A another look at matrix factorization for the pancreas data
Peter Carbonetto
Last updated: 2024-12-13
Checks: 7 0
Knit directory:
single-cell-jamboree/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(1) was run prior to running the
code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version b6c1981. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Untracked files:
Untracked: data/Immune_ALL_human.h5ad
Untracked: scripts/pancreas_smartseq2_factors.RData
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/pancreas_another_look.Rmd)
and HTML (docs/pancreas_another_look.html) files. If you’ve
configured a remote Git repository (see ?wflow_git_remote),
click on the hyperlinks in the table below to view the files as they
were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | b6c1981 | Peter Carbonetto | 2024-12-13 | workflowr::wflow_publish("analysis/pancreas_another_look.Rmd", |
| Rmd | 3283b84 | Peter Carbonetto | 2024-12-13 | Added some structure plots to the pancreas_another_look analysis. |
| Rmd | e264e5b | Peter Carbonetto | 2024-12-12 | Created draft analysis in pancreas_celseq2.R; this will be incorporated into pancreas_another_look.Rmd. |
| html | c01f832 | Peter Carbonetto | 2024-12-12 | First build of the pancreas_another_look analysis. |
| Rmd | 04b172c | Peter Carbonetto | 2024-12-12 | workflowr::wflow_publish("pancreas_another_look.Rmd", view = FALSE) |
The previous analysis applied different matrix factorization approaches to the full pancreas data set. A key challenge in analyzing the full pancreas data set is that there are large batch or data-set effects, which some matrix factorization approaches have difficulty dealing with (particularly the topic model). Here we look more closely at a couple of the individual data sets to highlight better how the different factorizations yield different representations of the underlying structure in the cells without the added complication of dealing with the batch effects.
First, load the packages needed for this analysis.
library(Matrix)
library(fastTopics)
library(ggplot2)
library(cowplot)Set the seed for reproducibility.
set.seed(1)CEL-Seq2 data
Let’s start with the “CEL-Seq2” data from the Muraro et al 2016 paper. (The data were generated using the CEL-Seq2 protocol.)
First load the CEL-Seq2 pancreas data and the outputs generated by
running the compute_pancreas_celseq2_factors.R script.
load("../data/pancreas.RData")
load("../output/pancreas_celseq2_factors.RData")
i <- which(sample_info$tech == "celseq2")
sample_info <- sample_info[i,]
counts <- counts[i,]
sample_info <- transform(sample_info,celltype = factor(celltype))For fair comparison all the matrix factorizations were generated with 9 factors or topics.
Topic model (fastTopics)
Here is the topic model with 9 topics:
celltype <- sample_info$celltype
celltype <-
factor(celltype,
c("acinar","ductal","activated_stellate","quiescent_stellate",
"endothelial","macrophage","mast","schwann","t_cell","alpha",
"beta","delta","gamma","epsilon"))
L <- poisson2multinom(pnmf)$L
structure_plot(L,grouping = celltype,gap = 20,perplexity = 70,n = Inf)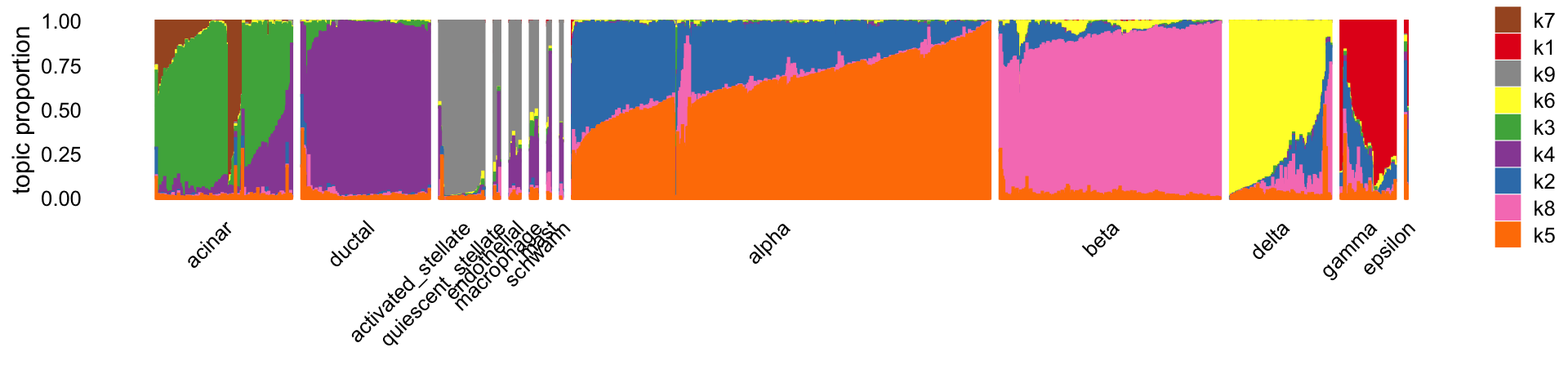
Most of the topics identify individual cell types or subsets of similar cell types. The topics also identify some substructures within and across cell types, e.g., topics 2 and 7. Most of the smaller cell types are not captured as a separate topic.
Flashier NMF
Let’s now compare the topic model to the empirical Bayes NMF result (with 9 factors).
I omit the first factor from the Structure plot because it is a “baseline” factor, and therefore not interesting to look at:
L <- fl_nmf_ldf$L
k <- ncol(L)
colnames(L) <- paste0("k",1:k)
structure_plot(L[,-1],grouping = celltype,gap = 20,perplexity = 70,n = Inf) +
labs(y = "membership",fill = "factor",color = "factor")
It is interesting that the EBNMF factors distinguish some of the rare cell types, but do not distinguish as well among some of the islet cells (e.g., delta and gamma), even though they are quite abundant. It seems that these methods are each adept at identifying different types of structure.
NMF (NNLM)
Let’s now have a look at the “vanilla” NMF (produced by the NNLM package). As before, this NMF has 9 factors.
scale_cols <- function (A, b)
t(t(A) * b)
W <- nmf$W
k <- ncol(W)
d <- apply(W,2,max)
W <- scale_cols(W,1/d)
colnames(W) <- paste0("k",1:k)
structure_plot(W,grouping = celltype,gap = 20,perplexity = 70,n = Inf) +
labs(y = "membership",fill = "factor",color = "factor")
Unlike the EBNMF results, there is no single factor that acts as a “baseline”. Some of the rarer cell types are missed by this NMF. It is also interesting that it has identified a single factor (k = 4) corresponding to all islet cells. Oddly, it seems to have identified factors that are active in the same cells, such as factors 3 and 6, as well as 7 and 8. So the NMF and EBNMF results are surprisingly different.
Flashier semi-NMF
The semi-NMF decomposition produced by flashier is interesting because it identifies not only cell-type-specific factors (e.g., factors 6, 7, 9), but also factors capturing expression programs common to several similar cell types (e.g., factors 2, 4, 8).
L <- fl_snmf_ldf$L
k <- ncol(L)
colnames(L) <- paste0("k",1:k)
structure_plot(L[,-c(1,5)],grouping = celltype,gap = 20,
perplexity = 70,n = Inf)
In other words, the semi-NMF is capturing structure at different levels of cell-type-specifity, achieving a cell-type “hierarchy” of sorts. Note that I removed two factors (1 and 5) from the Structure plot because they were active to varying degreees in all cells.
Smart-seq2 data
sessionInfo()
# R version 4.3.3 (2024-02-29)
# Platform: aarch64-apple-darwin20 (64-bit)
# Running under: macOS Sonoma 14.7.1
#
# Matrix products: default
# BLAS: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRblas.0.dylib
# LAPACK: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
#
# locale:
# [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
#
# time zone: America/Chicago
# tzcode source: internal
#
# attached base packages:
# [1] stats graphics grDevices utils datasets methods base
#
# other attached packages:
# [1] cowplot_1.1.3 ggplot2_3.5.0 fastTopics_0.6-193 Matrix_1.6-5
#
# loaded via a namespace (and not attached):
# [1] gtable_0.3.4 xfun_0.42 bslib_0.6.1
# [4] htmlwidgets_1.6.4 ggrepel_0.9.5 lattice_0.22-5
# [7] quadprog_1.5-8 vctrs_0.6.5 tools_4.3.3
# [10] generics_0.1.3 parallel_4.3.3 tibble_3.2.1
# [13] fansi_1.0.6 highr_0.10 pkgconfig_2.0.3
# [16] data.table_1.15.2 SQUAREM_2021.1 RcppParallel_5.1.7
# [19] lifecycle_1.0.4 truncnorm_1.0-9 farver_2.1.1
# [22] compiler_4.3.3 stringr_1.5.1 git2r_0.33.0
# [25] progress_1.2.3 munsell_0.5.0 RhpcBLASctl_0.23-42
# [28] httpuv_1.6.14 htmltools_0.5.7 sass_0.4.8
# [31] yaml_2.3.8 lazyeval_0.2.2 plotly_4.10.4
# [34] crayon_1.5.2 later_1.3.2 pillar_1.9.0
# [37] jquerylib_0.1.4 whisker_0.4.1 tidyr_1.3.1
# [40] uwot_0.2.2.9000 cachem_1.0.8 gtools_3.9.5
# [43] tidyselect_1.2.1 digest_0.6.34 Rtsne_0.17
# [46] stringi_1.8.3 dplyr_1.1.4 purrr_1.0.2
# [49] ashr_2.2-66 labeling_0.4.3 rprojroot_2.0.4
# [52] fastmap_1.1.1 grid_4.3.3 colorspace_2.1-0
# [55] cli_3.6.2 invgamma_1.1 magrittr_2.0.3
# [58] utf8_1.2.4 withr_3.0.0 prettyunits_1.2.0
# [61] scales_1.3.0 promises_1.2.1 rmarkdown_2.26
# [64] httr_1.4.7 workflowr_1.7.1 hms_1.1.3
# [67] pbapply_1.7-2 evaluate_0.23 knitr_1.45
# [70] viridisLite_0.4.2 irlba_2.3.5.1 rlang_1.1.3
# [73] Rcpp_1.0.12 mixsqp_0.3-54 glue_1.7.0
# [76] jsonlite_1.8.8 R6_2.5.1 fs_1.6.3