Visualize and interpret droplet and pulse-seq topics
Peter Carbonetto
Last updated: 2020-08-19
Checks: 7 0
Knit directory: single-cell-topics/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2.9000). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(1) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version fb91075. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: data/droplet.RData
Ignored: data/pbmc_68k.RData
Ignored: data/pbmc_purified.RData
Ignored: data/pulseseq.RData
Ignored: output/droplet/fits-droplet.RData
Ignored: output/droplet/rds/
Ignored: output/pbmc-68k/fits-pbmc-68k.RData
Ignored: output/pbmc-68k/rds/
Ignored: output/pbmc-purified/fits-pbmc-purified.RData
Ignored: output/pbmc-purified/rds/
Ignored: output/pulseseq/fits-pulseseq.RData
Ignored: output/pulseseq/rds/
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/plots_tracheal_epithelium.Rmd) and HTML (docs/plots_tracheal_epithelium.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | fb91075 | Peter Carbonetto | 2020-08-19 | wflow_publish(“plots_tracheal_epithelium.Rmd”) |
| html | 8b9b528 | Peter Carbonetto | 2020-08-19 | Added more PCA plots to plots_tracheal_epithelium analysis. |
| Rmd | ee7cbf1 | Peter Carbonetto | 2020-08-19 | wflow_publish(“plots_tracheal_epithelium.Rmd”) |
| html | c517ea2 | Peter Carbonetto | 2020-08-18 | Small fix to one of the PCA plots in plots_tracheal_epithelium. |
| Rmd | 8f5c210 | Peter Carbonetto | 2020-08-18 | wflow_publish(“plots_tracheal_epithelium.Rmd”) |
| html | 01afbd2 | Peter Carbonetto | 2020-08-18 | Added some PC plots to the plots_tracheal_epithelium analysis. |
| Rmd | f1c7d02 | Peter Carbonetto | 2020-08-18 | wflow_publish(“plots_tracheal_epithelium.Rmd”) |
| html | 0a04fc1 | Peter Carbonetto | 2020-08-18 | Added abundance plots to plots_tracheal_epithelium analysis. |
| Rmd | f914f7e | Peter Carbonetto | 2020-08-18 | wflow_publish(“plots_tracheal_epithelium.Rmd”) |
| Rmd | 61917ad | Peter Carbonetto | 2020-08-18 | Working on new analysis, plots_tracheal_epithelium.Rmd. |
TO DO: Add introductory text here.
Load the packages used in the analysis below.
# library(fastTopics)
devtools::load_all("~/git/fastTopics")
library(ggplot2)
library(cowplot)
source("../code/plots.R")Load the sample annotations. (The count data are no longer needed at this stage of the analysis.)
load("../data/droplet.RData")
samples_droplet <- samples
load("../data/pulseseq.RData")
samples_pulseseq <- samples
rm(samples,counts)Load the \(k = 7\) Poisson NMF model fit for the droplet data, and the \(k = 11\) Poisson NMF fit for the pulse-seq data.
fit_droplet <- readRDS("../output/droplet/rds/fit-droplet-scd-ex-k=7.rds")$fit
fit_pulseseq <- readRDS("../output/pulseseq/rds/fit-pulseseq-scd-ex-k=11.rds")$fitRare and abundant cell types in the droplet data:
p1 <- create_abundance_plot(fit_droplet)
print(p1)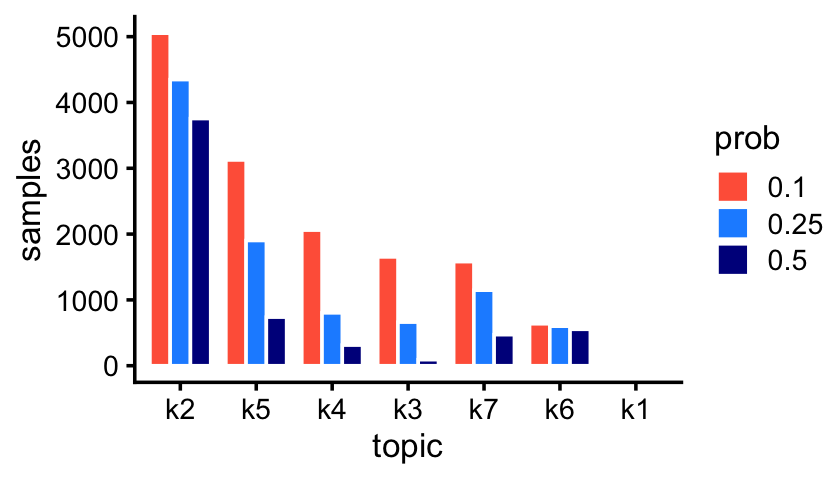
| Version | Author | Date |
|---|---|---|
| 0a04fc1 | Peter Carbonetto | 2020-08-18 |
The first topic is indeed very rare; only 43 samples have a greater than 10% contribution from this topic.
sum(poisson2multinom(fit_droplet)$L[,1] > 0.1)
# [1] 43Rare and abundant cell types in the pulse-seq data:
p2 <- create_abundance_plot(fit_pulseseq)
print(p2)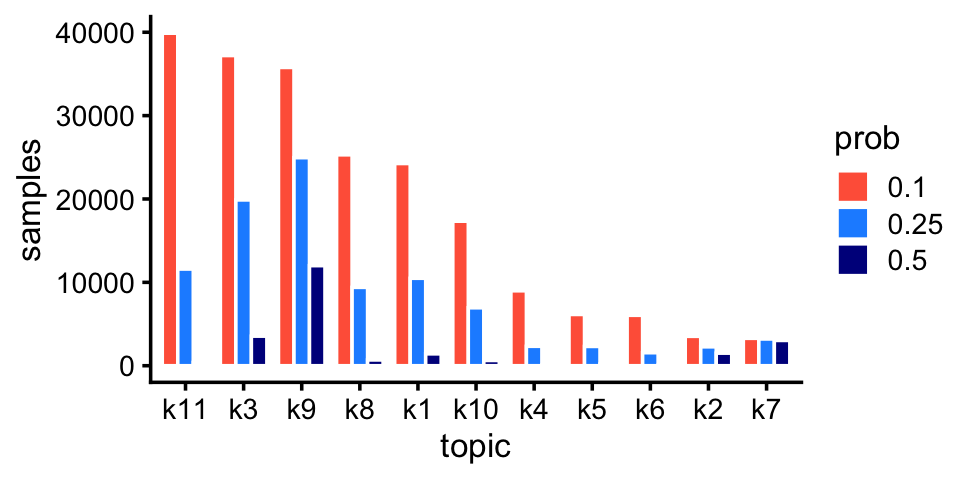
| Version | Author | Date |
|---|---|---|
| 0a04fc1 | Peter Carbonetto | 2020-08-18 |
[Explain why we don’t use t-SNE.] Substructure is evident from principal components of droplet topic proportions:
p3 <- basic_pca_plot(fit_droplet,c("PC1","PC2"))
p4 <- basic_pca_plot(fit_droplet,c("PC4","PC5"))
p5 <- basic_pca_plot(fit_droplet,c("PC5","PC6"))
plot_grid(p3,p4,p5,nrow = 1)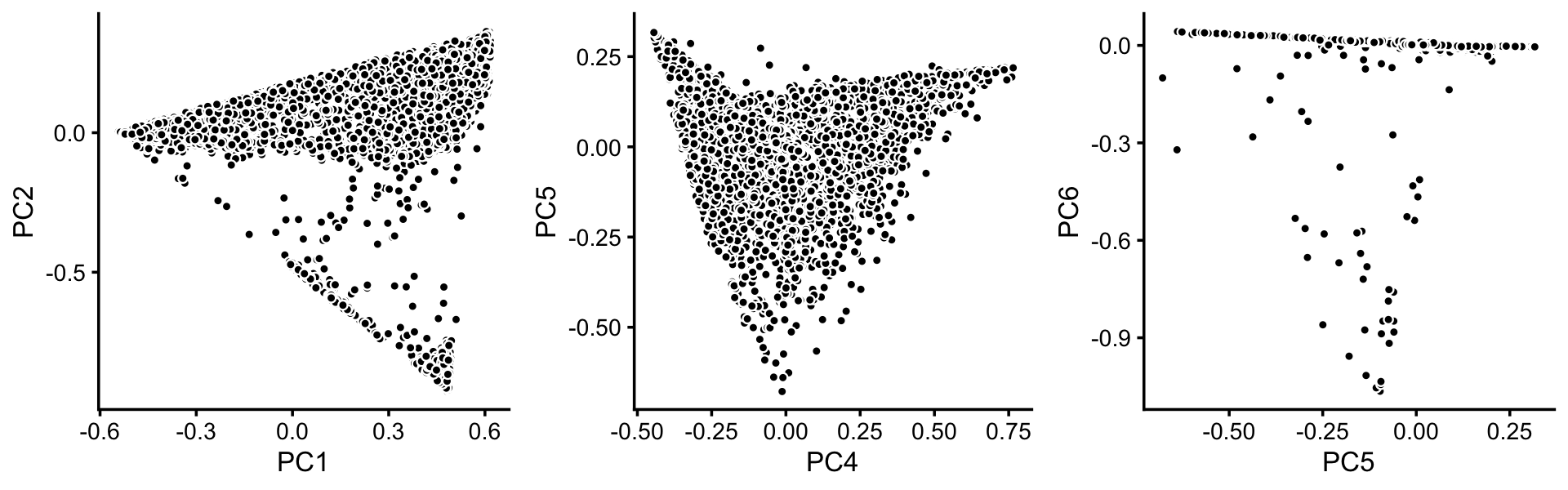
TO DO: Add PC plots with topic proportions here.
p6 <- pca_plot(poisson2multinom(fit_droplet),pcs = 1:2,k = c(2,5,6),
plot_grid_call = function (plots)
do.call(plot_grid,c(lapply(plots,
function (x) x + guides(fill = "none")),list(nrow = 1))))
print(p6)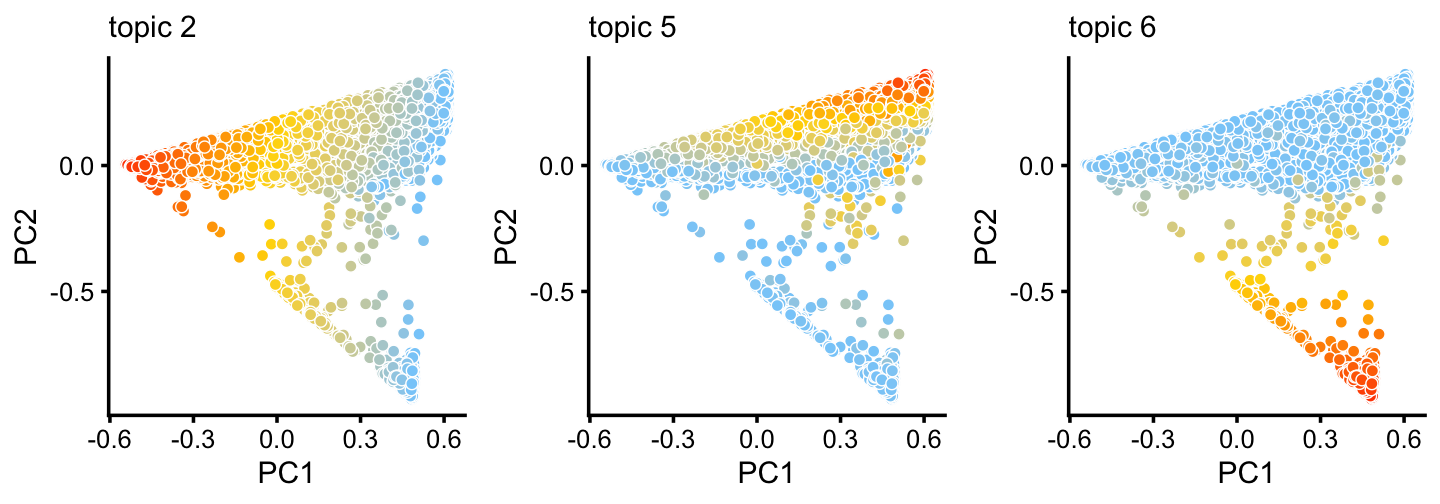
| Version | Author | Date |
|---|---|---|
| 8b9b528 | Peter Carbonetto | 2020-08-19 |
Principal components 4 and 5:
p7 <- pca_plot(poisson2multinom(fit_droplet),pcs = 4:5,k = c(3,4,7),
plot_grid_call = function (plots)
do.call(plot_grid,c(lapply(plots,
function (x) x + guides(fill = "none")),list(nrow = 1))))
print(p7)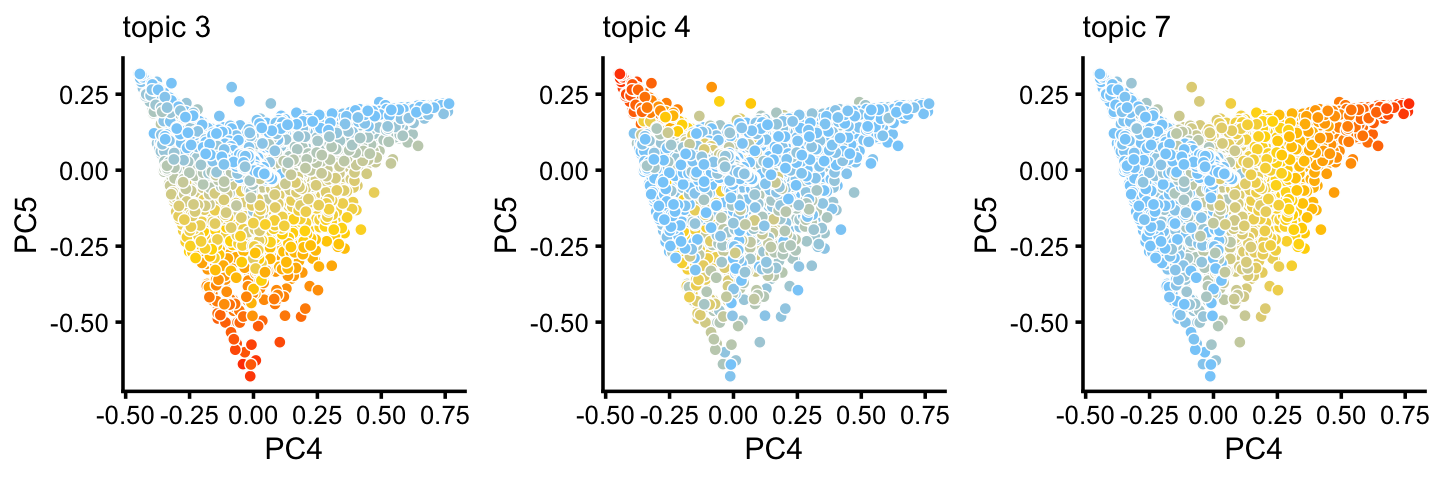
| Version | Author | Date |
|---|---|---|
| 8b9b528 | Peter Carbonetto | 2020-08-19 |
Principal components 5 and 6:
p8 <- pca_plot(poisson2multinom(fit_droplet),pcs = 5:6,k = 1) +
guides(fill = "none")
print(p8)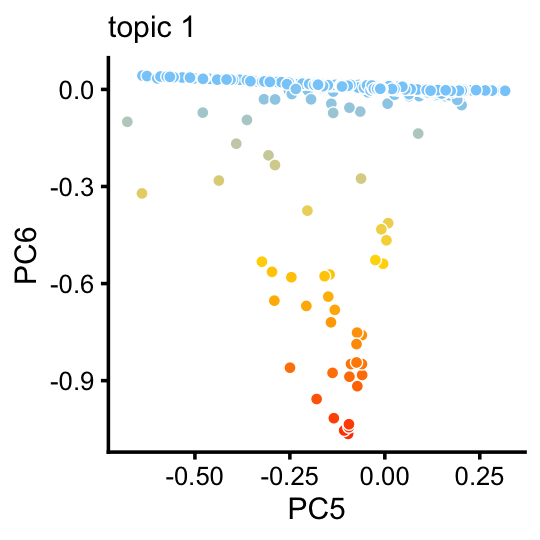
| Version | Author | Date |
|---|---|---|
| 8b9b528 | Peter Carbonetto | 2020-08-19 |
[Note that PCs 3 and 7 does not reveal any additional substructure, so are not included in our plots.]
We won’t dwell much on the clustering reported in the Montoro et al (2018) paper, but for comparison we layer the 7 clusters on top of these PCs:
droplet_celltype_colors <-
c("royalblue", # Basal
"firebrick", # Ciliated
"forestgreen", # Club
"gold", # Goblet
"darkmagenta", # Ionocyte
"darkorange", # Neuroendocrine
"lightsteelblue") # Tuft
p9 <- pca_plot_with_labels(fit_droplet,c("PC1","PC2"),samples_droplet$tissue,
droplet_celltype_colors) + labs(fill = "celltype")
p10 <- pca_plot_with_labels(fit_droplet,c("PC4","PC5"),samples_droplet$tissue,
droplet_celltype_colors) + labs(fill = "celltype")
p11 <- pca_plot_with_labels(fit_droplet,c("PC5","PC6"),samples_droplet$tissue,
droplet_celltype_colors) + labs(fill = "celltype")
plot_grid(p9,p10,p11)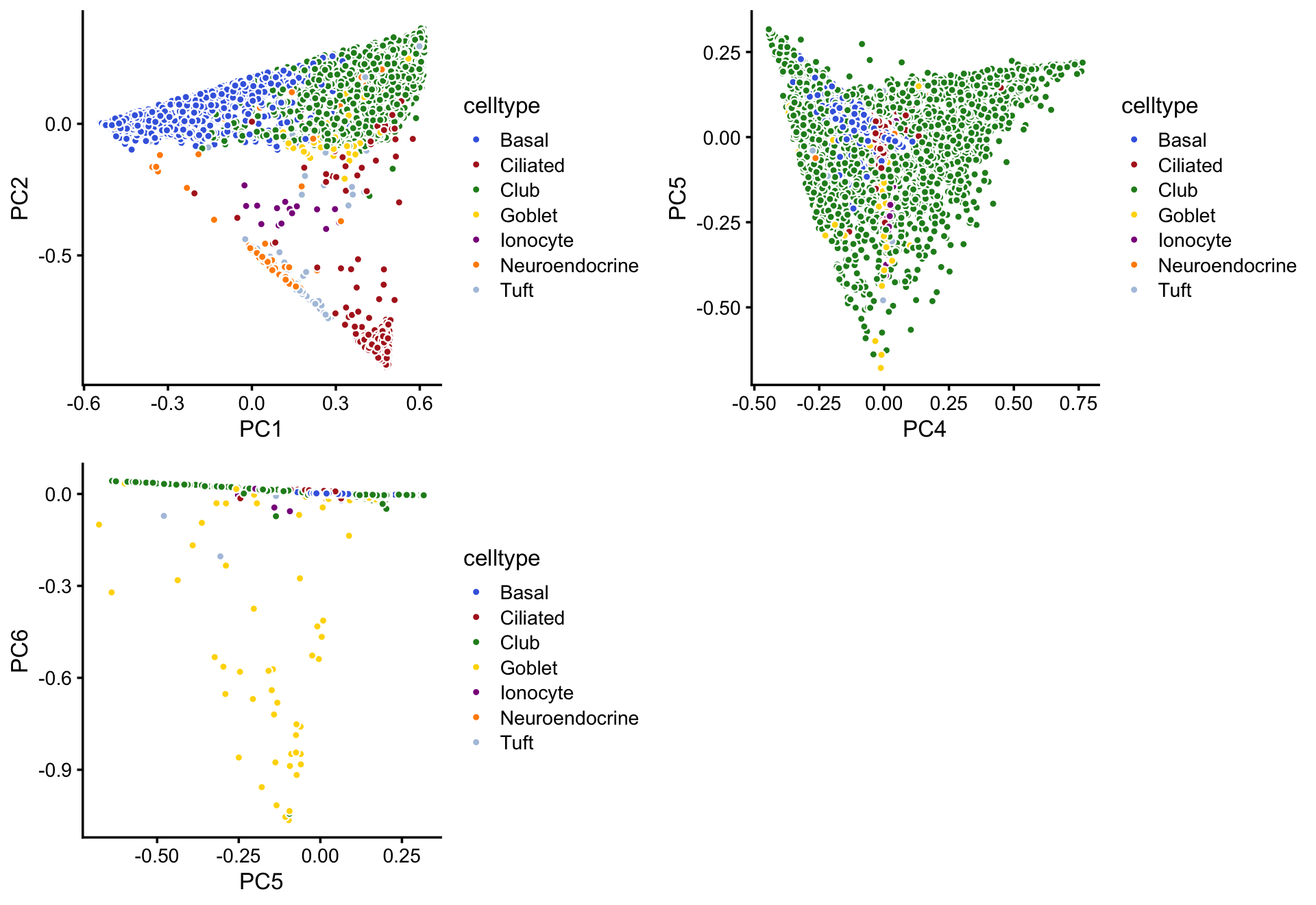
And likewise in the pulse-seq data:
p5 <- basic_pca_plot(fit_pulseseq,c("PC3","PC4"))
p6 <- basic_pca_plot(fit_pulseseq,c("PC5","PC6"))
plot_grid(p5,p6)ggplot(cbind(pca$x,data.frame(k2 = fit$L[,2])),
aes(x = PC1,y = PC2,fill = k2)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_gradient2(low = "deepskyblue",mid = "gold",high = "orangered",
midpoint = 0.5) +
theme_cowplot(font_size = 12)
ggplot(cbind(pca$x,data.frame(k5 = fit$L[,5])),
aes(x = PC1,y = PC2,fill = k5)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_gradient2(low = "deepskyblue",mid = "gold",high = "orangered",
midpoint = 0.5) +
theme_cowplot(font_size = 12)
ggplot(cbind(pca$x,data.frame(k6 = fit$L[,6])),
aes(x = PC1,y = PC2,fill = k6)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_gradient2(low = "deepskyblue",mid = "gold",high = "orangered",
midpoint = 0.5) +
theme_cowplot(font_size = 12)
ggplot(cbind(pca$x,data.frame(k1 = fit$L[,1])),
aes(x = PC5,y = PC6,fill = k1)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_gradient2(low = "deepskyblue",mid = "gold",high = "orangered",
midpoint = 0.5) +
theme_cowplot(font_size = 12)ggplot(cbind(pca$x,data.frame(k3 = fit$L[,3])),
aes(x = PC1,y = PC2,fill = k3)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_gradient2(low = "deepskyblue",mid = "gold",high = "orangered",
midpoint = 0.5) +
theme_cowplot(font_size = 12)
ggplot(cbind(pca$x,data.frame(k4 = fit$L[,4])),
aes(x = PC1,y = PC2,fill = k4)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_gradient2(low = "deepskyblue",mid = "gold",high = "orangered",
midpoint = 0.5) +
theme_cowplot(font_size = 12)ggplot(cbind(samples_droplet,pca$x),aes(x = PC1,y = PC2,fill = tissue)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_manual(values = clrs) +
theme_cowplot(font_size = 12)
ggplot(cbind(samples_droplet,pca$x),aes(x = PC5,y = PC6,fill = tissue)) +
geom_point(shape = 21,color = "white",,size = 2) +
scale_fill_manual(values = clrs) +
theme_cowplot(font_size = 12)clrs <- c("royalblue", # basal
"firebrick", # ciliated
"forestgreen", # club
"gold", # goblet
"darkmagenta", # ionocyte
"darkorange", # neuroendocrine
"tomato", # proliferating
"darkgray") # tuft
ggplot(cbind(samples_droplet,pca$x),aes(x = PC1,y = PC2,fill = tissue)) +
geom_point(shape = 21,color = "white",size = 2) +
scale_fill_manual(values = clrs) +
theme_cowplot(font_size = 12)
ggplot(cbind(samples_droplet,pca$x),aes(x = PC5,y = PC6,fill = tissue)) +
geom_point(shape = 21,color = "white",,size = 2) +
scale_fill_manual(values = clrs) +
theme_cowplot(font_size = 12)
sessionInfo()
# R version 3.6.2 (2019-12-12)
# Platform: x86_64-apple-darwin15.6.0 (64-bit)
# Running under: macOS Catalina 10.15.5
#
# Matrix products: default
# BLAS: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRblas.0.dylib
# LAPACK: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRlapack.dylib
#
# locale:
# [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
#
# attached base packages:
# [1] stats graphics grDevices utils datasets methods base
#
# other attached packages:
# [1] cowplot_1.0.0 ggplot2_3.3.0 fastTopics_0.3-160 testthat_2.3.1
#
# loaded via a namespace (and not attached):
# [1] httr_1.4.1 tidyr_1.0.0 pkgload_1.0.2
# [4] viridisLite_0.3.0 jsonlite_1.6 RcppParallel_4.4.4
# [7] assertthat_0.2.1 yaml_2.2.0 remotes_2.1.0
# [10] progress_1.2.2 ggrepel_0.9.0 sessioninfo_1.1.1
# [13] pillar_1.4.3 backports_1.1.5 lattice_0.20-38
# [16] quantreg_5.54 glue_1.3.1 quadprog_1.5-8
# [19] digest_0.6.23 promises_1.1.0 colorspace_1.4-1
# [22] htmltools_0.4.0 httpuv_1.5.2 Matrix_1.2-18
# [25] pkgconfig_2.0.3 devtools_2.2.1 SparseM_1.78
# [28] purrr_0.3.3 scales_1.1.0 processx_3.4.1
# [31] whisker_0.4 later_1.0.0 Rtsne_0.15
# [34] MatrixModels_0.4-1 git2r_0.26.1 tibble_2.1.3
# [37] farver_2.0.1 usethis_1.6.0 ellipsis_0.3.0
# [40] withr_2.1.2 lazyeval_0.2.2 cli_2.0.0
# [43] magrittr_1.5 crayon_1.3.4 memoise_1.1.0
# [46] mcmc_0.9-6 evaluate_0.14 ps_1.3.0
# [49] fs_1.3.1 fansi_0.4.0 MASS_7.3-51.4
# [52] pkgbuild_1.0.6 tools_3.6.2 data.table_1.12.8
# [55] prettyunits_1.1.1 hms_0.5.2 lifecycle_0.1.0
# [58] stringr_1.4.0 MCMCpack_1.4-5 plotly_4.9.2
# [61] munsell_0.5.0 irlba_2.3.3 callr_3.4.0
# [64] compiler_3.6.2 rlang_0.4.5 grid_3.6.2
# [67] rstudioapi_0.10 htmlwidgets_1.5.1 labeling_0.3
# [70] rmarkdown_2.3 gtable_0.3.0 R6_2.4.1
# [73] knitr_1.26 dplyr_0.8.3 zeallot_0.1.0
# [76] workflowr_1.6.2.9000 rprojroot_1.3-2 desc_1.2.0
# [79] stringi_1.4.3 Rcpp_1.0.3 vctrs_0.2.1
# [82] tidyselect_0.2.5 xfun_0.11 coda_0.19-3