eMLR data preparation
Jens Daniel Müller
19 December, 2020
Last updated: 2020-12-19
Checks: 7 0
Knit directory: emlr_mod_v_XXX/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20200707) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version b5fedce. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rproj.user/
Unstaged changes:
Modified: analysis/_site.yml
Modified: code/Workflowr_project_managment.R
Deleted: code/old_cstar_adjustment.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/eMLR_data_preparation.Rmd) and HTML (docs/eMLR_data_preparation.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | b5fedce | jens-daniel-mueller | 2020-12-19 | first build after creating model template |
| Rmd | 8e8abf5 | Jens Müller | 2020-12-18 | Initial commit |
1 Required data
Required are:
- GLODAPv2.2020
- cleaned data file
- Cant from Sabine 2004 (S04)
- Cant from Gruber 2019 (G19)
- annual mean atmospheric pCO2
GLODAP <-
read_csv(paste(path_version_data,
"GLODAPv2.2020_clean.csv",
sep = ""))
S04_cant_3d <-
read_csv(paste(path_preprocessing,
"S04_cant_3d.csv",
sep = ""))
G19_cant_3d <-
read_csv(paste(path_preprocessing,
"G19_cant_3d.csv",
sep = ""))
co2_atm <-
read_csv(paste(path_preprocessing,
"co2_atm.csv",
sep = ""))2 PO4*
2.1 Calculation
The predictor PO4* was be calculated according to Clement and Gruber (2018), ie based on oxygen. Please note that an erroneous equations for PO4* calculation is given in the supplement of Gruber et al (2019), based on nitrate.
Here we use following equation:
print(b_phosphate_star)function (phosphate, oxygen)
{
phosphate_star = phosphate + (oxygen/params_local$rPO) -
params_local$rPO_offset
return(phosphate_star)
}GLODAP <- GLODAP %>%
mutate(phosphate_star = b_phosphate_star(phosphate, oxygen))3 C*
C* serves as a conservative tracer of anthropogenic CO2 uptake. It is derived from measured DIC by removing the impact of
- organic matter formation and respiration
- calcification and calcium carbonate dissolution
Contributions of those processes are estimated from phosphate and alkalinity concentrations.
3.1 Stoichiometric ratios
The stoichiometric nutrient ratios for the production and mineralization of organic matter were set to:
- C/P: 117
- N/P: 16
3.2 Calculation
C* was calculated as:
print(b_cstar)function (tco2, phosphate, talk)
{
cstar = tco2 - (params_local$rCP * phosphate) - 0.5 * (talk -
(params_local$rNP * phosphate))
return(cstar)
}GLODAP <- GLODAP %>%
mutate(rCP_phosphate = -params_local$rCP * phosphate,
talk_05 = -0.5 * talk,
rNP_phosphate_05 = -0.5 * params_local$rNP * phosphate,
cstar = b_cstar(tco2, phosphate, talk))3.3 Reference year adjustment
To adjust C* values to the reference year of each observation period, we assume a transient steady state change of cant between the time of sampling the reference year. The adjustment requires an approximation of the cant concentration at the reference year. We approximate this concentration by adding the delta cant signal estimated by Gruber et al (2019) to the “base line” total cant concentration determined for 1994 by Sabine et al (2004):
Cant(tref) = S04 + (tref-1994)/13 * G19
This way, we use exactly S04+G19 for tref=2007. For all other tref we scale Cant with the observed anomalous change over the 1994-2007 period, rather than assuming a transient steady state. However, one assumes a linear behaviour of the anomalous change over time, which might be wrong in particular for the years past 2007.
3.3.1 Join Cant fields
Join Cant fields of G19 and S04
G19_cant_3d <- G19_cant_3d %>%
select(lon, lat, depth, cant_pos_G19 = cant_pos)
S04_cant_3d <- S04_cant_3d %>%
select(lon, lat, depth, cant_pos_S04 = cant_pos)
cant_3d <- inner_join(S04_cant_3d, G19_cant_3d)3.3.2 Cant at tref
Calculate Cant at tref by adding G19, scaled for the time since 1994.
# calculate reference year
tref <- GLODAP %>%
group_by(era) %>%
summarise(year = median(year)) %>%
ungroup()
# join cant with tref
cant_3d <- expand_grid(cant_3d, tref)
# calculate cant fields for all tref
cant_3d <- cant_3d %>%
mutate(cant_pos =
cant_pos_S04 +
((year - 1994) / 13 * cant_pos_G19))
# remove columns
cant_3d <- cant_3d %>%
select(lon, lat, depth, era, cant_pos)3.3.3 Combine GLODAP + Cant
# observations grid per era
GLODAP_obs_grid_era <- GLODAP %>%
distinct(lat, lon, era)
# cant data at observations grid
cant_3d_obs <- left_join(
GLODAP_obs_grid_era,
cant_3d)
# calculate number of cant data points per grid cell
cant_3d_obs <- cant_3d_obs %>%
group_by(lon, lat, era) %>%
mutate(n = n()) %>%
ungroup()
cant_3d_obs %>%
filter(n <= 1) %>%
ggplot(aes(lon,lat)) +
geom_point(data = GLODAP_obs_grid_era, aes(lon, lat)) +
geom_point(col = "red") +
facet_wrap(~era)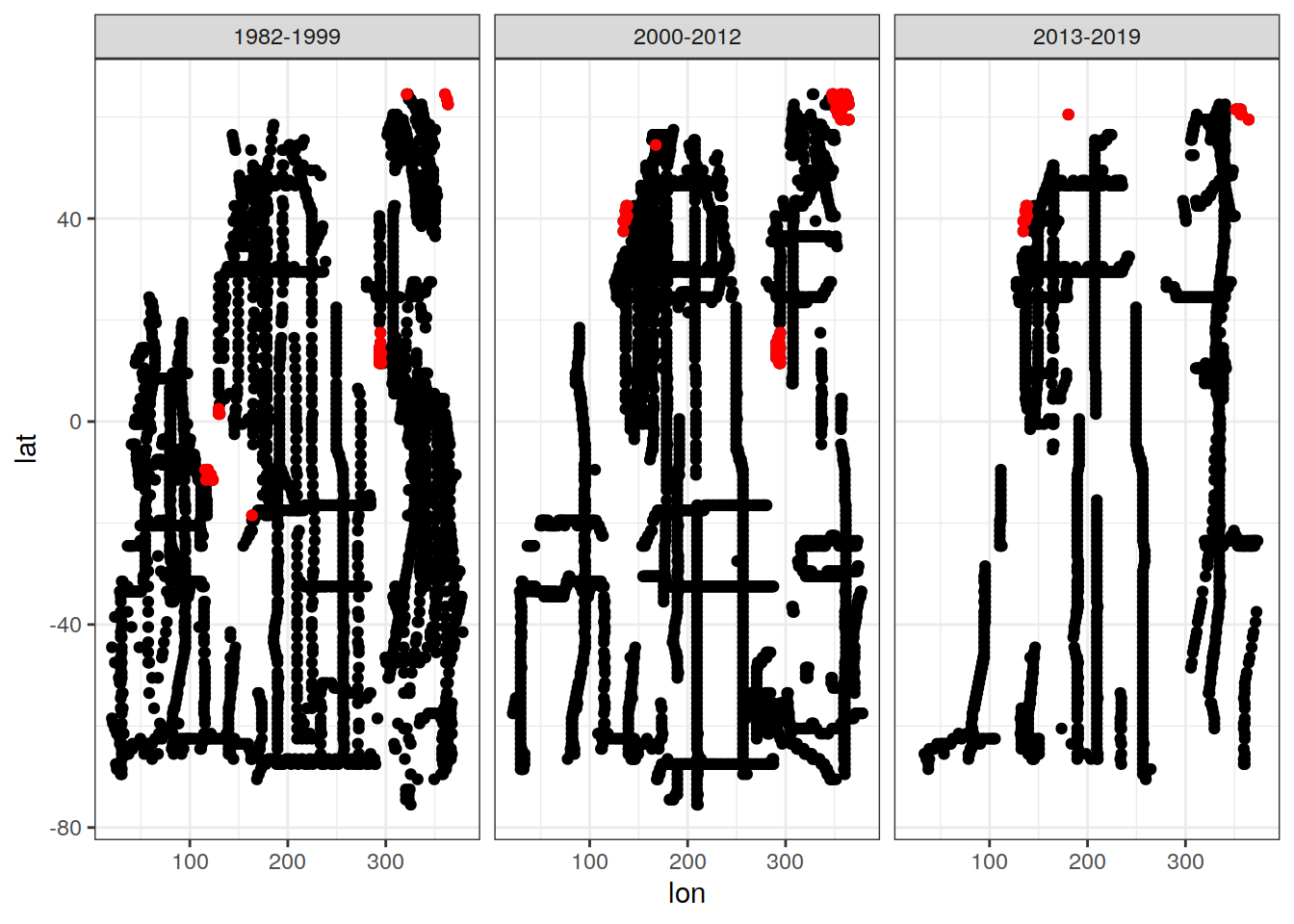
rm(cant_3d, GLODAP_obs_grid_era)
GLODAP_cant_obs <- full_join(GLODAP, cant_3d_obs)
rm(GLODAP, cant_3d_obs)
# fill number of cant data points per grid cell to all observations
GLODAP_cant_obs <- GLODAP_cant_obs %>%
group_by(lon, lat, era) %>%
fill(n, .direction = "updown") %>%
ungroup()The mapped Cant product was merged with GLODAP observation by:
- using an identical 1x1° horizontal grid
- linear interpolation of Cant from standard to sampling depth
# interpolate cant to observation depth
GLODAP_cant_obs_int <- GLODAP_cant_obs %>%
filter(n > 1) %>%
group_by(lat, lon, era) %>%
arrange(depth) %>%
mutate(cant_pos_int = approxfun(depth, cant_pos, rule = 2)(depth)) %>%
ungroup()
# set cant for observation depth if only one cant available
GLODAP_cant_obs_set <- GLODAP_cant_obs %>%
filter(n == 1) %>%
group_by(lat, lon, era) %>%
mutate(cant_pos_int = mean(cant_pos, na.rm = TRUE)) %>%
ungroup()
# bin data sets with interpolated and set cant
GLODAP_cant_obs <- bind_rows(GLODAP_cant_obs_int, GLODAP_cant_obs_set)
rm(GLODAP_cant_obs_int, GLODAP_cant_obs_set)
ggplot() +
geom_path(
data = GLODAP_cant_obs %>%
filter(lat == 48.5, lon == 165.5,!is.na(cant_pos)) %>%
arrange(depth),
aes(cant_pos, depth, col = "mapped")
) +
geom_point(
data = GLODAP_cant_obs %>%
filter(lat == 48.5, lon == 165.5,!is.na(cant_pos)) %>%
arrange(depth),
aes(cant_pos, depth, col = "mapped")
) +
geom_point(
data = GLODAP_cant_obs %>%
filter(lat == 48.5, lon == 165.5, date == ymd("2018-06-27")),
aes(cant_pos_int, depth, col = "interpolated")
) +
scale_y_reverse() +
facet_wrap(~era) +
scale_color_brewer(palette = "Dark2", name = "") +
labs(title = "Cant interpolation to sampling depth - example profile")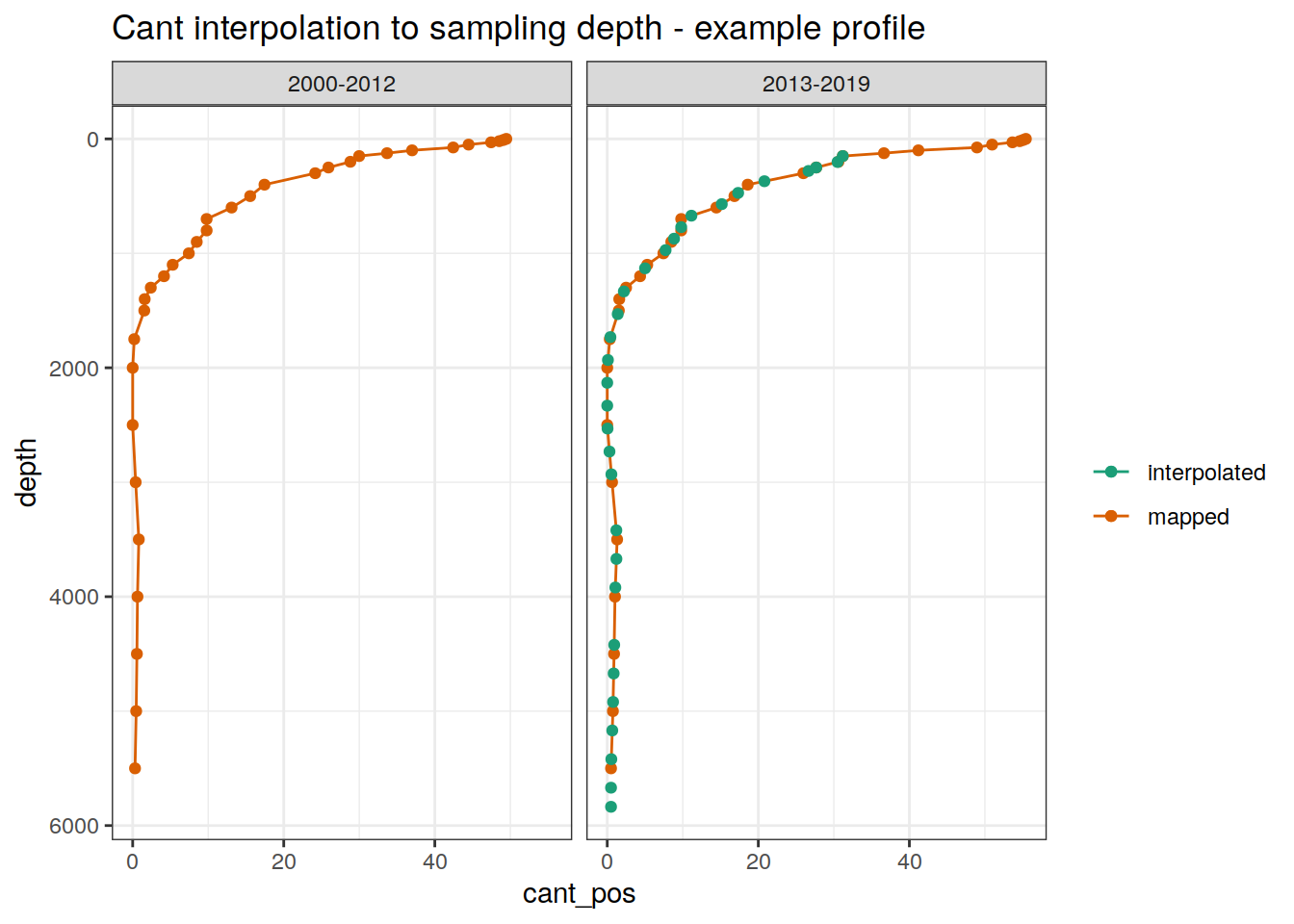
# remove cant data at grid cells without observations
GLODAP <- GLODAP_cant_obs %>%
filter(!is.na(cstar)) %>%
mutate(cant_pos = cant_pos_int) %>%
select(-cant_pos_int, n)
rm(GLODAP_cant_obs)3.3.4 Merge GLODAP + atm. pCO2
GLODAP observations were merged with mean annual atmospheric pCO2 levels by year.
GLODAP <- left_join(GLODAP, co2_atm)3.3.5 Calculation
# assign reference year
GLODAP <- GLODAP %>%
group_by(era) %>%
mutate(tref = median(year)) %>%
ungroup()
# extract atm pCO2 at reference year
co2_atm_tref <- right_join(co2_atm, tref) %>%
select(-year) %>%
rename(pCO2_tref = pCO2)
# merge atm pCO2 at tref with GLODAP
GLODAP <- full_join(GLODAP, co2_atm_tref)
rm(co2_atm, tref)
# calculate cstar for reference year
GLODAP <- GLODAP %>%
mutate(
cstar_tref_delta =
((pCO2 - pCO2_tref) / (pCO2_tref - params_local$preind_atm_pCO2)) * cant_pos,
cstar_tref = cstar - cstar_tref_delta)3.4 Control plots
GLODAP %>%
ggplot(aes(cstar_tref_delta)) +
geom_histogram(binwidth = 1) +
labs(title = "Histogramm with binwidth = 1")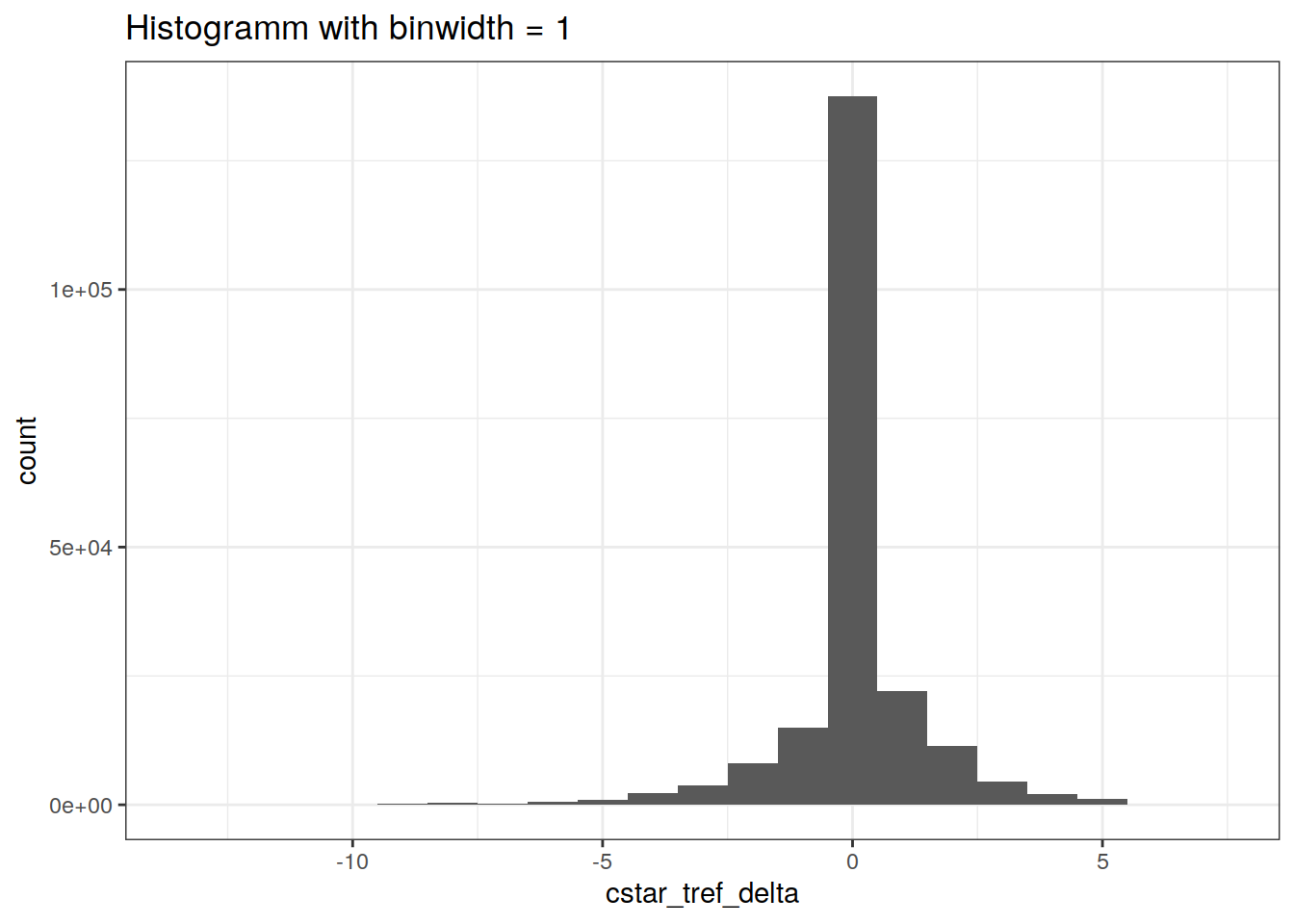
GLODAP %>%
sample_n(1e4) %>%
ggplot(aes(year, cstar_tref_delta, col = cant_pos)) +
geom_point() +
scale_color_viridis_c() +
labs(title = "Time series of random subsample 1e4")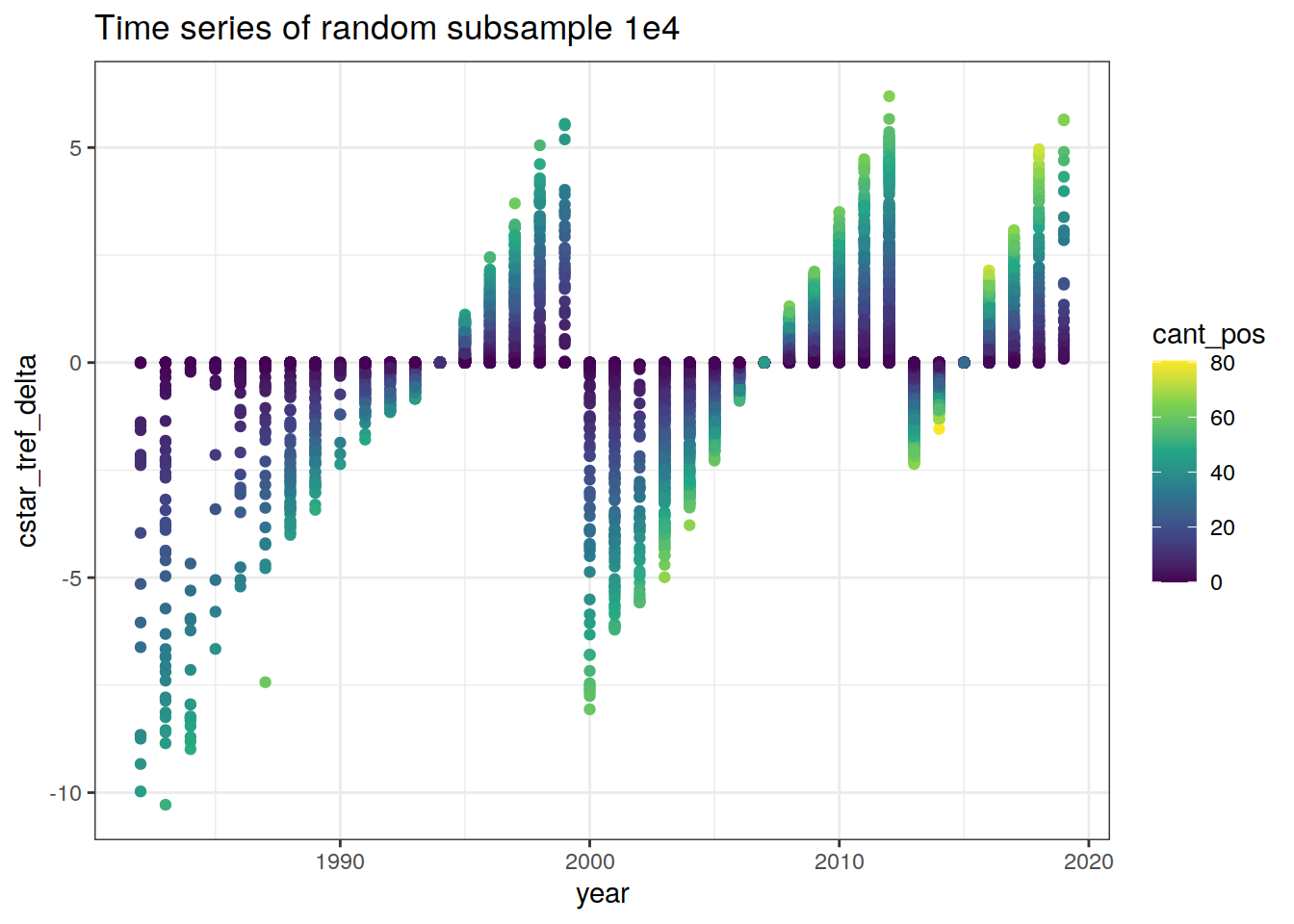
GLODAP %>%
ggplot(aes(year, cstar_tref_delta)) +
geom_bin2d(binwidth = 1) +
scale_fill_viridis_c(trans = "log10") +
labs(title = "Heatmap with binwidth = 1")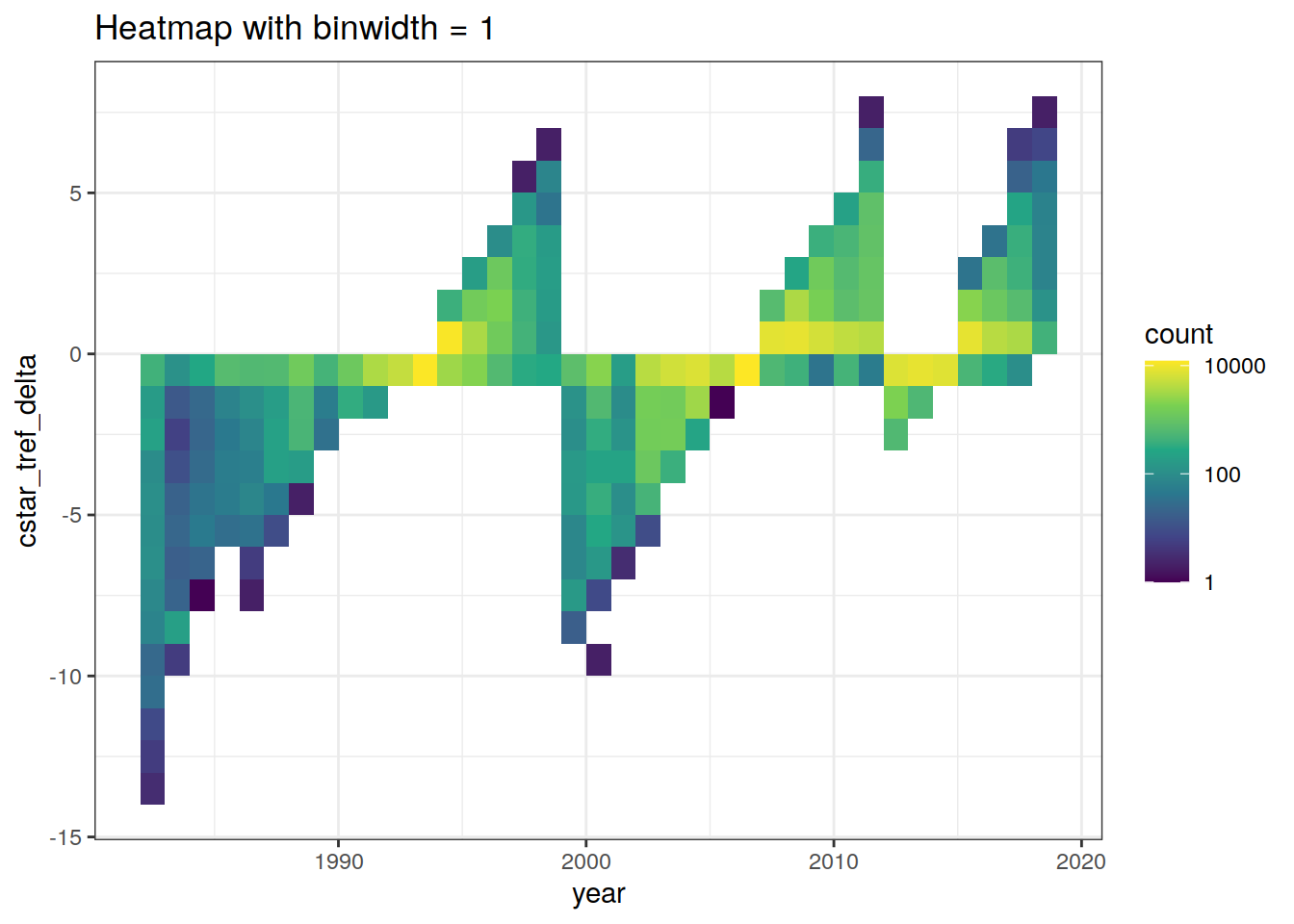
4 Selected section plots
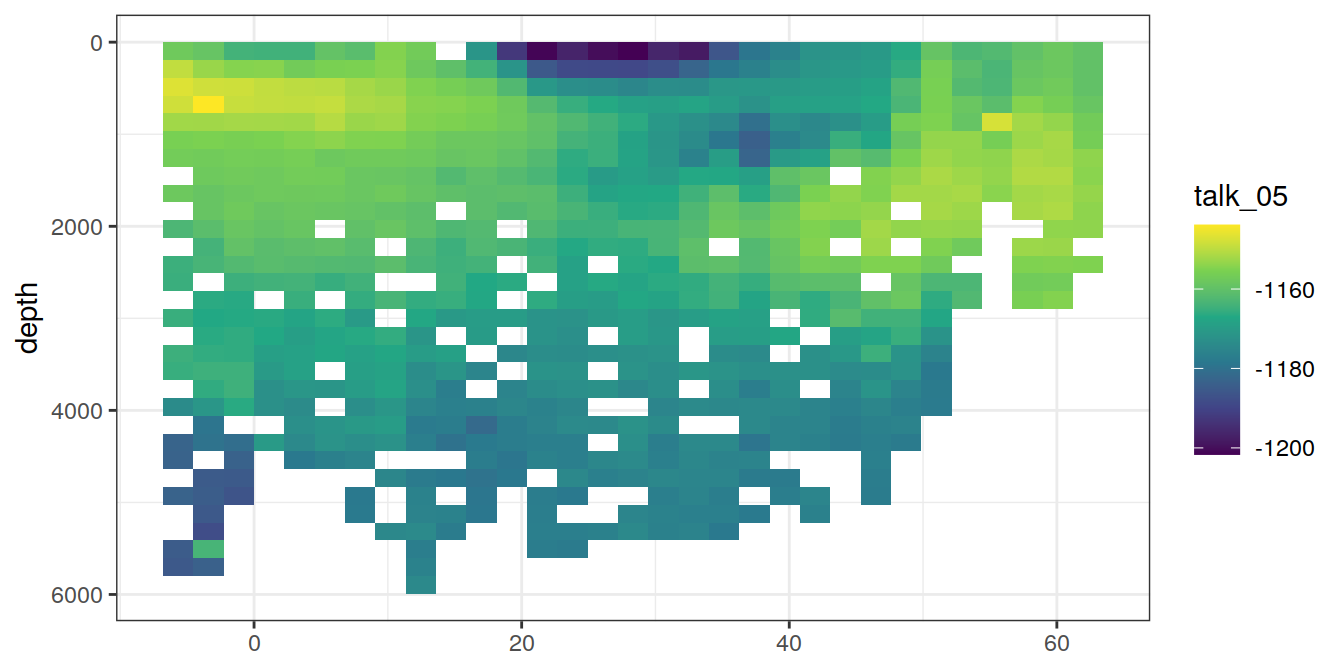
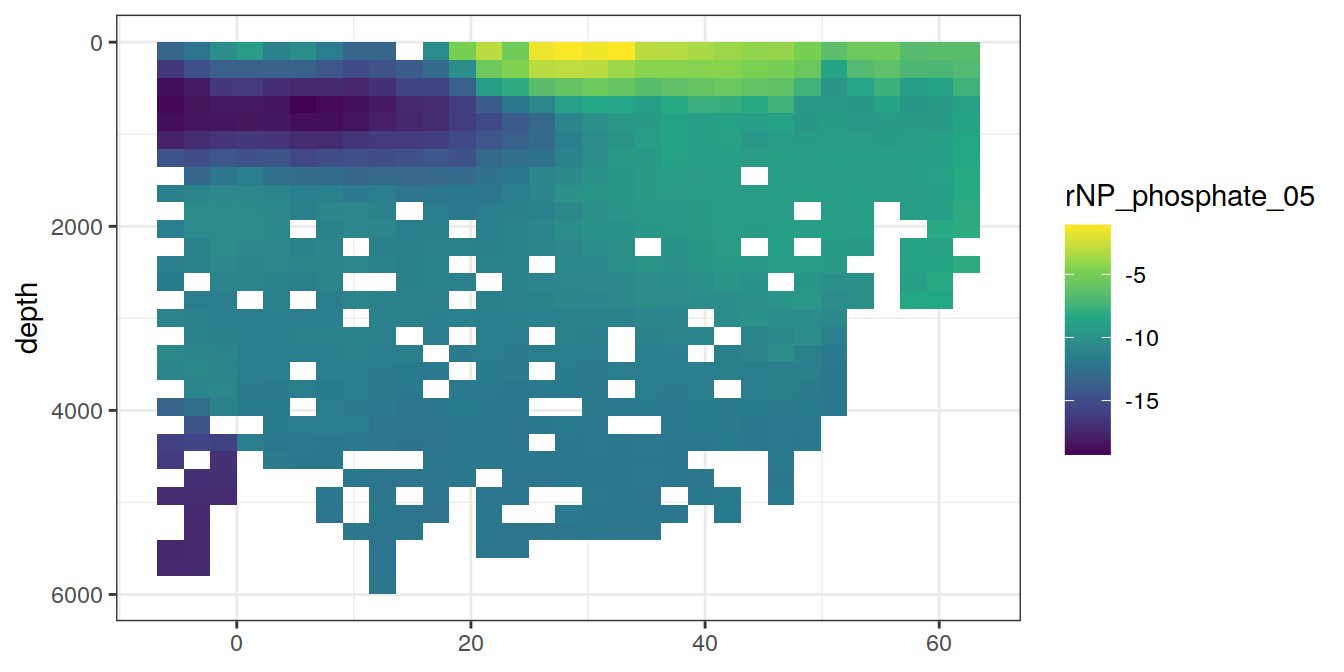
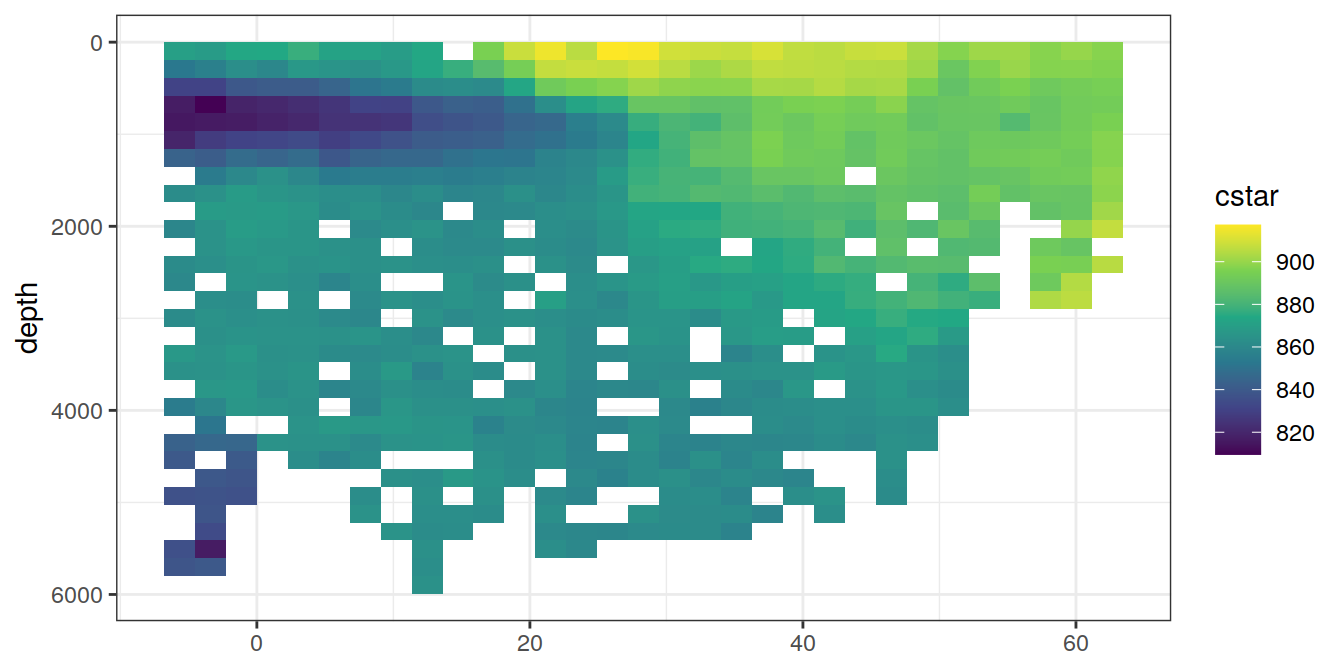
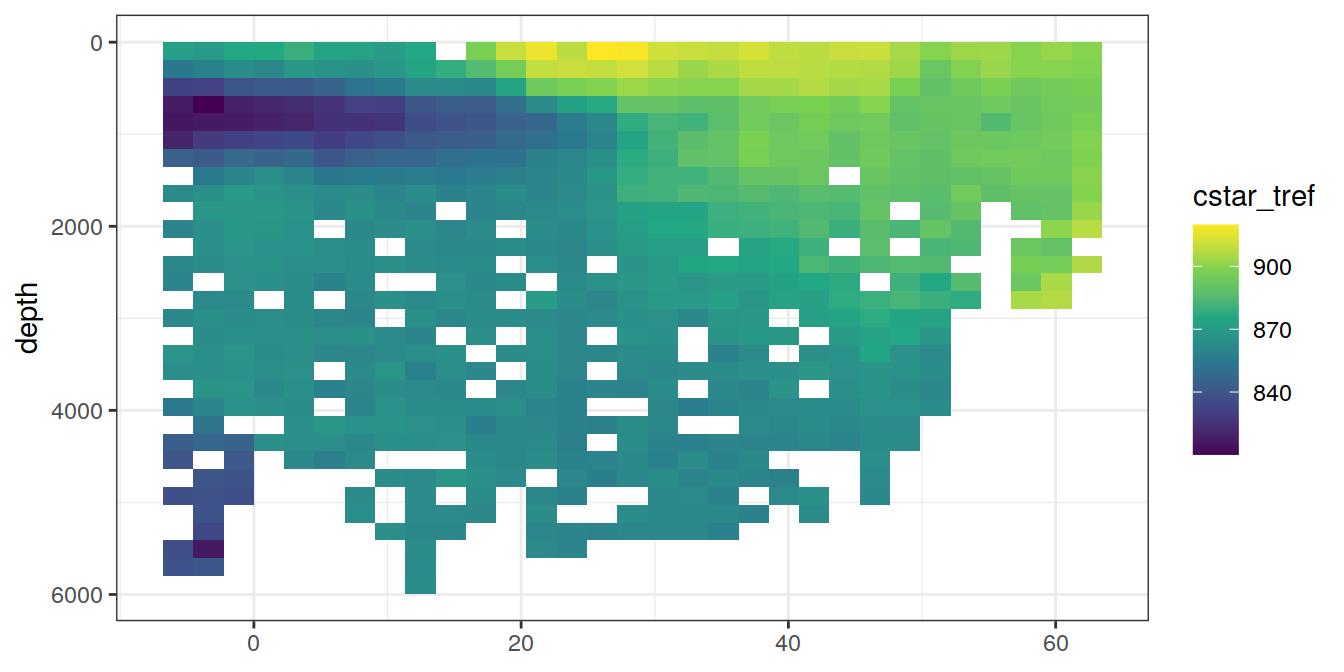
A selected section is plotted to demonstrate the magnitude of various parameters and corrections relevant to C*.
GLODAP_cruise <- GLODAP %>%
filter(cruise %in% params_global$cruises_meridional)map +
geom_path(data = GLODAP_cruise %>%
arrange(date),
aes(lon, lat)) +
geom_point(data = GLODAP_cruise %>%
arrange(date),
aes(lon, lat, col = date)) +
scale_color_viridis_c(trans = "date") +
labs(title = paste("Cruise year:", mean(GLODAP_cruise$year)))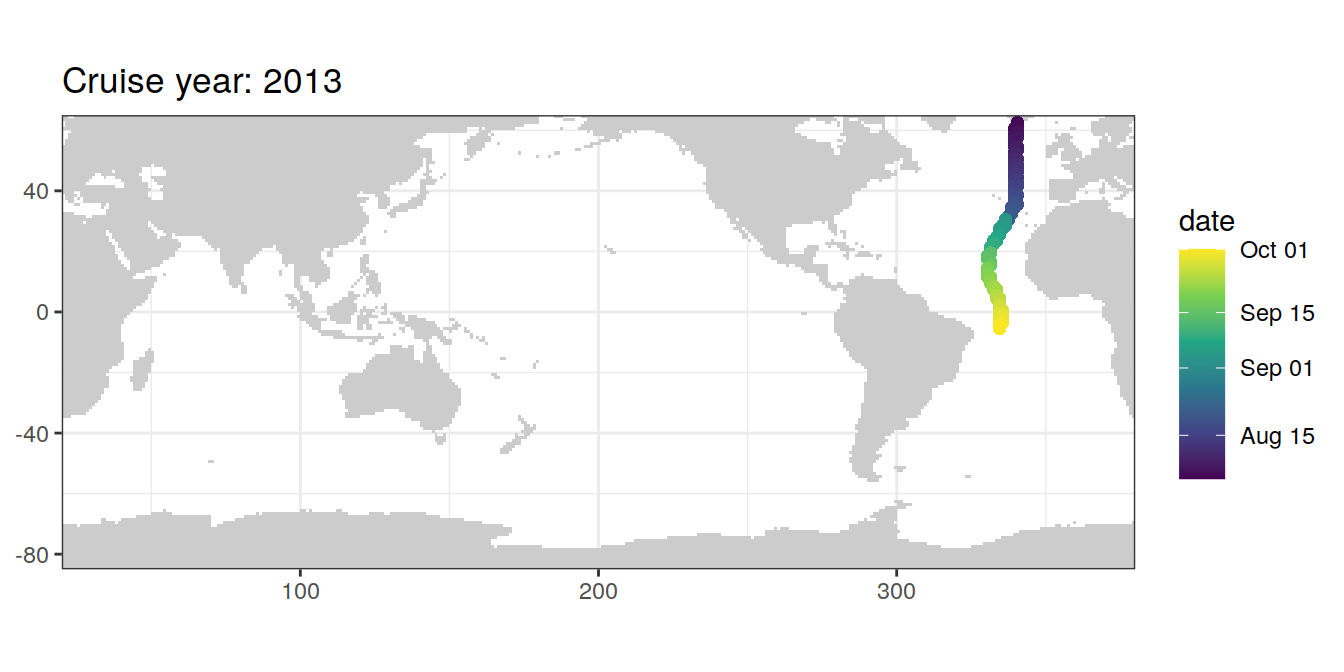
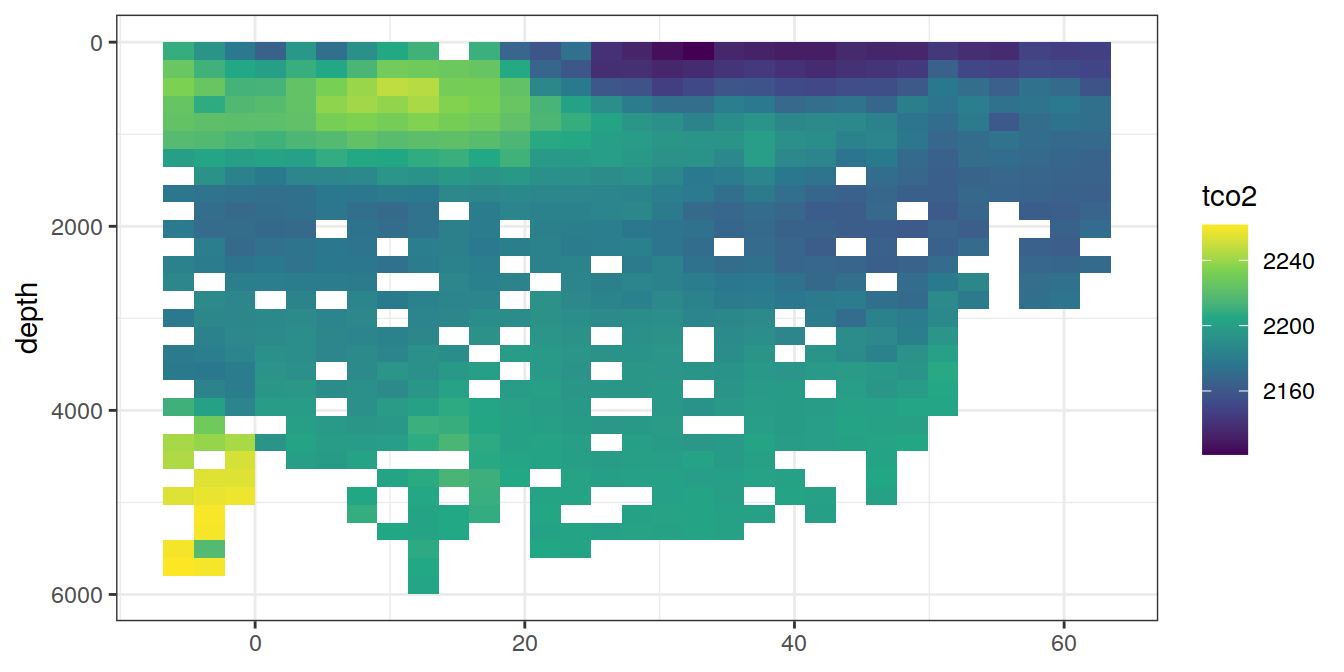
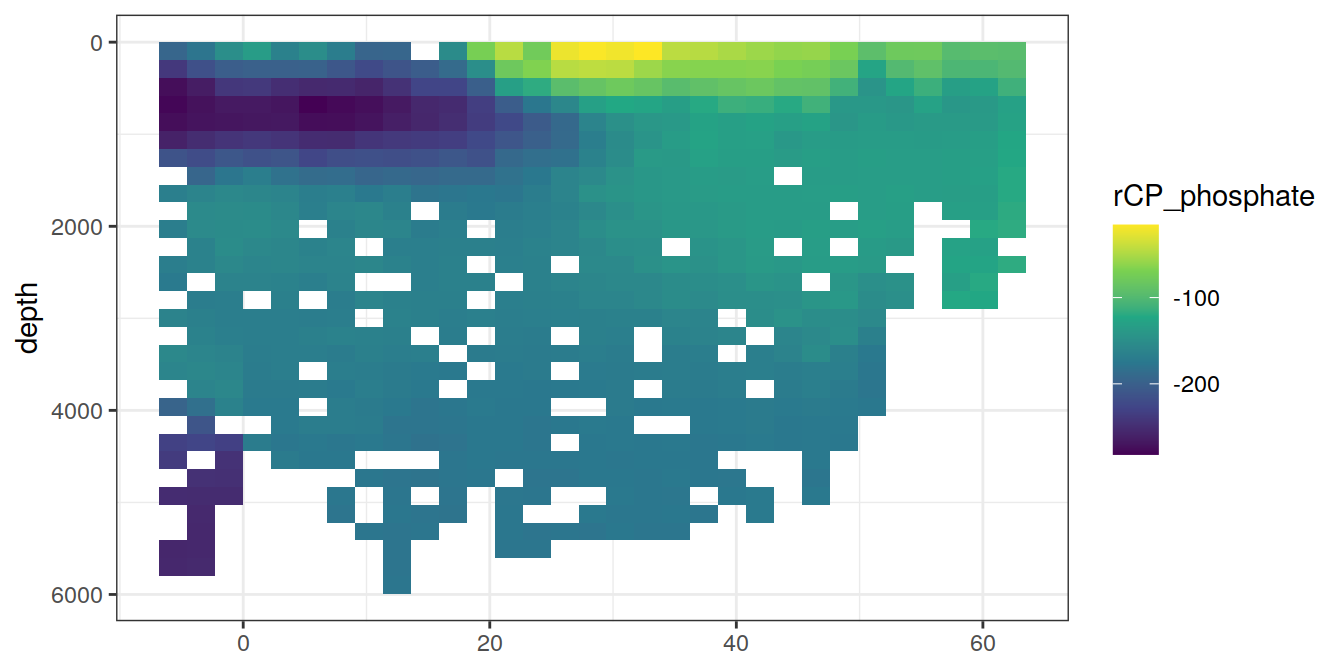
lat_section <-
GLODAP_cruise %>%
ggplot(aes(lat, depth)) +
scale_y_reverse() +
scale_fill_viridis_c() +
theme(axis.title.x = element_blank())
for (i_var in c("tco2",
"rCP_phosphate",
"talk_05",
"rNP_phosphate_05",
"cstar",
"cstar_tref")) {
print(lat_section +
stat_summary_2d(aes(z = !!sym(i_var))) +
scale_fill_viridis_c(name = i_var)
)
}
rm(lat_section, GLODAP_cruise)5 Isoneutral slabs
The following boundaries for isoneutral slabs were defined:
- Atlantic: -, 26, 26.5, 26.75, 27, 27.25, 27.5, 27.75, 27.85, 27.95, 28.05, 28.1, 28.15, 28.2,
- Indo-Pacific: -, 26, 26.5, 26.75, 27, 27.25, 27.5, 27.75, 27.85, 27.95, 28.05, 28.1,
Continuous neutral densities (gamma) values from GLODAP are grouped into isoneutral slabs.
GLODAP <- m_cut_gamma(GLODAP, "gamma")GLODAP_cruise <- GLODAP %>%
filter(cruise %in% params_global$cruises_meridional)
lat_section <-
GLODAP_cruise %>%
ggplot(aes(lat, depth)) +
scale_y_reverse() +
theme(legend.position = "bottom")
lat_section +
geom_point(aes(col = gamma_slab)) +
scale_color_viridis_d()
rm(lat_section, GLODAP_cruise)# this section was only used to calculate gamma locally, and compare it to the value provided in GLODAP data set
GLODAP_cruise <- GLODAP %>%
filter(cruise %in% params_global$cruises_meridional)
library(oce)
library(gsw)
# calculate pressure from depth
GLODAP_cruise <- GLODAP_cruise %>%
mutate(CTDPRS = gsw_p_from_z(-depth,
lat))
GLODAP_cruise <- GLODAP_cruise %>%
mutate(THETA = swTheta(salinity = sal,
temperature = temp,
pressure = CTDPRS,
referencePressure = 0,
longitude = lon-180,
latitude = lat))
GLODAP_cruise <- GLODAP_cruise %>%
rename(LATITUDE = lat,
LONGITUDE = lon,
SALNTY = sal,
gamma_provided = gamma)
library(reticulate)
source_python(here::here("code/python_scripts",
"Gamma_GLODAP_python.py"))
GLODAP_cruise <- calculate_gamma(GLODAP_cruise)
GLODAP_cruise <- GLODAP_cruise %>%
mutate(gamma_delta = gamma_provided - GAMMA)
lat_section <-
GLODAP_cruise %>%
ggplot(aes(LATITUDE, CTDPRS)) +
scale_y_reverse() +
theme(legend.position = "bottom")
lat_section +
stat_summary_2d(aes(z = gamma_delta)) +
scale_color_viridis_c()
GLODAP_cruise %>%
ggplot(aes(gamma_delta))+
geom_histogram()
rm(lat_section, GLODAP_cruise, cruises_meridional)6 Observations coverage
GLODAP <- GLODAP %>%
mutate(gamma_slab = factor(gamma_slab),
gamma_slab = factor(gamma_slab, levels = rev(levels(gamma_slab))))
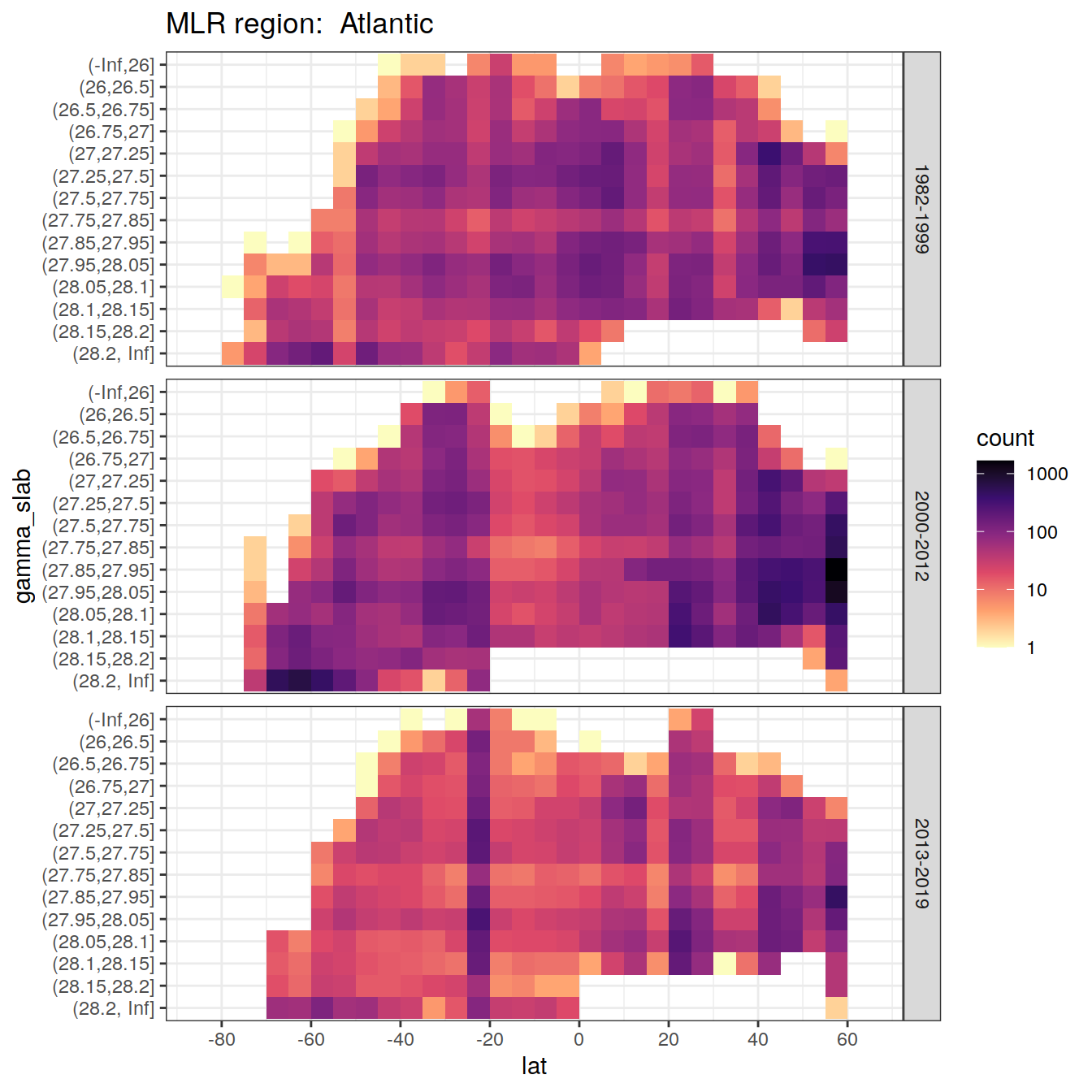
for (i_basin in unique(GLODAP$basin)) {
# i_basin <- unique(GLODAP$basin)[3]
print(
GLODAP %>%
filter(basin == i_basin) %>%
ggplot(aes(lat, gamma_slab)) +
geom_bin2d(binwidth = 5) +
scale_fill_viridis_c(
option = "magma",
direction = -1,
trans = "log10"
) +
scale_x_continuous(breaks = seq(-100, 100, 20),
limits = c(params_global$lat_min,
params_global$lat_max)) +
facet_grid(era ~ .) +
labs(title = paste("MLR region: ", i_basin))
)
}
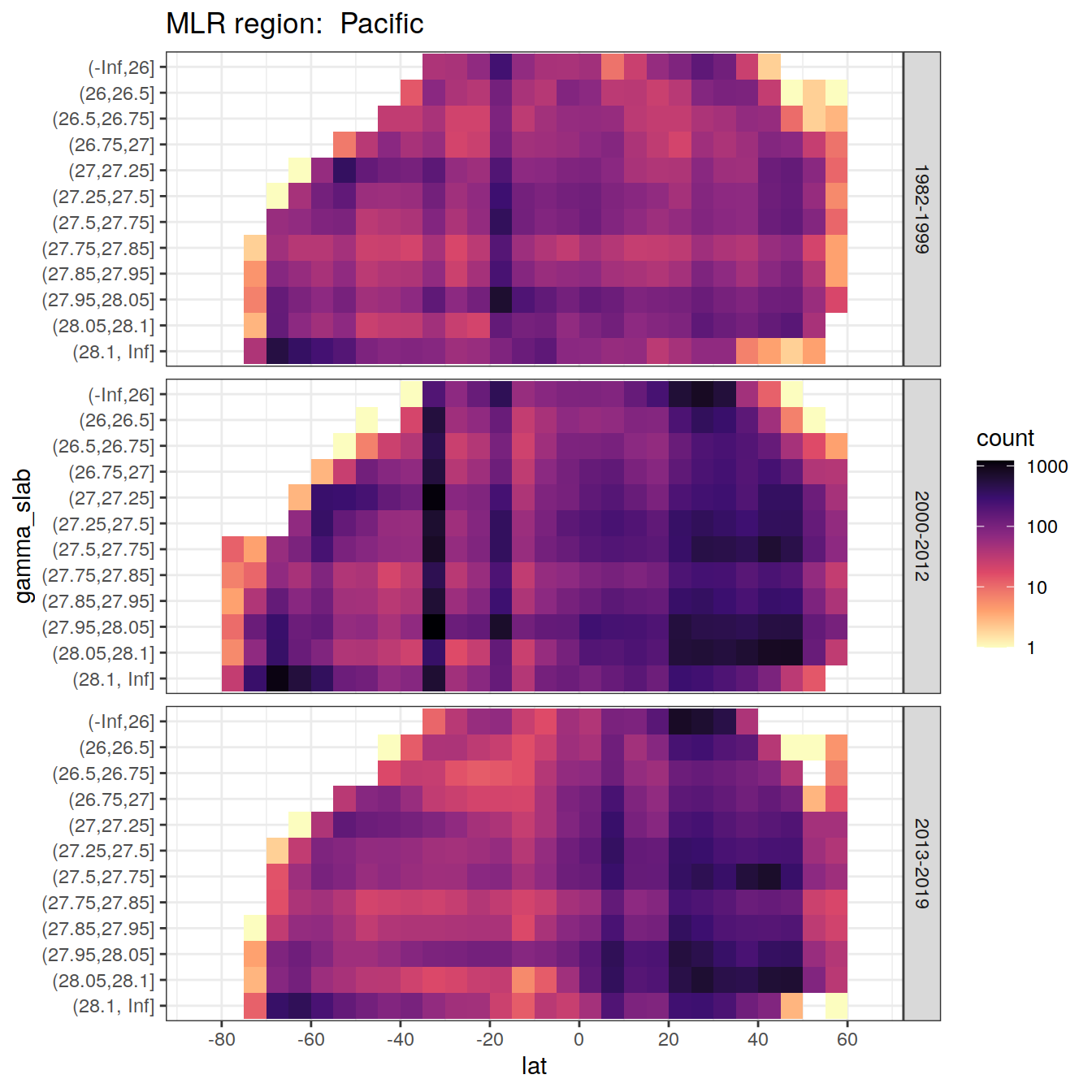
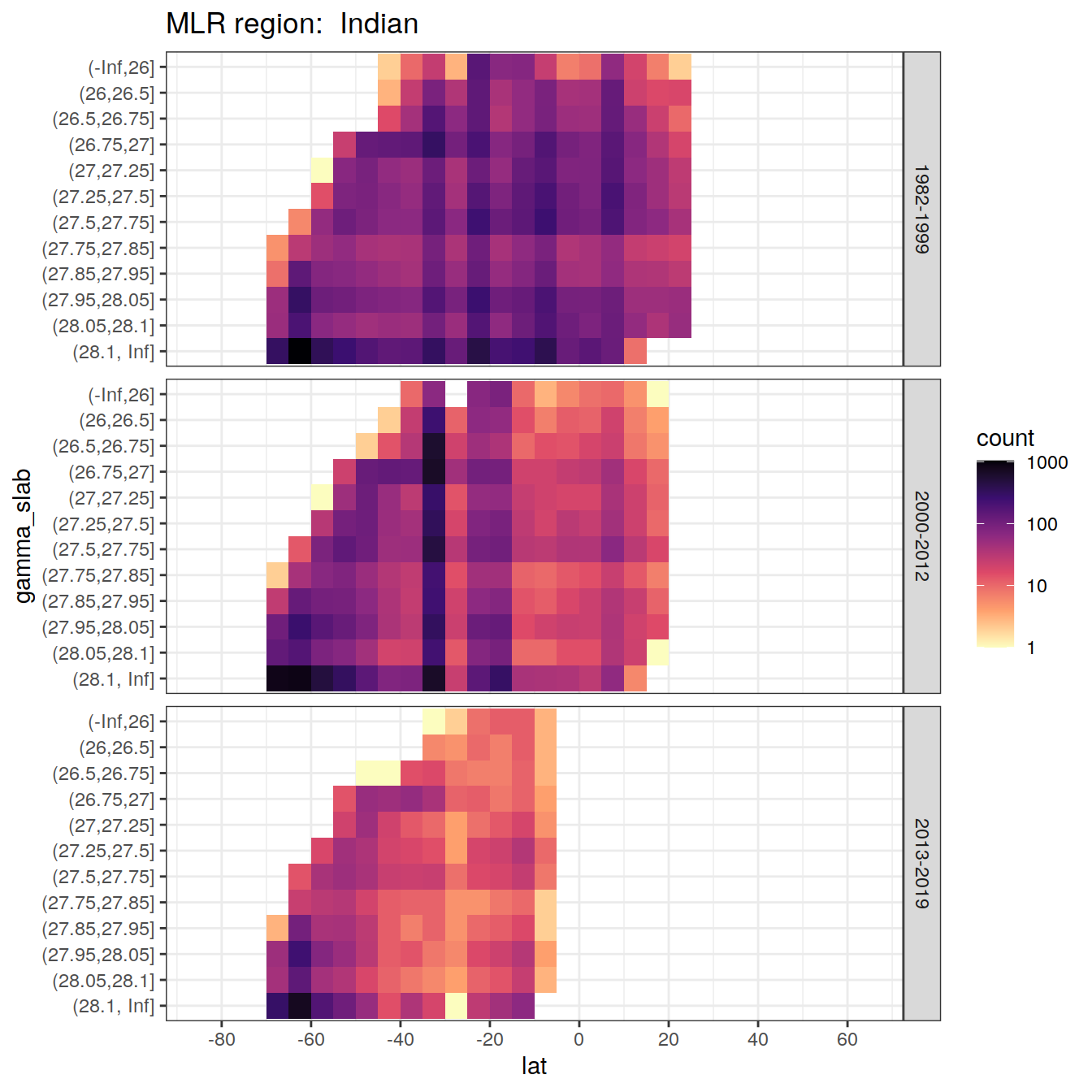
6.1 Histograms
GLODAP_vars <- GLODAP %>%
select(cstar_tref,
sal,
temp,
oxygen,
aou,
silicate,
phosphate,
phosphate_star)
GLODAP_vars_long <- GLODAP_vars %>%
pivot_longer(cstar_tref:phosphate_star, names_to = "variable", values_to = "value")
GLODAP_vars_long %>%
ggplot(aes(value)) +
geom_histogram() +
facet_wrap(~ variable,
ncol = 2,
scales = "free")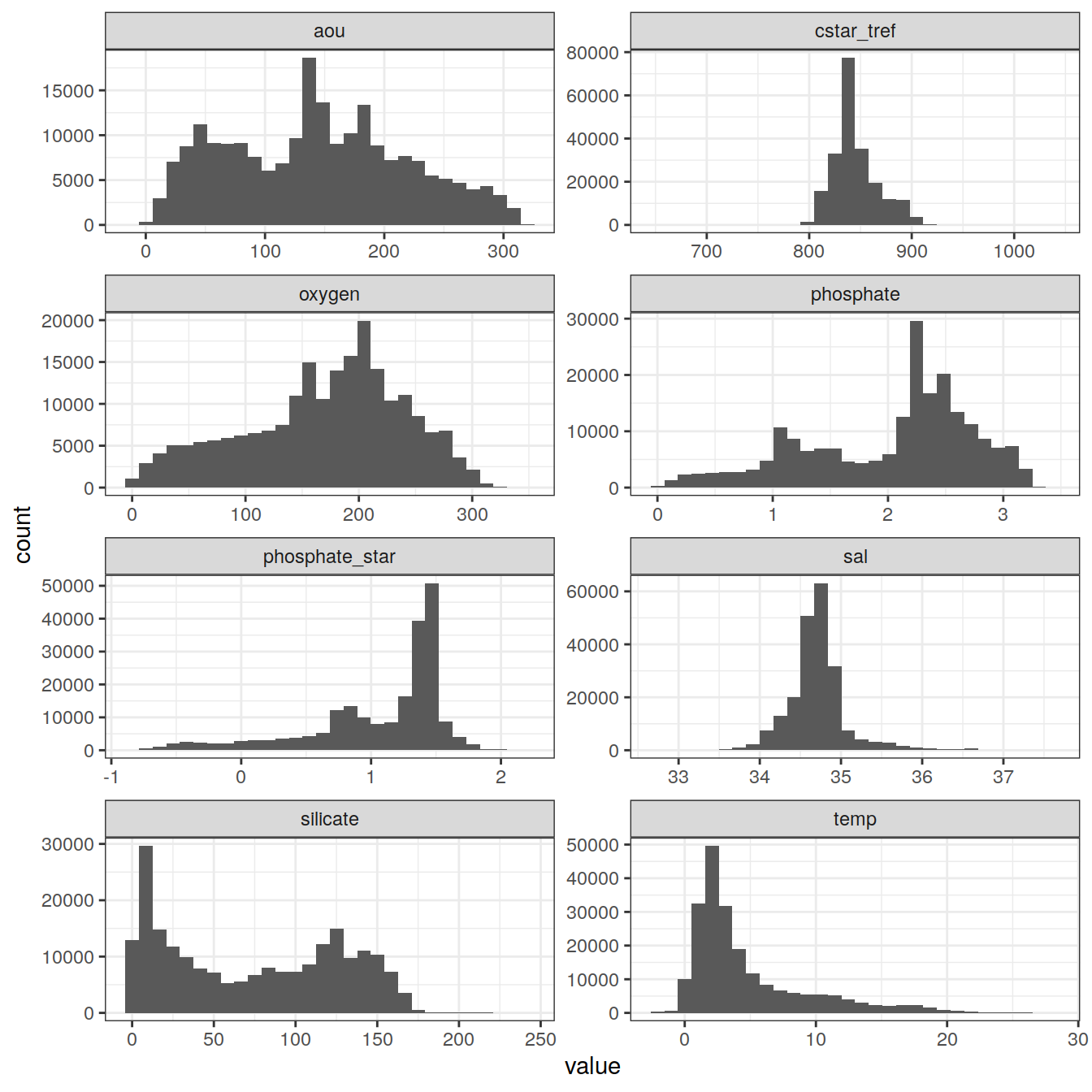
rm(GLODAP_vars, GLODAP_vars_long)7 Individual cruise sections
Zonal and meridional section plots are produce for each cruise individually and are available under:
/nfs/kryo/work/jenmueller/emlr_cant/model/v_XXX/figures/Cruise_sections_histograms/
if (params_local$plot_all_figures == "y") {
cruises <- GLODAP %>%
group_by(cruise) %>%
summarise(date_mean = mean(date, na.rm = TRUE),
n = n()) %>%
ungroup() %>%
arrange(date_mean)
GLODAP <- full_join(GLODAP, cruises)
n <- 0
for (i_cruise in unique(cruises$cruise)) {
# i_cruise <- unique(cruises$cruise)[1]
# n <- n + 1
# print(n)
GLODAP_cruise <- GLODAP %>%
filter(cruise == i_cruise) %>%
arrange(date)
cruises_cruise <- cruises %>%
filter(cruise == i_cruise)
map_plot <-
map +
geom_point(data = GLODAP_cruise,
aes(lon, lat, col = date)) +
scale_color_viridis_c(trans = "date") +
labs(title = paste("Mean date:", cruises_cruise$date_mean,
"| cruise:", cruises_cruise$cruise,
"| n(samples):", cruises_cruise$n))
lon_section <- GLODAP_cruise %>%
ggplot(aes(lon, depth)) +
scale_y_reverse() +
scale_fill_viridis_c()
lon_tco2 <- lon_section+
stat_summary_2d(aes(z=tco2))
lon_talk <- lon_section+
stat_summary_2d(aes(z=talk))
lon_phosphate <- lon_section+
stat_summary_2d(aes(z=phosphate))
lon_oxygen <- lon_section+
stat_summary_2d(aes(z=oxygen))
lon_aou <- lon_section+
stat_summary_2d(aes(z=aou))
lon_phosphate_star <- lon_section+
stat_summary_2d(aes(z=phosphate_star))
lon_nitrate <- lon_section+
stat_summary_2d(aes(z=nitrate))
lon_cstar <- lon_section+
stat_summary_2d(aes(z=cstar_tref))
lat_section <- GLODAP_cruise %>%
ggplot(aes(lat, depth)) +
scale_y_reverse() +
scale_fill_viridis_c()
lat_tco2 <- lat_section+
stat_summary_2d(aes(z=tco2))
lat_talk <- lat_section+
stat_summary_2d(aes(z=talk))
lat_phosphate <- lat_section+
stat_summary_2d(aes(z=phosphate))
lat_oxygen <- lat_section+
stat_summary_2d(aes(z=oxygen))
lat_aou <- lat_section+
stat_summary_2d(aes(z=aou))
lat_phosphate_star <- lat_section+
stat_summary_2d(aes(z=phosphate_star))
lat_nitrate <- lat_section+
stat_summary_2d(aes(z=nitrate))
lat_cstar <- lat_section+
stat_summary_2d(aes(z=cstar_tref))
hist_tco2 <- GLODAP_cruise %>%
ggplot(aes(tco2)) +
geom_histogram()
hist_talk <- GLODAP_cruise %>%
ggplot(aes(talk)) +
geom_histogram()
hist_phosphate <- GLODAP_cruise %>%
ggplot(aes(phosphate)) +
geom_histogram()
hist_oxygen <- GLODAP_cruise %>%
ggplot(aes(oxygen)) +
geom_histogram()
hist_aou <- GLODAP_cruise %>%
ggplot(aes(aou)) +
geom_histogram()
hist_phosphate_star <- GLODAP_cruise %>%
ggplot(aes(phosphate_star)) +
geom_histogram()
hist_nitrate <- GLODAP_cruise %>%
ggplot(aes(nitrate)) +
geom_histogram()
hist_cstar <- GLODAP_cruise %>%
ggplot(aes(cstar_tref)) +
geom_histogram()
(map_plot /
((hist_tco2 / hist_talk / hist_phosphate / hist_cstar) |
(hist_oxygen / hist_phosphate_star / hist_nitrate / hist_aou)
)) |
((lat_tco2 / lat_talk / lat_phosphate / lat_oxygen / lat_aou / lat_phosphate_star / lat_nitrate / lat_cstar) |
(lon_tco2 / lon_talk / lon_phosphate / lon_oxygen / lon_aou /lon_phosphate_star / lon_nitrate / lon_cstar))
ggsave(
path = paste(path_version_figures, "Cruise_sections_histograms/", sep = ""),
filename = paste(
"Cruise_date",
cruises_cruise$date_mean,
"count",
cruises_cruise$n,
"cruiseID",
cruises_cruise$cruise,
".png",
sep = "_"
),
width = 20, height = 12)
rm(map_plot,
lon_section, lat_section,
lat_tco2, lat_talk, lat_phosphate, lon_tco2, lon_talk, lon_phosphate,
GLODAP_cruise, cruises_cruise)
}
}8 Write files
# select relevant columns
GLODAP <- GLODAP %>%
select(
year,
date,
era,
basin,
basin_AIP,
lat,
lon,
depth,
gamma,
gamma_slab,
params_local$MLR_predictors,
params_local$MLR_target
)
GLODAP %>% write_csv(paste(
path_version_data,
"GLODAPv2.2020_MLR_fitting_ready.csv",
sep = ""
))
co2_atm_tref %>% write_csv(paste(path_version_data,
"co2_atm_tref.csv",
sep = ""))
sessionInfo()R version 4.0.3 (2020-10-10)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: openSUSE Leap 15.1
Matrix products: default
BLAS: /usr/local/R-4.0.3/lib64/R/lib/libRblas.so
LAPACK: /usr/local/R-4.0.3/lib64/R/lib/libRlapack.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] lubridate_1.7.9 marelac_2.1.10 shape_1.4.5 metR_0.9.0
[5] scico_1.2.0 patchwork_1.1.0 collapse_1.4.2 forcats_0.5.0
[9] stringr_1.4.0 dplyr_1.0.2 purrr_0.3.4 readr_1.4.0
[13] tidyr_1.1.2 tibble_3.0.4 ggplot2_3.3.2 tidyverse_1.3.0
[17] workflowr_1.6.2
loaded via a namespace (and not attached):
[1] httr_1.4.2 viridisLite_0.3.0 jsonlite_1.7.1
[4] here_0.1 modelr_0.1.8 assertthat_0.2.1
[7] blob_1.2.1 cellranger_1.1.0 yaml_2.2.1
[10] pillar_1.4.7 backports_1.1.10 lattice_0.20-41
[13] glue_1.4.2 RcppEigen_0.3.3.7.0 digest_0.6.27
[16] RColorBrewer_1.1-2 promises_1.1.1 checkmate_2.0.0
[19] rvest_0.3.6 colorspace_2.0-0 htmltools_0.5.0
[22] httpuv_1.5.4 Matrix_1.2-18 pkgconfig_2.0.3
[25] broom_0.7.2 seacarb_3.2.14 haven_2.3.1
[28] scales_1.1.1 whisker_0.4 later_1.1.0.1
[31] git2r_0.27.1 farver_2.0.3 generics_0.0.2
[34] ellipsis_0.3.1 withr_2.3.0 cli_2.2.0
[37] magrittr_2.0.1 crayon_1.3.4 readxl_1.3.1
[40] evaluate_0.14 fs_1.5.0 fansi_0.4.1
[43] xml2_1.3.2 RcppArmadillo_0.10.1.2.0 oce_1.2-0
[46] tools_4.0.3 data.table_1.13.2 hms_0.5.3
[49] lifecycle_0.2.0 munsell_0.5.0 reprex_0.3.0
[52] gsw_1.0-5 compiler_4.0.3 rlang_0.4.9
[55] grid_4.0.3 rstudioapi_0.13 labeling_0.4.2
[58] rmarkdown_2.5 testthat_3.0.0 gtable_0.3.0
[61] DBI_1.1.0 R6_2.5.0 knitr_1.30
[64] rprojroot_2.0.2 stringi_1.5.3 parallel_4.0.3
[67] Rcpp_1.0.5 vctrs_0.3.5 dbplyr_1.4.4
[70] tidyselect_1.1.0 xfun_0.18