symebcovmf_tree
Annie Xie
2025-04-08
Last updated: 2025-04-08
Checks: 7 0
Knit directory:
symmetric_covariance_decomposition/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20250408) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 1be0238. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .DS_Store
Unstaged changes:
Modified: analysis/index.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/symebcovmf_tree.Rmd) and
HTML (docs/symebcovmf_tree.html) files. If you’ve
configured a remote Git repository (see ?wflow_git_remote),
click on the hyperlinks in the table below to view the files as they
were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 1be0238 | Annie Xie | 2025-04-08 | Add symebcovmf analysis files |
Introduction
In this example, we test out symEBcovMF on tree-structured data.
Example
library(ebnm)
library(pheatmap)
library(ggplot2)source('code/symebcovmf_functions.R')
source('code/visualization_functions.R')Data Generation
sim_4pops <- function(args) {
set.seed(args$seed)
n <- sum(args$pop_sizes)
p <- args$n_genes
FF <- matrix(rnorm(7 * p, sd = rep(args$branch_sds, each = p)), ncol = 7)
# if (args$constrain_F) {
# FF_svd <- svd(FF)
# FF <- FF_svd$u
# FF <- t(t(FF) * branch_sds * sqrt(p))
# }
LL <- matrix(0, nrow = n, ncol = 7)
LL[, 1] <- 1
LL[, 2] <- rep(c(1, 1, 0, 0), times = args$pop_sizes)
LL[, 3] <- rep(c(0, 0, 1, 1), times = args$pop_sizes)
LL[, 4] <- rep(c(1, 0, 0, 0), times = args$pop_sizes)
LL[, 5] <- rep(c(0, 1, 0, 0), times = args$pop_sizes)
LL[, 6] <- rep(c(0, 0, 1, 0), times = args$pop_sizes)
LL[, 7] <- rep(c(0, 0, 0, 1), times = args$pop_sizes)
E <- matrix(rnorm(n * p, sd = args$indiv_sd), nrow = n)
Y <- LL %*% t(FF) + E
YYt <- (1/p)*tcrossprod(Y)
return(list(Y = Y, YYt = YYt, LL = LL, FF = FF, K = ncol(LL)))
}sim_args = list(pop_sizes = rep(40, 4), n_genes = 1000, branch_sds = rep(2,7), indiv_sd = 1, seed = 1)
sim_data <- sim_4pops(sim_args)This is a heatmap of the scaled Gram matrix:
plot_heatmap(sim_data$YYt, colors_range = c('blue','gray96','red'), brks = seq(-max(abs(sim_data$YYt)), max(abs(sim_data$YYt)), length.out = 50))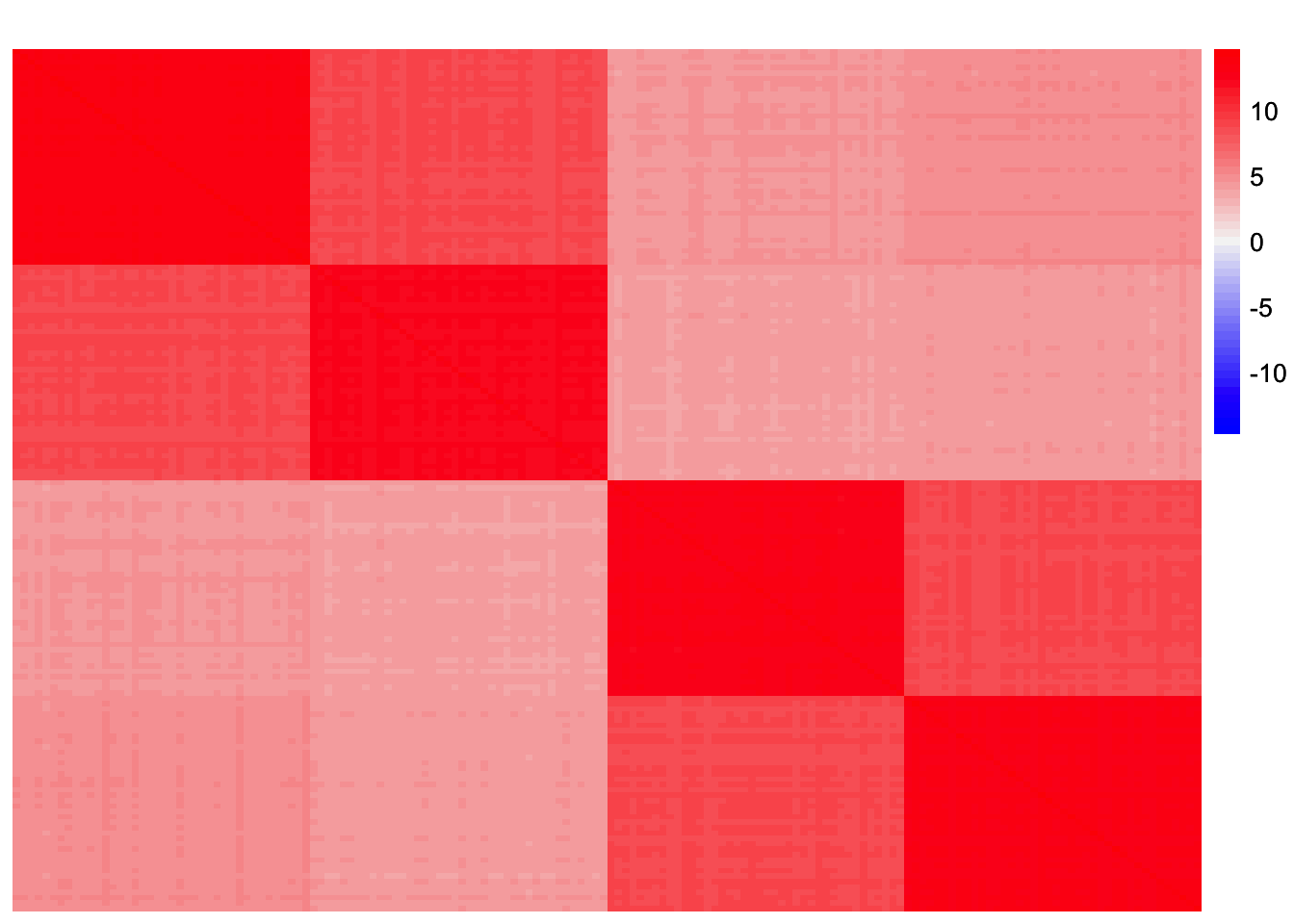
This is a scatter plot of the true loadings matrix:
pop_vec <- c(rep('A', 40), rep('B', 40), rep('C', 40), rep('D', 40))
plot_loadings(sim_data$LL, pop_vec)
symEBcovMF
symebcovmf_tree_fit <- sym_ebcovmf_fit(S = sim_data$YYt, ebnm_fn = ebnm_point_exponential, K = 7, maxiter = 100, rank_one_tol = 10^(-8), tol = 10^(-8))[1] "Warning: scaling factor is zero"
[1] "Adding factor 4 does not improve the objective function"Progression of ELBO
symebcovmf_tree_full_elbo_vec <- symebcovmf_tree_fit$vec_elbo_full[!(symebcovmf_tree_fit$vec_elbo_full %in% c(1:length(symebcovmf_tree_fit$vec_elbo_K)))]
ggplot() + geom_line(data = NULL, aes(x = 1:length(symebcovmf_tree_full_elbo_vec), y = symebcovmf_tree_full_elbo_vec)) + xlab('Iter') + ylab('ELBO')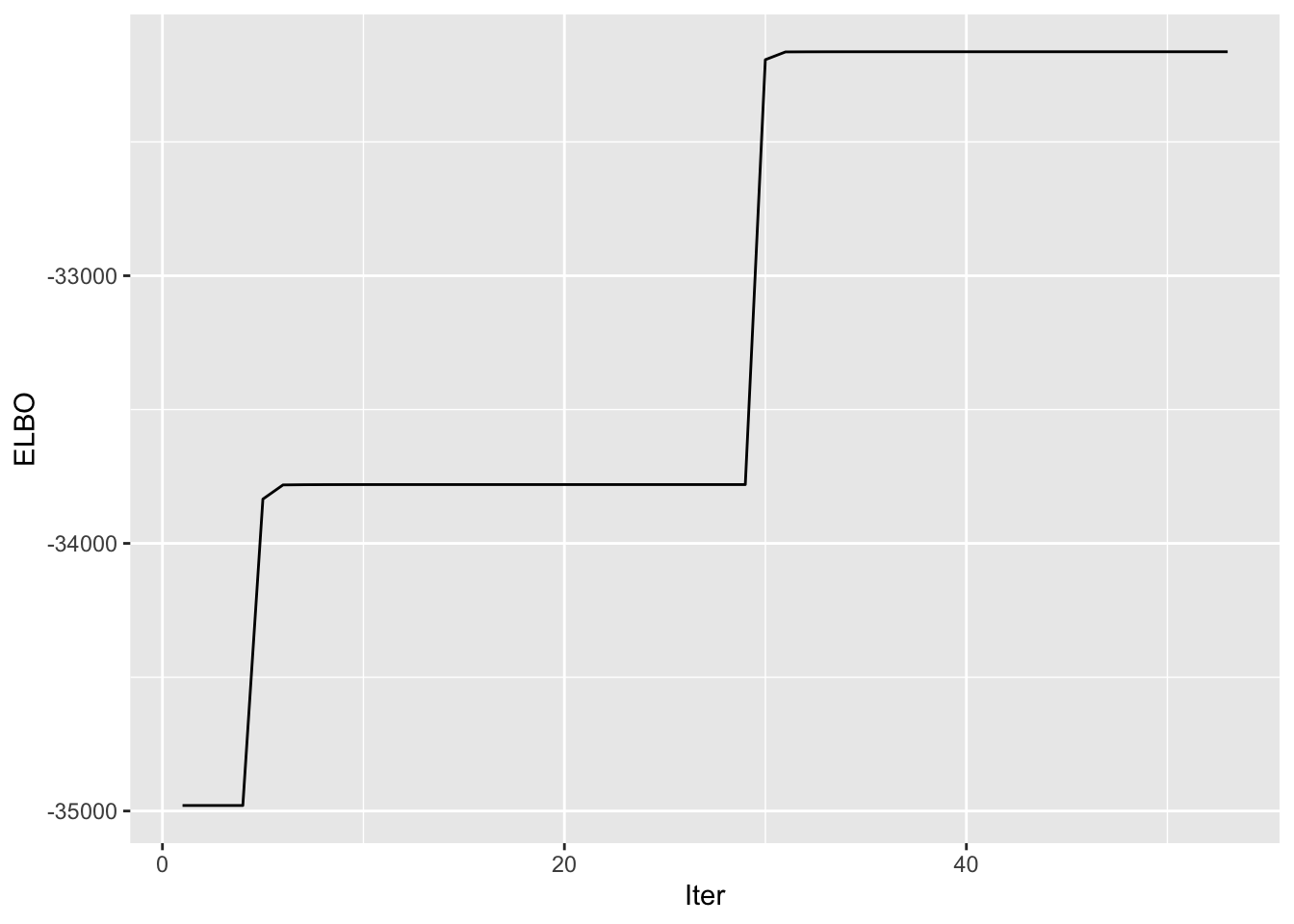
Visualization of Estimate
This is a scatter plot of \(\hat{L}\), the estimate from symEBcovMF:
bal_pops <- c(rep('A', 40), rep('B', 40), rep('C', 40), rep('D', 40))
plot_loadings(symebcovmf_tree_fit$L_pm %*% diag(sqrt(symebcovmf_tree_fit$lambda)), bal_pops)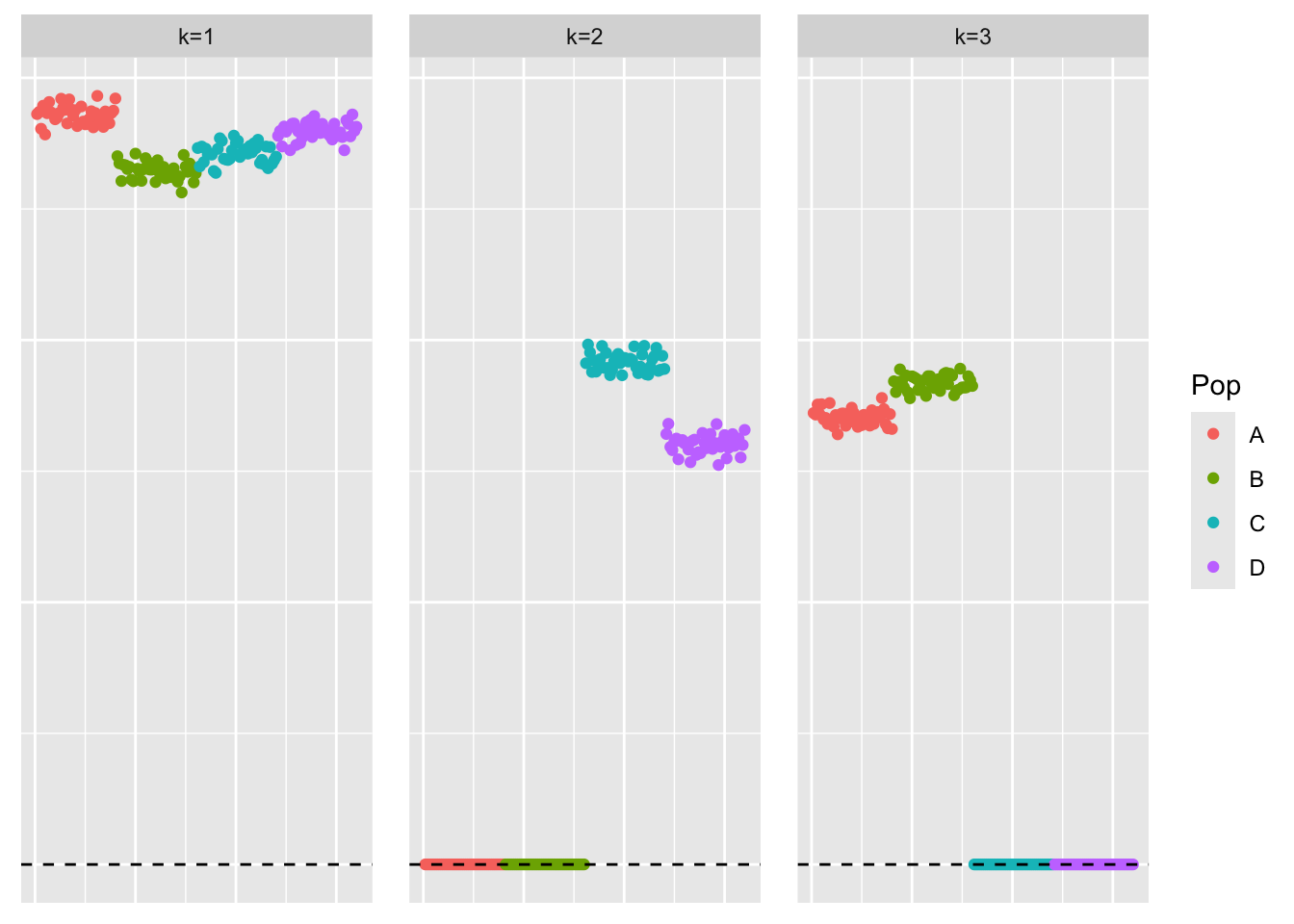
This is the objective function value attained:
symebcovmf_tree_fit$elbo[1] -32163.04Visualization of Fit
This is a heatmap of \(\hat{L}\hat{\Lambda}\hat{L}'\):
symebcovmf_tree_fitted_vals <- tcrossprod(symebcovmf_tree_fit$L_pm %*% diag(sqrt(symebcovmf_tree_fit$lambda)))
plot_heatmap(symebcovmf_tree_fitted_vals, brks = seq(0, max(symebcovmf_tree_fitted_vals), length.out = 50))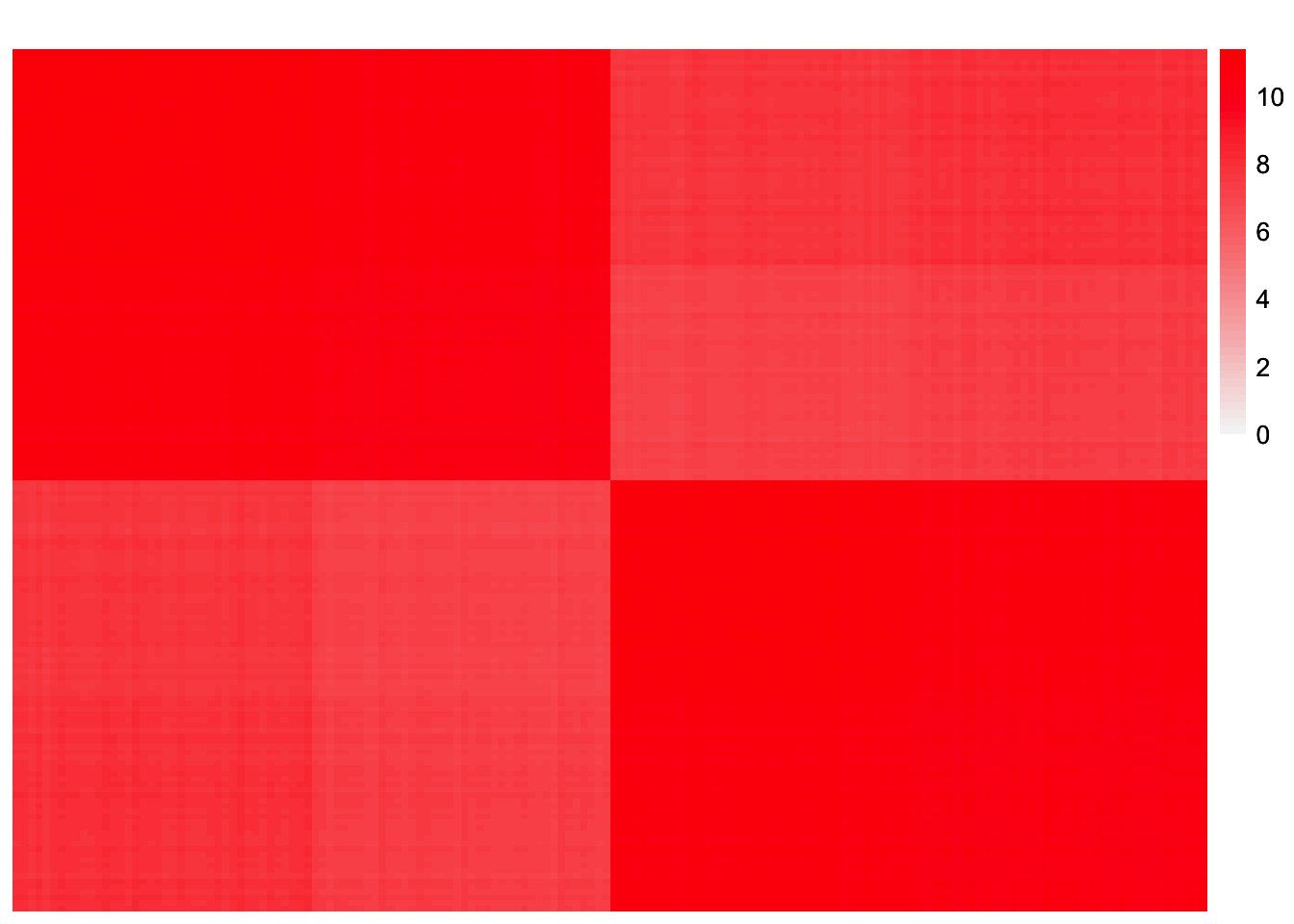
This is a scatter plot of fitted values vs. observed values for the off-diagonal entries:
diag_idx <- seq(1, prod(dim(sim_data$YYt)), length.out = ncol(sim_data$YYt))
off_diag_idx <- setdiff(c(1:prod(dim(sim_data$YYt))), diag_idx)
ggplot(data = NULL, aes(x = c(sim_data$YYt)[off_diag_idx], y = c(symebcovmf_tree_fitted_vals)[off_diag_idx])) + geom_point() + ylim(-1, 15) + xlim(-1,15) + xlab('Observed Values') + ylab('Fitted Values') + geom_abline(slope = 1, intercept = 0, color = 'red')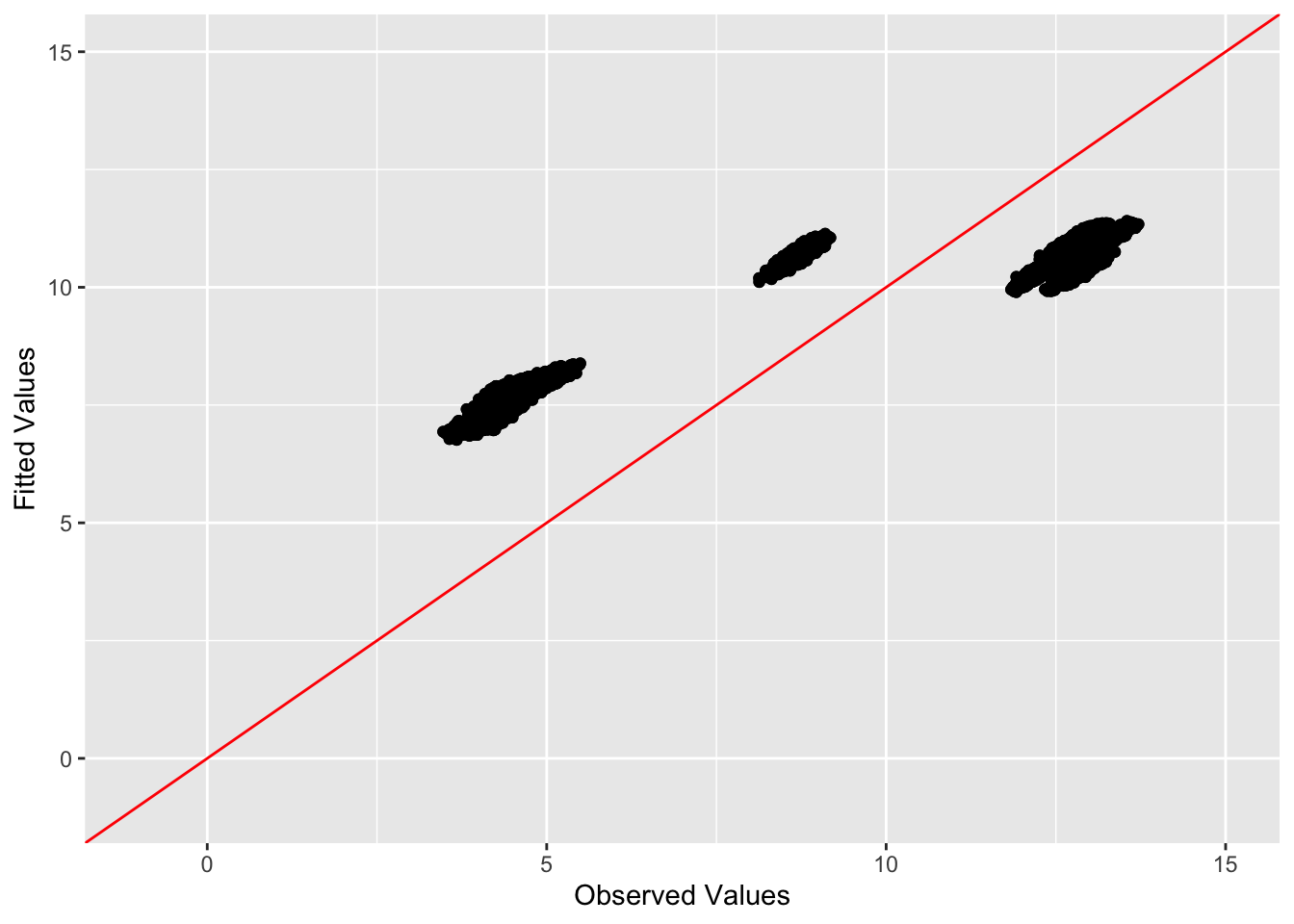
Visualization of Residual Matrix
symebcovmf_tree_resid <- sim_data$YYt - symebcovmf_tree_fitted_vals
plot_heatmap(symebcovmf_tree_resid, colors_range = c('blue','gray96','red'), brks = seq(-max(abs(symebcovmf_tree_resid)), max(abs(symebcovmf_tree_resid)), length.out = 50))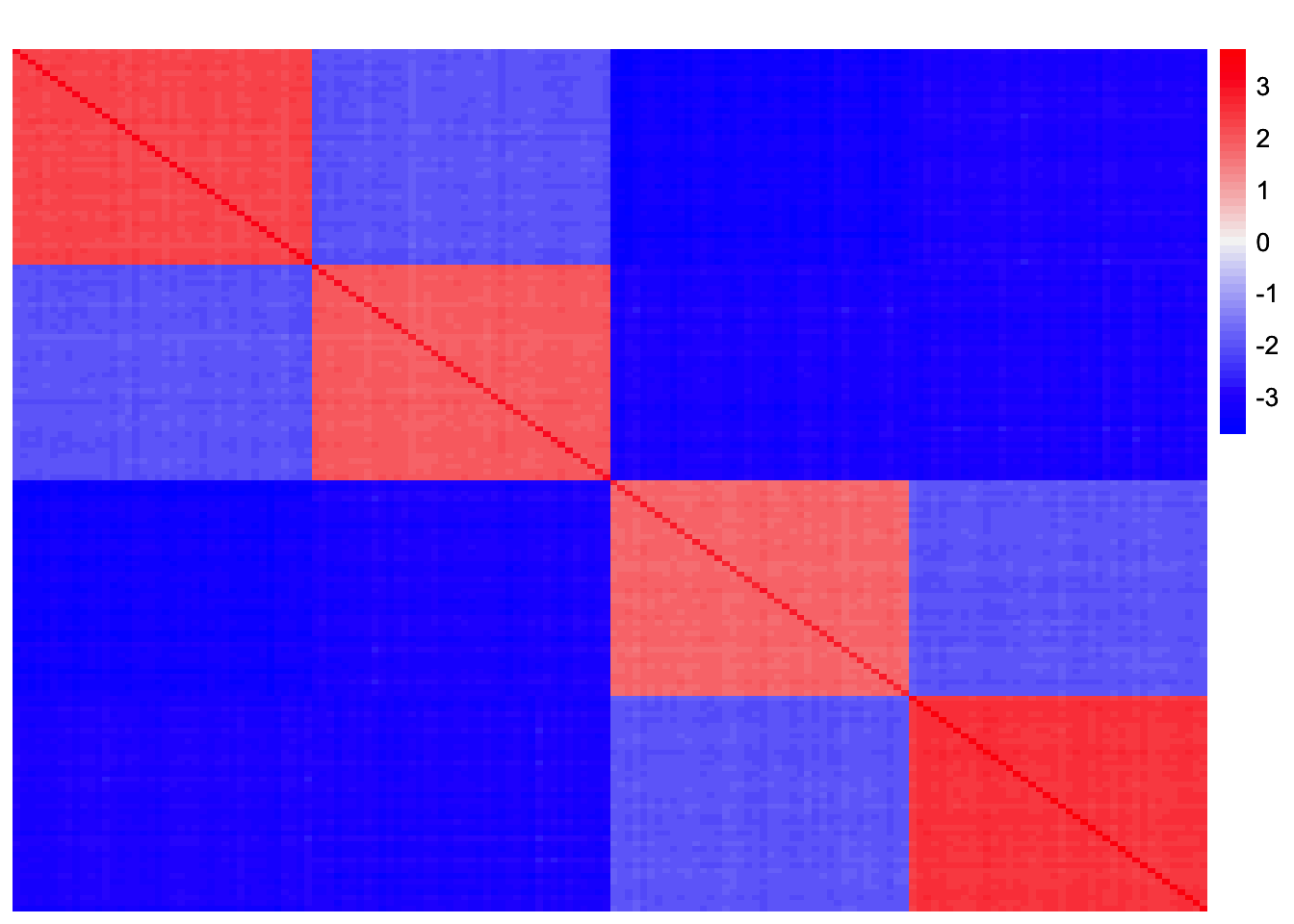
Observations
symEBcovMF struggles in the tree setting. Similar to EBMFcov, symEBcovMF only recovers three factors, one intercept factor and two subtype specific factors. I suspect symEBcovMF has similar issues to EBMFcov where the residual matrix has large chunks of negative entries that cause it to not add anymore factors.
symEBcovMF with refit step
symebcovmf_tree_refit_fit <- sym_ebcovmf_fit(S = sim_data$YYt, ebnm_fn = ebnm_point_exponential, K = 7, maxiter = 100, rank_one_tol = 10^(-8), tol = 10^(-8), refit_lam = TRUE)Progression of ELBO
symebcovmf_tree_refit_full_elbo_vec <- symebcovmf_tree_refit_fit$vec_elbo_full[!(symebcovmf_tree_refit_fit$vec_elbo_full %in% c(1:length(symebcovmf_tree_refit_fit$vec_elbo_K)))]
ggplot() + geom_line(data = NULL, aes(x = 1:length(symebcovmf_tree_refit_full_elbo_vec), y = symebcovmf_tree_refit_full_elbo_vec)) + xlab('Iter') + ylab('ELBO')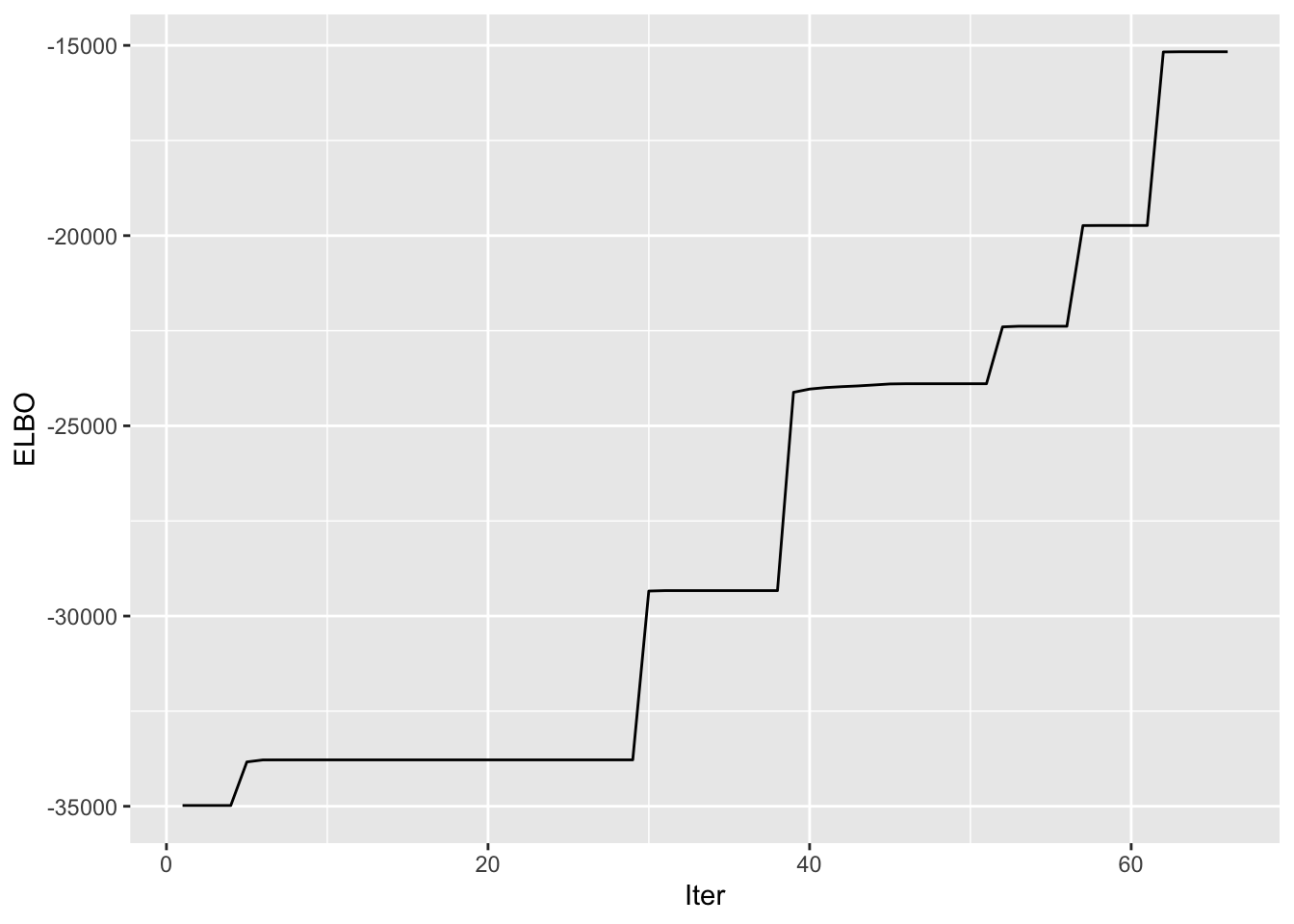 A note: I don’t think I save the ELBO value after the refitting step in
vec_elbo_full. But the refitting does change this vector since it
changes the residual matrix that is used when you add a new vector.
A note: I don’t think I save the ELBO value after the refitting step in
vec_elbo_full. But the refitting does change this vector since it
changes the residual matrix that is used when you add a new vector.
Visualization of Estimate
This is a scatter plot of \(\hat{L}_{refit}\), the estimate from symEBcovMF:
bal_pops <- c(rep('A', 40), rep('B', 40), rep('C', 40), rep('D', 40))
plot_loadings(symebcovmf_tree_refit_fit$L_pm %*% diag(sqrt(symebcovmf_tree_refit_fit$lambda)), bal_pops)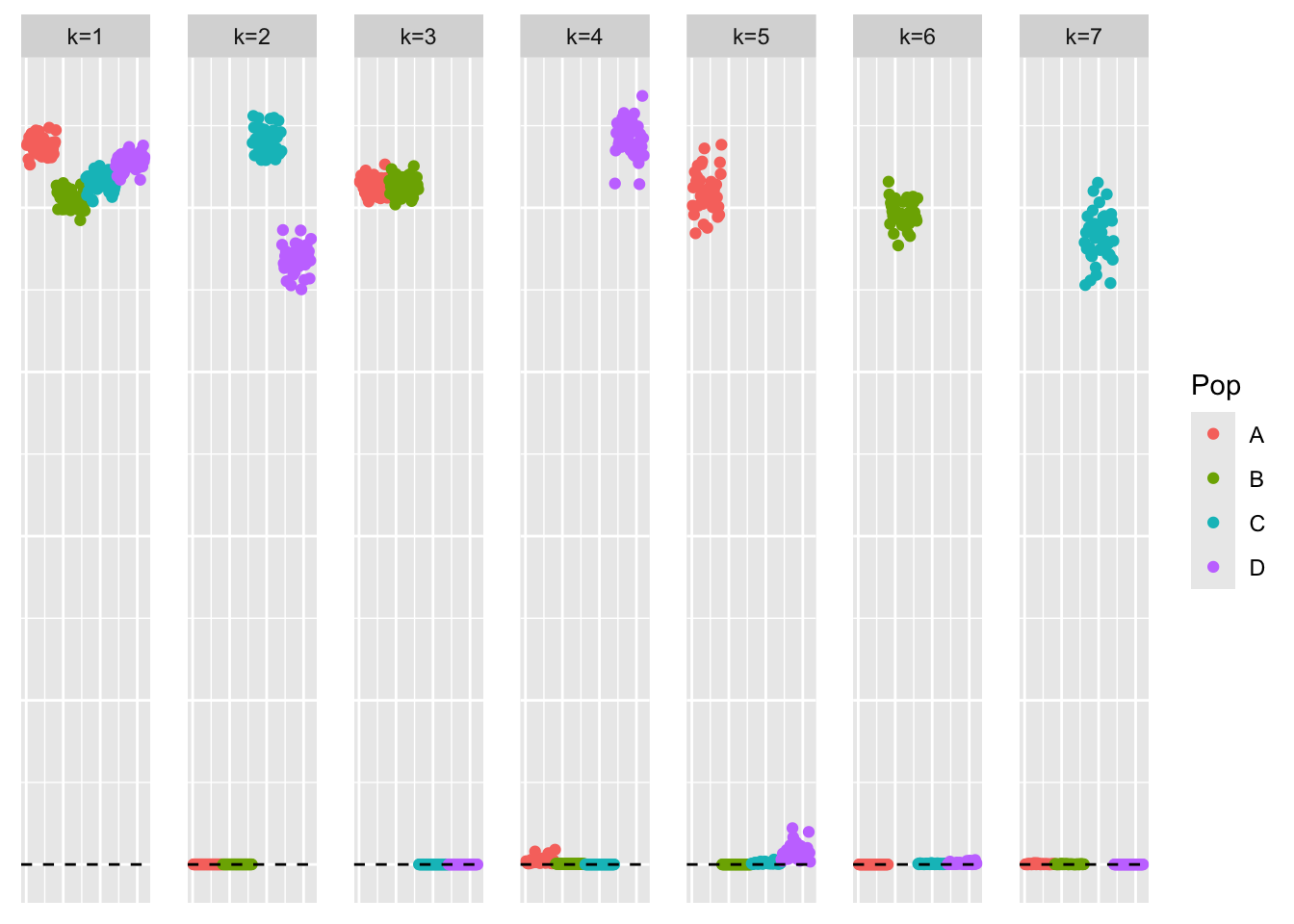
This is the objective function value attained:
symebcovmf_tree_refit_fit$elbo[1] -11576.24Visualization of Fit
This is a heatmap of \(\hat{L}_{refit}\hat{\Lambda}_{refit}\hat{L}_{refit}'\):
symebcovmf_tree_refit_fitted_vals <- tcrossprod(symebcovmf_tree_refit_fit$L_pm %*% diag(sqrt(symebcovmf_tree_refit_fit$lambda)))
plot_heatmap(symebcovmf_tree_refit_fitted_vals, brks = seq(0, max(symebcovmf_tree_refit_fitted_vals), length.out = 50))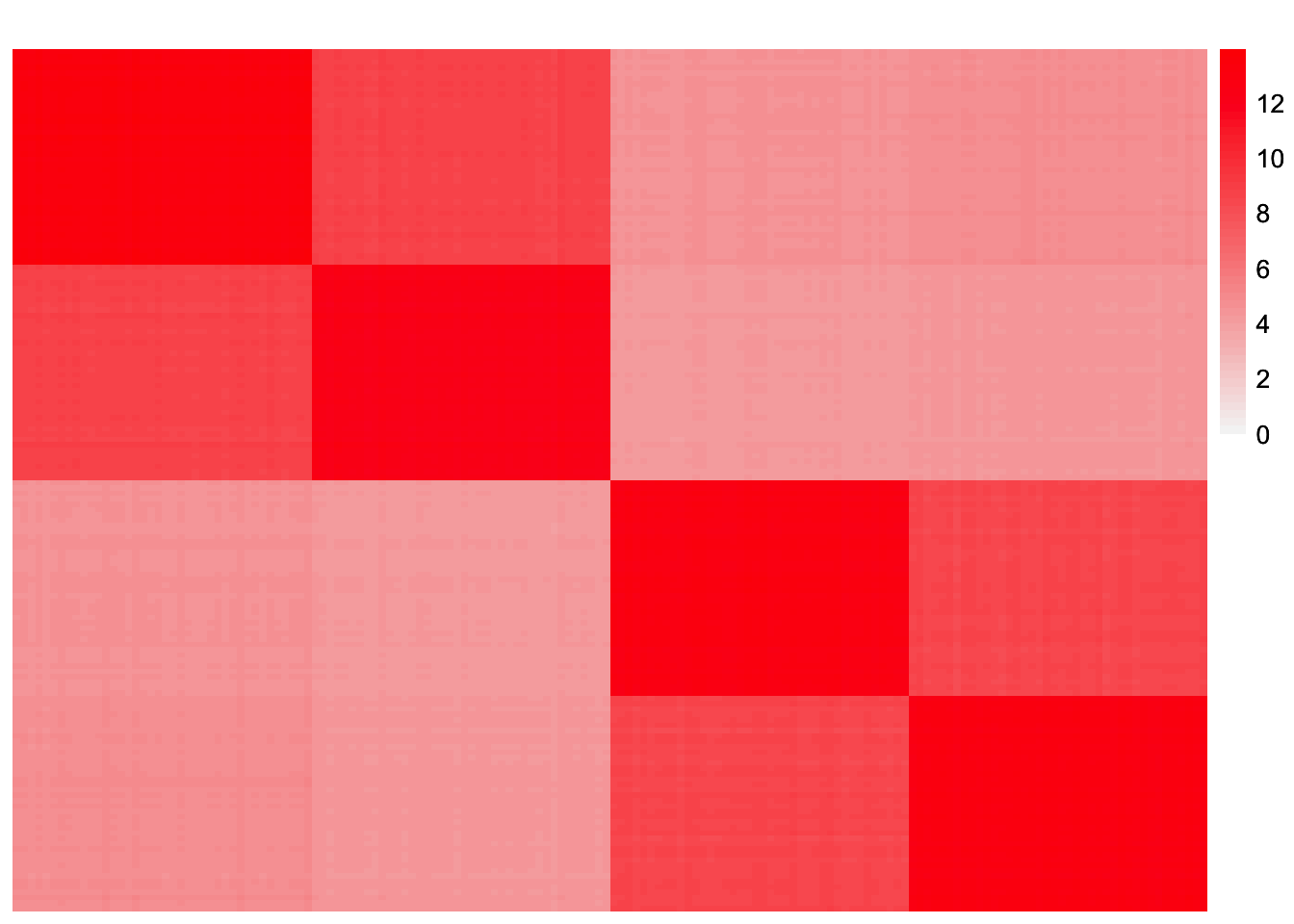
This is a scatter plot of fitted values vs. observed values for the off-diagonal entries:
diag_idx <- seq(1, prod(dim(sim_data$YYt)), length.out = ncol(sim_data$YYt))
off_diag_idx <- setdiff(c(1:prod(dim(sim_data$YYt))), diag_idx)
ggplot(data = NULL, aes(x = c(sim_data$YYt)[off_diag_idx], y = c(symebcovmf_tree_refit_fitted_vals)[off_diag_idx])) + geom_point() + ylim(-1, 15) + xlim(-1,15) + xlab('Observed Values') + ylab('Fitted Values') + geom_abline(slope = 1, intercept = 0, color = 'red')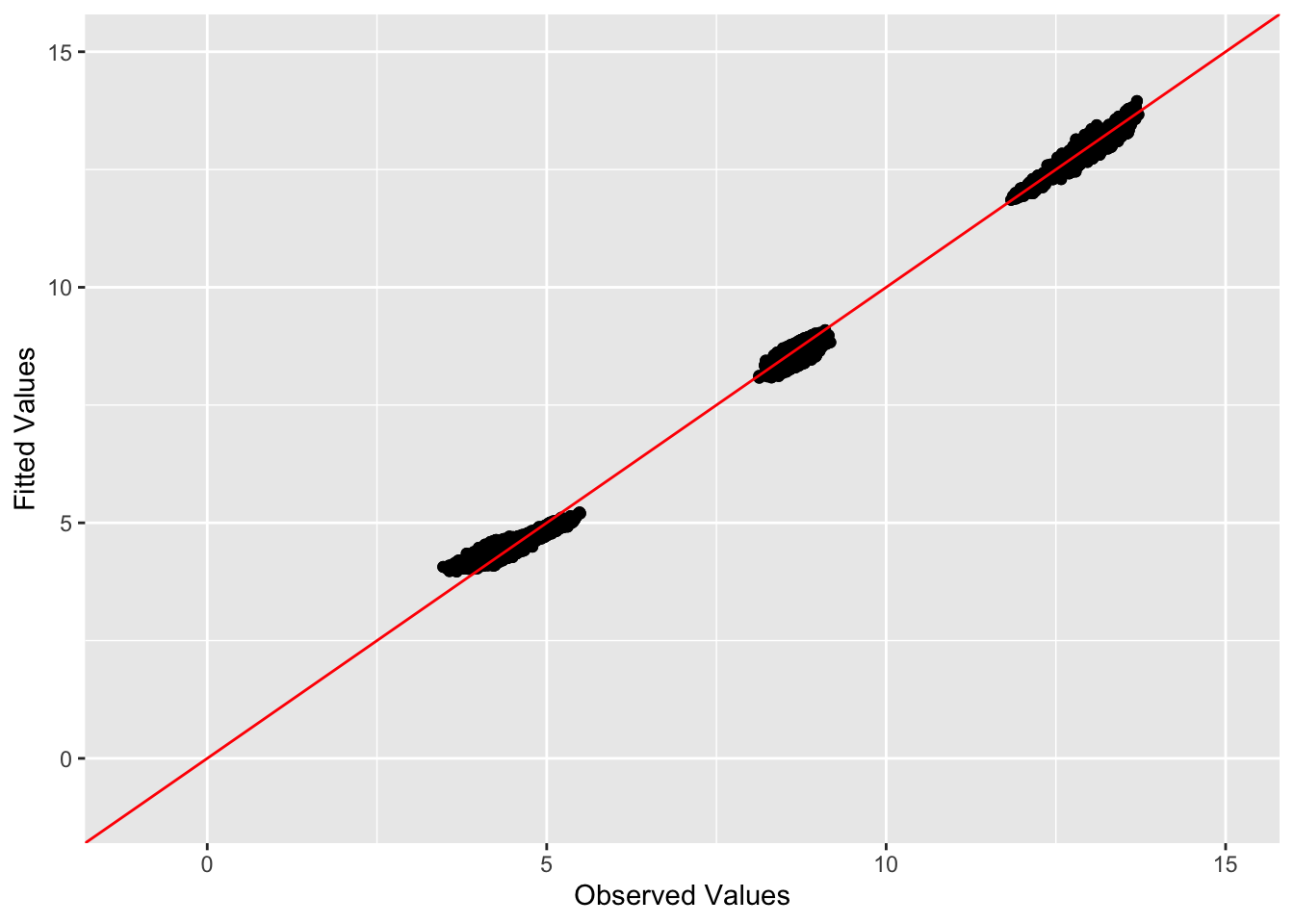
Visualization of Residual Matrix
For comparison, I also plot a heatmap of \(S - \sum_{k=1}^{3} \hat{\lambda}_k \hat{\ell}_k \hat{\ell}_k\) where \(\hat{\lambda}_k\) and \(\hat{\ell}_k\) are estimates from symEBcovMF with the refitting step:
symebcovmf_tree_refit_resid <- sim_data$YYt - tcrossprod(symebcovmf_tree_refit_fit$L_pm[,c(1:3)] %*% diag(sqrt(symebcovmf_tree_refit_fit$lambda[1:3])))
plot_heatmap(symebcovmf_tree_refit_resid, colors_range = c('blue','gray96','red'), brks = seq(-max(abs(symebcovmf_tree_refit_resid)), max(abs(symebcovmf_tree_resid)), length.out = 50))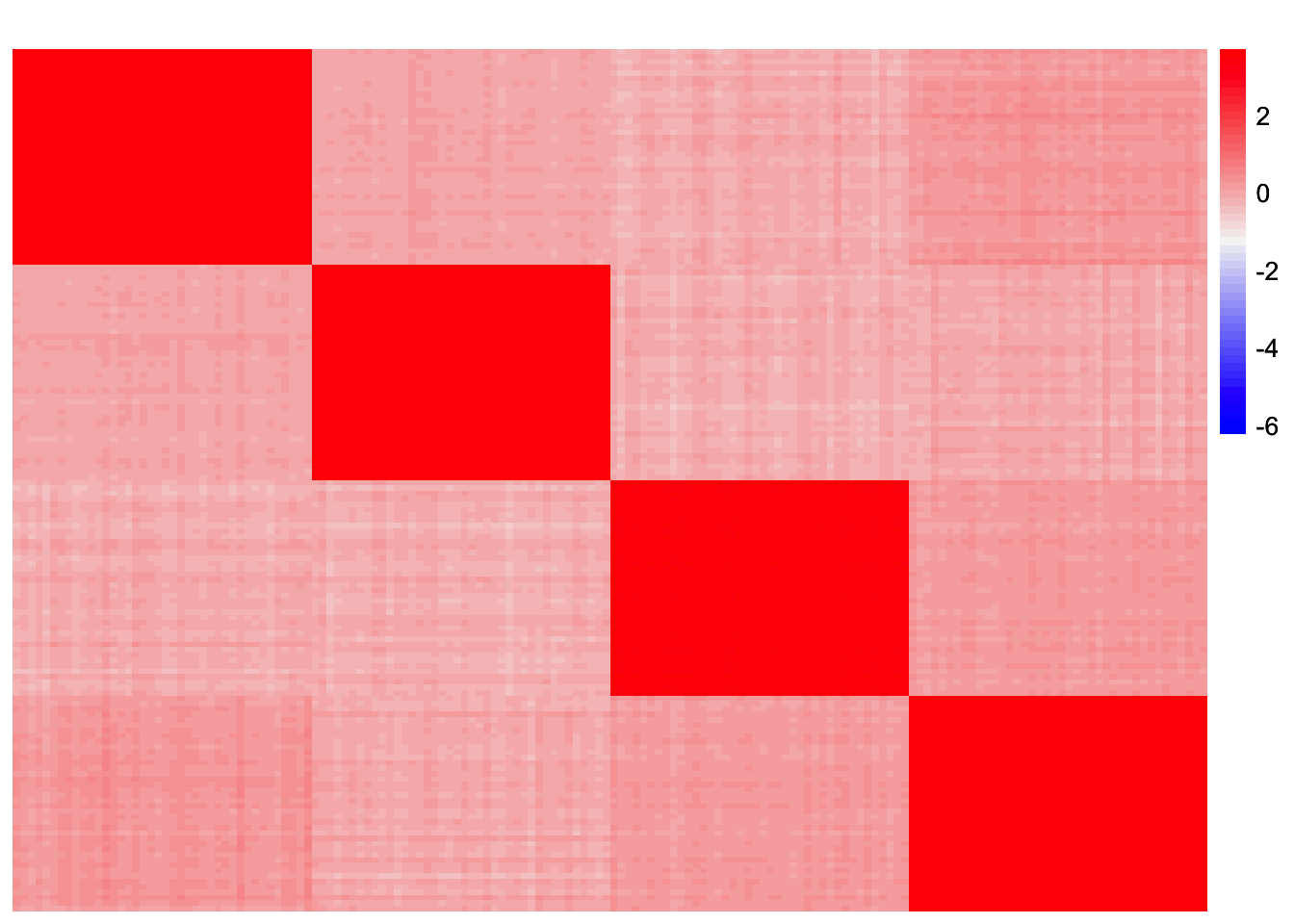
Observations
We see that symEBcovMF with the refitting step does a better job at recovering the hierarchical structure of the data. The loadings estimate does not look entirely binary, but this is to be expected since we used the point-exponential prior. One could imagine a procedure where you start with a fit using point-exponential prior, and then refine the estimates using the generalized binary prior.
I did try symEBcovMF with refitting with the generalized binary factor, and it yields a different representation. The first factor is an intercept like factor and the next two factors are subtype-effect factors. Factor 5 is a population effect factor. However, factors 4, 6, and 7 are not population effects. I think the method found a different representation for the four population effects (which is something I’ve seen in other examples). I’m not sure why the point-exponential prior and the generalized binary prior led to different representations. Perhaps the point-exponential prior is better at yielding sparser solutions? This would also motivate a procedure that uses point-exponential as an initialization and then uses generalized binary to make the loadings more binary.
sessionInfo()R version 4.3.2 (2023-10-31)
Platform: aarch64-apple-darwin20 (64-bit)
Running under: macOS Sonoma 14.4.1
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: America/Chicago
tzcode source: internal
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] ggplot2_3.5.1 pheatmap_1.0.12 ebnm_1.1-34 workflowr_1.7.1
loaded via a namespace (and not attached):
[1] gtable_0.3.5 xfun_0.48 bslib_0.8.0 processx_3.8.4
[5] lattice_0.22-6 callr_3.7.6 vctrs_0.6.5 tools_4.3.2
[9] ps_1.7.7 generics_0.1.3 tibble_3.2.1 fansi_1.0.6
[13] highr_0.11 pkgconfig_2.0.3 Matrix_1.6-5 SQUAREM_2021.1
[17] RColorBrewer_1.1-3 lifecycle_1.0.4 truncnorm_1.0-9 farver_2.1.2
[21] compiler_4.3.2 stringr_1.5.1 git2r_0.33.0 munsell_0.5.1
[25] getPass_0.2-4 httpuv_1.6.15 htmltools_0.5.8.1 sass_0.4.9
[29] yaml_2.3.10 later_1.3.2 pillar_1.9.0 jquerylib_0.1.4
[33] whisker_0.4.1 cachem_1.1.0 trust_0.1-8 RSpectra_0.16-2
[37] tidyselect_1.2.1 digest_0.6.37 stringi_1.8.4 dplyr_1.1.4
[41] ashr_2.2-66 labeling_0.4.3 splines_4.3.2 rprojroot_2.0.4
[45] fastmap_1.2.0 grid_4.3.2 colorspace_2.1-1 cli_3.6.3
[49] invgamma_1.1 magrittr_2.0.3 utf8_1.2.4 withr_3.0.1
[53] scales_1.3.0 promises_1.3.0 horseshoe_0.2.0 rmarkdown_2.28
[57] httr_1.4.7 deconvolveR_1.2-1 evaluate_1.0.0 knitr_1.48
[61] irlba_2.3.5.1 rlang_1.1.4 Rcpp_1.0.13 mixsqp_0.3-54
[65] glue_1.8.0 rstudioapi_0.16.0 jsonlite_1.8.9 R6_2.5.1
[69] fs_1.6.4