Single-cell PBMC Example
karltayeb
2022-03-16
Last updated: 2022-03-29
Checks: 7 0
Knit directory: logistic-susie-gsea/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20220105) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 524d94b. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .RData
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/figure/
Ignored: library/
Ignored: renv/library/
Ignored: renv/staging/
Ignored: staging/
Untracked files:
Untracked: _targets.R
Untracked: _targets.html
Untracked: _targets.md
Untracked: _targets/
Untracked: _targets_r/
Untracked: analysis/fetal_reference_cellid_gsea.Rmd
Untracked: analysis/fixed_intercept.Rmd
Untracked: analysis/iDEA_examples.Rmd
Untracked: analysis/latent_gene_list.Rmd
Untracked: analysis/latent_logistic_susie.Rmd
Untracked: analysis/libra_setup.Rmd
Untracked: analysis/linear_method_failure_modes.Rmd
Untracked: analysis/linear_regression_failure_regime.Rmd
Untracked: analysis/logistic_susie_veb_boost_vs_vb.Rmd
Untracked: analysis/references.bib
Untracked: analysis/simulations.Rmd
Untracked: analysis/test.Rmd
Untracked: analysis/wenhe_baboon_example.Rmd
Untracked: build_site.R
Untracked: cache/
Untracked: code/latent_logistic_susie.R
Untracked: code/marginal_sumstat_gsea_collapsed.R
Untracked: data/adipose_2yr_topsnp.txt
Untracked: data/fetal_reference_cellid_gene_sets.RData
Untracked: data/pbmc-purified/
Untracked: docs.zip
Untracked: index.md
Untracked: latent_logistic_susie_cache/
Untracked: simulation_targets/
Untracked: single_cell_pbmc_cache/
Untracked: summary_stat_gsea_exploration_cache/
Unstaged changes:
Modified: _simulation_targets.R
Modified: _targets.Rmd
Modified: analysis/gseabenchmark_tcga.Rmd
Modified: code/fit_baselines.R
Modified: code/fit_logistic_susie.R
Modified: code/fit_mr_ash.R
Modified: code/fit_susie.R
Modified: code/load_gene_sets.R
Modified: code/marginal_sumstat_gsea.R
Modified: code/simulate_gene_lists.R
Modified: code/utils.R
Modified: target_components/factories.R
Modified: target_components/methods.R
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/single_cell_pbmc.Rmd) and HTML (docs/single_cell_pbmc.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 524d94b | karltayeb | 2022-03-29 | wflow_publish(“analysis/single_cell_pbmc.Rmd”) |
| html | 61540e8 | karltayeb | 2022-03-29 | Build site. |
| Rmd | 54209af | karltayeb | 2022-03-29 | wflow_publish(“analysis/single_cell_pbmc.Rmd”) |
| html | 67bf8cf | karltayeb | 2022-03-29 | Build site. |
| Rmd | 170e480 | karltayeb | 2022-03-29 | wflow_publish(“analysis/single_cell_pbmc.Rmd”) |
| html | 90c6006 | karltayeb | 2022-03-29 | Build site. |
| Rmd | 8159b83 | karltayeb | 2022-03-29 | wflow_publish(“analysis/single_cell_pbmc.Rmd”) |
| html | 9d7fd29 | karltayeb | 2022-03-29 | Build site. |
| Rmd | 51647d4 | karltayeb | 2022-03-29 | wflow_publish(“analysis/single_cell_pbmc.Rmd”) |
| html | 3da7c5b | karltayeb | 2022-03-29 | Build site. |
| Rmd | fc1f3c1 | karltayeb | 2022-03-29 | wflow_publish(“analysis/single_cell_pbmc.Rmd”) |
| html | 8646723 | karltayeb | 2022-03-29 | Build site. |
| Rmd | 6143c44 | karltayeb | 2022-03-29 | wflow_publish(“analysis/single_cell_pbmc.Rmd”) |
| html | 56f8130 | karltayeb | 2022-03-29 | Build site. |
| html | a2bdb56 | karltayeb | 2022-03-29 | Build site. |
| Rmd | 122deec | karltayeb | 2022-03-29 | wflow_publish(pages) |
Introduction
Our goals here are to run Logistic SuSiE on differential expression results from TCGA. We want to assess:
- If the resulting enrichment results look good/interpretable across multiple/concatenated gene sets
- Assess sensitivity to a range of p-value thresholds
- Evaluate the potential of the summary stat latent model
library(GSEABenchmarkeR)
library(EnrichmentBrowser)
library(tidyverse)
library(susieR)
library(DT)
source('code/load_gene_sets.R')
source('code/utils.R')
source('code/logistic_susie_vb.R')
source('code/logistic_susie_veb_boost.R')
source('code/latent_logistic_susie.R')Setup
Load Gene Sets
loadGeneSetX uniformly formats gene sets and generates the \(X\) matrix We can source any gene set from WebGestaltR::listGeneSet()
gs_list <- WebGestaltR::listGeneSet()
gobp <- loadGeneSetX('geneontology_Biological_Process', min.size=50) # just huge number of gene sets
gobp_nr <- loadGeneSetX('geneontology_Biological_Process_noRedundant', min.size=1)
gomf <- loadGeneSetX('geneontology_Molecular_Function', min.size=1)
kegg <- loadGeneSetX('pathway_KEGG', min.size=1)
reactome <- loadGeneSetX('pathway_Reactome', min.size=1)
wikipathway_cancer <- loadGeneSetX('pathway_Wikipathway_cancer', min.size=1)
wikipathway <- loadGeneSetX('pathway_Wikipathway', min.size=1)
genesets <- list(
gobp=gobp,
gobp_nr=gobp_nr,
gomf=gomf,
kegg=kegg,
reactome=reactome,
wikipathway_cancer=wikipathway_cancer,
wikipathway=wikipathway
)load('data/pbmc-purified/deseq2-pbmc-purified.RData')
convert_labels <- function(y, from='SYMBOL', to='ENTREZID'){
hs <- org.Hs.eg.db::org.Hs.eg.db
gene_symbols <- names(y)
symbol2entrez <- AnnotationDbi::select(hs, keys=gene_symbols, columns=c(to, from), keytype = from)
symbol2entrez <- symbol2entrez[!duplicated(symbol2entrez[[from]]),]
symbol2entrez <- symbol2entrez[!is.na(symbol2entrez[[to]]),]
symbol2entrez <- symbol2entrez[!is.na(symbol2entrez[[from]]),]
rownames(symbol2entrez) <- symbol2entrez[[from]]
ysub <- y[names(y) %in% symbol2entrez[[from]]]
names(ysub) <- symbol2entrez[names(ysub),][[to]]
return(ysub)
}
par(mfrow=c(1,1))
deseq$`CD19+ B` %>% .$padj %>% hist(main='CD19+B p-values')Loading required package: DESeq2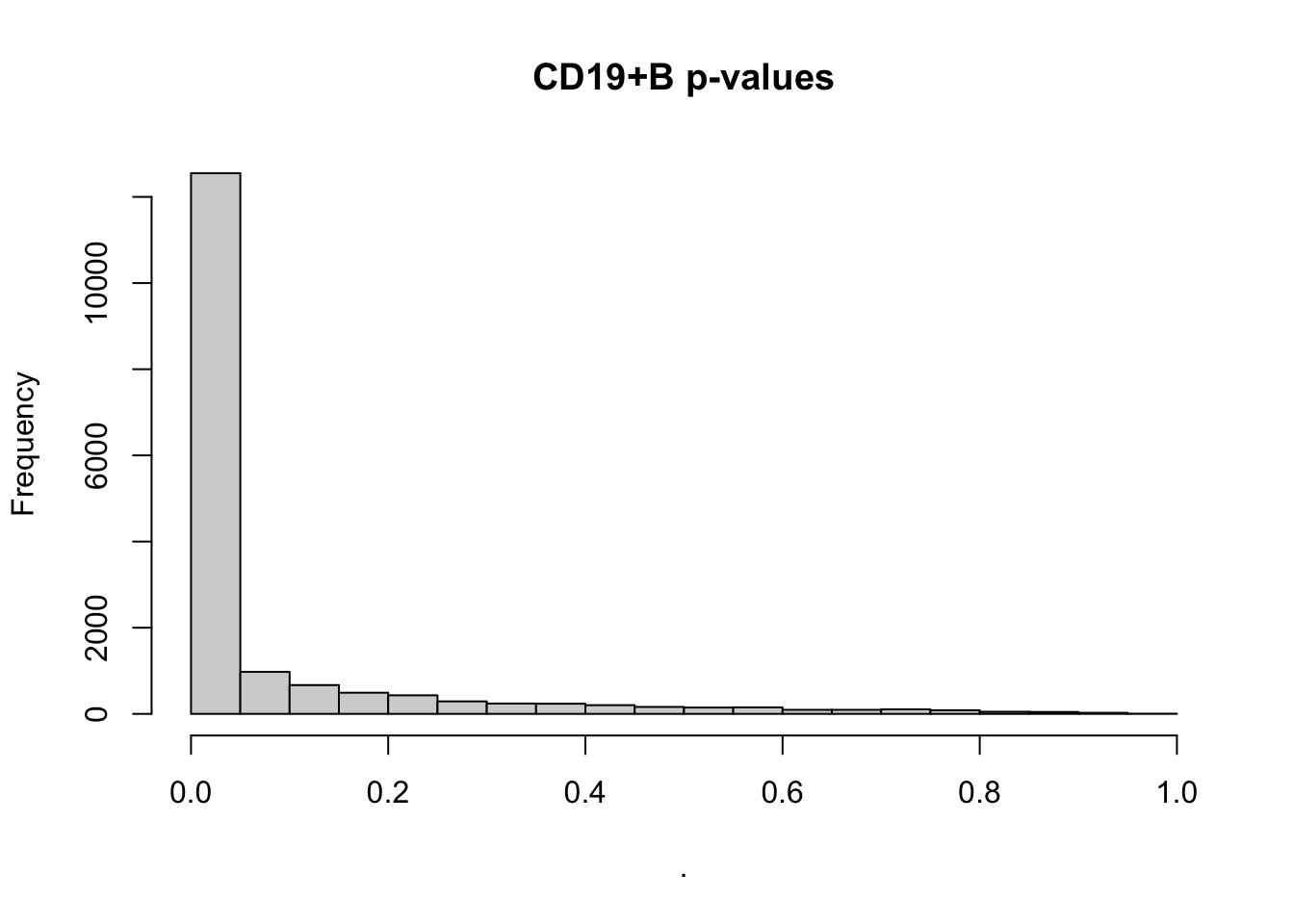
| Version | Author | Date |
|---|---|---|
| a2bdb56 | karltayeb | 2022-03-29 |
Fit logistic SuSiE
logistic_susie_driver = function(db, celltype, thresh){
gs <- genesets[[db]]
data <- deseq[[celltype]]
# set up binary y
y <- data %>%
as.data.frame %>%
rownames_to_column('gene') %>%
dplyr::select(gene, padj) %>%
filter(!is.na(padj)) %>%
mutate(y = as.integer(padj < thresh)) %>%
select(gene, y) %>%
tibble2namedlist %>%
convert_labels('ENSEMBL')
u <- process_input(gs$X, y) # subset to common genes
vb.fit <- logistic.susie( # fit model
u$X, u$y, L=10, init.intercept = 0, verbose=1, maxit=100)
# summarise results
set.summary <- vb.fit$pip %>%
as_tibble(rownames='geneSet') %>%
rename(pip=value) %>%
mutate(
top_component = apply(vb.fit$alpha, 2, which.max),
active_set = top_component %in% vb.fit$sets$cs_index,
top_component = paste0('L', top_component),
cs = purrr::map(top_component, ~tryCatch(
colnames(gs$X)[get(.x, vb.fit$sets$cs)], error = function(e) list())),
in_cs = geneSet %in% cs,
beta = colSums(vb.fit$mu * vb.fit$alpha),
geneListSize = sum(u$y),
geneSetSize = colSums(u$X),
overlap = (u$y %*% u$X)[1,],
nGenes = length(u$y),
propSetInList = overlap / geneSetSize,
oddsRatio = (overlap / (geneListSize - overlap)) / (
(geneSetSize - overlap) / (nGenes - geneSetSize + overlap)),
pValueHypergeometric = phyper(
overlap-1, geneListSize, nGenes, geneSetSize, lower.tail= FALSE),
db = db,
celltype = celltype,
thresh = thresh
) %>% left_join(gs$geneSet$geneSetDes)
return(list(fit = vb.fit, set.summary=set.summary))
}For each celltype, we fit logistic SuSiE using multiple gene set sources at various threshold of padj.
celltypes <- names(deseq)
pthresh <- c(0.1, 0.01, 0.001, 0.0001, 0.00001, 0.000001)
db_name <- names(genesets)
crossed <- cross3(db_name, celltypes, pthresh)
pbmc_res <- xfun::cache_rds({
res <- purrr::map(crossed, purrr::lift_dl(logistic_susie_driver))
for (i in 1:length(res)){ # save some space
res[[i]]$fit$dat <- NULL
}
res
}, file = 'logistic_susie_pbmc_genesets_pthresh.rds'
)
pbmc_res_set_summary <- dplyr::bind_rows(purrr::map(pbmc_res, ~ pluck(.x, 'set.summary')))Summary functions
Just a few functions to help streamline looking at output
pval_focussed_table = function(thresh=1e-3, filter_db=NULL, filter_celltype=NULL, top.n=50){
pbmc_res_set_summary %>%
filter(
case_when(
is.null(filter_db) ~ TRUE,
!is.null(filter_db) ~ db %in% filter_db
) &
thresh == thresh &
case_when(
is.null(filter_celltype) ~ TRUE,
!is.null(filter_celltype) ~ celltype %in% filter_celltype
)
) %>%
dplyr::arrange(celltype, db, pValueHypergeometric) %>%
group_by(celltype, db) %>% slice(1:top.n) %>%
select(celltype, db, geneSet, description, pip, top_component, oddsRatio, propSetInList, pValueHypergeometric) %>%
mutate_at(vars(celltype, db), factor) %>%
datatable(filter = 'top')
}
set_focussed_table = function(thresh=1e-3, filter_db=NULL, filter_celltype=NULL){
pbmc_res_set_summary %>%
filter(
case_when(
is.null(filter_db) ~ TRUE,
!is.null(filter_db) ~ db %in% filter_db
) &
thresh == 1e-3 &
in_cs & active_set &
case_when(
is.null(filter_celltype) ~ TRUE,
!is.null(filter_celltype) ~ celltype %in% filter_celltype
)
) %>%
dplyr::arrange(celltype, db, desc(pip)) %>%
select(celltype, db, geneSet, description, pip, top_component, oddsRatio, propSetInList, pValueHypergeometric) %>%
mutate_at(vars(celltype, geneSet, db), factor) %>%
datatable(filter = 'top')
}Results/Explore enrichments
Our goal is to assess 1. The quality of the gene set enrichments we get from each celltype - do reported gene set enrichments seem celltype specific/celltype relevant? - how much “interesting” marginal enrichment do we fail to capture in the multivariate model - how sensitive are we to the choice of pvalue threshold
Results
Lets take a look at what enrichment we’re getting across cell-types.
CD19+ B
pbmc_res_set_summary %>%
filter(celltype == 'CD19+ B') %>%
filter(active_set, thresh==1e-4) %>%
group_by(db, celltype, top_component) %>%
arrange(db, celltype, top_component, desc(pip)) %>%
select(geneSet, description, pip) %>% chop(c(geneSet, description, pip)) %>%
knitr::kable()Adding missing grouping variables: `db`, `celltype`, `top_component`| db | celltype | top_component | geneSet | description | pip |
|---|---|---|---|---|---|
| gobp | CD19+ B | L1 | GO:0002376 | immune system process | 0.9999575 |
| gobp | CD19+ B | L2 | GO:0045047, GO:0006613, GO:0072599, GO:0006614 | protein targeting to ER , cotranslational protein targeting to membrane , establishment of protein localization to endoplasmic reticulum, SRP-dependent cotranslational protein targeting to membrane | 0.96205208, 0.02165616, 0.01453688, 0.00410197 |
| gobp | CD19+ B | L3 | GO:0042773, GO:0042775, GO:0006119 | ATP synthesis coupled electron transport , mitochondrial ATP synthesis coupled electron transport, oxidative phosphorylation | 0.62449631, 0.36002221, 0.01752387 |
| gobp | CD19+ B | L5 | GO:0001775, GO:0045321, GO:0002366, GO:0002263 | cell activation , leukocyte activation , leukocyte activation involved in immune response, cell activation involved in immune response | 0.9780541751, 0.0218972721, 0.0005957131, 0.0005770067 |
| gobp | CD19+ B | L6 | GO:0008380, GO:0000377, GO:0000398, GO:0000375, GO:0006397, GO:0050684, GO:0043484, GO:0000380, GO:0048024, GO:0016071 | RNA splicing , RNA splicing, via transesterification reactions with bulged adenosine as nucleophile, mRNA splicing, via spliceosome , RNA splicing, via transesterification reactions , mRNA processing , regulation of mRNA processing , regulation of RNA splicing , alternative mRNA splicing, via spliceosome , regulation of mRNA splicing, via spliceosome , mRNA metabolic process | 0.822736133, 0.049644134, 0.049644134, 0.033005117, 0.017234053, 0.009417204, 0.007220671, 0.002939483, 0.001834918, 0.001051637 |
| gobp_nr | CD19+ B | L1 | GO:0002446, GO:0036230 | neutrophil mediated immunity, granulocyte activation | 0.5151440, 0.4857358 |
| gobp_nr | CD19+ B | L2 | GO:0070972 | protein localization to endoplasmic reticulum | 0.999958 |
| gobp_nr | CD19+ B | L4 | GO:0009123, GO:0009141 | nucleoside monophosphate metabolic process, nucleoside triphosphate metabolic process | 0.98449756, 0.01648675 |
| gobp_nr | CD19+ B | L5 | GO:0002764 | immune response-regulating signaling pathway | 0.9961853 |
| gomf | CD19+ B | L1 | GO:0003723 | RNA binding | 0.999744 |
| gomf | CD19+ B | L2 | GO:0000981, GO:0003700 | DNA-binding transcription factor activity, RNA polymerase II-specific, DNA-binding transcription factor activity | 0.995003783, 0.005058241 |
| gomf | CD19+ B | L3 | GO:0000977, GO:0001012, GO:0000987, GO:0000978, GO:0000976, GO:0003690, GO:1990837, GO:0001067, GO:0044212 | RNA polymerase II regulatory region sequence-specific DNA binding, RNA polymerase II regulatory region DNA binding , proximal promoter sequence-specific DNA binding , RNA polymerase II proximal promoter sequence-specific DNA binding, transcription regulatory region sequence-specific DNA binding , double-stranded DNA binding , sequence-specific double-stranded DNA binding , regulatory region nucleic acid binding , transcription regulatory region DNA binding | 0.4872513913, 0.4313993168, 0.0365953412, 0.0243679373, 0.0132413813, 0.0059278956, 0.0013679037, 0.0004652721, 0.0003557059 |
| gomf | CD19+ B | L6 | GO:0003735 | structural constituent of ribosome | 0.9953441 |
| gomf | CD19+ B | L7 | GO:0003954, GO:0008137, GO:0050136, GO:0016655, GO:0016651 | NADH dehydrogenase activity , NADH dehydrogenase (ubiquinone) activity , NADH dehydrogenase (quinone) activity , oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor, oxidoreductase activity, acting on NAD(P)H | 0.32595362, 0.21858318, 0.21858318, 0.17726552, 0.03879761 |
| kegg | CD19+ B | L1 | hsa00190, hsa05012 | Oxidative phosphorylation, Parkinson disease | 0.9979902, 0.0125303 |
| kegg | CD19+ B | L2 | hsa04640 | Hematopoietic cell lineage | 0.9997951 |
| kegg | CD19+ B | L3 | hsa03010 | Ribosome | 1 |
| reactome | CD19+ B | L1 | R-HSA-168256 | Immune System | 1 |
| reactome | CD19+ B | L2 | R-HSA-156842 , R-HSA-156902 , R-HSA-192823 , R-HSA-2408557, R-HSA-72764 , R-HSA-72689 , R-HSA-1799339, R-HSA-72706 , R-HSA-156827 | Eukaryotic Translation Elongation , Peptide chain elongation , Viral mRNA Translation , Selenocysteine synthesis , Eukaryotic Translation Termination , Formation of a pool of free 40S subunits , SRP-dependent cotranslational protein targeting to membrane , GTP hydrolysis and joining of the 60S ribosomal subunit , L13a-mediated translational silencing of Ceruloplasmin expression | 0.760651464, 0.124389740, 0.067132923, 0.026006681, 0.017267678, 0.003305376, 0.003131980, 0.001226762, 0.001000830 |
| reactome | CD19+ B | L3 | R-HSA-74160 , R-HSA-212436, R-HSA-73857 | Gene expression (Transcription), Generic Transcription Pathway , RNA Polymerase II Transcription | 0.88567935, 0.07266257, 0.04183476 |
| reactome | CD19+ B | L4 | R-HSA-163200 , R-HSA-1428517, R-HSA-611105 | Respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by uncoupling proteins., The citric acid (TCA) cycle and respiratory electron transport , Respiratory electron transport | 0.845323000, 0.149383366, 0.005965744 |
| reactome | CD19+ B | L5 | R-HSA-983168, R-HSA-983169 | Antigen processing: Ubiquitination & Proteasome degradation, Class I MHC mediated antigen processing & presentation | 0.997049696, 0.003118792 |
| reactome | CD19+ B | L6 | R-HSA-180585 , R-HSA-1234176, R-HSA-211733 , R-HSA-69601 , R-HSA-69610 , R-HSA-69613 , R-HSA-69541 , R-HSA-69229 , R-HSA-75815 , R-HSA-180534 , R-HSA-169911 , R-HSA-349425 , R-HSA-9604323, R-HSA-8854050, R-HSA-1236978, R-HSA-174154 , R-HSA-1169091, R-HSA-174084 , R-HSA-1234174, R-HSA-2262749, R-HSA-983705 , R-HSA-174113 , R-HSA-450408 , R-HSA-69563 , R-HSA-69580 , R-HSA-174184 , R-HSA-351202 , R-HSA-68867 , R-HSA-176409 , R-HSA-350562 , R-HSA-69615 , R-HSA-5362768, R-HSA-174178 , R-HSA-179419 , R-HSA-5610780 | Vif-mediated degradation of APOBEC3G , Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha , Regulation of activated PAK-2p34 by proteasome mediated degradation , Ubiquitin Mediated Degradation of Phosphorylated Cdc25A , p53-Independent DNA Damage Response , p53-Independent G1/S DNA damage checkpoint , Stabilization of p53 , Ubiquitin-dependent degradation of Cyclin D1 , Ubiquitin-dependent degradation of Cyclin D , Vpu mediated degradation of CD4 , Regulation of Apoptosis , Autodegradation of the E3 ubiquitin ligase COP1 , Negative regulation of NOTCH4 signaling , FBXL7 down-regulates AURKA during mitotic entry and in early mitosis , Cross-presentation of soluble exogenous antigens (endosomes) , APC/C:Cdc20 mediated degradation of Securin , Activation of NF-kappaB in B cells , Autodegradation of Cdh1 by Cdh1:APC/C , Regulation of Hypoxia-inducible Factor (HIF) by oxygen , Cellular response to hypoxia , Signaling by the B Cell Receptor (BCR) , SCF-beta-TrCP mediated degradation of Emi1 , AUF1 (hnRNP D0) binds and destabilizes mRNA , p53-Dependent G1 DNA Damage Response , p53-Dependent G1/S DNA damage checkpoint , Cdc20:Phospho-APC/C mediated degradation of Cyclin A , Metabolism of polyamines , Assembly of the pre-replicative complex , APC/C:Cdc20 mediated degradation of mitotic proteins , Regulation of ornithine decarboxylase (ODC) , G1/S DNA Damage Checkpoints , Hh mutants that don’t undergo autocatalytic processing are degraded by ERAD , APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1, APC:Cdc20 mediated degradation of cell cycle proteins prior to satisfation of the cell cycle checkpoint , Degradation of GLI1 by the proteasome | 0.7490393108, 0.0319137209, 0.0256472437, 0.0242873384, 0.0242873384, 0.0242873384, 0.0225422403, 0.0120133659, 0.0120133659, 0.0119846233, 0.0108046763, 0.0101911528, 0.0071579856, 0.0070377887, 0.0040655484, 0.0038589122, 0.0037679238, 0.0036141487, 0.0030579708, 0.0030579708, 0.0028683640, 0.0024109404, 0.0017126798, 0.0015009738, 0.0015009738, 0.0013319088, 0.0013173159, 0.0012105047, 0.0011649631, 0.0010246799, 0.0009578296, 0.0009514080, 0.0008622334, 0.0007207196, 0.0006610228 |
| reactome | CD19+ B | L7 | R-HSA-72163 , R-HSA-72172 , R-HSA-109688, R-HSA-73856 , R-HSA-72203 | mRNA Splicing - Major Pathway , mRNA Splicing , Cleavage of Growing Transcript in the Termination Region, RNA Polymerase II Transcription Termination , Processing of Capped Intron-Containing Pre-mRNA | 0.5457887505, 0.4439546364, 0.0021482288, 0.0021482288, 0.0006461411 |
| reactome | CD19+ B | L8 | R-HSA-76005 , R-HSA-114608, R-HSA-76002 , R-HSA-109582 | Response to elevated platelet cytosolic Ca2+ , Platelet degranulation , Platelet activation, signaling and aggregation, Hemostasis | 0.7063848487, 0.2314322026, 0.0252623002, 0.0004075344 |
| wikipathway | CD19+ B | L1 | WP477 | Cytoplasmic Ribosomal Proteins | 1 |
| wikipathway | CD19+ B | L2 | WP111 | Electron Transport Chain (OXPHOS system in mitochondria) | 0.9999189 |
| wikipathway_cancer | CD19+ B | L1 | WP619 | Type II interferon signaling (IFNG) | 0.9998636 |
CD56+ NK
pbmc_res_set_summary %>%
filter(celltype == 'CD56+ NK') %>%
filter(active_set, thresh==1e-4) %>%
group_by(db, celltype, top_component) %>%
arrange(db, celltype, top_component, desc(pip)) %>%
select(geneSet, description, pip) %>% chop(c(geneSet, description, pip)) %>%
knitr::kable()Adding missing grouping variables: `db`, `celltype`, `top_component`| db | celltype | top_component | geneSet | description | pip |
|---|---|---|---|---|---|
| gobp | CD56+ NK | L1 | GO:0002376 | immune system process | 0.9999984 |
| gobp | CD56+ NK | L2 | GO:0045047, GO:0072599, GO:0006614, GO:0006613 | protein targeting to ER , establishment of protein localization to endoplasmic reticulum, SRP-dependent cotranslational protein targeting to membrane , cotranslational protein targeting to membrane | 0.68278614, 0.20978513, 0.07123053, 0.03822676 |
| gobp | CD56+ NK | L4 | GO:0006119, GO:0009123, GO:0042773, GO:0042775, GO:0009161, GO:0009126, GO:0009167, GO:0022900, GO:0022904, GO:0046034 | oxidative phosphorylation , nucleoside monophosphate metabolic process , ATP synthesis coupled electron transport , mitochondrial ATP synthesis coupled electron transport, ribonucleoside monophosphate metabolic process , purine nucleoside monophosphate metabolic process , purine ribonucleoside monophosphate metabolic process , electron transport chain , respiratory electron transport chain , ATP metabolic process | 0.9815754083, 0.0075172568, 0.0050461669, 0.0035658370, 0.0031462719, 0.0018470112, 0.0018470112, 0.0017166414, 0.0015391764, 0.0009472366 |
| gobp | CD56+ NK | L5 | GO:0008380, GO:0006397, GO:0000375, GO:0000377, GO:0000398, GO:0016071 | RNA splicing , mRNA processing , RNA splicing, via transesterification reactions , RNA splicing, via transesterification reactions with bulged adenosine as nucleophile, mRNA splicing, via spliceosome , mRNA metabolic process | 0.59074452, 0.22252471, 0.05825611, 0.05441422, 0.05441422, 0.01452235 |
| gobp_nr | CD56+ NK | L1 | GO:0036230, GO:0002446 | granulocyte activation , neutrophil mediated immunity | 0.6655074, 0.3354429 |
| gobp_nr | CD56+ NK | L2 | GO:0006413 | translational initiation | 0.9954877 |
| gobp_nr | CD56+ NK | L3 | GO:0042110, GO:0007159 | T cell activation , leukocyte cell-cell adhesion | 0.985660130, 0.008171918 |
| gobp_nr | CD56+ NK | L4 | GO:0009123, GO:0009141, GO:0009259 | nucleoside monophosphate metabolic process, nucleoside triphosphate metabolic process , ribonucleotide metabolic process | 0.981592710, 0.015904774, 0.001372449 |
| gobp_nr | CD56+ NK | L5 | GO:0042113 | B cell activation | 0.9972725 |
| gomf | CD56+ NK | L1 | GO:0003735 | structural constituent of ribosome | 1 |
| gomf | CD56+ NK | L2 | GO:0000981, GO:0003700 | DNA-binding transcription factor activity, RNA polymerase II-specific, DNA-binding transcription factor activity | 0.996770584, 0.003356549 |
| gomf | CD56+ NK | L3 | GO:0000977, GO:0000976, GO:0001012, GO:1990837, GO:0003690, GO:0043565, GO:0000987, GO:0001067, GO:0044212 | RNA polymerase II regulatory region sequence-specific DNA binding, transcription regulatory region sequence-specific DNA binding , RNA polymerase II regulatory region DNA binding , sequence-specific double-stranded DNA binding , double-stranded DNA binding , sequence-specific DNA binding , proximal promoter sequence-specific DNA binding , regulatory region nucleic acid binding , transcription regulatory region DNA binding | 0.5246638008, 0.2816905881, 0.1760785081, 0.0110809288, 0.0067171732, 0.0006260858, 0.0003679068, 0.0002517074, 0.0002268130 |
| kegg | CD56+ NK | L1 | hsa03010 | Ribosome | 1 |
| kegg | CD56+ NK | L2 | hsa05012 | Parkinson disease | 0.9999978 |
| kegg | CD56+ NK | L3 | hsa04640 | Hematopoietic cell lineage | 0.9978541 |
| reactome | CD56+ NK | L1 | R-HSA-168256 | Immune System | 1 |
| reactome | CD56+ NK | L2 | R-HSA-156842, R-HSA-156902, R-HSA-192823, R-HSA-72764 , R-HSA-72689 | Eukaryotic Translation Elongation , Peptide chain elongation , Viral mRNA Translation , Eukaryotic Translation Termination , Formation of a pool of free 40S subunits | 0.864742537, 0.106897566, 0.027158260, 0.002413175, 0.001418708 |
| reactome | CD56+ NK | L3 | R-HSA-212436, R-HSA-74160 , R-HSA-73857 | Generic Transcription Pathway , Gene expression (Transcription), RNA Polymerase II Transcription | 0.68127740, 0.29679295, 0.02222635 |
| reactome | CD56+ NK | L4 | R-HSA-163200 , R-HSA-1428517, R-HSA-611105 | Respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by uncoupling proteins., The citric acid (TCA) cycle and respiratory electron transport , Respiratory electron transport | 0.993324533, 0.006034017, 0.001575567 |
| reactome | CD56+ NK | L5 | R-HSA-8878171, R-HSA-157118 , R-HSA-8939236 | Transcriptional regulation by RUNX1 , Signaling by NOTCH , RUNX1 regulates transcription of genes involved in differentiation of HSCs | 0.97128181, 0.04393861, 0.01998012 |
| reactome | CD56+ NK | L6 | R-HSA-983168 , R-HSA-983169 , R-HSA-8951664 | Antigen processing: Ubiquitination & Proteasome degradation, Class I MHC mediated antigen processing & presentation , Neddylation | 0.8808248324, 0.1189949639, 0.0009720837 |
| wikipathway | CD56+ NK | L1 | WP477 | Cytoplasmic Ribosomal Proteins | 1 |
| wikipathway | CD56+ NK | L2 | WP111, WP623 | Electron Transport Chain (OXPHOS system in mitochondria), Oxidative phosphorylation | 0.99086202, 0.01897664 |
T cell
pbmc_res_set_summary %>%
filter(celltype == 'T cell') %>%
filter(active_set, thresh==1e-4) %>%
group_by(db, celltype, top_component) %>%
arrange(db, celltype, top_component, desc(pip)) %>%
select(geneSet, description, pip) %>% chop(c(geneSet, description, pip)) %>%
knitr::kable()Adding missing grouping variables: `db`, `celltype`, `top_component`| db | celltype | top_component | geneSet | description | pip |
|---|---|---|---|---|---|
| gobp | T cell | L1 | GO:0001775, GO:0045321 | cell activation , leukocyte activation | 0.998243491, 0.002864201 |
| gobp | T cell | L2 | GO:0006119, GO:0042773, GO:0042775 | oxidative phosphorylation , ATP synthesis coupled electron transport , mitochondrial ATP synthesis coupled electron transport | 0.998867321, 0.002306692, 0.002090733 |
| gobp | T cell | L3 | GO:0010941, GO:0008219, GO:0043067, GO:0042981, GO:0012501, GO:0006915, GO:0097190, GO:0048518, GO:0010942, GO:0048522, GO:0048519, GO:0043068, GO:0043065 | regulation of cell death , cell death , regulation of programmed cell death , regulation of apoptotic process , programmed cell death , apoptotic process , apoptotic signaling pathway , positive regulation of biological process , positive regulation of cell death , positive regulation of cellular process , negative regulation of biological process , positive regulation of programmed cell death, positive regulation of apoptotic process | 0.404035437, 0.367067203, 0.059812581, 0.059590178, 0.042848010, 0.038547811, 0.020693460, 0.009702335, 0.006246010, 0.006037632, 0.005468877, 0.002090144, 0.001933994 |
| gobp | T cell | L4 | GO:0045047, GO:0006614, GO:0072599, GO:0070972, GO:0006613 | protein targeting to ER , SRP-dependent cotranslational protein targeting to membrane , establishment of protein localization to endoplasmic reticulum, protein localization to endoplasmic reticulum , cotranslational protein targeting to membrane | 0.42251775, 0.37005939, 0.13809458, 0.04385614, 0.03174252 |
| gobp | T cell | L5 | GO:0002376, GO:0006955 | immune system process, immune response | 0.998468901, 0.002881243 |
| gobp_nr | T cell | L1 | GO:0036230, GO:0002446 | granulocyte activation , neutrophil mediated immunity | 0.7324297, 0.2703360 |
| gobp_nr | T cell | L2 | GO:0042110 | T cell activation | 0.9999906 |
| gobp_nr | T cell | L3 | GO:0070972 | protein localization to endoplasmic reticulum | 0.9998559 |
| gomf | T cell | L1 | GO:0005515 | protein binding | 1 |
| kegg | T cell | L1 | hsa05010 | Alzheimer disease | 0.9999993 |
| reactome | T cell | L1 | R-HSA-6798695 | Neutrophil degranulation | 1 |
| reactome | T cell | L2 | R-HSA-72706 , R-HSA-156827, R-HSA-72689 , R-HSA-72613 , R-HSA-72737 , R-HSA-192823, R-HSA-156902 | GTP hydrolysis and joining of the 60S ribosomal subunit , L13a-mediated translational silencing of Ceruloplasmin expression, Formation of a pool of free 40S subunits , Eukaryotic Translation Initiation , Cap-dependent Translation Initiation , Viral mRNA Translation , Peptide chain elongation | 0.586005479, 0.400596683, 0.009180040, 0.004668524, 0.004668524, 0.003496445, 0.003174071 |
CD14+ Monocyte
pbmc_res_set_summary %>%
filter(celltype == 'CD14+ Monocyte') %>%
filter(active_set, thresh==1e-4) %>%
group_by(db, celltype, top_component) %>%
arrange(db, celltype, top_component, desc(pip)) %>%
select(geneSet, description, pip) %>% chop(c(geneSet, description, pip)) %>%
knitr::kable()Adding missing grouping variables: `db`, `celltype`, `top_component`| db | celltype | top_component | geneSet | description | pip |
|---|---|---|---|---|---|
| gobp | CD14+ Monocyte | L1 | GO:0045321, GO:0001775 | leukocyte activation, cell activation | 0.9139560, 0.0861039 |
| gobp | CD14+ Monocyte | L2 | GO:0045047, GO:0006614, GO:0006613, GO:0072599, GO:0070972 | protein targeting to ER , SRP-dependent cotranslational protein targeting to membrane , cotranslational protein targeting to membrane , establishment of protein localization to endoplasmic reticulum, protein localization to endoplasmic reticulum | 0.8615391988, 0.0574691370, 0.0477713854, 0.0336165786, 0.0004015912 |
| gobp | CD14+ Monocyte | L3 | GO:0006119, GO:0042773, GO:0042775 | oxidative phosphorylation , ATP synthesis coupled electron transport , mitochondrial ATP synthesis coupled electron transport | 0.9990484928, 0.0006152697, 0.0004379516 |
| gobp | CD14+ Monocyte | L4 | GO:0016192, GO:0002376, GO:0006955, GO:0045055, GO:0006810, GO:0042119, GO:0006887, GO:0051234 | vesicle-mediated transport , immune system process , immune response , regulated exocytosis , transport , neutrophil activation , exocytosis , establishment of localization | 0.9851433899, 0.0210152324, 0.0059841488, 0.0010584611, 0.0009289568, 0.0008104012, 0.0007570762, 0.0007478103 |
| gobp | CD14+ Monocyte | L5 | GO:0006915, GO:0012501, GO:0043067, GO:0042981, GO:0010941, GO:0008219, GO:0097190, GO:0006950, GO:0010942, GO:0050790, GO:0048518, GO:0010033, GO:0070887, GO:2001233, GO:0043065, GO:0043068, GO:0043069, GO:0043066, GO:0051716, GO:0048522, GO:0060548, GO:0050896, GO:0048523, GO:0048519 | apoptotic process , programmed cell death , regulation of programmed cell death , regulation of apoptotic process , regulation of cell death , cell death , apoptotic signaling pathway , response to stress , positive regulation of cell death , regulation of catalytic activity , positive regulation of biological process , response to organic substance , cellular response to chemical stimulus , regulation of apoptotic signaling pathway , positive regulation of apoptotic process , positive regulation of programmed cell death, negative regulation of programmed cell death, negative regulation of apoptotic process , cellular response to stimulus , positive regulation of cellular process , negative regulation of cell death , response to stimulus , negative regulation of cellular process , negative regulation of biological process | 2.720176e-01, 1.948576e-01, 1.940882e-01, 1.583264e-01, 1.008376e-01, 5.132999e-02, 2.549188e-03, 9.307323e-04, 6.576479e-04, 5.735087e-04, 5.459875e-04, 4.811908e-04, 4.506495e-04, 3.491954e-04, 3.298411e-04, 2.900530e-04, 1.817724e-04, 1.476441e-04, 1.365127e-04, 1.359366e-04, 1.228290e-04, 1.125502e-04, 7.493961e-05, 6.920531e-05 |
| gobp | CD14+ Monocyte | L6 | GO:0006518, GO:0006412, GO:0043043, GO:0043603, GO:0043604 | peptide metabolic process , translation , peptide biosynthetic process , cellular amide metabolic process, amide biosynthetic process | 0.790995030, 0.119614037, 0.082857945, 0.002543295, 0.001245957 |
| gobp | CD14+ Monocyte | L7 | GO:0008380, GO:0000375, GO:0000377, GO:0000398, GO:0006397, GO:0016071 | RNA splicing , RNA splicing, via transesterification reactions , RNA splicing, via transesterification reactions with bulged adenosine as nucleophile, mRNA splicing, via spliceosome , mRNA processing , mRNA metabolic process | 0.422448316, 0.196794593, 0.178759471, 0.178759471, 0.021244858, 0.001609996 |
| gobp_nr | CD14+ Monocyte | L1 | GO:0036230, GO:0002446 | granulocyte activation , neutrophil mediated immunity | 0.97592803, 0.02469514 |
| gobp_nr | CD14+ Monocyte | L2 | GO:0006413 | translational initiation | 0.999994 |
| gobp_nr | CD14+ Monocyte | L3 | GO:0009123, GO:0009141 | nucleoside monophosphate metabolic process, nucleoside triphosphate metabolic process | 0.98835280, 0.01259324 |
| gobp_nr | CD14+ Monocyte | L4 | GO:0002521, GO:0042110, GO:0002694, GO:1903706 | leukocyte differentiation , T cell activation , regulation of leukocyte activation, regulation of hemopoiesis | 0.619222289, 0.358464783, 0.007935017, 0.004456591 |
| gobp_nr | CD14+ Monocyte | L5 | GO:0008380, GO:0006397, GO:1903311 | RNA splicing , mRNA processing , regulation of mRNA metabolic process | 0.876773133, 0.089632405, 0.004303895 |
| gobp_nr | CD14+ Monocyte | L6 | GO:0090150, GO:0070972, GO:0006605 | establishment of protein localization to membrane, protein localization to endoplasmic reticulum , protein targeting | 0.756201036, 0.243465201, 0.004950252 |
| gomf | CD14+ Monocyte | L1 | GO:0003723 | RNA binding | 0.9999998 |
| gomf | CD14+ Monocyte | L2 | GO:0000981, GO:0003700 | DNA-binding transcription factor activity, RNA polymerase II-specific, DNA-binding transcription factor activity | 0.9998274825, 0.0002279895 |
| gomf | CD14+ Monocyte | L3 | GO:0001012, GO:0000977, GO:0000987, GO:0000978, GO:0000976, GO:1990837, GO:0043565, GO:0044212 | RNA polymerase II regulatory region DNA binding , RNA polymerase II regulatory region sequence-specific DNA binding, proximal promoter sequence-specific DNA binding , RNA polymerase II proximal promoter sequence-specific DNA binding, transcription regulatory region sequence-specific DNA binding , sequence-specific double-stranded DNA binding , sequence-specific DNA binding , transcription regulatory region DNA binding | 0.6603205658, 0.2924363216, 0.0360620592, 0.0077244907, 0.0030829257, 0.0007025284, 0.0003868975, 0.0001017222 |
| gomf | CD14+ Monocyte | L4 | GO:0003735 | structural constituent of ribosome | 1 |
| gomf | CD14+ Monocyte | L5 | GO:0016655, GO:0008137, GO:0050136, GO:0003954, GO:0016651 | oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor, NADH dehydrogenase (ubiquinone) activity , NADH dehydrogenase (quinone) activity , NADH dehydrogenase activity , oxidoreductase activity, acting on NAD(P)H | 0.8270835588, 0.0778621792, 0.0778621792, 0.0178702201, 0.0003469471 |
| gomf | CD14+ Monocyte | L6 | GO:0045296, GO:0050839 | cadherin binding , cell adhesion molecule binding | 0.7890801, 0.1881604 |
| kegg | CD14+ Monocyte | L1 | hsa03010 | Ribosome | 1 |
| kegg | CD14+ Monocyte | L2 | hsa05012 | Parkinson disease | 0.9999021 |
| reactome | CD14+ Monocyte | L1 | R-HSA-6798695 | Neutrophil degranulation | 0.9999954 |
| reactome | CD14+ Monocyte | L2 | R-HSA-72766 | Translation | 1 |
| reactome | CD14+ Monocyte | L3 | R-HSA-163200, R-HSA-611105 | Respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by uncoupling proteins., Respiratory electron transport | 0.9996110591, 0.0008392269 |
| reactome | CD14+ Monocyte | L5 | R-HSA-72172, R-HSA-72163, R-HSA-72203 | mRNA Splicing , mRNA Splicing - Major Pathway , Processing of Capped Intron-Containing Pre-mRNA | 0.67495558, 0.30641144, 0.01826453 |
| reactome | CD14+ Monocyte | L6 | R-HSA-198933 | Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell | 0.9974877 |
| reactome | CD14+ Monocyte | L7 | R-HSA-379726 | Mitochondrial tRNA aminoacylation | 0.9972986 |
| wikipathway | CD14+ Monocyte | L1 | WP477 | Cytoplasmic Ribosomal Proteins | 1 |
| wikipathway | CD14+ Monocyte | L2 | WP111 | Electron Transport Chain (OXPHOS system in mitochondria) | 0.9998312 |
CD34+
pbmc_res_set_summary %>%
filter(celltype == 'CD14+') %>%
filter(active_set, thresh==1e-4) %>%
group_by(db, celltype, top_component) %>%
arrange(db, celltype, top_component, desc(pip)) %>%
select(geneSet, description, pip) %>% chop(c(geneSet, description, pip)) %>%
knitr::kable()Adding missing grouping variables: `db`, `celltype`, `top_component`| db | celltype | top_component | geneSet | description | pip |
|---|
knitr::knit_exit()