Effect of data transformation in null data
Joyce Hsiao
2019-05-07
Last updated: 2019-05-08
Checks: 6 0
Knit directory: dsc-log-fold-change/
This reproducible R Markdown analysis was created with workflowr (version 1.3.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20181115) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: .sos/
Ignored: analysis/.sos/
Ignored: dsc/.sos/
Ignored: dsc/benchmark/
Ignored: dsc/dsc_test/.sos/
Ignored: output/
Untracked files:
Untracked: analysis/eval_initial_type1_libsize.Rmd
Unstaged changes:
Modified: analysis/eval_initial.Rmd
Modified: dsc/modules/limma_voom.R
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | dd43766 | Joyce Hsiao | 2019-05-08 | update results |
| html | a4c5501 | Joyce Hsiao | 2019-05-08 | Build site. |
| Rmd | eafe173 | Joyce Hsiao | 2019-05-08 | downstream effects of data transformation methods in null datasets: initial assessment |
Introduction
Applying data transformation methods to single-cell gene expression count and computing type I error rate in null datasets.
Notations:
\(X_{ig}\): gene expression count in cell \(i\) from gene \(g\)
\(X_{i+}\): gene expression count in cell \(i\), i.e., \(X_{i+} = \sum_g X_{ig}\)
\(S_i\): library size normalization factor for cell \(i\)
\(p\): a positive pseudo-count added to expression matrix; traditionally it is used to ensure log-transformation of the expression matrix is well-defined. For now we use pseudo-count of 1.
Data transformation compared:
log2_none_p1: \(log2(X_{ig} + p)\) where \(p=1\).log2_libsum_p1: \(log2(X_{ig}/X_{i+} + p)\) where \(p=1\)log2_libscale_TMM_p1: \(log2(X_{ig}/S_{i} + p)\) where \(p=1\) using edgeR TMM method to estimate \(S_i\).log2_libscale_RLE_p1: \(log2(X_{ig}/S_{i} + p)\) where \(p=1\) using DESeq2 RLE method to estimate \(S_i\).counts_pearsons: Pearson’s residuals of expression counts, derived usingsctransform(Hafemeister and Satija, 2019).
Pipeline compared:
- t-test + all 5 data transformation methods
- DEseq2: count data + RLE transformation
- edgeR: count data + TMM transformation
More about counts_pearson:
For a given gene \(g\), use the sum of all molecules assigned to a cell as a proxy for sequencing depth, and use this cell attribute in a regression model with negative binomial distribution and log link function. Thus, let \(X_g\) be the vector of UMI counts assigned to gene \(g\), and \(m\) be the vector of molecules assigned to the cells, i.e., \(m_i = \sum_i X_{ig}\). For a given \(g\), we have
\(log(E(X_g)) = \beta_0 + \beta_1 log10 m\)
Using the NB parametrization with mean \(\mu\) and variance \(\mu + \mu^2/\theta\),
Pearson’s residuals are defined as:
\(z_{ig} = (X_{ig}-\mu_{ig})/\sigma_{ig}\)
where
\(\mu_{ig} = exp(\beta_{0g} + \beta_{1g}log10 m_i)\),
\(\sigma_{ig} = \sqrt(\mu_{ig} + \mu^2_{ig}/\theta_{g})\)
Data simulation parameters:
- 100 cells (50 vs 50)
- 1K genes randomly drawn
- 100% null genes
- 20 simulated datasets
Required packages
knitr::opts_chunk$set(warning=F, message=F)
library(dscrutils)
library(tidyverse)── Attaching packages ───────────────────────────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.1.0 ✔ purrr 0.3.2
✔ tibble 2.1.1 ✔ dplyr 0.8.0.1
✔ tidyr 0.8.3 ✔ stringr 1.3.1
✔ readr 1.3.1 ✔ forcats 0.3.0 ── Conflicts ──────────────────────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()Simulate data and run methods
#methods <- c("limma_voom", "sva_ttest", "t_test")
library(seqgendiff)
library(sctransform)
#source("dsc/modules/poisthin.R")
source("dsc/modules/filter_genes.R")
source("dsc/modules/transform_data.R")
source("dsc/modules/t_test.R")
source("dsc/modules/wilcoxon.R")
source("dsc/modules/limma_voom.R")
source("dsc/modules/edger.R")
source("dsc/modules/deseq2.R")
counts <- readRDS("dsc/data/pbmc_counts.rds")
nsamp <- 100
ngene <- 1000
prop_null <- 0
libsize_factor <- 0
signal_fun <- function(n) rep(libsize_factor, n)
signal_params <- list()
#pvals_thres <- .001
nsim <- 50
for (i in 1:nsim) {
set.seed(i)
data_obj <- poisthin(t(counts), nsamp=nsamp, ngene=ngene,
signal_params=signal_params, signal_fun=signal_fun,
prop_null = prop_null)
saveRDS(data_obj, file = paste0("output/transform_null.Rmd/data_obj_",i,".rds"))
}
nsim <- 50
transform_methods_list <- c("log2_none_p1", "log2_libsum_p1", "log2_libscale_TMM_p1",
"log2_libscale_RLE_p1", "counts_pearsons")
de_methods_list <- c("edger", "deseq2", "limma_voom", "t_test")
transform_methods_list <- c("log2_none_p1")
de_methods_list <- c("t_test")
out <- do.call(rbind, lapply(1:nsim, function(i) {
data_obj <- readRDS(file = paste0("output/transform_null.Rmd/data_obj_",i,".rds"))
Y <- t(data_obj$Y)
X <- data_obj$X
keep_genes <- filter_genes(Y, min_cell_detected=5)
Y <- Y[keep_genes,]
foo_m <- do.call(rbind, lapply(1:length(de_methods_list), function(j) {
if (de_methods_list[j] == "edger") {
res <- edger(Y=Y, X=X)
pvals <- res$pval
return(data.frame(type1error_01=mean(pvals < .01, na.rm=TRUE),
type1error_005=mean(pvals < .005, na.rm=TRUE),
type1error_001=mean(pvals < .001, na.rm=TRUE),
transform_method = de_methods_list[j],
de_method = de_methods_list[j],
nsim = i))
}
if (de_methods_list[j] == "deseq2") {
res <- deseq2(Y=Y, X=X)
pvals <- res$pval
return(data.frame(type1error_01=mean(pvals < .01, na.rm=TRUE),
type1error_005=mean(pvals < .005, na.rm=TRUE),
type1error_001=mean(pvals < .001, na.rm=TRUE),
transform_method = de_methods_list[j],
de_method = de_methods_list[j],
nsim = i))
}
if (de_methods_list[j] == "limma_voom") {
res <- limma_voom(Y=Y, X=X)
pvals <- res$pval
return(data.frame(type1error_01=mean(pvals < .01, na.rm=TRUE),
type1error_005=mean(pvals < .005, na.rm=TRUE),
type1error_001=mean(pvals < .001, na.rm=TRUE),
transform_method = de_methods_list[j],
de_method = de_methods_list[j],
nsim = i))
}
if (de_methods_list[j] == "t_test") {
foo_t <- do.call(rbind, lapply(1:length(transform_methods_list), function(k) {
if (transform_methods_list[k] == "log2_none_p1") {
transformed_Y <- transform_data(Y, libscale_method = "none",
log="log2", pseudo_count=1)
}
if (transform_methods_list[k] == "log2_libsum_p1") {
transformed_Y <- transform_data(Y, libscale_method = "sum",
log="log2", pseudo_count=1)
}
if (transform_methods_list[k] == "log2_libscale_TMM_p1") {
transformed_Y <- transform_data(Y, libscale_method = "TMM",
log="log2", pseudo_count=1)
}
if (transform_methods_list[k] == "log2_libscale_RLE_p1") {
transformed_Y <- transform_data(Y, libscale_method = "RLE",
log="log2", pseudo_count=1)
}
if (transform_methods_list[k] == "counts_pearsons") {
transformed_Y <- transform_data(Y, libscale_method = "pearsons_residual",
log="none", pseudo_count=1)
}
res <- t_test(transformed_Y, X)
pvals <- res[2,]
return(data.frame(type1error_01=mean(pvals < .01, na.rm=TRUE),
type1error_005=mean(pvals < .005, na.rm=TRUE),
type1error_001=mean(pvals < .001, na.rm=TRUE),
transform_method = transform_methods_list[k],
de_method = de_methods_list[j],
nsim = i))
}) )
return(foo_t)
}
}) )
return(foo_m)
}))
saveRDS(out, file = "output/transform_null.Rmd/type1error.rds")Analysis
#alpha <- .001
out <- readRDS(file = "output/transform_null.Rmd/type1error.rds")
levels(out$transform_method)[1] "edger" "deseq2" "limma_voom"
[4] "log2_none_p1" "log2_libsum_p1" "log2_libscale_TMM_p1"
[7] "log2_libscale_RLE_p1" "counts_pearsons" labels_methods <- c("edger", "deseq2", "limma_voom", "ttest_log2_none_p1",
"ttest_log2_libsum_p1", "ttest_log2_libscale_TMM_p1",
"ttest_log2_libscale_RLE_p1", "ttest_counts_pearsons")
out %>% #filter(n1==50) %>%
group_by(de_method, transform_method) %>%
ggplot(., aes(x=transform_method, y=type1error_001, col=transform_method)) +
# facet_wrap(~de_method) +
geom_boxplot() + geom_point() + xlab("Type 1 error at alpha < .001") +
geom_hline(yintercept = .001, col="gray50") +
ylab("Type I error") +
scale_x_discrete(position = "top",
labels=labels_methods) +
theme(axis.text.x=element_text(angle = 20, vjust = -.3, hjust=.1)) +
stat_summary(fun.y=mean, geom="point", shape=4, size=4, col="black")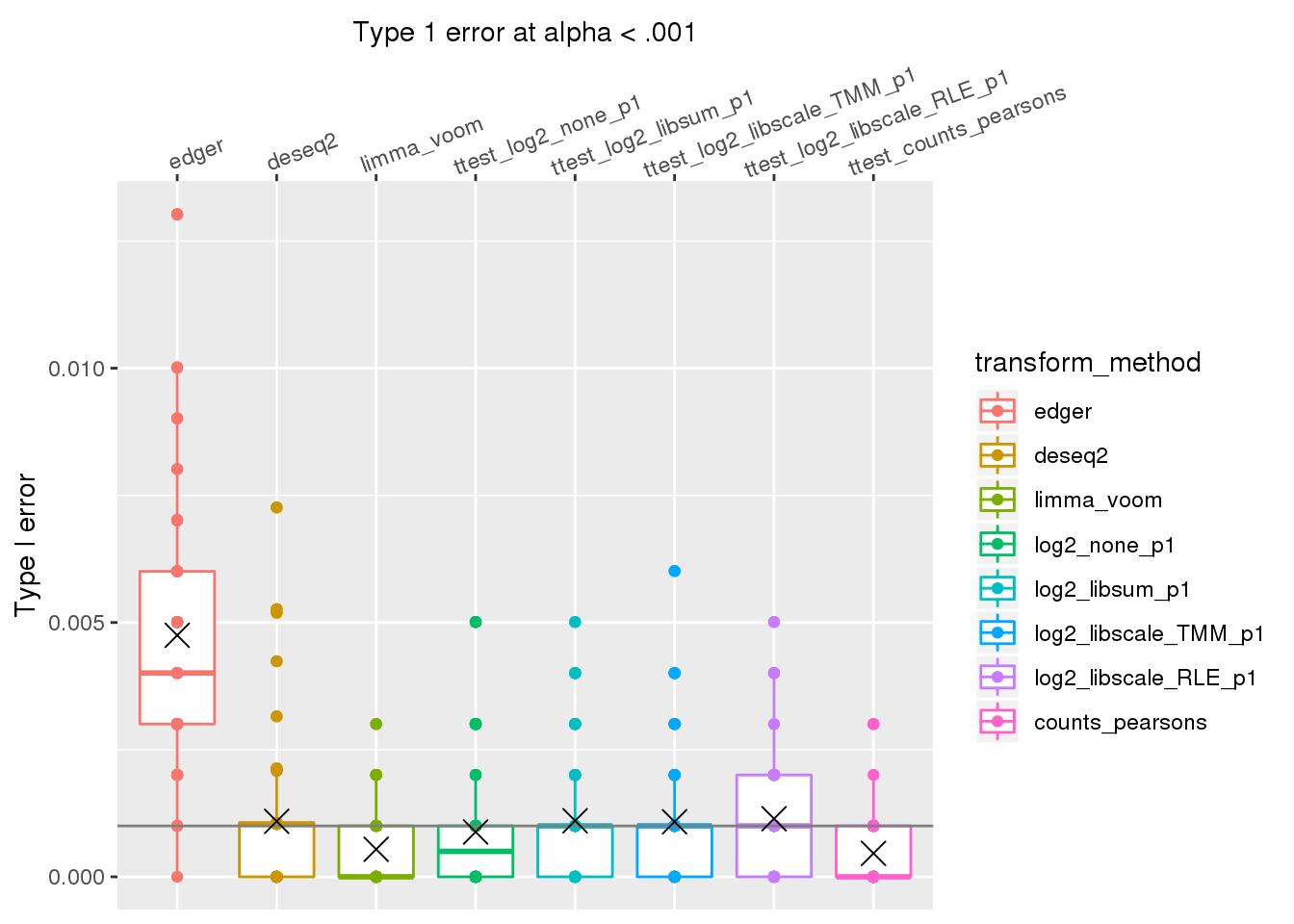
| Version | Author | Date |
|---|---|---|
| a4c5501 | Joyce Hsiao | 2019-05-08 |
out %>% #filter(n1==50) %>%
group_by(de_method, transform_method) %>%
ggplot(., aes(x=transform_method, y=type1error_01, col=transform_method)) +
# facet_wrap(~de_method) +
geom_boxplot() + geom_point() + xlab("Type 1 error at alpha < .01") +
geom_hline(yintercept = .01, col="gray50") +
ylab("Type I error") +
scale_x_discrete(position = "top",
labels=labels_methods) +
theme(axis.text.x=element_text(angle = 20, vjust = -.3, hjust=.1)) +
stat_summary(fun.y=mean, geom="point", shape=4, size=4, col="black")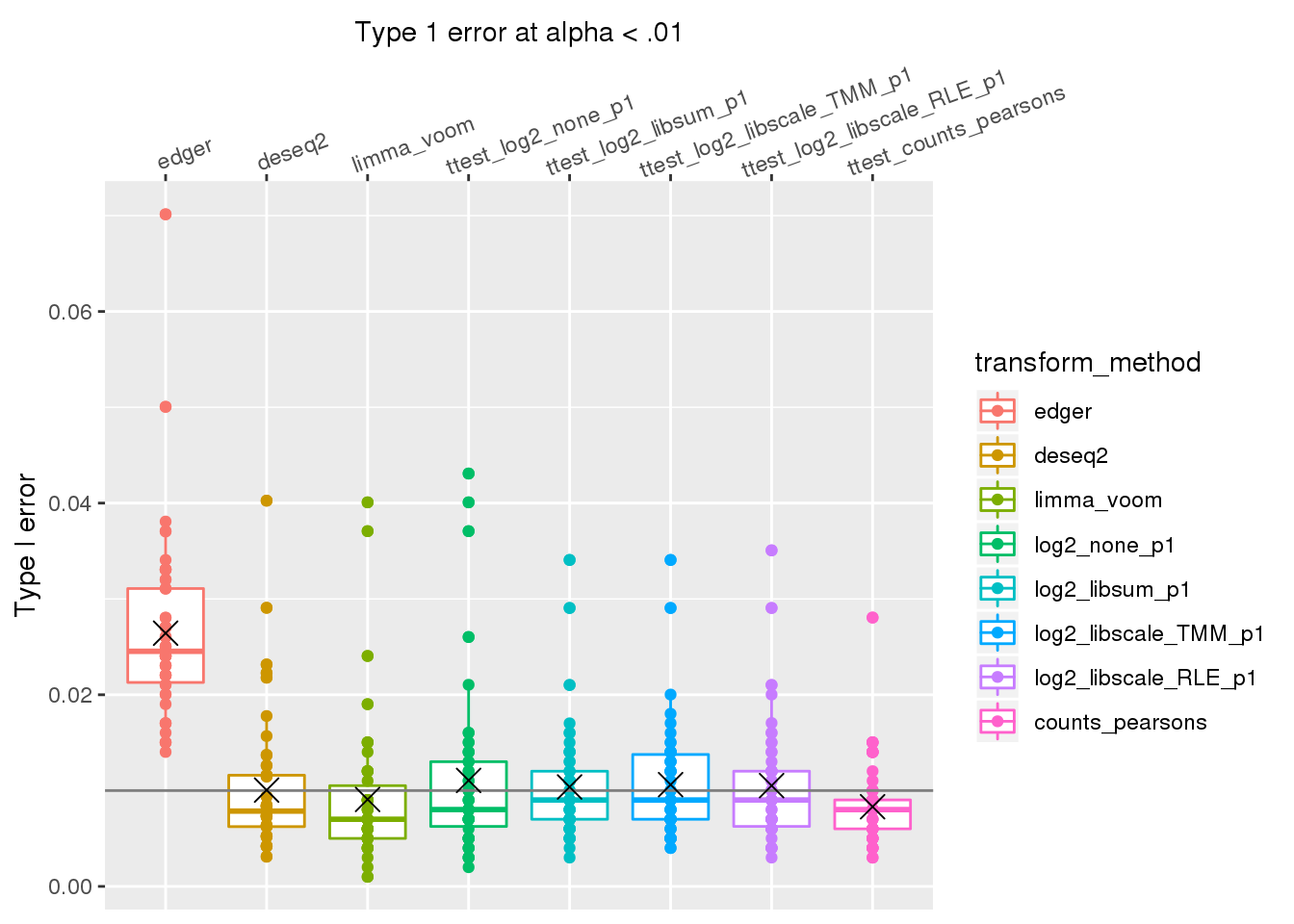
| Version | Author | Date |
|---|---|---|
| a4c5501 | Joyce Hsiao | 2019-05-08 |
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] forcats_0.3.0 stringr_1.3.1 dplyr_0.8.0.1 purrr_0.3.2
[5] readr_1.3.1 tidyr_0.8.3 tibble_2.1.1 ggplot2_3.1.0
[9] tidyverse_1.2.1 dscrutils_0.3.8
loaded via a namespace (and not attached):
[1] Rcpp_1.0.1 cellranger_1.1.0 plyr_1.8.4 compiler_3.5.1
[5] pillar_1.3.1 git2r_0.23.0 workflowr_1.3.0 tools_3.5.1
[9] digest_0.6.18 lubridate_1.7.4 jsonlite_1.6 evaluate_0.12
[13] nlme_3.1-137 gtable_0.2.0 lattice_0.20-38 pkgconfig_2.0.2
[17] rlang_0.3.4 cli_1.0.1 rstudioapi_0.10 yaml_2.2.0
[21] haven_1.1.2 withr_2.1.2 xml2_1.2.0 httr_1.3.1
[25] knitr_1.20 hms_0.4.2 generics_0.0.2 fs_1.2.6
[29] rprojroot_1.3-2 grid_3.5.1 tidyselect_0.2.5 glue_1.3.0
[33] R6_2.4.0 readxl_1.1.0 rmarkdown_1.10 modelr_0.1.2
[37] magrittr_1.5 whisker_0.3-2 backports_1.1.2 scales_1.0.0
[41] htmltools_0.3.6 rvest_0.3.2 assertthat_0.2.0 colorspace_1.3-2
[45] labeling_0.3 stringi_1.2.4 lazyeval_0.2.1 munsell_0.5.0
[49] broom_0.5.1 crayon_1.3.4