Autism - Brain Substantia nigra
sheng Qian
2021-2-6
Last updated: 2022-02-27
Checks: 6 1
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 3dd5b4c. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .ipynb_checkpoints/
Ignored: data/AF/
Untracked files:
Untracked: Rplot.png
Untracked: analysis/.ipynb_checkpoints/
Untracked: analysis/Autism_Brain_Amygdala.Rmd
Untracked: analysis/Autism_Brain_Anterior_cingulate_cortex_BA24.Rmd
Untracked: analysis/Autism_Brain_Caudate_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Cerebellar_Hemisphere.Rmd
Untracked: analysis/Autism_Brain_Cerebellum.Rmd
Untracked: analysis/Autism_Brain_Cortex.Rmd
Untracked: analysis/Autism_Brain_Frontal_Cortex_BA9.Rmd
Untracked: analysis/Autism_Brain_Hippocampus.Rmd
Untracked: analysis/Autism_Brain_Hypothalamus.Rmd
Untracked: analysis/Autism_Brain_Nucleus_accumbens_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Putamen_basal_ganglia.Rmd
Untracked: analysis/Autism_Brain_Spinal_cord_cervical_c-1.Rmd
Untracked: analysis/Autism_Brain_Substantia_nigra.Rmd
Untracked: analysis/Glucose_Adipose_Subcutaneous.Rmd
Untracked: analysis/Glucose_Adipose_Visceral_Omentum.Rmd
Untracked: analysis/Splicing_Test.Rmd
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: data/.ipynb_checkpoints/
Untracked: data/Autism/
Untracked: data/BMI/
Untracked: data/BMI_S/
Untracked: data/Glucose/
Untracked: data/LDL_S/
Untracked: data/SCZ/
Untracked: data/T2D/
Untracked: data/TEST/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Unstaged changes:
Modified: analysis/BMI_Brain_Amygdala_S.Rmd
Modified: analysis/BMI_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/BMI_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/BMI_Brain_Cerebellum_S.Rmd
Modified: analysis/BMI_Brain_Cortex.Rmd
Modified: analysis/BMI_Brain_Cortex_S.Rmd
Modified: analysis/BMI_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/BMI_Brain_Hippocampus_S.Rmd
Modified: analysis/BMI_Brain_Hypothalamus_S.Rmd
Modified: analysis/BMI_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/BMI_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/BMI_Brain_Substantia_nigra_S.Rmd
Modified: analysis/LDL_Liver_S.Rmd
Modified: analysis/index.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_Brain_Substantia_nigra.Rmd) and HTML (docs/SCZ_Brain_Substantia_nigra.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 3dd5b4c | sq-96 | 2022-02-27 | update |
Weight QC
#number of imputed weights
nrow(qclist_all)[1] 10096#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
970 724 606 388 494 522 474 399 398 404 602 577 222 334 344 461 597 153 768 314
21 22
112 233 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 8298#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8219Check convergence of parameters
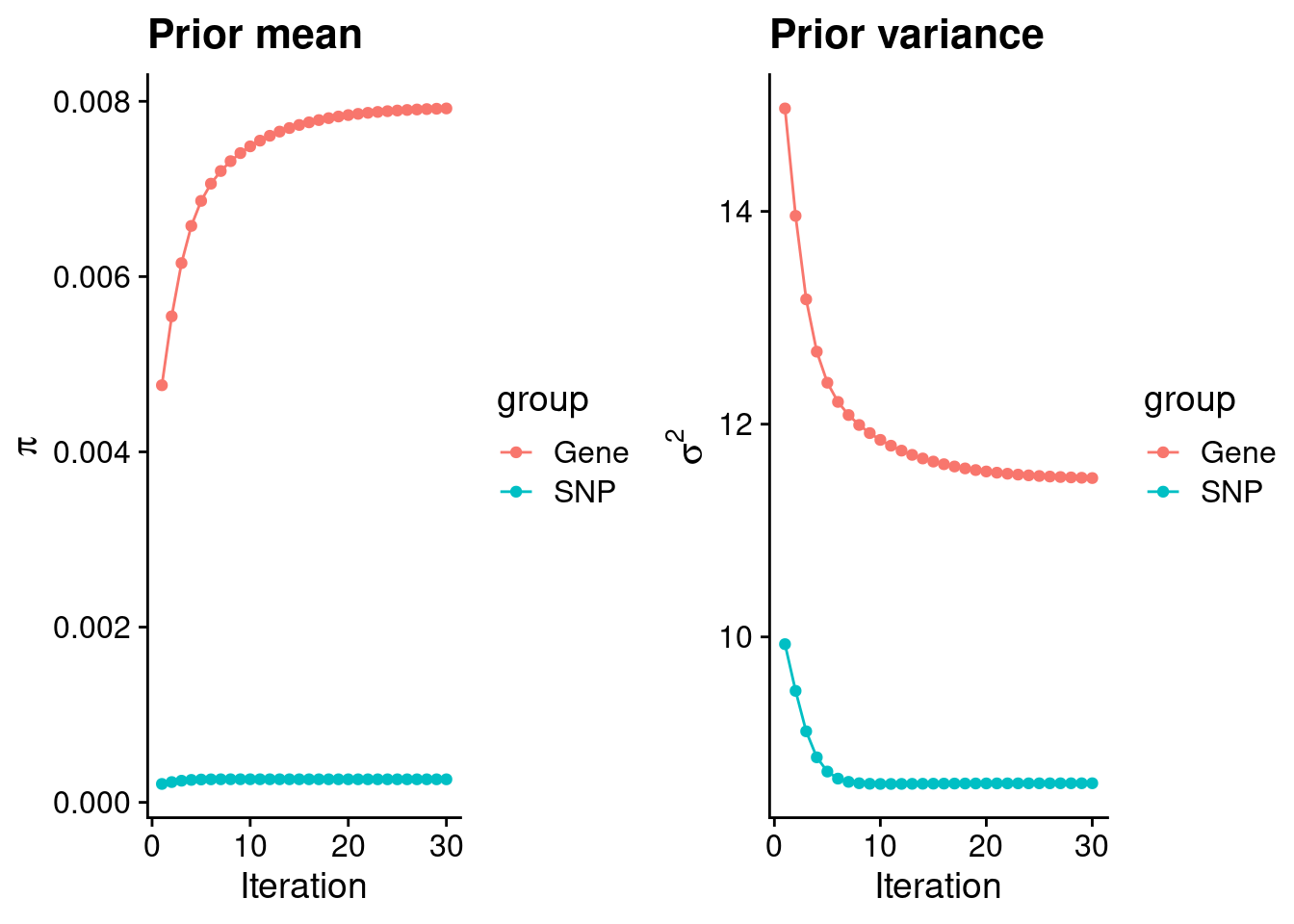
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0079186 0.0002633 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
11.493 8.624 #report sample size
print(sample_size)[1] 82315#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 10096 7573890#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.01116 0.20896 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.04909 1.56331Genes with highest PIPs
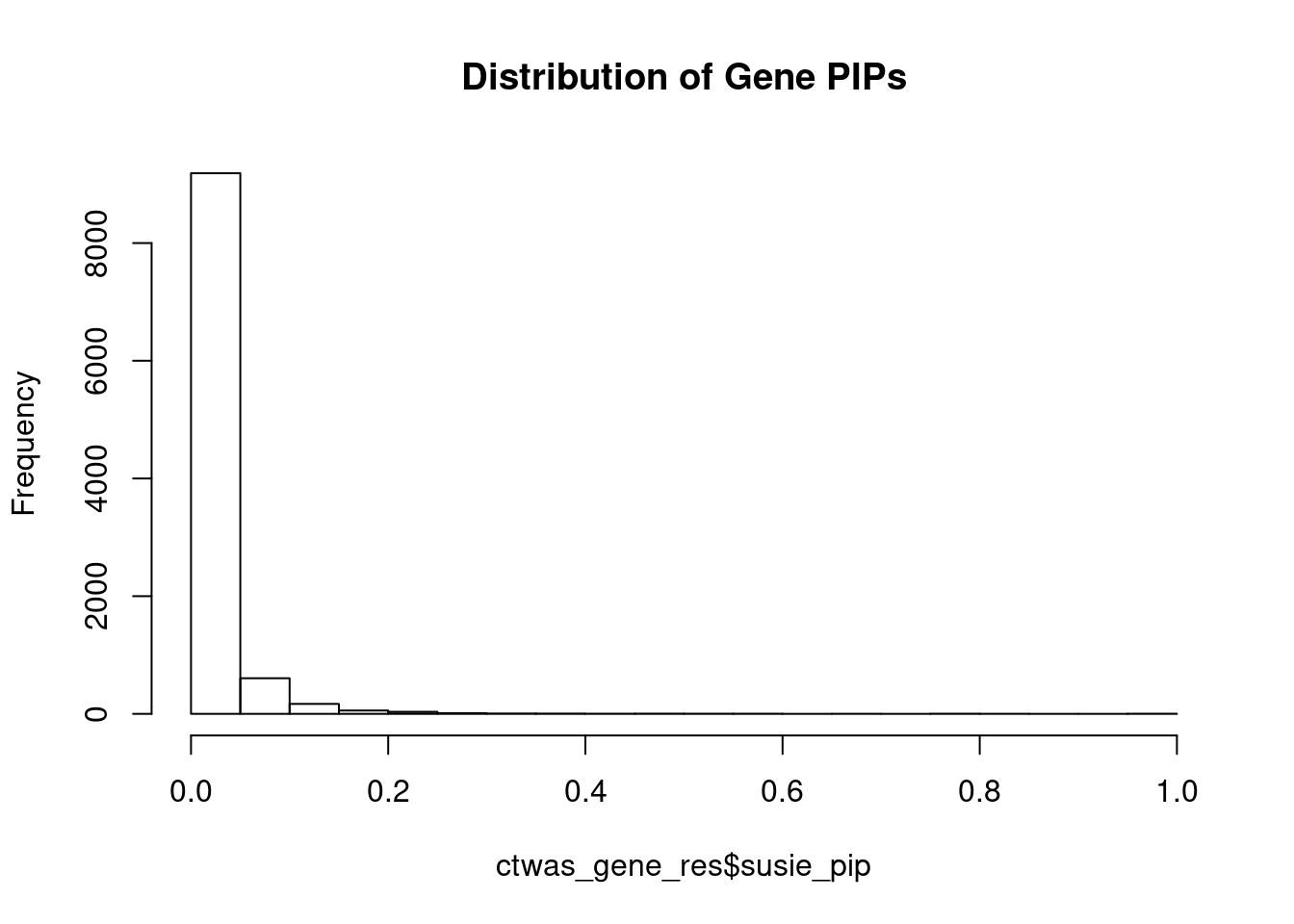
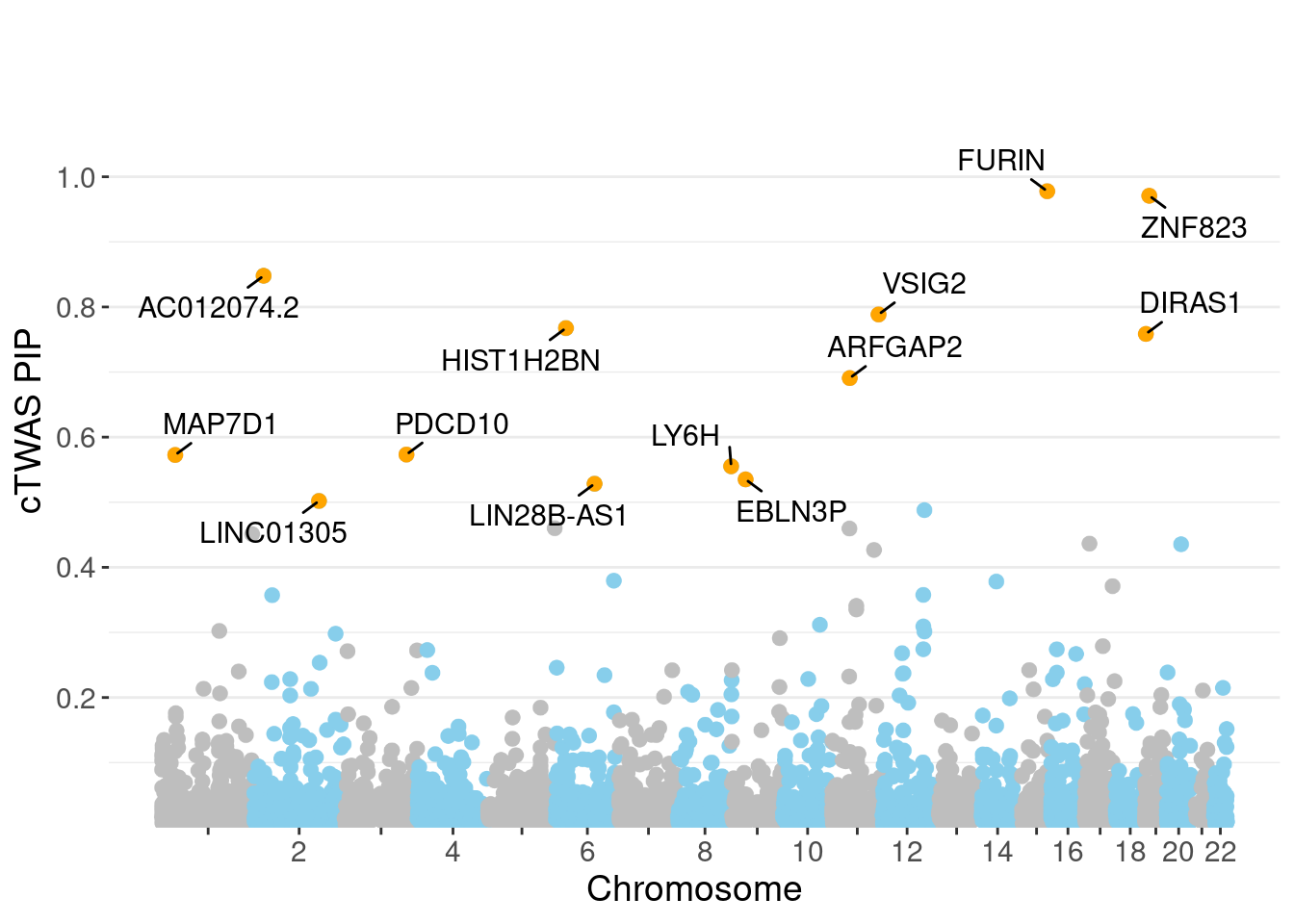
genename region_tag susie_pip mu2 PVE z num_eqtl
4961 FURIN 15_42 0.9778 46.16 0.0005483 -7.000 1
10000 ZNF823 19_10 0.9709 29.75 0.0003509 5.455 1
11036 AC012074.2 2_15 0.8479 22.88 0.0002357 4.620 2
247 VSIG2 11_77 0.7885 26.23 0.0002512 -3.818 1
10995 HIST1H2BN 6_21 0.7676 94.50 0.0008812 10.773 1
8494 DIRAS1 19_3 0.7586 25.35 0.0002337 4.867 2
5687 ARFGAP2 11_29 0.6907 24.40 0.0002047 4.740 1
2668 PDCD10 3_103 0.5732 21.86 0.0001522 -4.033 2
2900 MAP7D1 1_22 0.5726 24.30 0.0001691 4.907 1
8526 LY6H 8_94 0.5552 21.43 0.0001446 4.042 2
12597 EBLN3P 9_28 0.5351 23.06 0.0001499 -4.450 1
10218 LIN28B-AS1 6_70 0.5284 23.56 0.0001512 -4.651 1
10923 LINC01305 2_105 0.5020 23.25 0.0001418 4.523 1
5807 PLBD2 12_68 0.4878 21.18 0.0001255 3.986 1
8611 ZNF354C 5_108 0.4600 24.48 0.0001368 -3.965 1
2371 MDK 11_28 0.4596 39.12 0.0002184 -6.357 1
5246 CEP170 1_128 0.4510 25.69 0.0001408 -4.678 1
98 ELAC2 17_11 0.4364 30.58 0.0001622 4.227 1
1618 PPP1R16B 20_23 0.4354 35.67 0.0001887 6.091 1
11529 RP11-65M17.3 11_66 0.4267 22.40 0.0001161 4.330 1Genes with largest effect sizes
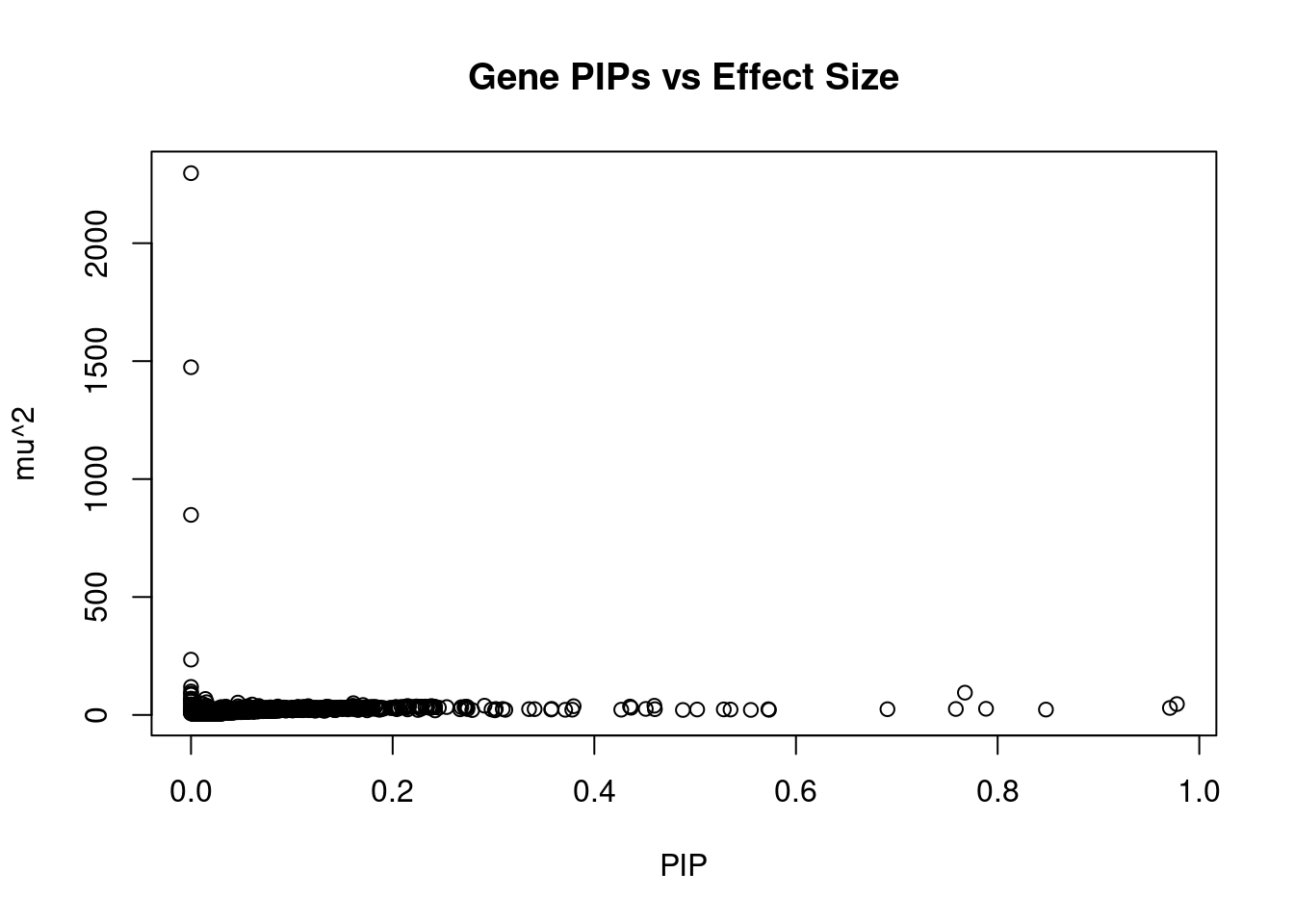
genename region_tag susie_pip mu2 PVE z num_eqtl
6375 ARHGAP27 17_27 0.000e+00 2296.94 0.000e+00 -2.0935 1
62 KMT2E 7_65 1.106e-05 1474.23 1.981e-07 -5.7816 2
8739 HLA-DQB1 6_26 5.596e-14 848.22 5.766e-16 4.1487 1
10292 HSPA1A 6_26 3.034e-13 234.54 8.645e-16 7.1259 1
8149 DCAKD 17_27 0.000e+00 119.02 0.000e+00 -2.8009 2
4321 SRPK2 7_65 0.000e+00 99.65 0.000e+00 -1.1604 1
10995 HIST1H2BN 6_21 7.676e-01 94.50 8.812e-04 10.7729 1
8901 ACBD4 17_27 0.000e+00 91.02 0.000e+00 0.1106 2
10526 CLIC1 6_26 2.818e-13 85.62 2.931e-16 0.4634 1
9454 HEXIM1 17_27 0.000e+00 69.44 0.000e+00 -2.8451 1
9418 BTN3A2 6_20 1.425e-02 67.98 1.177e-05 9.0770 3
2212 GOSR2 17_27 0.000e+00 67.64 0.000e+00 -2.5096 1
10868 SAPCD1 6_26 3.779e-12 64.23 2.949e-15 2.7814 1
10298 MSH5 6_26 5.906e-14 57.52 4.127e-17 0.7907 2
1217 PUS7 7_65 0.000e+00 56.76 0.000e+00 -2.8339 2
8834 HIST1H2BC 6_20 1.538e-02 54.00 1.009e-05 -8.0277 1
9552 ZSCAN23 6_22 4.653e-02 52.42 2.963e-05 -7.5541 2
4600 PGBD1 6_22 8.004e-03 50.72 4.932e-06 -6.3599 2
11951 LINC01415 18_30 1.610e-01 49.98 9.776e-05 -5.3243 1
4961 FURIN 15_42 9.778e-01 46.16 5.483e-04 -7.0004 1Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_eqtl
10995 HIST1H2BN 6_21 0.7676 94.50 0.0008812 10.773 1
4961 FURIN 15_42 0.9778 46.16 0.0005483 -7.000 1
10000 ZNF823 19_10 0.9709 29.75 0.0003509 5.455 1
247 VSIG2 11_77 0.7885 26.23 0.0002512 -3.818 1
11036 AC012074.2 2_15 0.8479 22.88 0.0002357 4.620 2
8494 DIRAS1 19_3 0.7586 25.35 0.0002337 4.867 2
2371 MDK 11_28 0.4596 39.12 0.0002184 -6.357 1
5687 ARFGAP2 11_29 0.6907 24.40 0.0002047 4.740 1
1618 PPP1R16B 20_23 0.4354 35.67 0.0001887 6.091 1
426 ARID1B 6_102 0.3795 37.06 0.0001709 -3.907 1
2900 MAP7D1 1_22 0.5726 24.30 0.0001691 4.907 1
98 ELAC2 17_11 0.4364 30.58 0.0001622 4.227 1
2668 PDCD10 3_103 0.5732 21.86 0.0001522 -4.033 2
10218 LIN28B-AS1 6_70 0.5284 23.56 0.0001512 -4.651 1
12597 EBLN3P 9_28 0.5351 23.06 0.0001499 -4.450 1
8526 LY6H 8_94 0.5552 21.43 0.0001446 4.042 2
10923 LINC01305 2_105 0.5020 23.25 0.0001418 4.523 1
5246 CEP170 1_128 0.4510 25.69 0.0001408 -4.678 1
12007 RP11-247A12.7 9_66 0.2911 39.33 0.0001391 4.243 2
8611 ZNF354C 5_108 0.4600 24.48 0.0001368 -3.965 1Genes with largest z scores
genename region_tag susie_pip mu2 PVE z num_eqtl
10995 HIST1H2BN 6_21 7.676e-01 94.50 8.812e-04 10.773 1
9418 BTN3A2 6_20 1.425e-02 67.98 1.177e-05 9.077 3
8834 HIST1H2BC 6_20 1.538e-02 54.00 1.009e-05 -8.028 1
9552 ZSCAN23 6_22 4.653e-02 52.42 2.963e-05 -7.554 2
2539 TRIM38 6_20 1.187e-02 45.99 6.630e-06 -7.478 2
6323 ZSCAN12 6_22 1.556e-02 39.73 7.511e-06 7.193 2
10292 HSPA1A 6_26 3.034e-13 234.54 8.645e-16 7.126 1
4961 FURIN 15_42 9.778e-01 46.16 5.483e-04 -7.000 1
5665 CYP17A1 10_66 4.682e-03 31.84 1.811e-06 -6.720 1
4600 PGBD1 6_22 8.004e-03 50.72 4.932e-06 -6.360 2
2371 MDK 11_28 4.596e-01 39.12 2.184e-04 -6.357 1
2778 KCNJ13 2_137 1.584e-01 35.34 6.800e-05 -6.333 1
1137 PPP1R13B 14_54 6.061e-02 44.26 3.259e-05 -6.297 1
8821 HARBI1 11_28 1.622e-01 36.44 7.181e-05 6.169 1
10439 DNAJC19 3_111 2.146e-01 37.89 9.879e-05 6.158 1
3921 C12orf65 12_75 3.691e-03 36.50 1.637e-06 -6.141 1
9960 NMB 15_39 1.706e-01 42.26 8.760e-05 6.132 1
9514 ZKSCAN4 6_22 1.111e-02 28.19 3.805e-06 -6.092 1
1618 PPP1R16B 20_23 4.354e-01 35.67 1.887e-04 6.091 1
10577 AS3MT 10_66 6.259e-03 31.85 2.422e-06 6.055 2Comparing z scores and PIPs
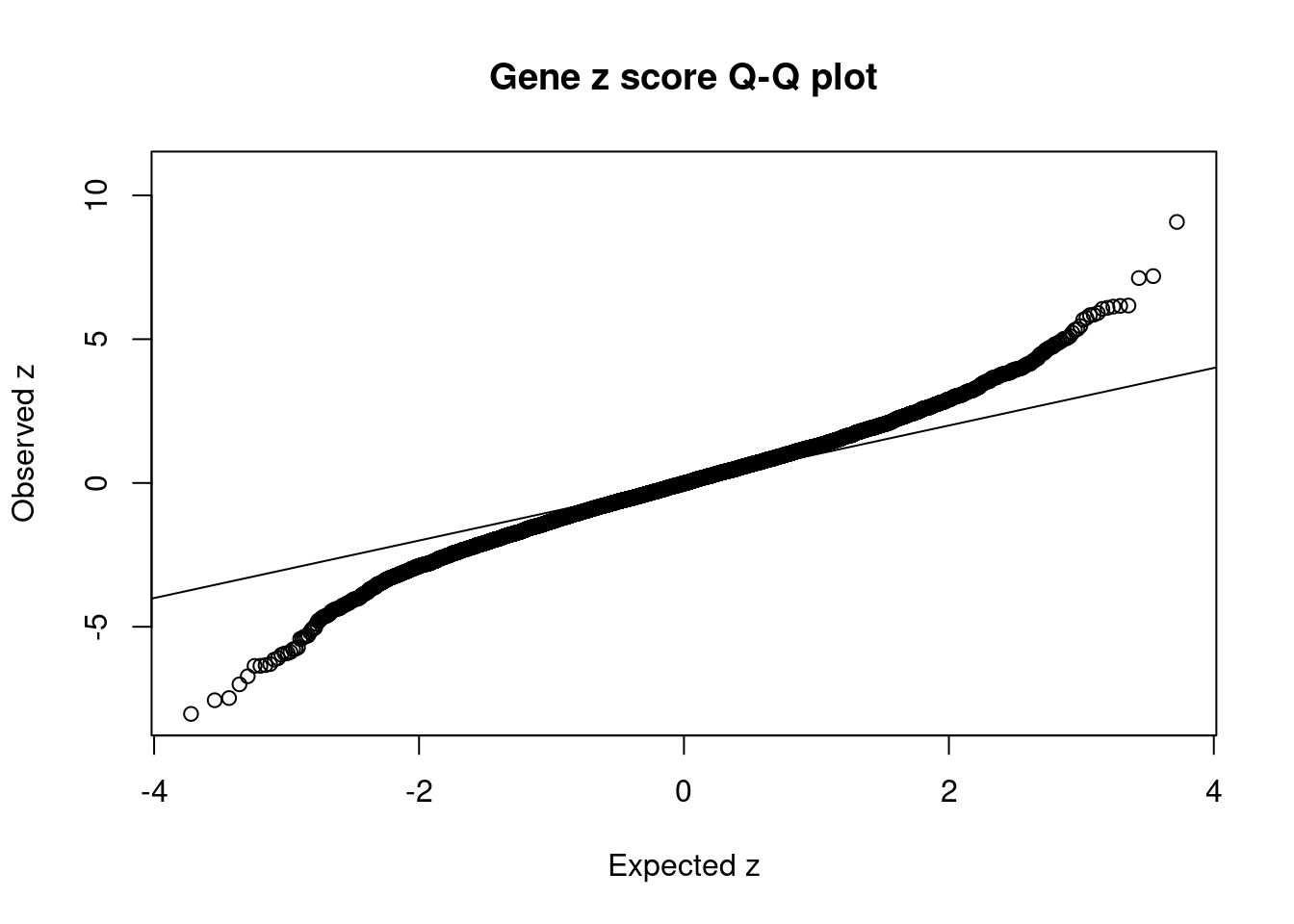
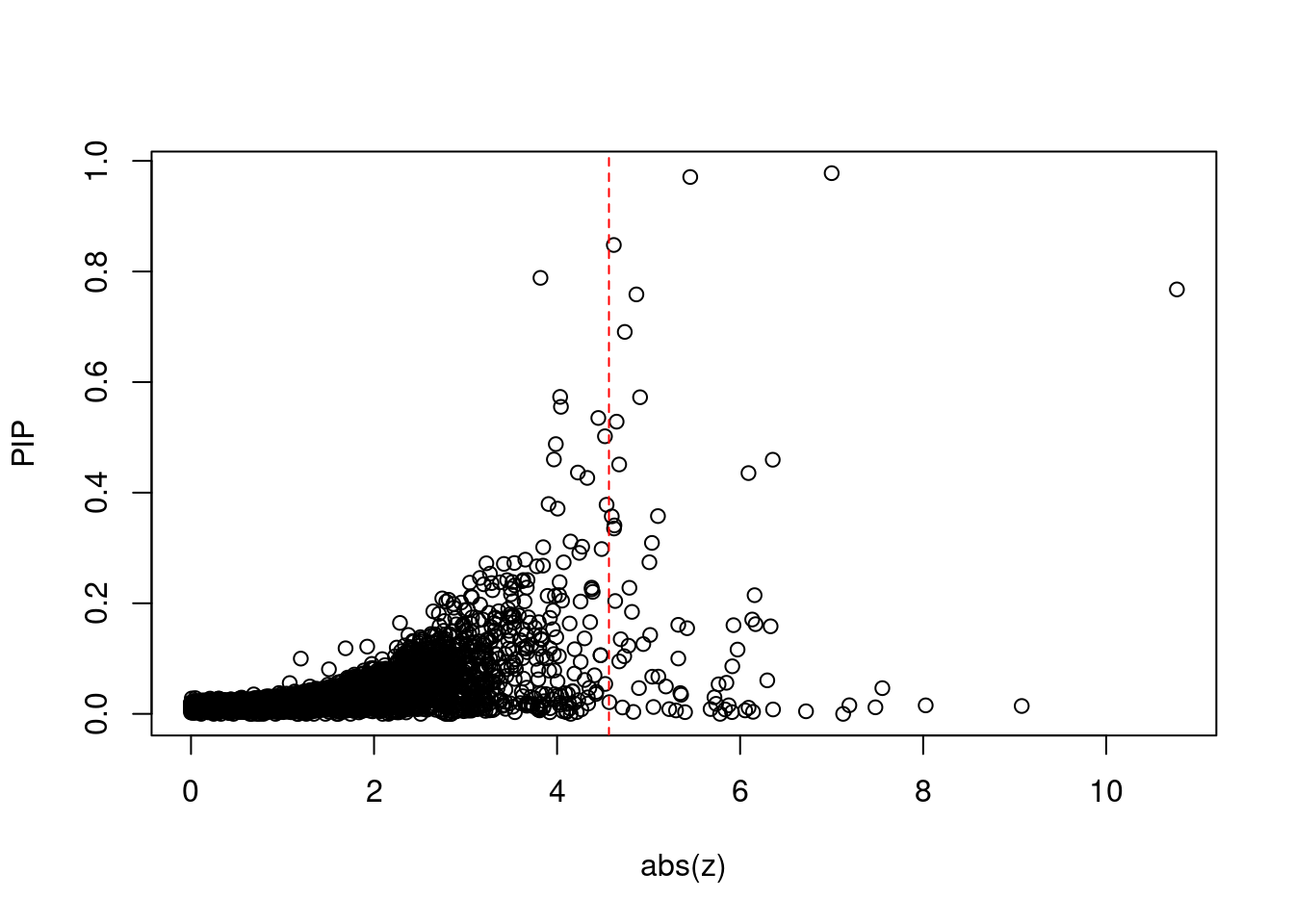
[1] 0.006933 genename region_tag susie_pip mu2 PVE z num_eqtl
10995 HIST1H2BN 6_21 7.676e-01 94.50 8.812e-04 10.773 1
9418 BTN3A2 6_20 1.425e-02 67.98 1.177e-05 9.077 3
8834 HIST1H2BC 6_20 1.538e-02 54.00 1.009e-05 -8.028 1
9552 ZSCAN23 6_22 4.653e-02 52.42 2.963e-05 -7.554 2
2539 TRIM38 6_20 1.187e-02 45.99 6.630e-06 -7.478 2
6323 ZSCAN12 6_22 1.556e-02 39.73 7.511e-06 7.193 2
10292 HSPA1A 6_26 3.034e-13 234.54 8.645e-16 7.126 1
4961 FURIN 15_42 9.778e-01 46.16 5.483e-04 -7.000 1
5665 CYP17A1 10_66 4.682e-03 31.84 1.811e-06 -6.720 1
4600 PGBD1 6_22 8.004e-03 50.72 4.932e-06 -6.360 2
2371 MDK 11_28 4.596e-01 39.12 2.184e-04 -6.357 1
2778 KCNJ13 2_137 1.584e-01 35.34 6.800e-05 -6.333 1
1137 PPP1R13B 14_54 6.061e-02 44.26 3.259e-05 -6.297 1
8821 HARBI1 11_28 1.622e-01 36.44 7.181e-05 6.169 1
10439 DNAJC19 3_111 2.146e-01 37.89 9.879e-05 6.158 1
3921 C12orf65 12_75 3.691e-03 36.50 1.637e-06 -6.141 1
9960 NMB 15_39 1.706e-01 42.26 8.760e-05 6.132 1
9514 ZKSCAN4 6_22 1.111e-02 28.19 3.805e-06 -6.092 1
1618 PPP1R16B 20_23 4.354e-01 35.67 1.887e-04 6.091 1
10577 AS3MT 10_66 6.259e-03 31.85 2.422e-06 6.055 2GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 13Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"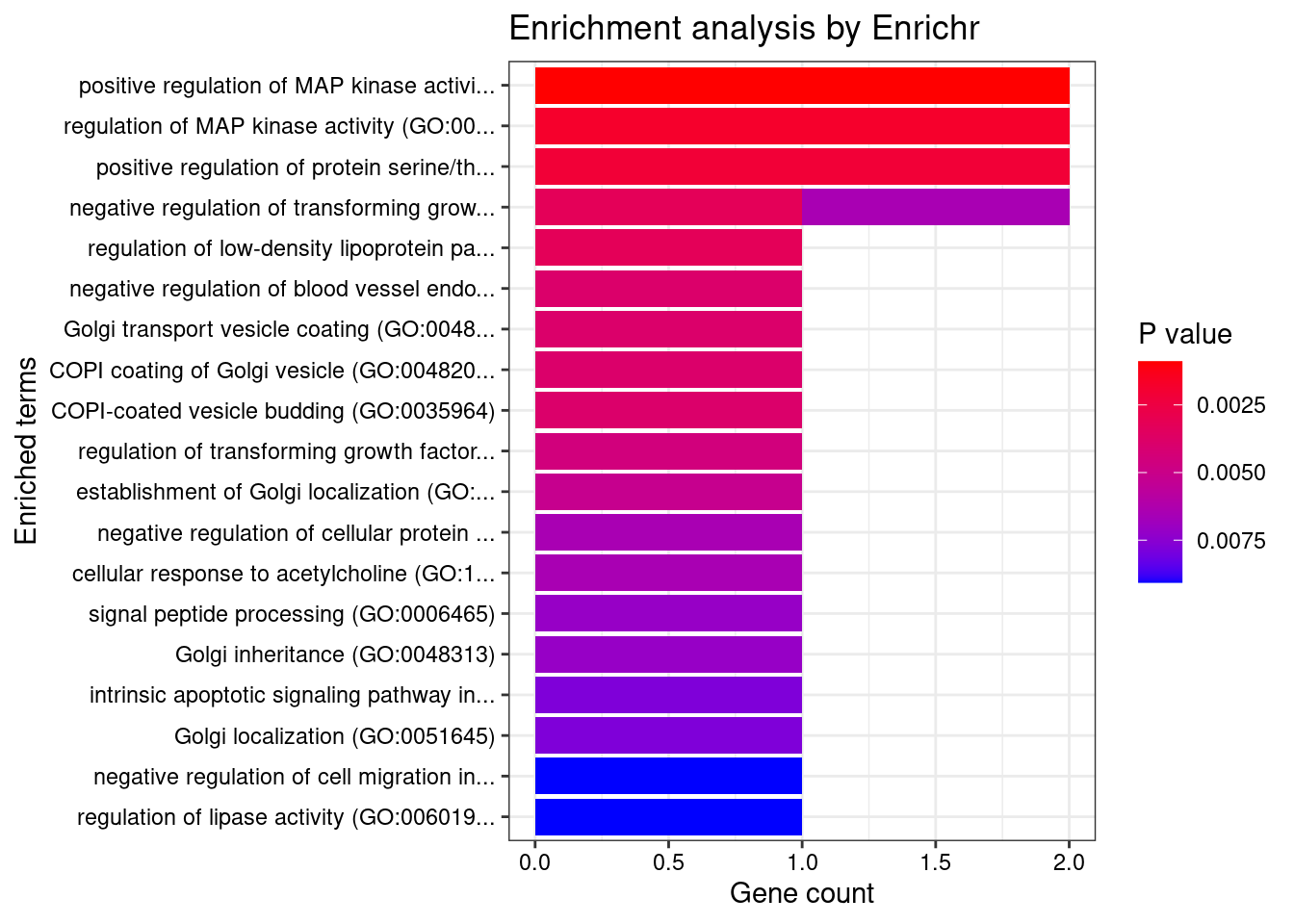
Term
1 positive regulation of MAP kinase activity (GO:0043406)
2 regulation of MAP kinase activity (GO:0043405)
3 positive regulation of protein serine/threonine kinase activity (GO:0071902)
4 negative regulation of transforming growth factor beta1 production (GO:0032911)
5 regulation of low-density lipoprotein particle receptor catabolic process (GO:0032803)
6 negative regulation of blood vessel endothelial cell proliferation involved in sprouting angiogenesis (GO:1903588)
7 Golgi transport vesicle coating (GO:0048200)
8 COPI coating of Golgi vesicle (GO:0048205)
9 COPI-coated vesicle budding (GO:0035964)
10 regulation of transforming growth factor beta1 production (GO:0032908)
11 establishment of Golgi localization (GO:0051683)
12 negative regulation of transforming growth factor beta production (GO:0071635)
13 negative regulation of cellular protein catabolic process (GO:1903363)
14 cellular response to acetylcholine (GO:1905145)
15 signal peptide processing (GO:0006465)
16 Golgi inheritance (GO:0048313)
17 intrinsic apoptotic signaling pathway in response to oxidative stress (GO:0008631)
18 Golgi localization (GO:0051645)
19 negative regulation of cell migration involved in sprouting angiogenesis (GO:0090051)
20 regulation of lipase activity (GO:0060191)
21 stress fiber assembly (GO:0043149)
22 positive regulation of membrane protein ectodomain proteolysis (GO:0051044)
23 contractile actin filament bundle assembly (GO:0030038)
24 epiboly involved in wound healing (GO:0090505)
25 regulation of blood vessel endothelial cell proliferation involved in sprouting angiogenesis (GO:1903587)
26 regulation of Golgi organization (GO:1903358)
27 regulation of catabolic process (GO:0009894)
28 positive regulation of stress-activated protein kinase signaling cascade (GO:0070304)
29 positive regulation of MAPK cascade (GO:0043410)
30 acetylcholine receptor signaling pathway (GO:0095500)
31 regulation of lipoprotein lipase activity (GO:0051004)
32 regulation of membrane protein ectodomain proteolysis (GO:0051043)
33 regulation of peptidase activity (GO:0052547)
34 wound healing, spreading of cells (GO:0044319)
Overlap Adjusted.P.value Genes
1 2/69 0.04473 PDCD10;DIRAS1
2 2/97 0.04473 PDCD10;DIRAS1
3 2/106 0.04473 PDCD10;DIRAS1
4 1/5 0.04473 FURIN
5 1/5 0.04473 FURIN
6 1/6 0.04473 PDCD10
7 1/6 0.04473 ARFGAP2
8 1/6 0.04473 ARFGAP2
9 1/6 0.04473 ARFGAP2
10 1/7 0.04473 FURIN
11 1/8 0.04473 PDCD10
12 1/10 0.04473 FURIN
13 1/10 0.04473 FURIN
14 1/10 0.04473 LY6H
15 1/11 0.04473 FURIN
16 1/11 0.04473 PDCD10
17 1/12 0.04473 PDCD10
18 1/12 0.04473 PDCD10
19 1/14 0.04473 PDCD10
20 1/14 0.04473 FURIN
21 1/15 0.04473 PDCD10
22 1/15 0.04473 FURIN
23 1/15 0.04473 PDCD10
24 1/16 0.04473 PDCD10
25 1/16 0.04473 PDCD10
26 1/17 0.04490 PDCD10
27 1/18 0.04490 FURIN
28 1/18 0.04490 PDCD10
29 2/274 0.04727 PDCD10;DIRAS1
30 1/21 0.04727 LY6H
31 1/21 0.04727 FURIN
32 1/22 0.04796 FURIN
33 1/23 0.04861 FURIN
34 1/24 0.04921 PDCD10
[1] "GO_Cellular_Component_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"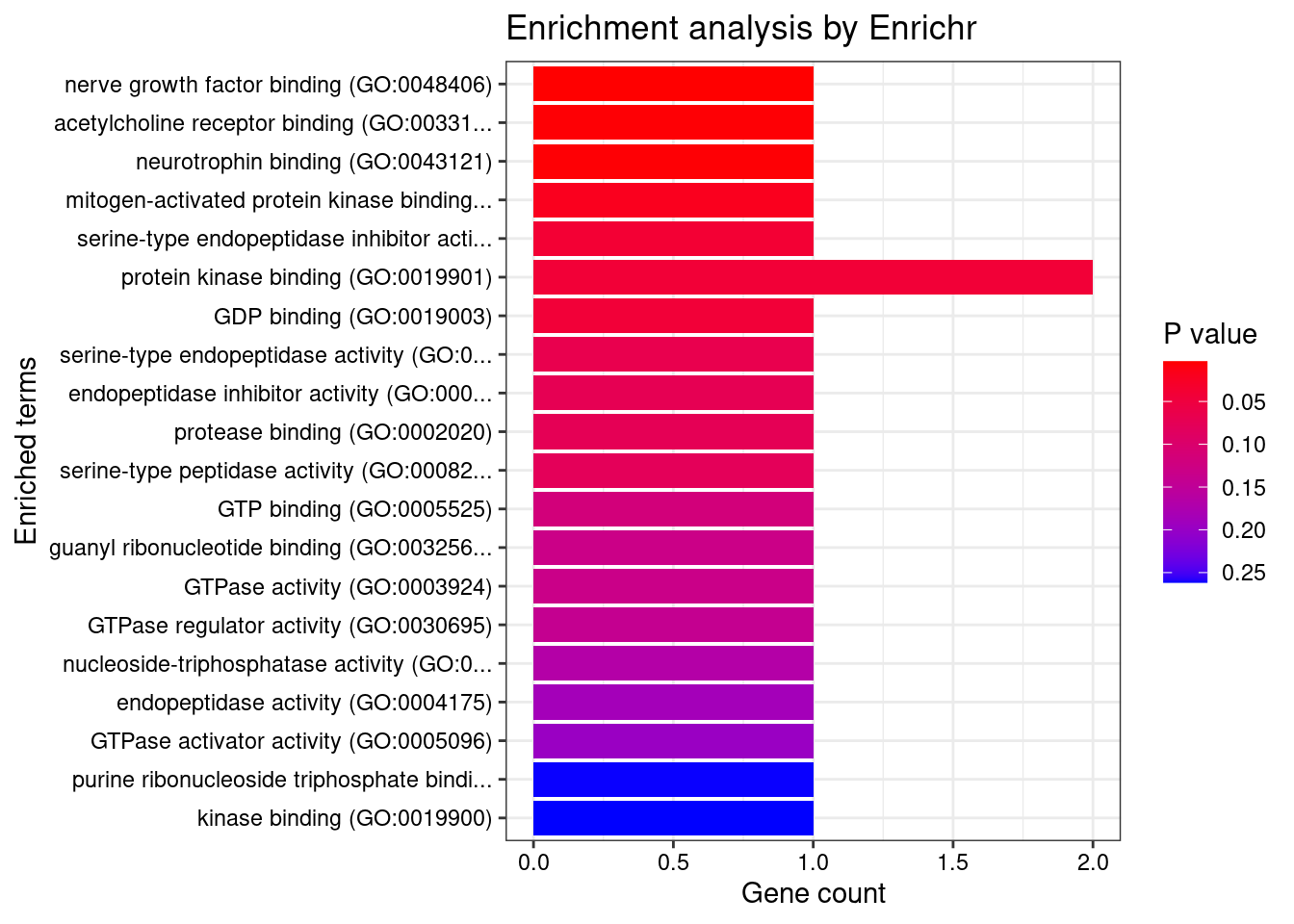
Term Overlap Adjusted.P.value Genes
1 nerve growth factor binding (GO:0048406) 1/5 0.03978 FURIN
2 acetylcholine receptor binding (GO:0033130) 1/8 0.03978 LY6H
3 neurotrophin binding (GO:0043121) 1/8 0.03978 FURINDisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio BgRatio
13 Cerebral Cavernous Malformations 3 0.002319 1/3 1/9703
15 Familial cerebral cavernous malformation 0.002319 1/3 1/9703
14 Cavernous Hemangioma of Brain 0.004637 1/3 3/9703
1 Carcinoma 0.062703 1/3 164/9703
3 Animal Mammary Neoplasms 0.062703 1/3 142/9703
4 Mammary Neoplasms, Experimental 0.062703 1/3 155/9703
6 Anaplastic carcinoma 0.062703 1/3 163/9703
7 Carcinoma, Spindle-Cell 0.062703 1/3 163/9703
8 Undifferentiated carcinoma 0.062703 1/3 163/9703
9 Carcinomatosis 0.062703 1/3 163/9703WebGestalt enrichment analysis for genes with PIP>0.5
Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 41#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 20#significance threshold for TWAS
print(sig_thresh)[1] 4.567#number of ctwas genes
length(ctwas_genes)[1] 3#number of TWAS genes
length(twas_genes)[1] 70#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols][1] genename region_tag susie_pip mu2 PVE z num_eqtl
<0 rows> (or 0-length row.names)#sensitivity / recall
print(sensitivity)ctwas TWAS
0 0 #specificity
print(specificity) ctwas TWAS
0.9997 0.9931 #precision / PPV
print(precision)ctwas TWAS
0 0 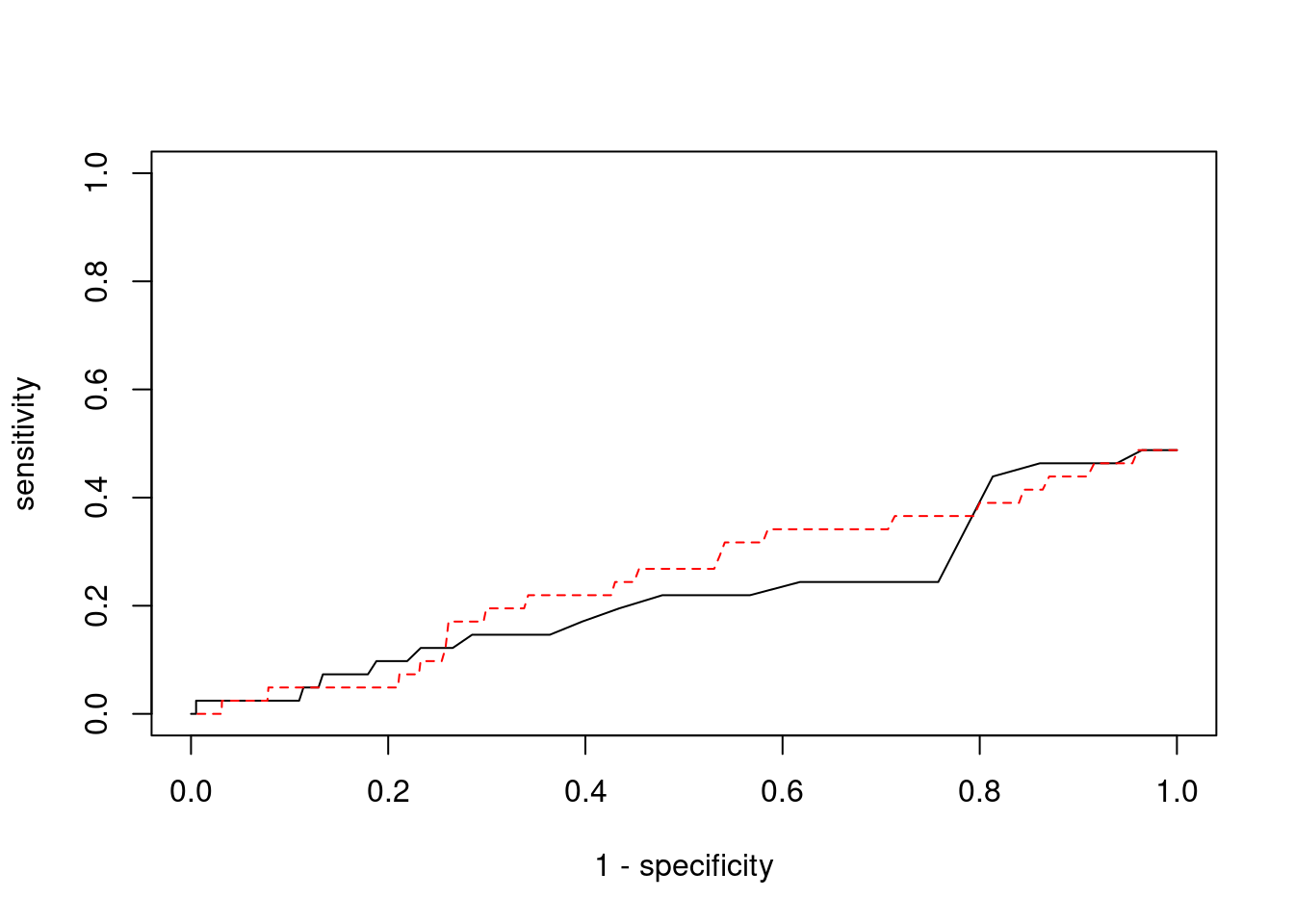
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.3.1 forcats_0.5.1 stringr_1.4.0 dplyr_1.0.7
[5] purrr_0.3.4 readr_2.1.1 tidyr_1.1.4 tidyverse_1.3.1
[9] tibble_3.1.6 WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0
[13] cowplot_1.0.0 ggplot2_3.3.5 workflowr_1.6.2
loaded via a namespace (and not attached):
[1] fs_1.5.2 lubridate_1.8.0 bit64_4.0.5 doParallel_1.0.17
[5] httr_1.4.2 rprojroot_2.0.2 tools_3.6.1 backports_1.4.1
[9] doRNG_1.8.2 utf8_1.2.2 R6_2.5.1 vipor_0.4.5
[13] DBI_1.1.2 colorspace_2.0-2 withr_2.4.3 ggrastr_1.0.1
[17] tidyselect_1.1.1 bit_4.0.4 curl_4.3.2 compiler_3.6.1
[21] git2r_0.26.1 rvest_1.0.2 cli_3.1.0 Cairo_1.5-12.2
[25] xml2_1.3.3 labeling_0.4.2 scales_1.1.1 apcluster_1.4.8
[29] digest_0.6.29 rmarkdown_2.11 svglite_1.2.2 pkgconfig_2.0.3
[33] htmltools_0.5.2 dbplyr_2.1.1 fastmap_1.1.0 highr_0.9
[37] rlang_1.0.1 rstudioapi_0.13 RSQLite_2.2.8 jquerylib_0.1.4
[41] farver_2.1.0 generics_0.1.1 jsonlite_1.7.2 vroom_1.5.7
[45] magrittr_2.0.2 Matrix_1.2-18 ggbeeswarm_0.6.0 Rcpp_1.0.8
[49] munsell_0.5.0 fansi_1.0.2 gdtools_0.1.9 lifecycle_1.0.1
[53] stringi_1.7.6 whisker_0.3-2 yaml_2.2.1 plyr_1.8.6
[57] grid_3.6.1 blob_1.2.2 ggrepel_0.9.1 parallel_3.6.1
[61] promises_1.0.1 crayon_1.5.0 lattice_0.20-38 haven_2.4.3
[65] hms_1.1.1 knitr_1.36 pillar_1.6.4 igraph_1.2.10
[69] rjson_0.2.20 rngtools_1.5.2 reshape2_1.4.4 codetools_0.2-16
[73] reprex_2.0.1 glue_1.6.2 evaluate_0.14 data.table_1.14.2
[77] modelr_0.1.8 vctrs_0.3.8 tzdb_0.2.0 httpuv_1.5.1
[81] foreach_1.5.2 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] cachem_1.0.6 xfun_0.29 broom_0.7.10 later_0.8.0
[89] iterators_1.0.14 beeswarm_0.2.3 memoise_2.0.1 ellipsis_0.3.2