index]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>=sig_thresh_plot]
sensitivity[index] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
}
lines(1-specificity, sensitivity, xlim=c(0,1), ylim=c(0,1), col="red", lty=2)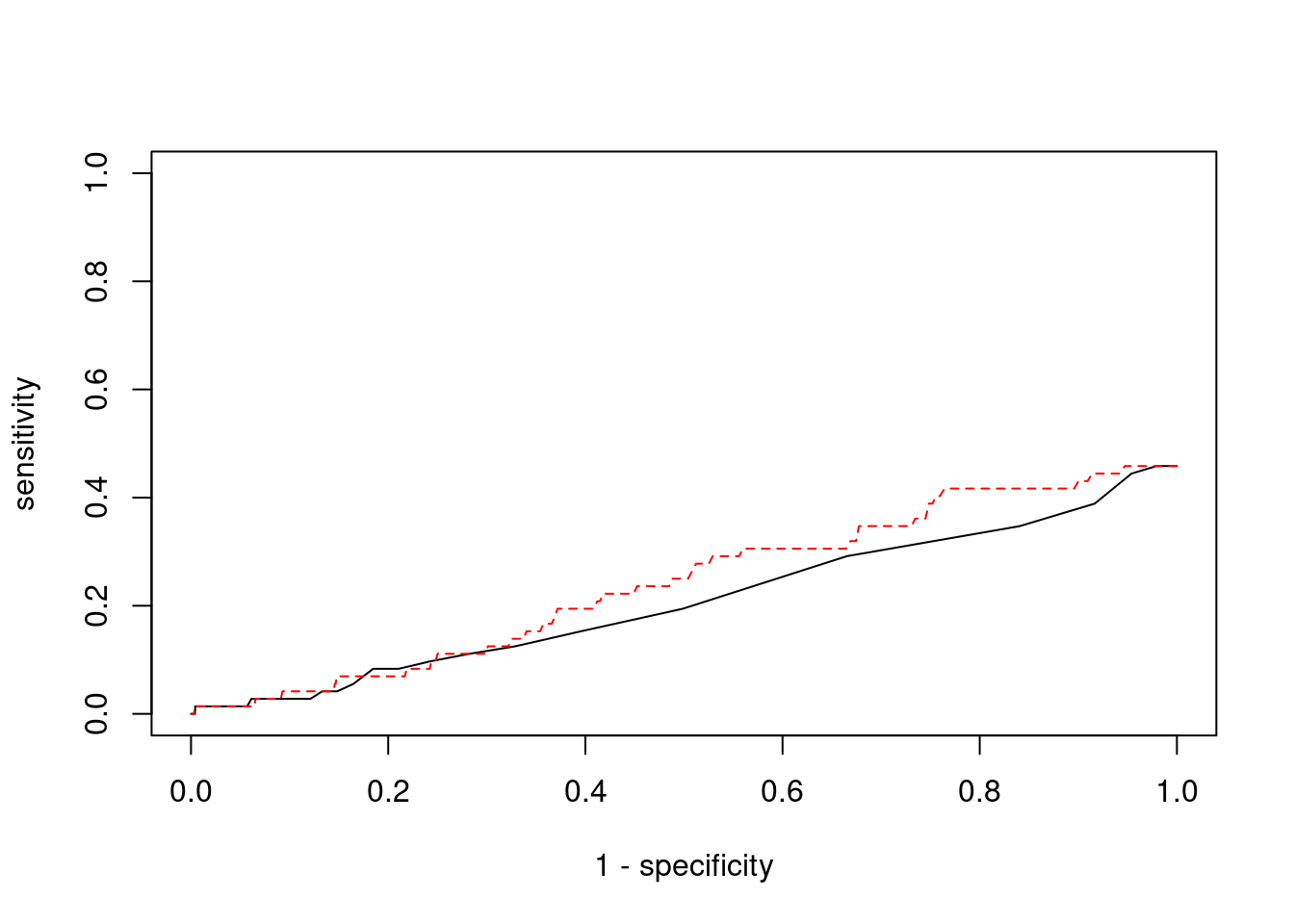
Sensitivity, specificity and precision for silver standard genes - bystanders only
This section first uses all silver standard genes to identify bystander genes within 1Mb. The silver standard and bystander gene lists are then subset to only genes with imputed expression in this analysis. Then, the ctwas and TWAS gene lists from this analysis are subset to only genes that are in the (subset) silver standard and bystander genes. These gene lists are then used to compute sensitivity, specificity and precision for ctwas and TWAS.
library(biomaRt)
library(GenomicRanges)Loading required package: stats4Loading required package: BiocGenericsLoading required package: parallel
Attaching package: 'BiocGenerics'The following objects are masked from 'package:parallel':
clusterApply, clusterApplyLB, clusterCall, clusterEvalQ,
clusterExport, clusterMap, parApply, parCapply, parLapply,
parLapplyLB, parRapply, parSapply, parSapplyLBThe following objects are masked from 'package:stats':
IQR, mad, sd, var, xtabsThe following objects are masked from 'package:base':
anyDuplicated, append, as.data.frame, basename, cbind, colnames,
dirname, do.call, duplicated, eval, evalq, Filter, Find, get, grep,
grepl, intersect, is.unsorted, lapply, Map, mapply, match, mget,
order, paste, pmax, pmax.int, pmin, pmin.int, Position, rank,
rbind, Reduce, rownames, sapply, setdiff, sort, table, tapply,
union, unique, unsplit, which, which.max, which.minLoading required package: S4Vectors
Attaching package: 'S4Vectors'The following object is masked from 'package:base':
expand.gridLoading required package: IRangesLoading required package: GenomeInfoDbensembl <- useEnsembl(biomart="ENSEMBL_MART_ENSEMBL", dataset="hsapiens_gene_ensembl")
G_list <- getBM(filters= "chromosome_name", attributes= c("hgnc_symbol","chromosome_name","start_position","end_position","gene_biotype"), values=1:22, mart=ensembl)
G_list <- G_list[G_list$hgnc_symbol!="",]
G_list <- G_list[G_list$gene_biotype %in% c("protein_coding","lncRNA"),]
G_list$start <- G_list$start_position
G_list$end <- G_list$end_position
G_list_granges <- makeGRangesFromDataFrame(G_list, keep.extra.columns=T)
known_annotations_positions <- G_list[G_list$hgnc_symbol %in% known_annotations,]
half_window <- 1000000
known_annotations_positions$start <- known_annotations_positions$start_position - half_window
known_annotations_positions$end <- known_annotations_positions$end_position + half_window
known_annotations_positions$start[known_annotations_positions$start<1] <- 1
known_annotations_granges <- makeGRangesFromDataFrame(known_annotations_positions, keep.extra.columns=T)
bystanders <- findOverlaps(known_annotations_granges,G_list_granges)
bystanders <- unique(subjectHits(bystanders))
bystanders <- G_list$hgnc_symbol[bystanders]
bystanders <- bystanders[!(bystanders %in% known_annotations)]
unrelated_genes <- bystanders
#number of genes in known annotations
print(length(known_annotations))[1] 72#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 33#number of bystander genes
print(length(unrelated_genes))[1] 1847#number of bystander genes with imputed expression
print(sum(unrelated_genes %in% ctwas_gene_res$genename))[1] 799#remove genes without imputed expression from gene lists
known_annotations <- known_annotations[known_annotations %in% ctwas_gene_res$genename]
unrelated_genes <- unrelated_genes[unrelated_genes %in% ctwas_gene_res$genename]
#assign ctwas and TWAS genes
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>sig_thresh]
#significance threshold for TWAS
print(sig_thresh)[1] 4.570782#number of ctwas genes
length(ctwas_genes)[1] 1#number of ctwas genes in known annotations or bystanders
sum(ctwas_genes %in% c(known_annotations, unrelated_genes))[1] 0#number of ctwas genes
length(twas_genes)[1] 2#number of TWAS genes
sum(twas_genes %in% c(known_annotations, unrelated_genes))[1] 0#remove genes not in known or bystander lists from results
ctwas_genes <- ctwas_genes[ctwas_genes %in% c(known_annotations, unrelated_genes)]
twas_genes <- twas_genes[twas_genes %in% c(known_annotations, unrelated_genes)]
#sensitivity / recall
sensitivity <- rep(NA,2)
names(sensitivity) <- c("ctwas", "TWAS")
sensitivity["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
sensitivity["TWAS"] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
sensitivityctwas TWAS
0 0 #specificity
specificity <- rep(NA,2)
names(specificity) <- c("ctwas", "TWAS")
specificity["ctwas"] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
specificity["TWAS"] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
specificityctwas TWAS
1 1 #precision / PPV
precision <- rep(NA,2)
names(precision) <- c("ctwas", "TWAS")
precision["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
precision["TWAS"] <- sum(twas_genes %in% known_annotations)/length(twas_genes)
precisionctwas TWAS
NaN NaN
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] parallel stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] GenomicRanges_1.36.1 GenomeInfoDb_1.20.0 IRanges_2.18.1
[4] S4Vectors_0.22.1 BiocGenerics_0.30.0 biomaRt_2.40.1
[7] readxl_1.3.1 cowplot_1.0.0 ggplot2_3.3.5
[10] workflowr_1.6.2
loaded via a namespace (and not attached):
[1] Rcpp_1.0.7 prettyunits_1.1.1 assertthat_0.2.1
[4] rprojroot_2.0.2 digest_0.6.29 utf8_1.2.2
[7] R6_2.5.1 cellranger_1.1.0 RSQLite_2.2.8
[10] evaluate_0.14 httr_1.4.2 highr_0.9
[13] pillar_1.6.4 zlibbioc_1.30.0 progress_1.2.2
[16] rlang_0.4.12 curl_4.3.2 data.table_1.14.2
[19] whisker_0.3-2 jquerylib_0.1.4 blob_1.2.2
[22] rmarkdown_2.11 labeling_0.4.2 stringr_1.4.0
[25] RCurl_1.98-1.5 bit_4.0.4 munsell_0.5.0
[28] compiler_3.6.1 httpuv_1.5.1 xfun_0.29
[31] pkgconfig_2.0.3 htmltools_0.5.2 tidyselect_1.1.1
[34] GenomeInfoDbData_1.2.1 tibble_3.1.6 XML_3.99-0.3
[37] fansi_0.5.0 crayon_1.4.2 dplyr_1.0.7
[40] withr_2.4.3 later_0.8.0 bitops_1.0-7
[43] grid_3.6.1 gtable_0.3.0 lifecycle_1.0.1
[46] DBI_1.1.1 git2r_0.26.1 magrittr_2.0.1
[49] scales_1.1.1 stringi_1.7.6 cachem_1.0.6
[52] XVector_0.24.0 farver_2.1.0 fs_1.5.2
[55] promises_1.0.1 ellipsis_0.3.2 generics_0.1.1
[58] vctrs_0.3.8 tools_3.6.1 bit64_4.0.5
[61] Biobase_2.44.0 glue_1.5.1 purrr_0.3.4
[64] hms_1.1.1 fastmap_1.1.0 yaml_2.2.1
[67] AnnotationDbi_1.46.0 colorspace_2.0-2 memoise_2.0.1
[70] knitr_1.36