Read raw data
Jens Daniel Müller
30 April, 2020
Last updated: 2020-04-30
Checks: 7 0
Knit directory: BloomSail/
This reproducible R Markdown analysis was created with workflowr (version 1.6.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20191021) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version fe72316. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: data/Finnmaid_2018/
Ignored: data/GETM/
Ignored: data/Maps/
Ignored: data/Ostergarnsholm/
Ignored: data/TinaV/
Ignored: data/_merged_data_files/
Ignored: data/_summarized_data_files/
Ignored: data/backup/
Ignored: output/Plots/Figures_publication/.tmp.drivedownload/
Untracked files:
Untracked: output/Plots/response_time/
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/read-in.Rmd) and HTML (docs/read-in.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | fe72316 | jens-daniel-mueller | 2020-04-30 | revised variable and object names, used temp-dependent tau only, rerun code |
| html | d9248a6 | jens-daniel-mueller | 2020-04-29 | Build site. |
| Rmd | 70bd3f0 | jens-daniel-mueller | 2020-04-29 | correct interpolation, new d pco2 plot |
| html | aa52c73 | jens-daniel-mueller | 2020-04-28 | Build site. |
| Rmd | 3044ec0 | jens-daniel-mueller | 2020-04-28 | completely revised |
| html | 57f7231 | jens-daniel-mueller | 2020-04-28 | Build site. |
| Rmd | 5ebd364 | jens-daniel-mueller | 2020-04-28 | revised |
| html | b5722a7 | jens-daniel-mueller | 2020-04-28 | Build site. |
| html | 472c2b4 | jens-daniel-mueller | 2020-04-21 | Build site. |
| html | f8fcf50 | jens-daniel-mueller | 2020-04-19 | created pub figures for time series |
| html | 87658c3 | jens-daniel-mueller | 2020-04-14 | Build site. |
| Rmd | 5c96a65 | jens-daniel-mueller | 2020-04-14 | temperature penetration depth |
| html | 624835e | jens-daniel-mueller | 2020-04-02 | Build site. |
| Rmd | a7ac65d | jens-daniel-mueller | 2020-04-02 | BloomSail data 1-5m and sd in time series plots |
| html | 26cf733 | jens-daniel-mueller | 2020-04-02 | Build site. |
| Rmd | 57b77af | jens-daniel-mueller | 2020-04-02 | corrected Finnmaid lat borders and plotted fm track in map |
| html | a6c4c22 | jens-daniel-mueller | 2020-03-30 | Build site. |
| html | 80c78b3 | jens-daniel-mueller | 2020-03-30 | Build site. |
| html | 5f8ca30 | jens-daniel-mueller | 2020-03-20 | Build site. |
| Rmd | 1ebd01a | jens-daniel-mueller | 2020-03-20 | reorganitzed filenames and navbar |
library(tidyverse)
library(data.table)
library(lubridate)
library(DataExplorer)
library(leaflet)
library(readxl)
library(gsubfn)In this document, raw data files are read, merged into one file with harmonized column names and written as summarized data file.
1 Sea-Bird SBE 16 sensor data (ts)
CTD sensor data including recordings from auxiliary pH, O2, Chla and pCO2 sensors were recorded with a measurement frequency of 15 sec. (In addition, pCO2 data were also internally recorded on the Contros HydroC instrument with higher temporal resolution and will later be used for further analysis after merging with CTD data.)
1.1 Read regular profiles and transects
files <- list.files(path = "data/TinaV/Sensor/Profiles_Transects/", pattern = "[.]cnv$")
#file <- files[1]
for (file in files){
start.date <- data.table(read.delim(here::here("data/TinaV/Sensor/Profiles_Transects/", file),
sep="#", nrows = 160))[[78,1]]
start.date <- substr(start.date, 15, 34)
start.date <- mdy_hms(start.date, tz="UTC")
tempo <- read.delim(here::here("data/TinaV/Sensor/Profiles_Transects/", file),
sep="", skip = 160, header = FALSE)
tempo <- data.table(tempo[,c(2,3,4,5,6,7,9,11,13)])
names(tempo) <- c("date_time", "dep", "tem", "sal", "V_pH", "pH", "Chl", "O2", "pCO2_analog")
tempo$start.date <- start.date
tempo$date_time <- tempo$date_time + tempo$start.date
tempo$ID <- substr(file, 1, 6)
tempo$type <- substr(file, 8,8)
tempo$station <- substr(file, 8,10)
tempo$cast <- "up"
tempo[date_time < mean(tempo[dep == max(tempo$dep)]$date_time)]$cast <- "down"
if (exists("dataset")){
dataset <- rbind(dataset, tempo)
}
if (!exists("dataset")){
dataset <- tempo
}
rm(start.date)
rm(tempo)
}
ts <- dataset
rm(dataset, file, files)1.2 Read profiles and transects around Ostergarnsholm
files <- list.files(path = "data/TinaV/Sensor/Ostergarnsholm/", pattern = "[.]cnv$")
for (file in files){
start.date <- data.table(read.delim(here::here("data/TinaV/Sensor/Ostergarnsholm/", file),
sep="#", nrows = 160))[[78,1]]
start.date <- substr(start.date, 15, 34)
start.date <- mdy_hms(start.date, tz="UTC")
tempo <- read.delim(here::here("data/TinaV/Sensor/Ostergarnsholm/", file),
sep="", skip = 160, header = FALSE)
tempo <- data.table(tempo[,c(2,3,4,5,6,7,9,11,13)])
names(tempo) <- c("date_time", "dep", "tem", "sal", "V_pH", "pH", "Chl", "O2", "pCO2_analog")
tempo$start.date <- start.date
tempo$date_time <- tempo$date_time + tempo$start.date
tempo$ID <- substr(file, 1, 6)
tempo$type <- substr(file, 8,8)
tempo$station <- substr(file, 11,12)
tempo$cast <- "up"
tempo[date_time < mean(tempo[dep == max(tempo$dep)]$date_time)]$cast <- "down"
if (exists("dataset")){
dataset <- rbind(dataset, tempo)
}
if (!exists("dataset")){
dataset <- tempo
}
rm(start.date)
rm(tempo)
}
ts_OGB <- dataset
rm(dataset, file, files)
ts_OGB <- ts_OGB %>%
mutate(type = if_else(station=="bo", "P", "T"),
station = if_else(station == "bo", "P14", station),
station = if_else(station == "in", "T14", station),
station = if_else(station == "ou", "T15", station))ts <- bind_rows(ts, ts_OGB) %>%
arrange(date_time)
rm(ts_OGB)1.3 EDA raw data
source("code/eda.R")
eda(ts, "ts-raw")
rm(eda)The output of an automated Exploratory Data Analysis (EDA) performed with the package DataExplorer can be accessed here:
1.4 Clean data set
Sensor recordings were cleaned from obviously erroneous readings, by setting suspecious values to NA.
class(ts)
ts <- data.table(ts)
# Profiling data
# temperature
# ts %>%
# filter(type == "P") %>%
# ggplot(aes(tem, dep, col=station, linetype = cast))+
# geom_line()+
# scale_y_reverse()+
# geom_vline(xintercept = c(10, 20))+
# facet_wrap(~ID)
ts[ID == "180723" & station == "P07" & dep < 2 & cast == "up"]$tem <- NA
# salinity
# ts %>%
# filter(type == "P") %>%
# ggplot(aes(sal, dep, col=station, linetype = cast))+
# geom_path()+
# scale_y_reverse()+
# facet_wrap(~ID)
ts[sal < 6]$sal <- NA
# pH
# ts %>%
# filter(type == "P") %>%
# ggplot(aes(pH, dep, col=station, linetype=cast))+
# geom_path()+
# scale_y_reverse()+
# facet_wrap(~ID)
#
# ts %>%
# filter(type == "P") %>%
# ggplot(aes(V_pH, dep, col=station, linetype=cast))+
# geom_path()+
# scale_y_reverse()+
# facet_wrap(~ID)
ts[pH < 7.5]$V_pH <- NA
ts[pH < 7.5]$pH <- NA
ts[ID == "180709" & station == "P03" & dep < 5 & cast == "down"]$pH <- NA
ts[ID == "180709" & station == "P05" & dep < 10 & cast == "down"]$pH <- NA
ts[ID == "180718" & station == "P10" & dep < 3 & cast == "down"]$pH <- NA
ts[ID == "180815" & station == "P03" & dep < 2 & cast == "down"]$pH <- NA
ts[ID == "180820" & station == "P11" & dep < 15 & cast == "down"]$pH <- NA
ts[ID == "180709" & station == "P03" & dep < 5 & cast == "down"]$V_pH <- NA
ts[ID == "180709" & station == "P05" & dep < 10 & cast == "down"]$V_pH <- NA
ts[ID == "180718" & station == "P10" & dep < 3 & cast == "down"]$V_pH <- NA
ts[ID == "180815" & station == "P03" & dep < 2 & cast == "down"]$V_pH <- NA
ts[ID == "180820" & station == "P11" & dep < 15 & cast == "down"]$V_pH <- NA
# pCO2
# ts %>%
# filter(type == "P") %>%
# ggplot(aes(pCO2, dep, col=station, linetype = cast))+
# geom_path()+
# scale_y_reverse()+
# facet_wrap(~ID)
ts[ID == "180616"]$pCO2_analog <- NA
# O2
# ts %>%
# filter(type == "P") %>%
# ggplot(aes(O2, dep, col=station, linetype = cast))+
# geom_path()+
# scale_y_reverse()+
# facet_wrap(~ID)
# Chlorophyll
# ts %>%
# filter(type == "P") %>%
# ggplot(aes(Chl, dep, col=station, linetype = cast))+
# geom_path()+
# scale_y_reverse()+
# facet_wrap(~ID)
ts[Chl > 100]$Chl <- NA
#### Surface transect data
# ts %>%
# filter(type == "T") %>%
# ggplot(aes(date, dep, col=station))+
# geom_point()+
# scale_y_reverse()+
# facet_wrap(~ID, scales = "free_x")
#
# ts %>%
# filter(type == "T") %>%
# ggplot(aes(date, tem, col=station))+
# geom_point()+
# facet_wrap(~ID, scales = "free_x")
#
# ts %>%
# filter(type == "T") %>%
# ggplot(aes(date, sal, col=station))+
# geom_point()+
# facet_wrap(~ID, scales = "free_x")
#
# ts %>%
# filter(type == "T") %>%
# ggplot(aes(date, pCO2, col=station))+
# geom_point()+
# facet_wrap(~ID, scales = "free_x")
#
# ts %>%
# filter(type == "T") %>%
# ggplot(aes(date, pH, col=station))+
# geom_point()+
# facet_wrap(~ID, scales = "free_x")
#
# ts %>%
# filter(type == "T") %>%
# ggplot(aes(date, Chl, col=station))+
# geom_point()+
# facet_wrap(~ID, scales = "free_x")
ts[type == "T" & Chl > 10]$Chl <- NA
# ts %>%
# filter(type == "T") %>%
# ggplot(aes(date, O2, col=station))+
# geom_point()+
# facet_wrap(~ID, scales = "free_x")1.5 Write summary file
Relevant columns were selected and renamed, only observations from regular stations (P01-P13) and transects (T01-T13) were selected and summarized data were written to file.
ts <- ts %>%
select(date_time,
ID,
type,
station,
dep,
sal,
tem,
pCO2_analog)
# ts <- ts %>%
# filter( !(station %in% c("PX1", "PX2", "TX1", "TX2") ))
ts %>%
write_csv(here::here("data/_summarized_data_files", "ts.csv"))
rm(ts)1.6 EDA clean data
ts <- read_csv(here::here("data/_summarized_data_files", "ts.csv"),
col_types = cols(pCO2_analog = col_double()))source("code/eda.R")
eda(ts, "ts_clean")
rm(eda)The output of an automated Exploratory Data Analysis (EDA) performed with the package DataExplorer can be accessed here:
1.7 Overview plots
ts %>%
arrange(date_time) %>%
filter(type == "P", !(station %in% c("PX1", "PX2"))) %>%
ggplot(aes(tem, dep, col=ymd(ID), group=ID))+
geom_path()+
scale_y_reverse()+
scale_color_viridis_c(trans = "date", name="")+
labs(x="temperature (°C)", y="Depth (m)")+
facet_wrap(~station)
temperature profiles by stations. Color refers to the starting date of each cruise.
ts %>%
arrange(date_time) %>%
filter(type == "P", !(station %in% c("PX1", "PX2"))) %>%
ggplot(aes(pCO2_analog, dep, col=ymd(ID), group=ID))+
geom_path()+
scale_y_reverse()+
scale_color_viridis_c(trans = "date", name="")+
labs(x="temperature (°C)", y="Depth (m)")+
facet_wrap(~station)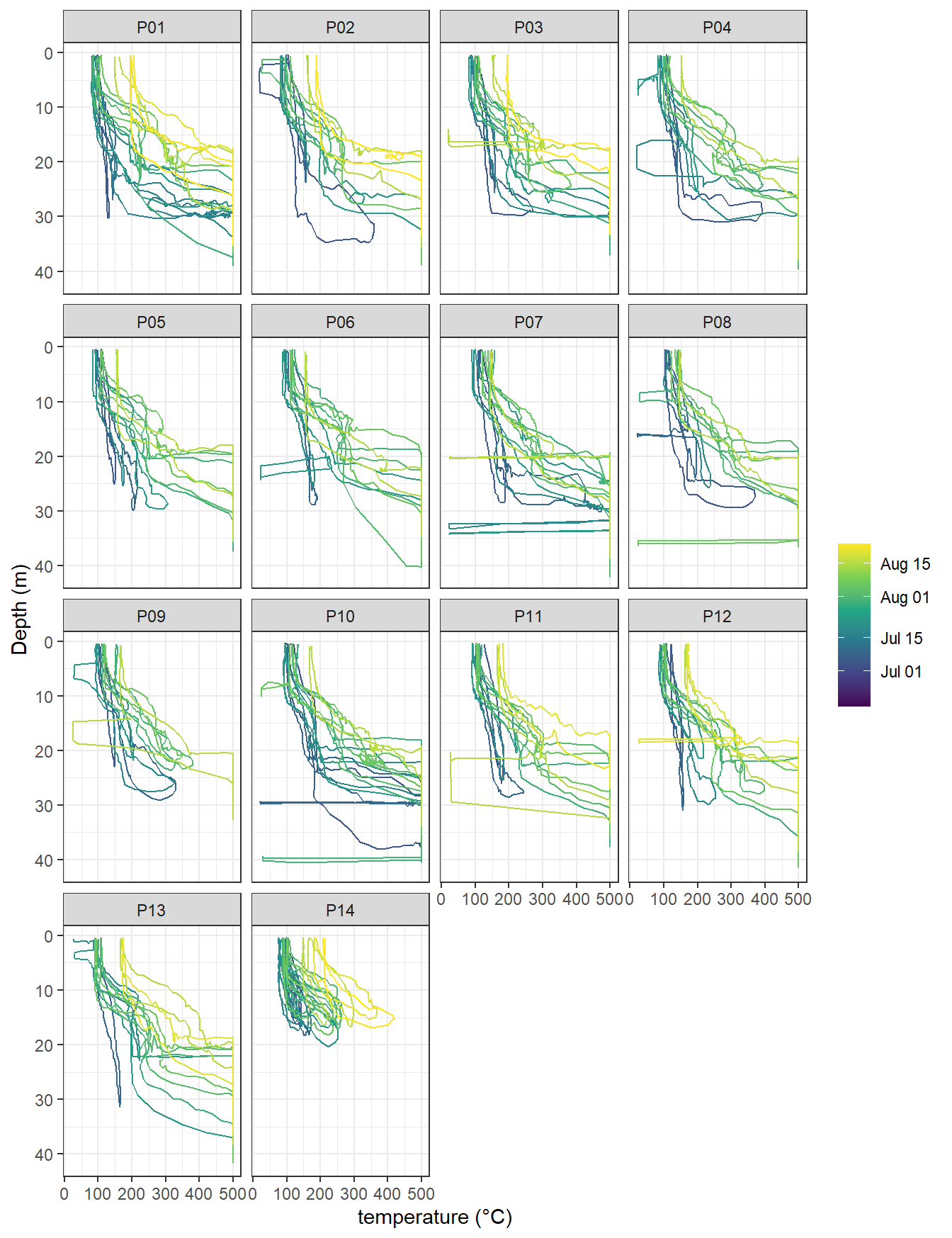
pCO2 (analog signal) profiles by stations. Color refers to the starting date of each cruise.
2 HydroC CO2 data (th)
2.1 Read data
HydroC pCO2 data were provided by KM Contros after applying a drift correction to the raw data, which was based on pre- and post-deployment calibration results.
# Read Contros corrected data file
th <-
read_csv2(here::here("Data/TinaV/Sensor/HydroC-pCO2/corrected_Contros",
"parameter&pCO2s(method 43).txt"),
col_names = c("date_time", "Zero", "Flush", "p_NDIR",
"p_in", "T_control", "T_gas", "%rH_gas",
"Signal_raw", "Signal_ref", "T_sensor",
"pCO2_corr", "Runtime", "nr.ave")) %>%
mutate(date_time = dmy_hms(date_time),
Flush = as.factor(as.character(Flush)),
Zero = as.factor(as.character(Zero)))2.2 Deployment identification and subsetting
Individual deployments (periods of observations with less than 30 sec between recordings) were identified and relevant deployment periods were subsetted. This procedure removes only recordings attributable to sensor testing and set-up.
th <- th %>%
arrange(date_time) %>%
mutate(deployment = cumsum(c(TRUE,diff(date_time)>=30)))
th <- th %>%
filter(deployment %in% c(2,6,9,14,17,21,23,27,31,33,34,35,37))2.3 Removal of duplicated time stamps
# add counter for date_time observations
th <- th %>%
add_count(date_time)
# find triplicated time stamp and select only first observation, and merge
th_no_triple <- th %>%
filter(n <= 2)
th_triple_clean <- th %>%
filter(n > 2) %>%
slice(1)
th <- full_join(th_no_triple, th_triple_clean)
rm(list=setdiff(ls(), "th"))
# find duplicated time stamps and shift first by one second backward, and merge
th %>%
distinct(date_time)
th <- th %>%
select(-n) %>%
add_count(date_time)
unique(th$n)
th_no_duplicated <- th %>%
filter(n == 1)
th_duplicated <- th %>%
filter(n == 2)
th_duplicated_first <- th_duplicated %>%
group_by(date_time) %>%
slice(1) %>%
ungroup() %>%
mutate(date_time = date_time - 1)
th_duplicated_second <- th_duplicated %>%
group_by(date_time) %>%
slice(2) %>%
ungroup()
th_duplicated_clean <- full_join(th_duplicated_first, th_duplicated_second) %>%
arrange(date_time)
th <- full_join(th_no_duplicated, th_duplicated_clean)
th %>%
distinct(date_time)
rm(list=setdiff(ls(), "th"))
# find duplicated time stamps and shift first by two seconds forward, and merge
th %>%
distinct(date_time)
th <- th %>%
select(-n) %>%
add_count(date_time)
unique(th$n)
th_no_duplicated <- th %>%
filter(n == 1)
th_duplicated <- th %>%
filter(n == 2)
th_duplicated_first <- th_duplicated %>%
group_by(date_time) %>%
slice(1) %>%
ungroup() %>%
mutate(date_time = date_time + 2)
th_duplicated_second <- th_duplicated %>%
group_by(date_time) %>%
slice(2) %>%
ungroup()
th_duplicated_clean <- full_join(th_duplicated_first, th_duplicated_second) %>%
arrange(date_time)
th <- full_join(th_no_duplicated, th_duplicated_clean)
th %>%
distinct(date_time)
rm(list=setdiff(ls(), "th"))
# remaining duplicates are observations where other observations with a +/- 1 sec timestamp exist
# for those cases, only the first duplicated observation is selected (similar to triplicate treatment)
th %>%
distinct(date_time)
th <- th %>%
select(-n) %>%
add_count(date_time)
unique(th$n)
th_still_no_duplicated <- th %>%
filter(n == 1)
th_still_duplicated_first <- th %>%
filter(n == 2) %>%
group_by(date_time) %>%
slice(1)
th <- full_join(th_still_no_duplicated, th_still_duplicated_first)
th %>%
distinct(date_time)
rm(list=setdiff(ls(), "th"))
th <- th %>%
select(-n)2.4 Flush and Zeroing identification
# Zeroing ID labelling
th <- th %>%
arrange(date_time) %>%
group_by(Zero) %>%
mutate(Zero_ID = as.factor(cumsum(c(TRUE,diff(date_time)>=30)))) %>%
ungroup()
unique(th$Zero_ID)
# Flush: Identification
th <- th %>%
mutate(Flush = 0) %>%
group_by(Zero, Zero_ID) %>%
mutate(start = min(date_time),
duration = date_time - start,
Flush = if_else(Zero == 0 & duration < 600, "1", "0")) %>%
ungroup()
# Flush: Identify equilibration and internal gas mixing periods
th <- th %>%
mutate(mixing = if_else(duration < 20, "mixing", "equilibration"))2.5 Deployment plots
pdf(file=here::here("output/Plots/read_in",
"th_deployments.pdf"), onefile = TRUE, width = 7, height = 4)
for (i in unique(th$deployment)) {
#i <- unique(th$deployment)[3]
sub <- th %>%
filter(deployment == i)
start_date <- min(sub$date_time)
print(
sub %>%
ggplot(aes(date_time, pCO2_corr, col=Zero_ID))+
geom_line()+
labs(title = paste("Deployment: ",i, "| Start time: ", start_date))
)
}
dev.off()
rm(sub, start_date, i)A pdf with pCO2 timeseries plots of all deployments can be found here:
source("code/eda.R")
eda(th, "th")
rm(eda)The output of an automated Exploratory Data Analysis (EDA) performed with the package DataExplorer can be accessed here:
2.6 Write summary file
Summarized pCO2 date were written to file.
th %>% write_csv(here::here("Data/_summarized_data_files",
"th_full.csv"))
th %>%
select(date_time, Zero, Flush, pCO2_corr, deployment, Zero_ID, duration, mixing) %>%
write_csv(here::here("Data/_summarized_data_files",
"th.csv"))
rm(th)3 Bottle data / discrete samples (tb)
Discrete samples were collected with a Niskin bottle and analysed for C[T] and A[T] at IOW CO2 lab.
3.1 Read data
tb <- read_csv(here::here("Data/TinaV/Bottle/Tracegases", "BloomSail_bottle_CO2_all.csv"),
col_types = list("c","c","n","n","n","n","n"))
tb <- tb %>%
select(ID=transect.ID,
station=label,
dep=Dep,
sal=Sal,
CT, AT)3.2 Write summary file
tb %>% write_csv(here::here("Data/_summarized_data_files",
"tb.csv"))
rm(tb)4 GPS track (tt)
GPS track data were recorded with a Samsung Galaxy tablet.
4.1 Read data
files <- list.files(path = "data/TinaV/Track/GPS_Logger_Track/", pattern = "[.]txt$")
for (file in files){
# if the merged dataset does exist, append to it
if (exists("dataset")){
tempo<-data.table(read.delim(here::here("data/TinaV/Track/GPS_Logger_Track", file),
sep=",")[,c(2,3,4)])
names(tempo) <- c("date_time", "lat", "lon")
tempo$date_time<- ymd_hms(tempo$date, tz="UTC")
dataset<-rbind(dataset, tempo)
rm(tempo)
}
# if the merged dataset doesn't exist, create it
if (!exists("dataset")){
dataset<-data.table(read.delim(here::here("data/TinaV/Track/GPS_Logger_Track", file),
sep=",")[,c(2,3,4)])
names(dataset) <- c("date_time", "lat", "lon")
dataset$date_time<- ymd_hms(dataset$date_time, tz="UTC")
}
}
tt <- dataset
rm(dataset, file, files)4.2 Write summary file
tt %>%
write_csv(here::here("data/_summarized_data_files",
"tt.csv"))
rm(tt)5 Finnmaid
pCO2 data were recorded on VOS Finnmaid in summer 2018.
5.1 Read data
### June - August 2018
files <- list.files(path = "data/Finnmaid_2018", pattern = "[.]xls$")
#file <-files[1]
for (file in files){
df <- read_excel(here::here("data/Finnmaid_2018", file))
df <- df[c(1,2,3,12,7,4,15,8,5,17)]
names(df) <- c("date_time","lon","lat","pCO2","sal","tem","cO2","patm", "Teq","xCO2")
df <- df[-c(1),]
df$date_time <- as.POSIXct(as.numeric(df$date_time)*60*60*24, origin="1899-12-30", tz="GMT")
df$lon <- as.numeric(as.character(df$lon))
df$lat <- as.numeric(as.character(df$lat))
df$pCO2 <- as.numeric(as.character(df$pCO2))
df$sal <- as.numeric(as.character(df$sal))
df$tem <- as.numeric(as.character(df$tem))
df$cO2 <- as.numeric(as.character(df$cO2))
df$patm <- as.numeric(as.character(df$patm))
df$Teq <- as.numeric(as.character(df$Teq))
df$xCO2 <- as.numeric(as.character(df$xCO2))
df <- data.table(df)
df$route <- strapplyc(as.character(file), ".*(.).xls*", simplify = TRUE)
df$ID <- substr(as.character(file), 3, 10)
if (exists("temp")){
temp <- rbind (temp, df)
} else{temp <- df}
}
rm(df, files, file)
temp <- temp[pCO2 != 0]
#### Los Gatos data
files <- list.files(path = "data/Finnmaid_2018/LGR", pattern = "[.]xls$")
#file <-files[1]
for (file in files){
df <- read_excel(here::here("data/Finnmaid_2018/LGR", file))
df <- df[c(2,3,4,8,6,5,14,7,15,9)]
names(df) <- c("date_time","lon","lat","pCO2","sal","tem","cO2","patm", "Teq","xCO2")
df <- df[-c(1),]
df$date_time <- dmy_hms(df$date_time)
df <- data.table(df)
df$route <- substr(as.character(file), 12, 12)
df$ID <- substr(as.character(file), 3, 10)
if (exists("temp.LGR")){
temp.LGR <- rbind (temp.LGR, df)
} else{temp.LGR <- df}
}5.2 Convert O2 concentration units
source(here::here("code", "O2stoO2c.R"))
temp.LGR <- temp.LGR %>%
filter() %>%
mutate(cO2 = O2stoO2c(O2sat = cO2, T=tem, S=sal, P=3/10, p_atm = 1013.5))
rm(O2stoO2c, pH2Osat, sca_T, Scorr, TCorr, R, Vm)temp$sensor <- "LICOR"
temp.LGR$sensor <- "LosGatos"
fm <- bind_rows(temp, temp.LGR)
rm(temp, temp.LGR, df, file, files)
# temp$Area <- with(temp,
# ifelse(lon>12 & lon<12.6, "1.MEB",
# ifelse(lon>13.1 & lon<14.3, "2.ARK",
# ifelse(lat>57.5 & lat<58.5 & route %in% c("E", "G"), "4.EGS",
# ifelse(lat>57.3 & lat<57.5 & route %in% c("E"), "BS",
# ifelse(lat>56.8 & lat<57.5 & route=="W", "3.WGS",
# ifelse(lat>58.5 & lat<59 & lon>20, "5.NGS",
# ifelse(lon>22 & lon<24, "6.WGF",
# ifelse(lon>24 & lon<24.5, "7.HGF", "NaN")))))))))5.3 Write summary file
fm <-fm[complete.cases(fm[,pCO2]),]
fm %>%
write_csv(here::here("Data/_summarized_data_files",
"fm.csv"))
rm(fm)6 Interactive map
fm <- read_csv(here::here("Data/_summarized_data_files",
"fm.csv"))
fm_sub <- fm %>%
arrange(date_time) %>%
slice(which(row_number() %% 20 == 1))
tt <- read_csv(here::here("Data/_summarized_data_files", "tt.csv"))
tt_sub <- tt %>%
slice(which(row_number() %% 20 == 1))
rm(tt, fm)
leaflet() %>%
setView(lng = 20, lat = 57.3, zoom = 8) %>%
addLayersControl(baseGroups = c("Ocean Basemap",
"Satellite"),
overlayGroups = c("BloomSail", "Finnmaid"),
options = layersControlOptions(collapsed = FALSE),
position = 'topright') %>%
addProviderTiles("Esri.WorldImagery", group = "Satellite") %>%
addProviderTiles(providers$Esri.OceanBasemap, group = "Ocean Basemap") %>%
addScaleBar(position = 'topright') %>%
addMeasure(
primaryLengthUnit = "kilometers",
secondaryLengthUnit = 'miles',
primaryAreaUnit = "sqmeters",
secondaryAreaUnit="acres",
position = 'topleft') %>%
addCircles(data = fm_sub, ~lon, ~lat,
color = "white",
group = "Finnmaid") %>%
addPolylines(data = tt_sub, ~lon, ~lat,
color = "red",
group = "BloomSail")7 Tasks / open questions
sessionInfo()R version 3.6.3 (2020-02-29)
Platform: i386-w64-mingw32/i386 (32-bit)
Running under: Windows 10 x64 (build 18363)
Matrix products: default
locale:
[1] LC_COLLATE=English_Germany.1252 LC_CTYPE=English_Germany.1252
[3] LC_MONETARY=English_Germany.1252 LC_NUMERIC=C
[5] LC_TIME=English_Germany.1252
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] gsubfn_0.7 proto_1.0.0 readxl_1.3.1 leaflet_2.0.3
[5] DataExplorer_0.8.1 lubridate_1.7.4 data.table_1.12.8 forcats_0.5.0
[9] stringr_1.4.0 dplyr_0.8.5 purrr_0.3.3 readr_1.3.1
[13] tidyr_1.0.2 tibble_3.0.0 ggplot2_3.3.0 tidyverse_1.3.0
[17] workflowr_1.6.1
loaded via a namespace (and not attached):
[1] Rcpp_1.0.4 whisker_0.4 knitr_1.28
[4] xml2_1.3.0 magrittr_1.5 hms_0.5.3
[7] rvest_0.3.5 tidyselect_1.0.0 viridisLite_0.3.0
[10] here_0.1 colorspace_1.4-1 lattice_0.20-41
[13] R6_2.4.1 rlang_0.4.5 fansi_0.4.1
[16] tcltk_3.6.3 parallel_3.6.3 broom_0.5.5
[19] xfun_0.12 dbplyr_1.4.2 modelr_0.1.6
[22] withr_2.1.2 git2r_0.26.1 ellipsis_0.3.0
[25] htmltools_0.4.0 assertthat_0.2.1 rprojroot_1.3-2
[28] digest_0.6.25 lifecycle_0.2.0 haven_2.2.0
[31] rmarkdown_2.1 compiler_3.6.3 cellranger_1.1.0
[34] pillar_1.4.3 leaflet.providers_1.9.0 scales_1.1.0
[37] backports_1.1.5 generics_0.0.2 jsonlite_1.6.1
[40] httpuv_1.5.2 pkgconfig_2.0.3 igraph_1.2.5
[43] rstudioapi_0.11 munsell_0.5.0 highr_0.8
[46] httr_1.4.1 tools_3.6.3 networkD3_0.4
[49] grid_3.6.3 nlme_3.1-145 gtable_0.3.0
[52] DBI_1.1.0 cli_2.0.2 crosstalk_1.1.0.1
[55] yaml_2.2.1 crayon_1.3.4 gridExtra_2.3
[58] farver_2.0.3 later_1.0.0 promises_1.1.0
[61] htmlwidgets_1.5.1 fs_1.4.0 vctrs_0.2.4
[64] glue_1.3.2 evaluate_0.14 labeling_0.3
[67] reprex_0.3.0 stringi_1.4.6