Evaluating KOMPUTE - Clinical Chemistry data
Coby Warkentin and Donghyung Lee
2023-06-23
- Load packages
- Preparing control phenotype
data
- Importing Clinical Chemistry Control Phenotype Dataset
- Visualizing measured phenotypes via a heatmap
- Exclude phenotypes with fewer than 20,000 observations
- Remove samples with fewer than 15 measured phenotypes
- Heapmap of filtered phenotypes
- Reforatting the dataset (long to wide)
- Visualizing phenotype distributions
- Rank Z transformation
- Conducting Principal Variance Component Analysis (PVCA)
- Batch effect removal using ComBat
- PVCA on ComBat residuals
- Computing phenotypic correlations
- Preparation of IMPC
summary statistics data
- Loading Clinical Chemistry summary stat (IMPCv16)
- Visualizing gene-phenotype pair duplicates
- Consolidating muliple z-scores of a gene-phenotype pair using Stouffer’s Method
- Generating Z-score matrix (reformatting)
- Visualization of Phenotype-Gene Coverage
- Distribution of Z-Scores Across Phenotypes
- Estimation of Genetic Correlation Matrix Using Z-Scores
- Comparison of Phenotypic Correlation and Genetic Correlation Among Phenotypes
- Correlation Analysis Between Genetic Correlation Matrices Using Mantel’s Test
- Evaluating the KOMPUTE Imputation Algorithm
Last updated: 2023-06-23
Checks: 7 0
Knit directory: komputeExamples/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20230110) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version fbd0f5c. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/.DS_Store
Ignored: code/.DS_Store
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/kompute_test_CC_v16.Rmd)
and HTML (docs/kompute_test_CC_v16.html) files. If you’ve
configured a remote Git repository (see ?wflow_git_remote),
click on the hyperlinks in the table below to view the files as they
were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | fbd0f5c | statsleelab | 2023-06-23 | kompute input format changed |
| html | dcb3b0d | statsleelab | 2023-06-21 | pheno.cor added |
| Rmd | e7a365f | statsleelab | 2023-06-21 | pheno.cor added |
| html | 9b680ec | statsleelab | 2023-06-20 | doc updated |
| Rmd | d343f6a | statsleelab | 2023-06-20 | doc updated |
| html | d56cc7a | statsleelab | 2023-06-19 | Build site. |
| html | f4e1ab9 | statsleelab | 2023-01-10 | v16 update |
| Rmd | 7685a09 | statsleelab | 2023-01-10 | first commit |
| html | 7685a09 | statsleelab | 2023-01-10 | first commit |
Load packages
rm(list=ls())
knitr::opts_chunk$set(message = FALSE, warning = FALSE)
library(data.table)
library(dplyr)
library(reshape2)
library(ggplot2)
library(tidyr) #spread
library(RColorBrewer)
library(circlize)
library(ComplexHeatmap)Preparing control phenotype data
Importing Clinical Chemistry Control Phenotype Dataset
CC.data <- readRDS("data/CC.data.rds")
dim(CC.data)[1] 679047 10Visualizing measured phenotypes via a heatmap
The heatmap below presents a visualization of the phenotypic measurements taken for each control mouse. The columns represent individual mice, while the rows correspond to the distinct phenotypes measured.
mtest <- table(CC.data$proc_param_name_stable_id, CC.data$biological_sample_id)
mtest <-as.data.frame.matrix(mtest)
dim(mtest)[1] 155 34952if(FALSE){
nmax <-max(mtest)
library(circlize)
col_fun = colorRamp2(c(0, nmax), c("white", "red"))
col_fun(seq(0, nmax))
ht = Heatmap(as.matrix(mtest), cluster_rows = FALSE, cluster_columns = FALSE, show_column_names = F, col = col_fun,
row_names_gp = gpar(fontsize = 8), name="Count")
draw(ht)
}Exclude phenotypes with fewer than 20,000 observations
To maintain data quality and robustness, we will discard any phenotypes that have fewer than 20,000 recorded observations.
mtest <- table(CC.data$proc_param_name, CC.data$biological_sample_id)
dim(mtest)[1] 36 34952#head(mtest[,1:10])
mtest0 <- mtest>0
#head(mtest0[,1:10])
rowSums(mtest0) CC_Alanine aminotransferase
34633
CC_Albumin
34594
CC_Alkaline phosphatase
34450
CC_Alpha-amylase
22077
CC_Aspartate aminotransferase
34078
CC_Calcium
34526
CC_Chloride
24604
CC_Cholesterol ratio
25
CC_Creatine kinase
15761
CC_Creatinine
29741
CC_Ferretin
150
CC_Free fatty acid
4338
CC_Free fatty acids
6147
CC_Fructosamine
12867
CC_Glucose
34118
CC_Glycerol
7509
CC_Glycosilated hemoglobin A1c (HbA1c)
1698
CC_HDL-cholesterol
28478
CC_Iron
25602
CC_Lactate dehydrogenase
8967
CC_LDL-cholesterol
11387
CC_Lipase
2777
CC_Magnesium
7682
CC_Phosphorus
34361
CC_Potassium
24401
CC_Sodium
24609
CC_Thyroxine
3540
CC_Total bilirubin
29694
CC_Total cholesterol
34394
CC_Total protein
34429
CC_Transferrin
169
CC_Triglycerides
33795
CC_UIBC (unsaturated iron binding capacity)
2801
CC_Urea
8296
CC_Urea (Blood Urea Nitrogen - BUN)
26272
CC_Uric acid
4299 rmv.pheno.list <- rownames(mtest)[rowSums(mtest0)<20000]
#rmv.pheno.list
dim(CC.data)[1] 679047 10CC.data <- CC.data %>% filter(!(proc_param_name %in% rmv.pheno.list))
dim(CC.data)[1] 580566 10# number of phenotypes left
length(unique(CC.data$proc_param_name))[1] 19Remove samples with fewer than 15 measured phenotypes
mtest <- table(CC.data$proc_param_name, CC.data$biological_sample_id)
dim(mtest)[1] 19 34925head(mtest[,1:10])
21 22 24 25 26 27 28 29 30 31
CC_Alanine aminotransferase 1 1 1 1 1 1 1 0 1 1
CC_Albumin 1 1 1 1 1 1 1 0 1 1
CC_Alkaline phosphatase 1 1 1 1 1 1 1 0 1 1
CC_Alpha-amylase 1 1 1 1 1 1 1 0 1 1
CC_Aspartate aminotransferase 1 1 1 1 1 1 1 1 1 1
CC_Calcium 1 1 1 1 1 1 1 1 1 1mtest0 <- mtest>0
head(mtest0[,1:10])
21 22 24 25 26 27 28 29 30
CC_Alanine aminotransferase TRUE TRUE TRUE TRUE TRUE TRUE TRUE FALSE TRUE
CC_Albumin TRUE TRUE TRUE TRUE TRUE TRUE TRUE FALSE TRUE
CC_Alkaline phosphatase TRUE TRUE TRUE TRUE TRUE TRUE TRUE FALSE TRUE
CC_Alpha-amylase TRUE TRUE TRUE TRUE TRUE TRUE TRUE FALSE TRUE
CC_Aspartate aminotransferase TRUE TRUE TRUE TRUE TRUE TRUE TRUE TRUE TRUE
CC_Calcium TRUE TRUE TRUE TRUE TRUE TRUE TRUE TRUE TRUE
31
CC_Alanine aminotransferase TRUE
CC_Albumin TRUE
CC_Alkaline phosphatase TRUE
CC_Alpha-amylase TRUE
CC_Aspartate aminotransferase TRUE
CC_Calcium TRUEsummary(colSums(mtest0)) Min. 1st Qu. Median Mean 3rd Qu. Max.
1.00 15.00 17.00 16.57 19.00 19.00 rmv.sample.list <- colnames(mtest)[colSums(mtest0)<15]
length(rmv.sample.list)[1] 8380dim(CC.data)[1] 580566 10CC.data <- CC.data %>% filter(!(biological_sample_id %in% rmv.sample.list))
dim(CC.data)[1] 472110 10# number of observations to use
length(unique(CC.data$biological_sample_id))[1] 26545Heapmap of filtered phenotypes
if(FALSE){
mtest <- table(CC.data$proc_param_name, CC.data$biological_sample_id)
dim(mtest)
mtest <-as.data.frame.matrix(mtest)
nmax <-max(mtest)
library(circlize)
col_fun = colorRamp2(c(0, nmax), c("white", "red"))
col_fun(seq(0, nmax))
pdf("~/Google Drive Miami/Miami_IMPC/output/measured_phenotypes_controls_after_filtering_CC.pdf", width = 10, height = 3)
ht = Heatmap(as.matrix(mtest), cluster_rows = FALSE, cluster_columns = FALSE, show_column_names = F, col = col_fun,
row_names_gp = gpar(fontsize = 7), name="Count")
draw(ht)
dev.off()
}Reforatting the dataset (long to wide)
We restructure our data from a long format to a wide one for further processing and analysis.
CC.mat <- CC.data %>%
dplyr::select(biological_sample_id, proc_param_name, data_point, sex, phenotyping_center, strain_name) %>%
##consider weight or age in weeks
arrange(biological_sample_id) %>%
distinct(biological_sample_id, proc_param_name, .keep_all=TRUE) %>% ## remove duplicates, maybe mean() is better.
spread(proc_param_name, data_point) %>%
tibble::column_to_rownames(var="biological_sample_id")
head(CC.mat) sex phenotyping_center strain_name CC_Alanine aminotransferase
21 male MRC Harwell 129S8/SvEv-Gpi1<c>/NimrH 85.9
22 male MRC Harwell 129S8/SvEv-Gpi1<c>/NimrH 110.9
24 male MRC Harwell 129S8/SvEv-Gpi1<c>/NimrH 32.1
25 male MRC Harwell 129S8/SvEv-Gpi1<c>/NimrH 33.7
26 male MRC Harwell 129S8/SvEv-Gpi1<c>/NimrH 37.2
27 male MRC Harwell 129S8/SvEv-Gpi1<c>/NimrH 39.7
CC_Albumin CC_Alkaline phosphatase CC_Alpha-amylase
21 25.3 90 759.2
22 26.9 86 844.3
24 26.5 103 822.9
25 26.2 81 799.9
26 28.4 95 810.5
27 27.3 93 821.4
CC_Aspartate aminotransferase CC_Calcium CC_Chloride CC_Creatinine
21 97.7 2.33 112 NA
22 114.7 2.41 113 NA
24 57.7 2.35 108 NA
25 64.0 2.35 110 NA
26 62.3 2.35 109 NA
27 58.3 2.37 109 NA
CC_Glucose CC_HDL-cholesterol CC_Iron CC_Phosphorus CC_Potassium CC_Sodium
21 8.46 NA 37.86 1.76 4.8 152
22 9.83 NA 39.78 1.82 5.7 153
24 8.36 NA 38.24 1.89 4.7 154
25 10.42 NA 36.28 2.10 4.8 153
26 9.79 NA 36.26 2.02 5.1 153
27 9.74 NA 38.30 1.57 4.5 153
CC_Total bilirubin CC_Total cholesterol CC_Total protein CC_Triglycerides
21 NA 3.27 50.6 1.04
22 NA 3.40 52.4 1.02
24 NA 3.63 52.4 1.43
25 NA 3.40 51.6 0.72
26 NA 3.53 51.9 1.15
27 NA 3.20 51.8 1.12
CC_Urea (Blood Urea Nitrogen - BUN)
21 NA
22 NA
24 NA
25 NA
26 NA
27 NAdim(CC.mat)[1] 26545 22summary(colSums(is.na(CC.mat[,-1:-3]))) Min. 1st Qu. Median Mean 3rd Qu. Max.
25 61 134 1784 2930 7853 Visualizing phenotype distributions
ggplot(melt(CC.mat), aes(x=value)) +
geom_histogram() +
facet_wrap(~variable, scales="free", ncol=5)+
theme(strip.text.x = element_text(size = 6))
| Version | Author | Date |
|---|---|---|
| 7685a09 | statsleelab | 2023-01-10 |
Rank Z transformation
In this step, we conduct a rank Z transformation on the phenotype data to ensure that the data is normally distributed
library(RNOmni)
CC.mat.rank <- CC.mat
dim(CC.mat.rank)[1] 26545 22CC.mat.rank <- CC.mat.rank[complete.cases(CC.mat.rank),]
dim(CC.mat.rank)[1] 11663 22dim(CC.mat)[1] 26545 22CC.mat <- CC.mat[complete.cases(CC.mat),]
dim(CC.mat)[1] 11663 22CC.mat.rank <- cbind(CC.mat.rank[,1:3], apply(CC.mat.rank[,-1:-3], 2, RankNorm))
ggplot(melt(CC.mat.rank), aes(x=value)) +
geom_histogram() +
facet_wrap(~variable, scales="free", ncol=5)+
theme(strip.text.x = element_text(size = 6))
| Version | Author | Date |
|---|---|---|
| 7685a09 | statsleelab | 2023-01-10 |
Conducting Principal Variance Component Analysis (PVCA)
In this step, we apply Principal Variance Component Analysis (PVCA) on the phenotype matrix data. PVCA is an approach that combines Principal Component Analysis (PCA) and Variance Component Analysis to quantify the proportion of total variance in the data attributed to each important covariate, in this case ‘sex’ and ‘phenotyping_center’.
First, we prepare our metadata which includes our chosen covariates. Any character variables in the metadata are then converted to factors. To avoid potential confounding, we check for associations between our covariates and drop ‘strain_name’ due to its strong association with ‘phenotyping_center’.
Next, we run PVCA on randomly chosen subsets of our phenotype data (for computational efficiency). Finally, we compute the average effect size across all random samples and visualize the results in a PVCA plot.
source("code/PVCA.R")
meta <- CC.mat.rank[,1:3] ## examining covariates sex, phenotyping_center, and strain_name
head(meta) sex phenotyping_center strain_name
39638 female MRC Harwell C57BL/6NTac
39639 female HMGU C57BL/6NCrl
39640 female HMGU C57BL/6NTac
39643 female HMGU C57BL/6NCrl
39650 female HMGU C57BL/6NTac
39657 male HMGU C57BL/6NCrldim(meta)[1] 11663 3summary(meta) # variables are still characters sex phenotyping_center strain_name
Length:11663 Length:11663 Length:11663
Class :character Class :character Class :character
Mode :character Mode :character Mode :character meta[sapply(meta, is.character)] <- lapply(meta[sapply(meta, is.character)], as.factor)
summary(meta) # now all variables are converted to factors sex phenotyping_center strain_name
female:5850 HMGU :2552 B6Brd;B6Dnk;B6N-Tyr<c-Brd>: 164
male :5813 MRC Harwell:4801 C57BL/6N :4146
WTSI :4310 C57BL/6NCrl : 891
C57BL/6NTac :6462 chisq.test(meta[,1],meta[,2])
Pearson's Chi-squared test
data: meta[, 1] and meta[, 2]
X-squared = 0.032984, df = 2, p-value = 0.9836chisq.test(meta[,2],meta[,3])
Pearson's Chi-squared test
data: meta[, 2] and meta[, 3]
X-squared = 14688, df = 6, p-value < 2.2e-16meta<-meta[,-3] # phenotyping_center and strain_name strongly associated which could cause confounding in the PVCA analysis, so we drop 'strain_name'.
G <- t(CC.mat.rank[,-1:-3]) ## preparing the phenotype matrix data
set.seed(09302021)
# Perform PVCA for 10 random samples of size 1000 (more computationally efficient)
pvca.res <- matrix(nrow=10, ncol=3)
for (i in 1:10){
sample <- sample(1:ncol(G), 1000, replace=FALSE)
pvca.res[i,] <- PVCA(G[,sample], meta[sample,], threshold=0.6, inter=FALSE)
}
# Compute average effect size across the 10 random samples
pvca.means <- colMeans(pvca.res)
names(pvca.means) <- c(colnames(meta), "resid")
# Create PVCA plot
pvca.plot <- PlotPVCA(pvca.means, "PVCA of Phenotype Matrix Data")
pvca.plot
| Version | Author | Date |
|---|---|---|
| 7685a09 | statsleelab | 2023-01-10 |
png(file="docs/figure/figures.Rmd/pvca_CC_1_v16.png", width=600, height=350)
pvca.plot
dev.off()quartz_off_screen
2 Batch effect removal using ComBat
We remove batch effects (the center effect) in the phenotype data set by using the ComBat method.
library(sva)
combat_komp = ComBat(dat=G, batch=meta$phenotyping_center, par.prior=TRUE, prior.plots=TRUE, mod=NULL)
| Version | Author | Date |
|---|---|---|
| 7685a09 | statsleelab | 2023-01-10 |
combat_komp[1:5,1:5] 39638 39639 39640 39643
CC_Alanine aminotransferase 0.9340988 -0.5544734 0.6666494 -0.9010928
CC_Albumin 2.5845338 1.2282189 0.2400336 0.5834969
CC_Alkaline phosphatase 1.8038375 0.7077080 0.6239891 0.4510424
CC_Alpha-amylase 0.3151157 -0.3139508 0.3021379 0.3236144
CC_Aspartate aminotransferase 0.9536882 0.2034362 1.0860320 0.5575948
39650
CC_Alanine aminotransferase -1.1876876
CC_Albumin -0.4044527
CC_Alkaline phosphatase 0.2895801
CC_Alpha-amylase -1.4941108
CC_Aspartate aminotransferase -2.4362255G[1:5,1:5] # for comparison, combat_komp is same form and same dimensions as G 39638 39639 39640 39643
CC_Alanine aminotransferase 0.6215108 -1.3455580 0.2437437 -1.7966860
CC_Albumin 2.8129728 2.0400251 0.9990476 1.3608599
CC_Alkaline phosphatase 1.8346659 1.1364171 1.0485945 0.8671703
CC_Alpha-amylase 0.3053479 -0.4417481 0.1869870 0.2089043
CC_Aspartate aminotransferase 0.5629829 0.1714807 1.1471404 0.5629829
39650
CC_Alanine aminotransferase -2.1696915
CC_Albumin 0.3201306
CC_Alkaline phosphatase 0.6977934
CC_Alpha-amylase -1.6461331
CC_Aspartate aminotransferase -2.7465161PVCA on ComBat residuals
After using ComBat to account for batch effects, we perform a PVCA on the residuals. We expect to observe a significantly reduced effect from the phenotyping centers.
set.seed(09302021)
# Perform PVCA for 10 samples (more computationally efficient)
pvca.res.nobatch <- matrix(nrow=10, ncol=3)
for (i in 1:10){
sample <- sample(1:ncol(combat_komp), 1000, replace=FALSE)
pvca.res.nobatch[i,] <- PVCA(combat_komp[,sample], meta[sample,], threshold=0.6, inter=FALSE)
}
# Compute average effect size across samples
pvca.means.nobatch <- colMeans(pvca.res.nobatch)
names(pvca.means.nobatch) <- c(colnames(meta), "resid")
# Generate PVCA plot
pvca.plot.nobatch <- PlotPVCA(pvca.means.nobatch, "PVCA of Phenotype Matrix Data with Reduced Batch Effect")
pvca.plot.nobatch
| Version | Author | Date |
|---|---|---|
| 7685a09 | statsleelab | 2023-01-10 |
png(file="docs/figure/figures.Rmd/pvca_CC_2_v16.png", width=600, height=350)
pvca.plot.nobatch
dev.off()quartz_off_screen
2 Computing phenotypic correlations
We compute the phenotype correlations using different methods and compare them.
CC.cor.rank <- cor(CC.mat.rank[,-1:-3], use="pairwise.complete.obs") # pearson correlation coefficient
CC.cor <- cor(CC.mat[,-1:-3], use="pairwise.complete.obs", method="spearman") # spearman
CC.cor.combat <- cor(t(combat_komp), use="pairwise.complete.obs")
pheno.list <- rownames(CC.cor)
ht1 = Heatmap(CC.cor, show_column_names = F, row_names_gp = gpar(fontsize = 9), name="Spearm. Corr.")
draw(ht1)
ht2 = Heatmap(CC.cor.rank, show_column_names = F, row_names_gp = gpar(fontsize = 9), name="Corr. RankZ")
draw(ht2)
ht3 = Heatmap(CC.cor.combat, show_column_names = F, row_names_gp = gpar(fontsize = 9), name="Corr. ComBat")
draw(ht3)
Preparation of IMPC summary statistics data
Loading Clinical Chemistry summary stat (IMPCv16)
CC.stat <- readRDS("data/CC.stat.v16.rds")
dim(CC.stat)[1] 118974 8table(CC.stat$parameter_name, CC.stat$procedure_name)
CC
Alanine aminotransferase 6312
Albumin 6312
Alkaline phosphatase 6297
Alpha-amylase 3310
Aspartate aminotransferase 6220
Calcium 6306
Chloride 3852
Cholesterol ratio 2269
Creatine kinase 2744
Creatinine 5701
Free fatty acids 1595
Fructosamine 2301
Glucose 6276
Glycerol 1617
HDL-cholesterol 5148
Iron 4025
LDL-cholesterol 1871
Magnesium 1656
Phosphorus 6306
Potassium 4459
Sodium 3852
Thyroxine 1129
Total bilirubin 5841
Total cholesterol 6301
Total protein 6278
Triglycerides 5473
Urea (Blood Urea Nitrogen - BUN) 5523length(unique(CC.stat$marker_symbol)) #3983[1] 5342length(unique(CC.stat$allele_symbol)) #4152[1] 5563length(unique(CC.stat$proc_param_name)) #27, number of phenotypes in association statistics data set[1] 27length(unique(CC.data$proc_param_name)) #19, number of phenotypes in final control data[1] 19pheno.list.stat <- unique(CC.stat$proc_param_name)
pheno.list.ctrl <- unique(CC.data$proc_param_name)
sum(pheno.list.stat %in% pheno.list.ctrl)[1] 19sum(pheno.list.ctrl %in% pheno.list.stat)[1] 19# Identifying common phenotypes between statistics and control data
common.pheno.list <- sort(intersect(pheno.list.ctrl, pheno.list.stat))
common.pheno.list [1] "CC_Alanine aminotransferase" "CC_Albumin"
[3] "CC_Alkaline phosphatase" "CC_Alpha-amylase"
[5] "CC_Aspartate aminotransferase" "CC_Calcium"
[7] "CC_Chloride" "CC_Creatinine"
[9] "CC_Glucose" "CC_HDL-cholesterol"
[11] "CC_Iron" "CC_Phosphorus"
[13] "CC_Potassium" "CC_Sodium"
[15] "CC_Total bilirubin" "CC_Total cholesterol"
[17] "CC_Total protein" "CC_Triglycerides"
[19] "CC_Urea (Blood Urea Nitrogen - BUN)"length(common.pheno.list)[1] 19# Filtering summary statistics to contain only common phenotypes
dim(CC.stat)[1] 118974 8CC.stat <- CC.stat %>% filter(proc_param_name %in% common.pheno.list)
dim(CC.stat)[1] 103792 8length(unique(CC.stat$proc_param_name))[1] 19Visualizing gene-phenotype pair duplicates
mtest <- table(CC.stat$proc_param_name, CC.stat$marker_symbol)
mtest <-as.data.frame.matrix(mtest)
nmax <-max(mtest)
col_fun = colorRamp2(c(0, nmax), c("white", "red"))
col_fun(seq(0, nmax)) [1] "#FFFFFFFF" "#FFF2EEFF" "#FFE6DCFF" "#FFD9CBFF" "#FFCCBBFF" "#FFBFAAFF"
[7] "#FFB299FF" "#FFA589FF" "#FF9779FF" "#FF8969FF" "#FF7B5AFF" "#FF6C4AFF"
[13] "#FF5B3AFF" "#FF482AFF" "#FF3118FF" "#FF0000FF"ht = Heatmap(as.matrix(mtest), cluster_rows = FALSE, cluster_columns = FALSE, show_column_names = F, col = col_fun,
row_names_gp = gpar(fontsize = 8), name="Count")
draw(ht)
| Version | Author | Date |
|---|---|---|
| 7685a09 | statsleelab | 2023-01-10 |
Consolidating muliple z-scores of a gene-phenotype pair using Stouffer’s Method
## sum(z-score)/sqrt(# of zscore)
sumz <- function(z){ sum(z)/sqrt(length(z)) }
CC.z = CC.stat %>%
dplyr::select(marker_symbol, proc_param_name, z_score) %>%
na.omit() %>%
group_by(marker_symbol, proc_param_name) %>%
summarize(zscore = sumz(z_score)) ## combine z-scores
dim(CC.z)[1] 88636 3Generating Z-score matrix (reformatting)
# Function to convert NaN to NA
nan2na <- function(df){
out <- data.frame(sapply(df, function(x) ifelse(is.nan(x), NA, x)))
colnames(out) <- colnames(df)
out
}
# Converting the long format of z-scores to a wide format matrix
CC.zmat = dcast(CC.z, marker_symbol ~ proc_param_name, value.var = "zscore",
fun.aggregate = mean) %>% tibble::column_to_rownames(var="marker_symbol")
CC.zmat = nan2na(CC.zmat) #convert nan to na
dim(CC.zmat)[1] 5342 19Visualization of Phenotype-Gene Coverage
The heatmap illustrates tested (red) and untested (white) gene-phenotype pairs.
# Generate a matrix indicating where z-scores are present
id.mat <- 1*(!is.na(CC.zmat)) # multiply 1 to make this matrix numeric
nrow(as.data.frame(colSums(id.mat)))[1] 19dim(id.mat)[1] 5342 19## heatmap of gene - phenotype (red: tested, white: untested)
ht = Heatmap(t(id.mat),
cluster_rows = T, clustering_distance_rows ="binary",
cluster_columns = T, clustering_distance_columns = "binary",
show_row_dend = F, show_column_dend = F, # do not show dendrogram
show_column_names = F, col = c("white","red"),
row_names_gp = gpar(fontsize = 10), name="Missing")
draw(ht)
Distribution of Z-Scores Across Phenotypes
The histogram presents the distribution of association Z-scores for each phenotype.
ggplot(melt(CC.zmat), aes(x=value)) +
geom_histogram() +
facet_wrap(~variable, scales="free", ncol=5)+
theme(strip.text.x = element_text(size = 6))
Estimation of Genetic Correlation Matrix Using Z-Scores
Here, we estimate the genetic correlations between phenotypes utilizing the association Z-score matrix.
# Select common phenotypes
CC.zmat <- CC.zmat[,common.pheno.list]
dim(CC.zmat)[1] 5342 19# Compute genetic correlations
CC.zcor = cor(CC.zmat, use="pairwise.complete.obs")
# Generate heatmap of the correlation matrix
ht = Heatmap(CC.zcor, cluster_rows = T, cluster_columns = T, show_column_names = F, #col = col_fun,
row_names_gp = gpar(fontsize = 10),
name="Genetic Corr (Z-score)"
)
draw(ht)
Comparison of Phenotypic Correlation and Genetic Correlation Among Phenotypes
We will compare the correlation matrix obtained from control mice phenotype data and the genetic correlation matrix estimated using association Z-scores. As depicted below, both correlation heatmaps show similar correlation patterns.
CC.cor.rank.fig <- CC.cor.rank[common.pheno.list,common.pheno.list]
CC.cor.fig <- CC.cor[common.pheno.list,common.pheno.list]
CC.cor.combat.fig <- CC.cor.combat[common.pheno.list, common.pheno.list]
CC.zcor.fig <- CC.zcor
ht = Heatmap(CC.cor.rank.fig, cluster_rows = TRUE, cluster_columns = TRUE, show_column_names = F, #col = col_fun,
show_row_dend = F, show_column_dend = F, # do not show dendrogram
row_names_gp = gpar(fontsize = 8), column_title="Phenotype Corr (RankZ, Pearson)", column_title_gp = gpar(fontsize = 8),
name="Corr")
pheno.order <- row_order(ht)
#draw(ht)
CC.cor.rank.fig <- CC.cor.rank.fig[pheno.order,pheno.order]
ht1 = Heatmap(CC.cor.rank.fig, cluster_rows = FALSE, cluster_columns = FALSE, show_column_names = F, #col = col_fun,
show_row_dend = F, show_column_dend = F, # do not show dendrogram
row_names_gp = gpar(fontsize = 8), column_title="Phenotype Corr (RankZ, Pearson)", column_title_gp = gpar(fontsize = 8),
name="Corr")
CC.cor.fig <- CC.cor.fig[pheno.order,pheno.order]
ht2 = Heatmap(CC.cor.fig, cluster_rows = FALSE, cluster_columns = FALSE, show_column_names = F, #col = col_fun,
row_names_gp = gpar(fontsize = 8), column_title="Phenotype Corr (Spearman)", column_title_gp = gpar(fontsize = 8),
name="Corr")
CC.cor.combat.fig <- CC.cor.combat.fig[pheno.order,pheno.order]
ht3 = Heatmap(CC.cor.combat.fig, cluster_rows = FALSE, cluster_columns = FALSE, show_column_names = F, #col = col_fun,
row_names_gp = gpar(fontsize = 8), column_title="Phenotype Corr (Combat, Pearson)", column_title_gp = gpar(fontsize = 8),
name="Corr")
CC.zcor.fig <- CC.zcor.fig[pheno.order,pheno.order]
ht4 = Heatmap(CC.zcor.fig, cluster_rows = FALSE, cluster_columns = FALSE, show_column_names = F, #col = col_fun,
row_names_gp = gpar(fontsize = 8), column_title="Genetic Corr (Pearson)", column_title_gp = gpar(fontsize = 8),
name="Corr"
)
draw(ht1+ht2+ht3+ht4)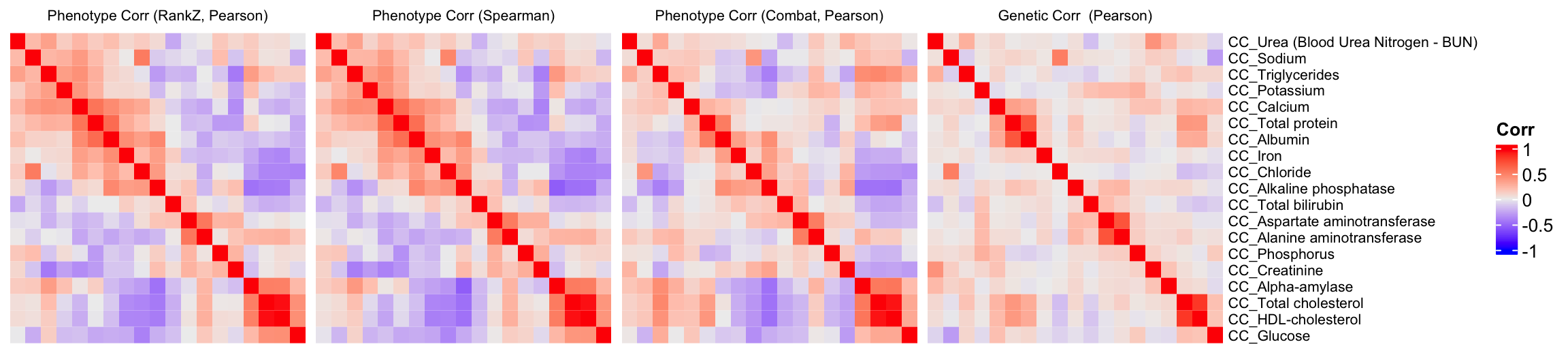
png(file="docs/figure/figures.Rmd/cors_CC.png", width=800, height=250)
draw(ht1+ht2+ht3+ht4)
dev.off()quartz_off_screen
2 Correlation Analysis Between Genetic Correlation Matrices Using Mantel’s Test
To evaluate the correlation between different genetic correlation matrices, we apply Mantel’s test, which measures the correlation between two distance matrices.
####################
# Use Mantel test
# https://stats.idre.ucla.edu/r/faq/how-can-i-perform-a-mantel-test-in-r/
# install.packages("ade4")
library(ade4)
to.upper<-function(X) X[upper.tri(X,diag=FALSE)]
a1 <- to.upper(CC.cor.fig)
a2 <- to.upper(CC.cor.rank.fig)
a3 <- to.upper(CC.cor.combat.fig)
a4 <- to.upper(CC.zcor.fig)
plot(a4, a1)
| Version | Author | Date |
|---|---|---|
| 9b680ec | statsleelab | 2023-06-20 |
plot(a4, a2)
| Version | Author | Date |
|---|---|---|
| 9b680ec | statsleelab | 2023-06-20 |
plot(a4, a3)
| Version | Author | Date |
|---|---|---|
| 9b680ec | statsleelab | 2023-06-20 |
mantel.rtest(as.dist(1-CC.cor.fig), as.dist(1-CC.zcor.fig), nrepet = 9999) #nrepet = number of permutationsMonte-Carlo test
Call: mantelnoneuclid(m1 = m1, m2 = m2, nrepet = nrepet)
Observation: 0.4065029
Based on 9999 replicates
Simulated p-value: 1e-04
Alternative hypothesis: greater
Std.Obs Expectation Variance
5.3342338350 0.0006783314 0.0057880553 mantel.rtest(as.dist(1-CC.cor.rank.fig), as.dist(1-CC.zcor.fig), nrepet = 9999)Monte-Carlo test
Call: mantelnoneuclid(m1 = m1, m2 = m2, nrepet = nrepet)
Observation: 0.4418449
Based on 9999 replicates
Simulated p-value: 1e-04
Alternative hypothesis: greater
Std.Obs Expectation Variance
5.775580172 0.001222770 0.005820245 mantel.rtest(as.dist(1-CC.cor.combat.fig), as.dist(1-CC.zcor.fig), nrepet = 9999)Monte-Carlo test
Call: mantelnoneuclid(m1 = m1, m2 = m2, nrepet = nrepet)
Observation: 0.5885487
Based on 9999 replicates
Simulated p-value: 1e-04
Alternative hypothesis: greater
Std.Obs Expectation Variance
7.818172131 -0.001408542 0.005694173 Evaluating the KOMPUTE Imputation Algorithm
Initializing the KOMPUTE Package
# Check if KOMPUTE is installed, if not, install it from GitHub using devtools
if(!"kompute" %in% rownames(installed.packages())){
library(devtools)
devtools::install_github("dleelab/kompute")
}
library(kompute)Simulation study - Comparison of imputed vs measured z-score values
In this section, we conduct a simulation study to compare the performance of the KOMPUTE method with the measured gene-phenotype association z-scores. We randomly select some of these measured z-scores, mask them, and then use the KOMPUTE method to impute them. We then compare the imputed z-scores with the measured ones.
zmat <-t(CC.zmat)
dim(zmat)[1] 19 5342# filter genes with less than 1 missing data point (na)
zmat0 <- is.na(zmat)
num.na<-colSums(zmat0)
summary(num.na) Min. 1st Qu. Median Mean 3rd Qu. Max.
0.000 0.000 2.000 2.408 5.000 17.000 dim(zmat)[1] 19 5342dim(zmat[,num.na<1])[1] 19 1641dim(zmat[,num.na<5])[1] 19 3995dim(zmat[,num.na<10])[1] 19 5336# filter genes with less than 1 missing data point (na)
zmat <- zmat[,num.na<1]
dim(zmat)[1] 19 1641# Set correlation method for phenotypes
#pheno.cor <- CC.cor.fig
#pheno.cor <- CC.cor.rank.fig
pheno.cor <- CC.cor.combat.fig
#pheno.cor <- CC.zcor.fig
zmat <- zmat[rownames(pheno.cor),,drop=FALSE]
rownames(zmat) [1] "CC_Urea (Blood Urea Nitrogen - BUN)" "CC_Sodium"
[3] "CC_Triglycerides" "CC_Potassium"
[5] "CC_Calcium" "CC_Total protein"
[7] "CC_Albumin" "CC_Iron"
[9] "CC_Chloride" "CC_Alkaline phosphatase"
[11] "CC_Total bilirubin" "CC_Aspartate aminotransferase"
[13] "CC_Alanine aminotransferase" "CC_Phosphorus"
[15] "CC_Creatinine" "CC_Alpha-amylase"
[17] "CC_Total cholesterol" "CC_HDL-cholesterol"
[19] "CC_Glucose" rownames(pheno.cor) [1] "CC_Urea (Blood Urea Nitrogen - BUN)" "CC_Sodium"
[3] "CC_Triglycerides" "CC_Potassium"
[5] "CC_Calcium" "CC_Total protein"
[7] "CC_Albumin" "CC_Iron"
[9] "CC_Chloride" "CC_Alkaline phosphatase"
[11] "CC_Total bilirubin" "CC_Aspartate aminotransferase"
[13] "CC_Alanine aminotransferase" "CC_Phosphorus"
[15] "CC_Creatinine" "CC_Alpha-amylase"
[17] "CC_Total cholesterol" "CC_HDL-cholesterol"
[19] "CC_Glucose" colnames(pheno.cor) [1] "CC_Urea (Blood Urea Nitrogen - BUN)" "CC_Sodium"
[3] "CC_Triglycerides" "CC_Potassium"
[5] "CC_Calcium" "CC_Total protein"
[7] "CC_Albumin" "CC_Iron"
[9] "CC_Chloride" "CC_Alkaline phosphatase"
[11] "CC_Total bilirubin" "CC_Aspartate aminotransferase"
[13] "CC_Alanine aminotransferase" "CC_Phosphorus"
[15] "CC_Creatinine" "CC_Alpha-amylase"
[17] "CC_Total cholesterol" "CC_HDL-cholesterol"
[19] "CC_Glucose" npheno <- nrow(zmat)
## calculate the percentage of missing Z-scores in the original data
100*sum(is.na(zmat))/(nrow(zmat)*ncol(zmat)) # 0%[1] 0nimp <- 1000 # # of missing/imputed Z-scores
set.seed(2222)
## find index of all measured zscores
all.i <- 1:(nrow(zmat)*ncol(zmat))
measured <- as.vector(!is.na(as.matrix(zmat)))
measured.i <- all.i[measured]
## mask 2000 measured z-scores
mask.i <- sort(sample(measured.i, nimp))
org.z = as.matrix(zmat)[mask.i]
zvec <- as.vector(as.matrix(zmat))
zvec[mask.i] <- NA
zmat.imp <- matrix(zvec, nrow=npheno)
rownames(zmat.imp) <- rownames(zmat)Run KOMPUTE method
kompute.res <- kompute(t(zmat.imp), pheno.cor, 0.01)
# Compare measured vs imputed z-scores
length(org.z)[1] 1000imp.z <- as.matrix(t(kompute.res$zmat))[mask.i]
imp.info <- as.matrix(t(kompute.res$infomat))[mask.i]
plot(imp.z, org.z)
# Create a dataframe with the original and imputed z-scores and the information of imputed z-scores
imp <- data.frame(org.z=org.z, imp.z=imp.z, info=imp.info)
dim(imp)[1] 1000 3imp <- imp[complete.cases(imp),]
imp <- subset(imp, info>=0 & info <= 1)
dim(imp)[1] 1000 3cor.val <- round(cor(imp$imp.z, imp$org.z), digits=3)
cor.val[1] 0.486plot(imp$imp.z, imp$org.z)
# Set a cutoff for information content and filter the data accordingly
info.cutoff <- 0.8
imp.sub <- subset(imp, info>info.cutoff)
dim(imp.sub)[1] 113 3summary(imp.sub$imp.z) Min. 1st Qu. Median Mean 3rd Qu. Max.
-5.0505 -1.1858 -0.1241 -0.2458 0.6759 5.2871 summary(imp.sub$info) Min. 1st Qu. Median Mean 3rd Qu. Max.
0.8724 0.8801 0.8801 0.8799 0.8804 0.8804 cor.val <- round(cor(imp.sub$imp.z, imp.sub$org.z), digits=3)
cor.val[1] 0.874g <- ggplot(imp.sub, aes(x=imp.z, y=org.z, col=info)) +
geom_point() +
labs(title=paste0("IMPC Behavior Data (CC), Info>", info.cutoff, ", Cor=",cor.val),
x="Imputed Z-scores", y = "Measured Z-scores", col="Info") +
theme_minimal()
g
# save plot
png(file="docs/figure/figures.Rmd/sim_results_CC_v16.png", width=600, height=350)
g
dev.off()quartz_off_screen
2 # Part 2 of Figure 2
fig2.2 <- ggplot(imp.sub, aes(x=imp.z, y=org.z, col=info)) +
geom_point() +
labs(title="Clinical Chemistry",
x="Imputed Z-scores", y = "", col="Info") +
scale_x_continuous(limits=c(-9,9), breaks=c(seq(-9,9,3)), minor_breaks = NULL) +
scale_y_continuous(limits=c(-9,9), breaks=c(seq(-9,9,3))) +
scale_color_gradient(limits=c(0.8,1), low="#98cdf9", high="#084b82") +
theme_bw() +
theme(legend.position="none", plot.title=element_text(hjust=0.5))
save(fig2.2, file="docs/figure/figures.Rmd/sim_CC_v16.rdata")Save z-score matrix and phenotype correlation matrix
plist <- sort(colnames(CC.zmat))
CC_Zscore_Mat <- as.matrix(CC.zmat[,plist])
save(CC_Zscore_Mat, file = "data/CC.zmat.v16.RData")
CC_Pheno_Cor <- pheno.cor[plist,plist]
save(CC_Pheno_Cor, file = "data/CC.pheno.cor.v16.RData")
sessionInfo()R version 4.2.1 (2022-06-23)
Platform: x86_64-apple-darwin17.0 (64-bit)
Running under: macOS Catalina 10.15.7
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.2/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.2/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] grid stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] kompute_0.1.0 ade4_1.7-20 sva_3.44.0
[4] BiocParallel_1.30.3 genefilter_1.78.0 mgcv_1.8-40
[7] nlme_3.1-158 lme4_1.1-31 Matrix_1.5-1
[10] RNOmni_1.0.1 ComplexHeatmap_2.12.1 circlize_0.4.15
[13] RColorBrewer_1.1-3 tidyr_1.2.0 ggplot2_3.4.1
[16] reshape2_1.4.4 dplyr_1.0.9 data.table_1.14.2
[19] workflowr_1.7.0.1
loaded via a namespace (and not attached):
[1] minqa_1.2.5 colorspace_2.1-0 rjson_0.2.21
[4] rprojroot_2.0.3 XVector_0.36.0 GlobalOptions_0.1.2
[7] fs_1.5.2 clue_0.3-62 rstudioapi_0.13
[10] farver_2.1.1 bit64_4.0.5 AnnotationDbi_1.58.0
[13] fansi_1.0.4 codetools_0.2-18 splines_4.2.1
[16] doParallel_1.0.17 cachem_1.0.6 knitr_1.39
[19] jsonlite_1.8.0 nloptr_2.0.3 annotate_1.74.0
[22] cluster_2.1.3 png_0.1-8 compiler_4.2.1
[25] httr_1.4.3 assertthat_0.2.1 fastmap_1.1.0
[28] limma_3.52.4 cli_3.6.0 later_1.3.0
[31] htmltools_0.5.3 tools_4.2.1 gtable_0.3.1
[34] glue_1.6.2 GenomeInfoDbData_1.2.8 Rcpp_1.0.10
[37] Biobase_2.56.0 jquerylib_0.1.4 vctrs_0.5.2
[40] Biostrings_2.64.0 iterators_1.0.14 xfun_0.31
[43] stringr_1.4.0 ps_1.7.1 lifecycle_1.0.3
[46] XML_3.99-0.10 edgeR_3.38.4 getPass_0.2-2
[49] MASS_7.3-58.1 zlibbioc_1.42.0 scales_1.2.1
[52] promises_1.2.0.1 parallel_4.2.1 yaml_2.3.5
[55] memoise_2.0.1 sass_0.4.2 stringi_1.7.8
[58] RSQLite_2.2.15 highr_0.9 S4Vectors_0.34.0
[61] foreach_1.5.2 BiocGenerics_0.42.0 boot_1.3-28
[64] shape_1.4.6 GenomeInfoDb_1.32.3 rlang_1.0.6
[67] pkgconfig_2.0.3 matrixStats_0.62.0 bitops_1.0-7
[70] evaluate_0.16 lattice_0.20-45 purrr_0.3.4
[73] labeling_0.4.2 bit_4.0.4 processx_3.7.0
[76] tidyselect_1.2.0 plyr_1.8.7 magrittr_2.0.3
[79] R6_2.5.1 IRanges_2.30.0 generics_0.1.3
[82] DBI_1.1.3 pillar_1.8.1 whisker_0.4
[85] withr_2.5.0 survival_3.3-1 KEGGREST_1.36.3
[88] RCurl_1.98-1.8 tibble_3.1.8 crayon_1.5.1
[91] utf8_1.2.3 rmarkdown_2.14 GetoptLong_1.0.5
[94] locfit_1.5-9.6 blob_1.2.3 callr_3.7.1
[97] git2r_0.30.1 digest_0.6.29 xtable_1.8-4
[100] httpuv_1.6.5 stats4_4.2.1 munsell_0.5.0
[103] bslib_0.4.0