Run_all_analysis
ERM
20230410
Last updated: 2023-04-11
Checks: 7 0
Knit directory: Cardiotoxicity/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20230109) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 0aaa63d. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .RData
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: data/ACresponse_cluster24h.csv
Ignored: data/Clamp_Summary.csv
Ignored: data/Cormotif_24_k1-5_raw.RDS
Ignored: data/DAtable1.csv
Ignored: data/DDE_reQTL.txt
Ignored: data/DDEresp_list.csv
Ignored: data/DEG_cormotif.RDS
Ignored: data/DF_Plate_Peak.csv
Ignored: data/Da24counts.txt
Ignored: data/Dx24counts.txt
Ignored: data/Dx_reQTL_specific.txt
Ignored: data/Ep24counts.txt
Ignored: data/GOplots.R
Ignored: data/K_cluster
Ignored: data/K_cluster_kisthree.csv
Ignored: data/K_cluster_kistwo.csv
Ignored: data/Mt24counts.txt
Ignored: data/RINsamplelist.txt
Ignored: data/Seonane2019supp1.txt
Ignored: data/TOP2Bi-24hoursGO_analysis.csv
Ignored: data/TR24counts.txt
Ignored: data/Top2biresp_cluster24h.csv
Ignored: data/Viabilitylistfull.csv
Ignored: data/allexpressedgenes.txt
Ignored: data/allgenes.txt
Ignored: data/allmatrix.RDS
Ignored: data/avgLD50.RDS
Ignored: data/backGL.txt
Ignored: data/cormotif_3hk1-8.RDS
Ignored: data/cormotif_ER_cluster.txt
Ignored: data/cormotif_ER_respint.txt
Ignored: data/cormotif_ER_respset.txt
Ignored: data/cormotif_LR_cluster.txt
Ignored: data/cormotif_LR_respint.txt
Ignored: data/cormotif_LR_respset.txt
Ignored: data/cormotif_NRset.txt
Ignored: data/cormotif_TI_cluster.txt
Ignored: data/cormotif_TI_respint.txt
Ignored: data/cormotif_TI_respset.txt
Ignored: data/cormotif_initalK5.RDS
Ignored: data/cormotif_initialK5.RDS
Ignored: data/cormotif_initialall.RDS
Ignored: data/counts24hours.RDS
Ignored: data/cpmnorm_counts.csv
Ignored: data/dat_cpm.RDS
Ignored: data/data_outline.txt
Ignored: data/efit2results.RDS
Ignored: data/ensembl_backup.RDS
Ignored: data/ensgtotal.txt
Ignored: data/filenameonly.txt
Ignored: data/filtered_cpm_counts.csv
Ignored: data/filtermatrix_x.RDS
Ignored: data/folder_05top/
Ignored: data/gene_prob_tran3h.RDS
Ignored: data/gene_probabilityk5.RDS
Ignored: data/heartgenes.csv
Ignored: data/individualDRCfile.RDS
Ignored: data/knowles56.GMT
Ignored: data/knowlesGMT.GMT
Ignored: data/mymatrix.RDS
Ignored: data/nonresponse_cluster24h.csv
Ignored: data/norm_LDH.csv
Ignored: data/norm_counts.csv
Ignored: data/plan2plot.png
Ignored: data/raw_counts.csv
Ignored: data/response_cluster24h.csv
Ignored: data/sigVDA24.txt
Ignored: data/sigVDA3.txt
Ignored: data/sigVDX24.txt
Ignored: data/sigVDX3.txt
Ignored: data/sigVEP24.txt
Ignored: data/sigVEP3.txt
Ignored: data/sigVMT24.txt
Ignored: data/sigVMT3.txt
Ignored: data/sigVTR24.txt
Ignored: data/sigVTR3.txt
Ignored: data/table3a.omar
Ignored: data/tvl24hour.txt
Ignored: data/tvl24hourw.txt
Ignored: data/venn_code.R
Untracked files:
Untracked: .RDataTmp
Untracked: .RDataTmp1
Untracked: code/extra_code.R
Untracked: output/output-old/
Untracked: reneebasecode.R
Unstaged changes:
Modified: code/Corrscripts.R
Modified: code/eQTLcodes.R
Deleted: output/Cormotif.svg
Deleted: output/Ctrxn24-3-23.svg
Deleted: output/Ctxnrate3-23.png
Deleted: output/Decay_Slope3-23.svg
Deleted: output/ERmotif5_LFC.svg
Deleted: output/GOBP_motif345.svg
Deleted: output/KEGGmotif_345.svg
Deleted: output/LD503-21-23.png
Deleted: output/LDH_24-3-23.svg
Deleted: output/LDH_243-23.svg
Deleted: output/LFCbytreatment3-25.svg
Deleted: output/LFCmotif4_LR.svg
Deleted: output/LR_RespMoti4.svg
Deleted: output/MeanAmp243-23.svg
Deleted: output/NRmotif1_LFC.svg
Deleted: output/Rise_Slope3-23.svg
Deleted: output/TI_LFC.svg
Deleted: output/TVLcorr3-23.svg
Deleted: output/TropI3-23.svg
Deleted: output/Venn24DEG-3-24.png
Deleted: output/motif1NR_LFC.svg
Deleted: output/motif3TIlfc3-25.svg
Deleted: output/motif4LR3-25LFC.svg
Deleted: output/motif5ER-LFC-3-25.svg
Deleted: output/nolegendLDH.svg
Deleted: output/resultsigVDA24.csv
Deleted: output/tropI_24-3-23.svg
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/run_all_analysis.Rmd) and
HTML (docs/run_all_analysis.html) files. If you’ve
configured a remote Git repository (see ?wflow_git_remote),
click on the hyperlinks in the table below to view the files as they
were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 0aaa63d | reneeisnowhere | 2023-04-11 | cormotif analysis update |
| Rmd | 575fd81 | reneeisnowhere | 2023-04-11 | updating cormotif |
| html | 4cd8ac4 | reneeisnowhere | 2023-04-11 | Build site. |
| html | 08936e7 | reneeisnowhere | 2023-04-10 | Build site. |
| Rmd | fa2cbeb | reneeisnowhere | 2023-04-10 | monday end |
| html | 85526c5 | reneeisnowhere | 2023-04-10 | Build site. |
| Rmd | 1444a85 | reneeisnowhere | 2023-04-10 | update push of new data |
| html | b266b76 | reneeisnowhere | 2023-04-10 | Build site. |
| Rmd | d3f8cf7 | reneeisnowhere | 2023-04-10 | update push of new data |
| html | f0a75e1 | reneeisnowhere | 2023-04-10 | Build site. |
| Rmd | 8ca4c7e | reneeisnowhere | 2023-04-10 | first rmd commit |
| Rmd | 2e69969 | reneeisnowhere | 2023-04-10 | adding data |
| Rmd | 0f1f1da | reneeisnowhere | 2023-04-10 | final run analysis |
This starts the documentation of the RNA-seq cardiotoxicity analysis for my manuscript
library(edgeR)#
library(limma)#
library(RColorBrewer)
library(mixOmics)
library(gridExtra)#
library(reshape2)#
#library(devtools)
library(AnnotationHub)
library(tidyverse)
library(scales)
library(biomaRt)#
library(Homo.sapiens)
library(cowplot)#
library(ggrepel)#
library(corrplot)
library(Hmisc) ###now we add genenames to the geneid###
geneid <- rownames(mymatrix) ### pulls the names we have in the counts file
genes <- select(Homo.sapiens, keys=geneid, columns=c("SYMBOL"),
keytype="ENTREZID")
genes <- genes[!duplicated(genes$ENTREZID),]
mymatrix$genes <- genesInitial histograms
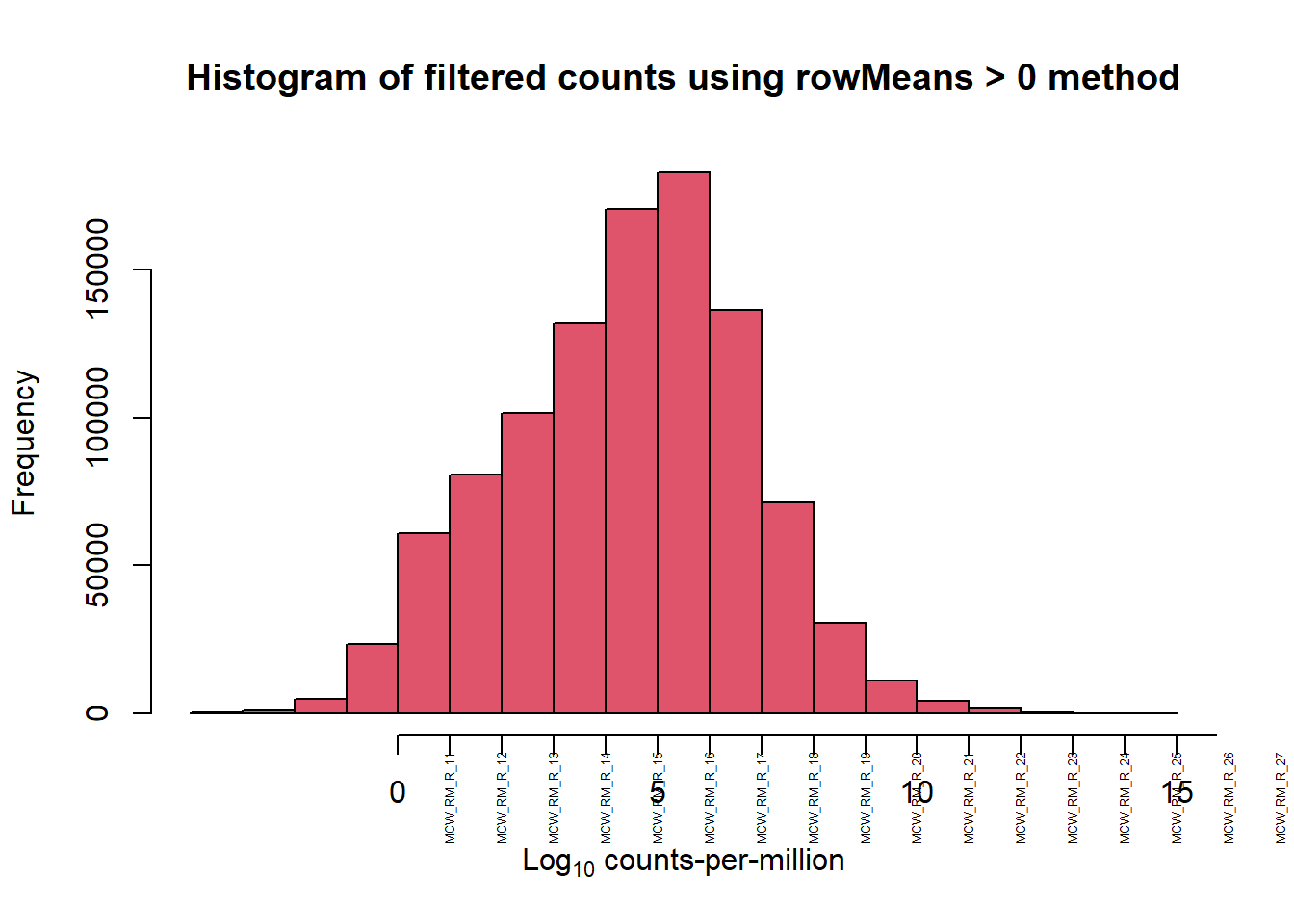
[1] 28395 72[1] 14086 72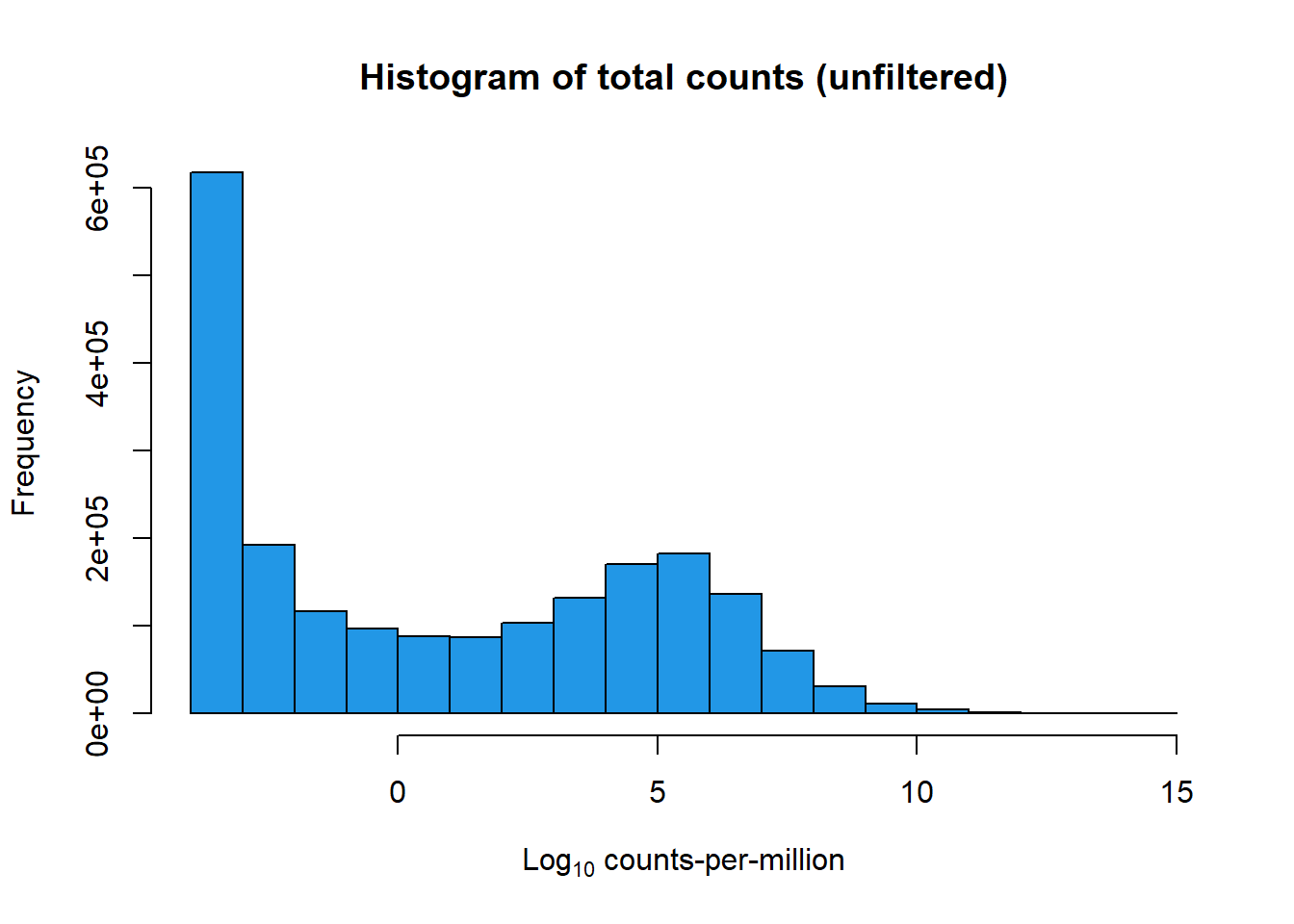
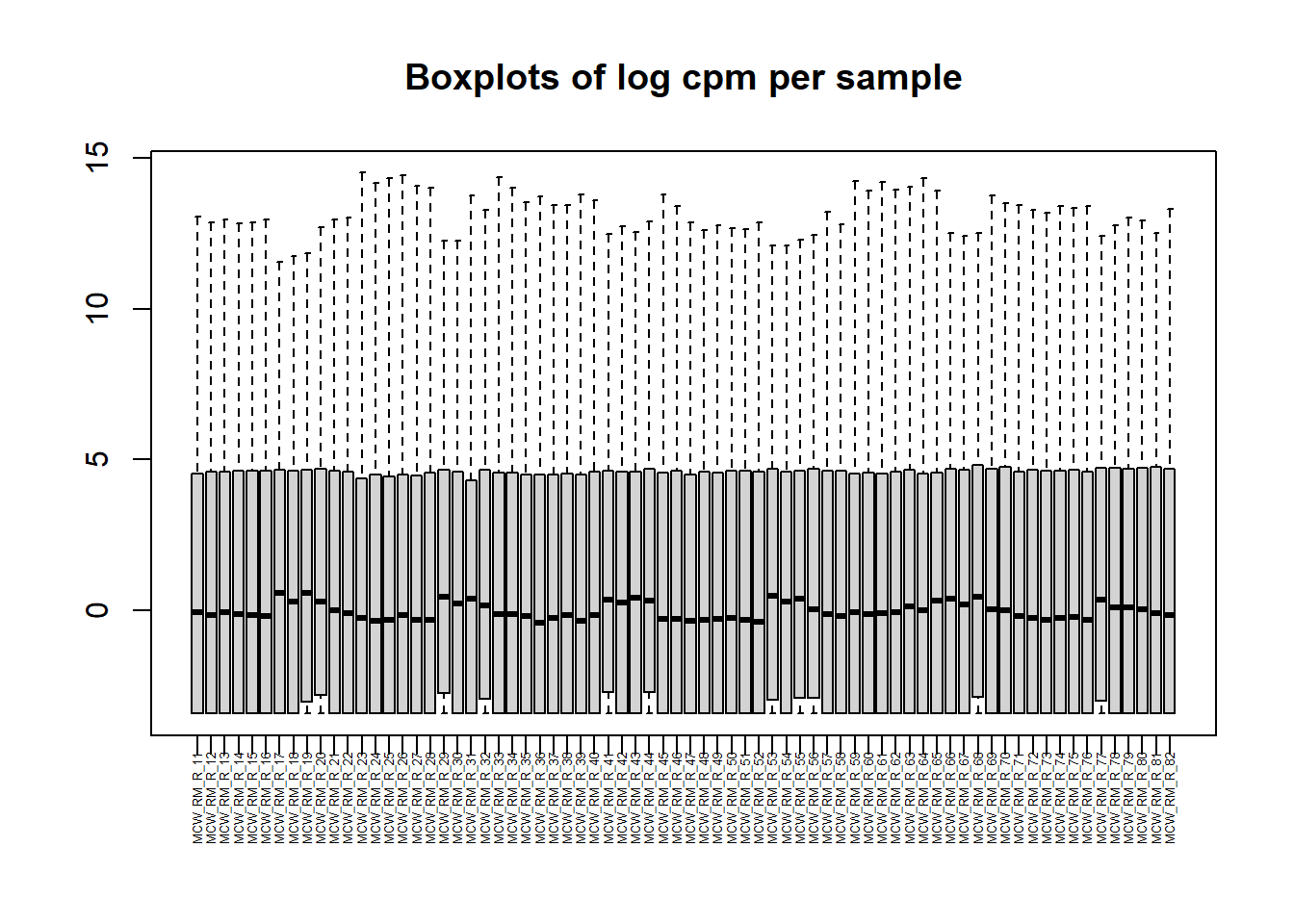
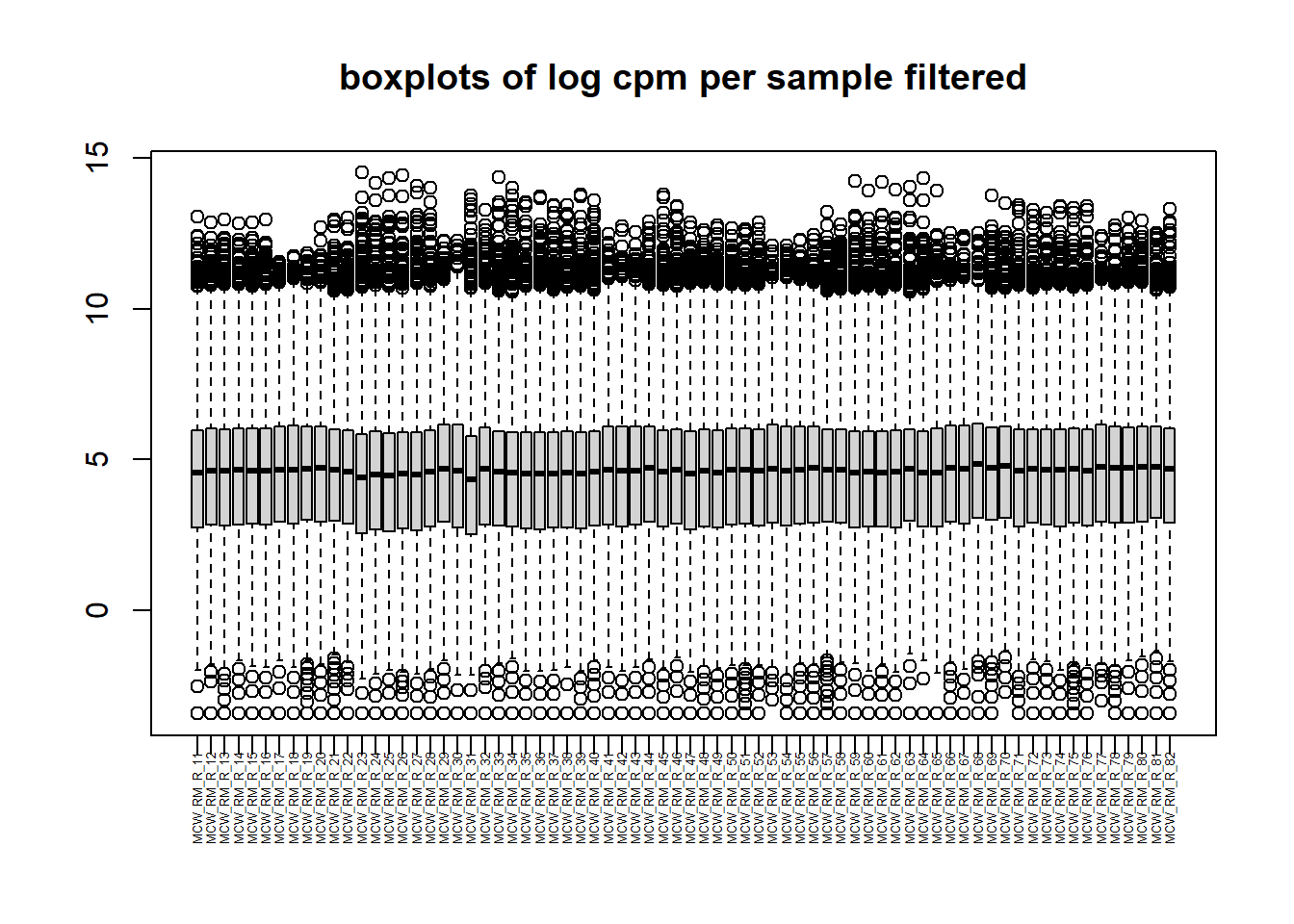
PCA by treatment and as a whole
### Vehicle 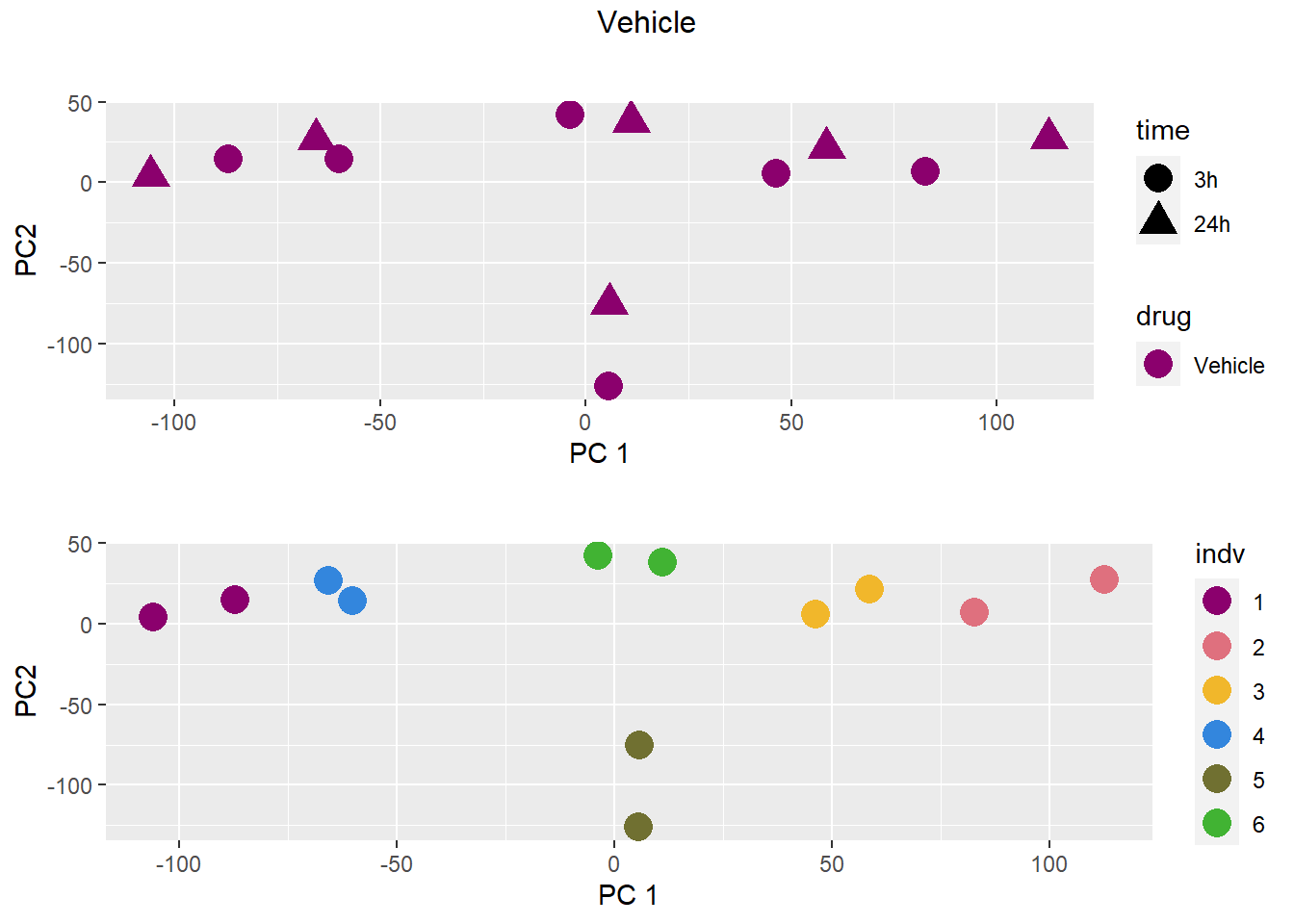
### Daunorubicin 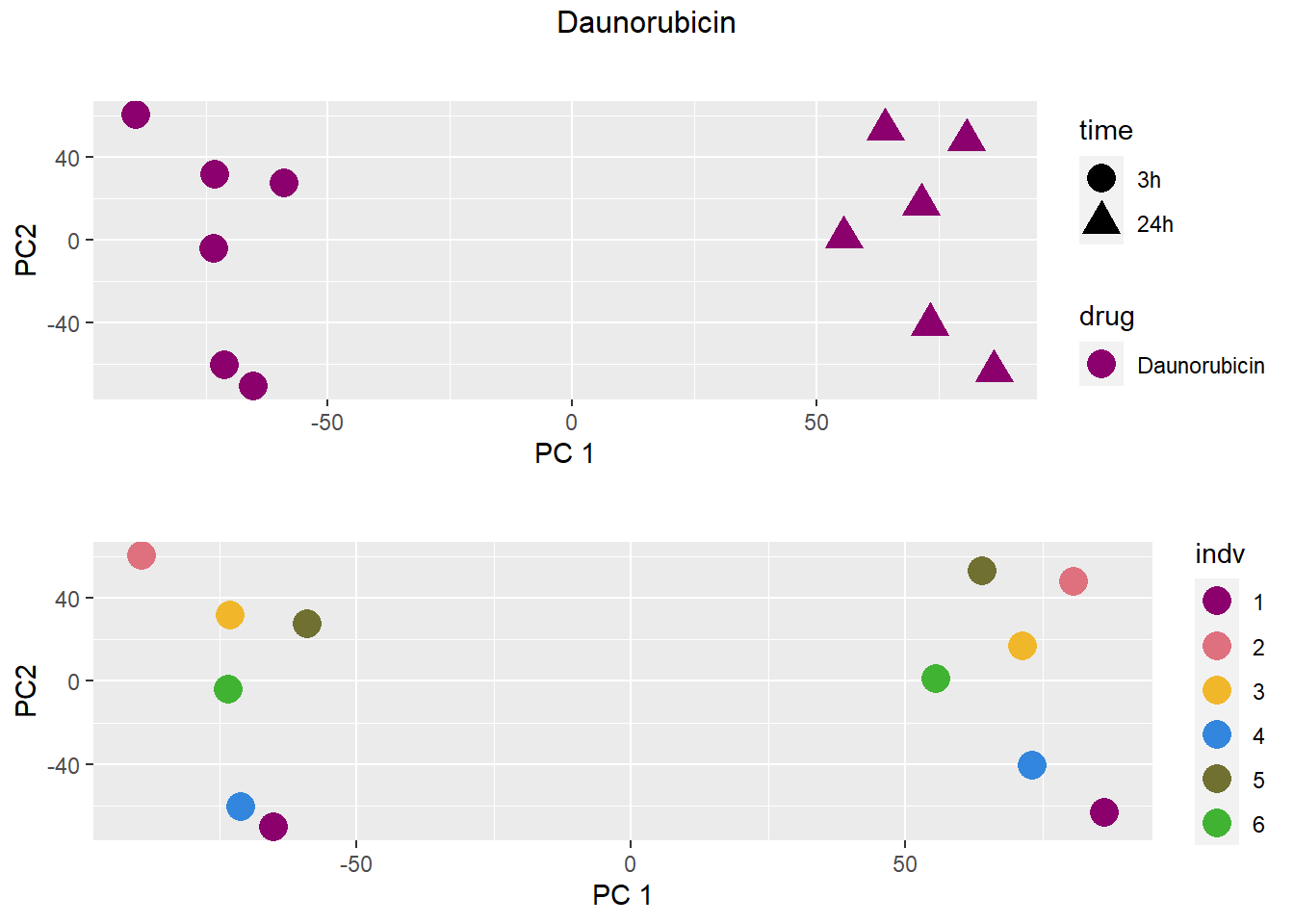
### Doxorubicin 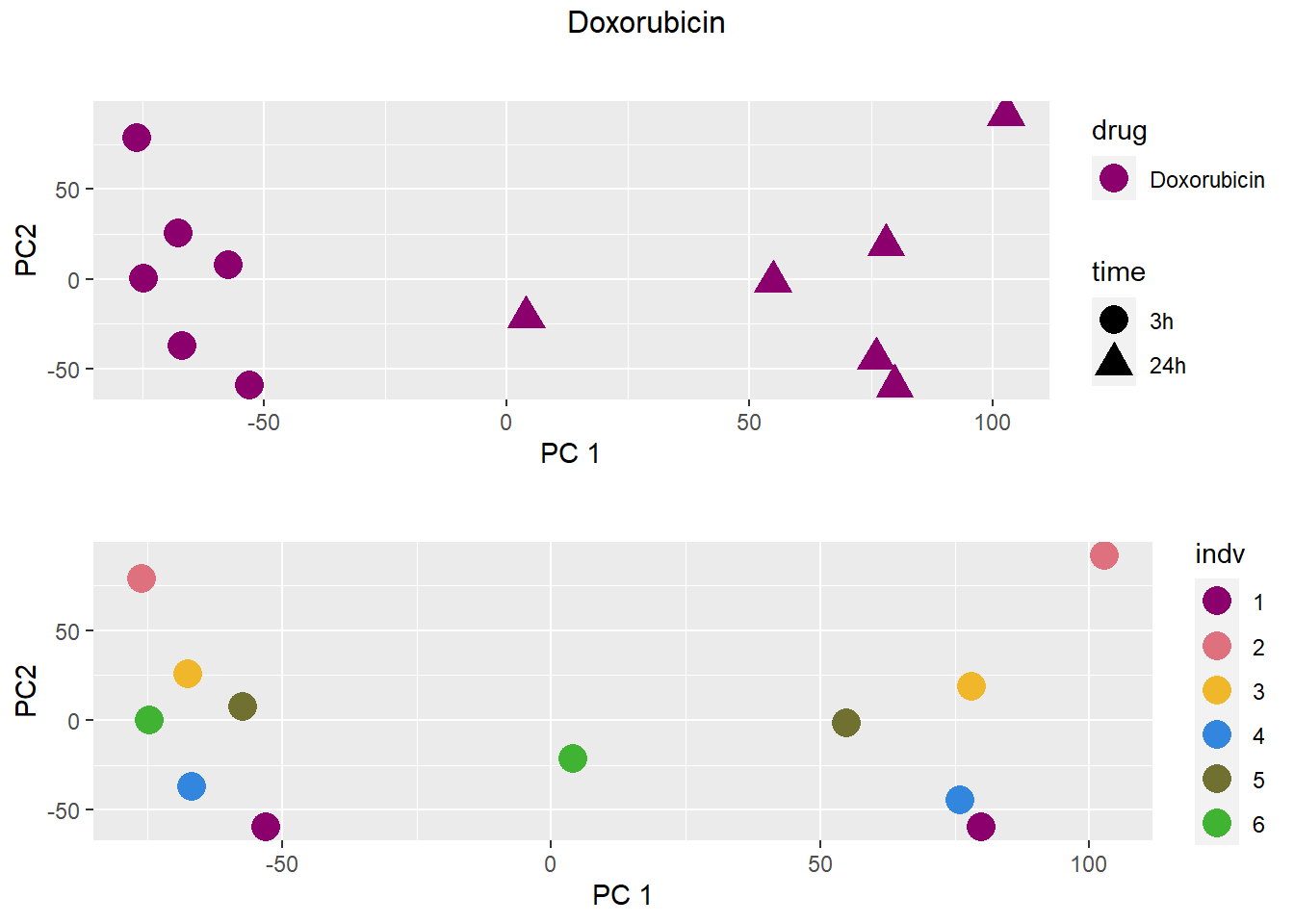
### Epirubicin 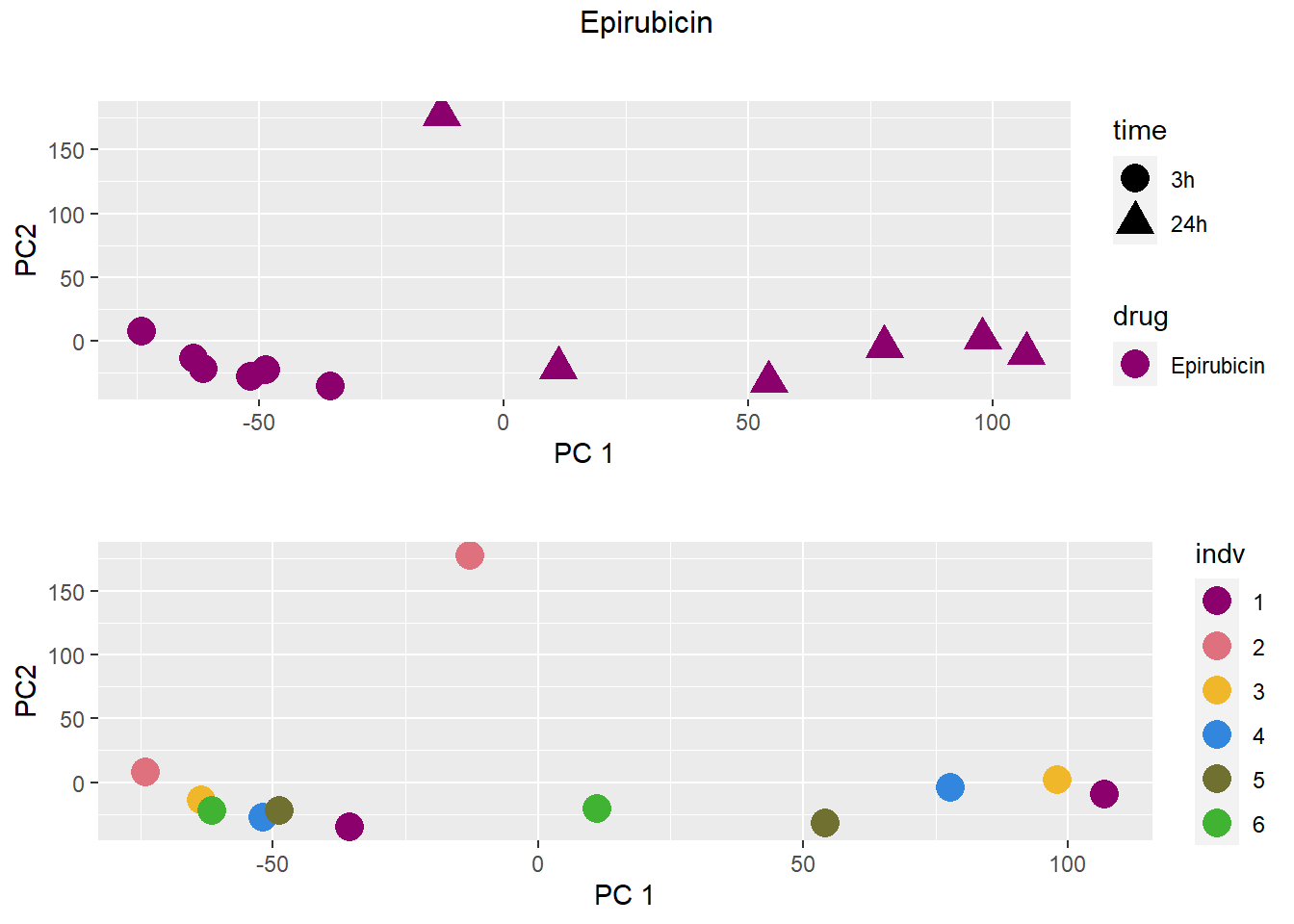
### Mitoxantrone 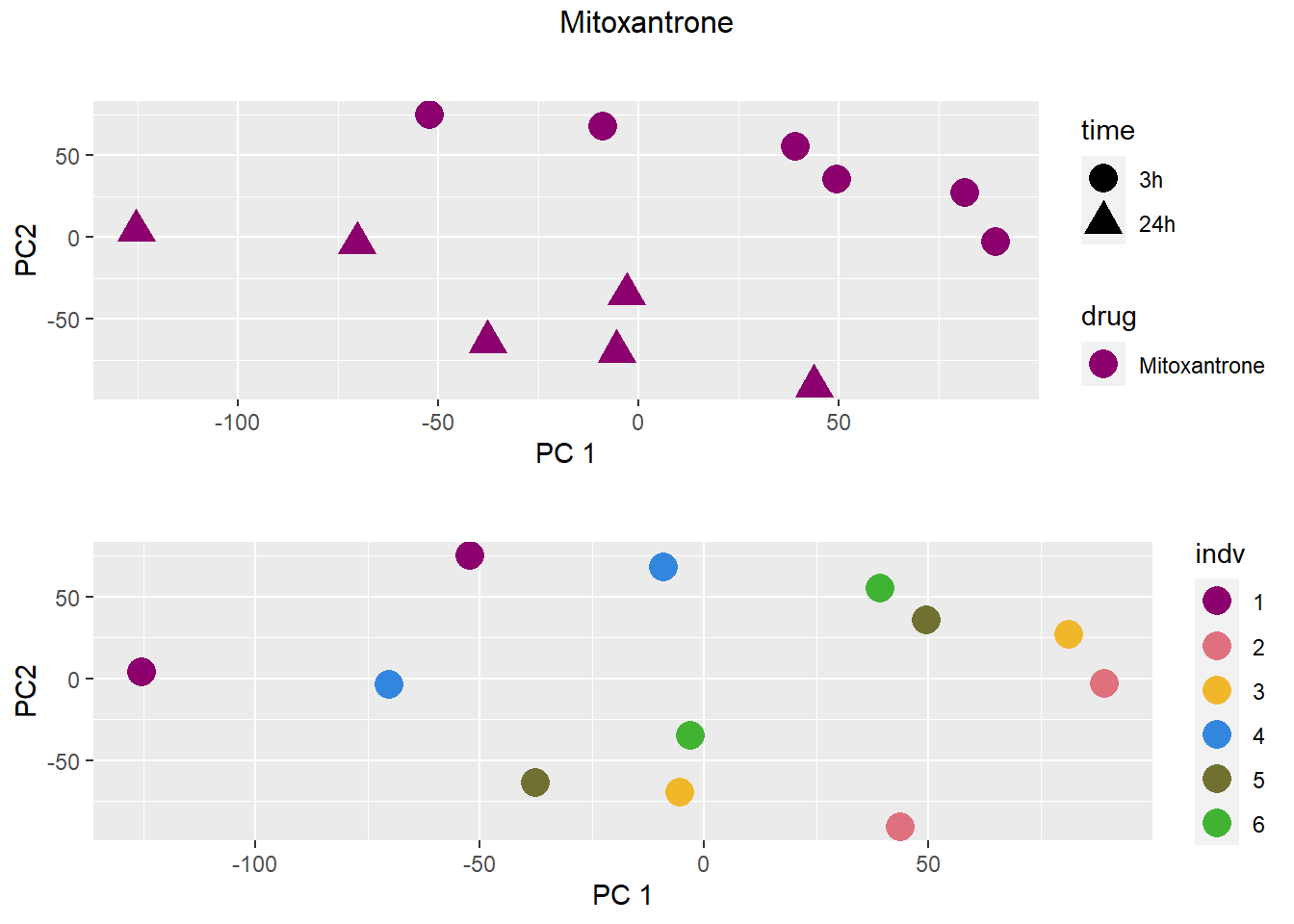
### Trastuzumab 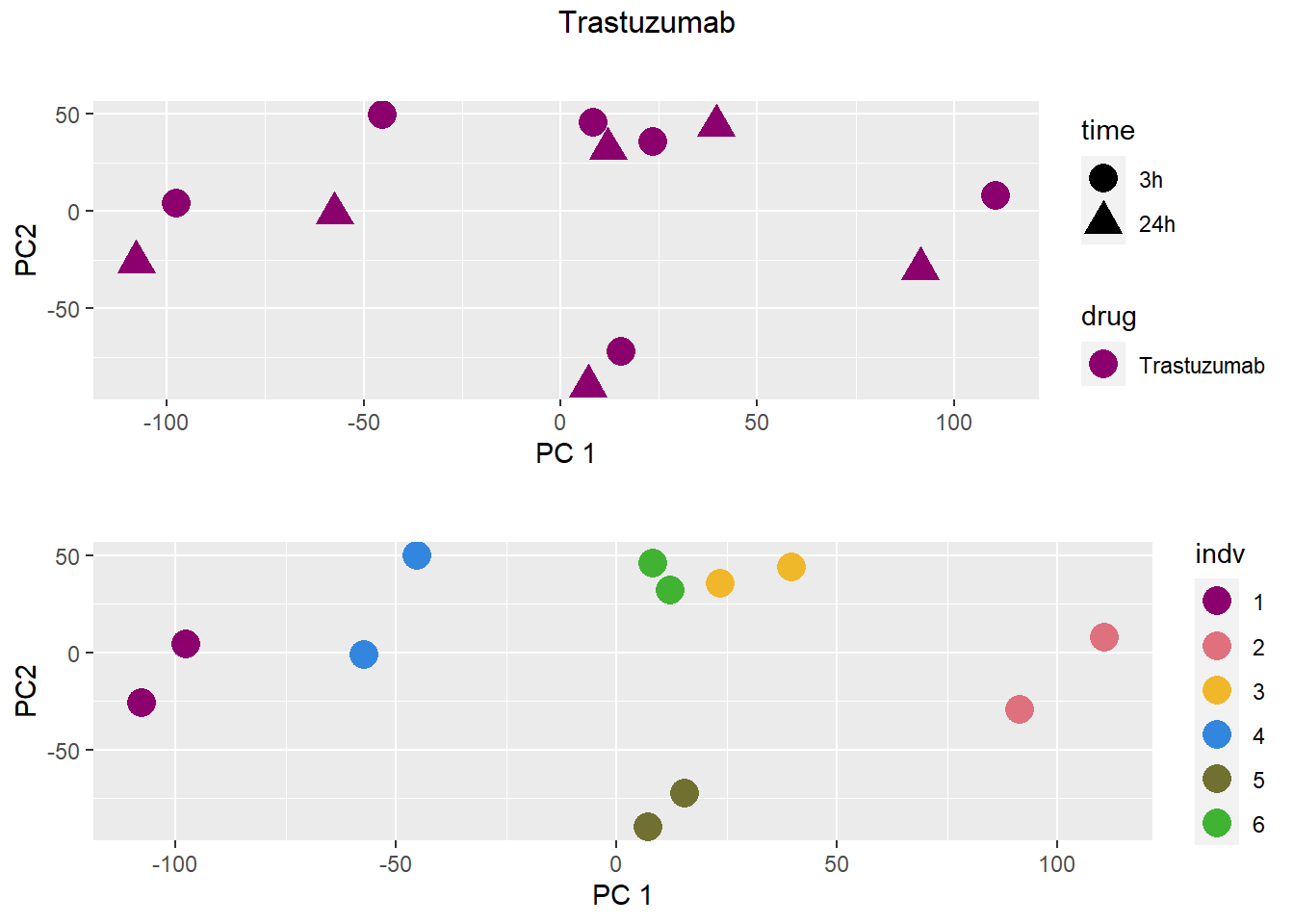
samplenames indv drug time RIN group PC1 PC2
Da.1.3h MCW_RM_R_11 1 Daunorubicin 3h 9.3 1 -18.53902 62.11914
Do.1.3h MCW_RM_R_12 1 Doxorubicin 3h 9.8 2 -12.37874 73.96424
Ep.1.3h MCW_RM_R_13 1 Epirubicin 3h 9.8 3 -11.19226 66.47554
Mi.1.3h MCW_RM_R_14 1 Mitoxantrone 3h 10 4 -10.24538 73.46900
Tr.1.3h MCW_RM_R_15 1 Trastuzumab 3h 9.6 5 -12.21578 79.97646
Ve.1.3h MCW_RM_R_16 1 Vehicle 3h 9.9 6 -15.01396 76.61739
PC3 PC4 PC5 PC6
Da.1.3h 44.793907 -4.229684 25.33474 -35.96203
Do.1.3h 24.565270 -8.522490 -19.86738 -19.00810
Ep.1.3h 33.103318 -9.146883 18.17321 -43.12911
Mi.1.3h 19.048074 -14.540036 -9.00047 -24.33769
Tr.1.3h 2.646249 -17.004856 -34.18428 -11.83222
Ve.1.3h 12.636907 -4.140009 -39.84065 -17.16481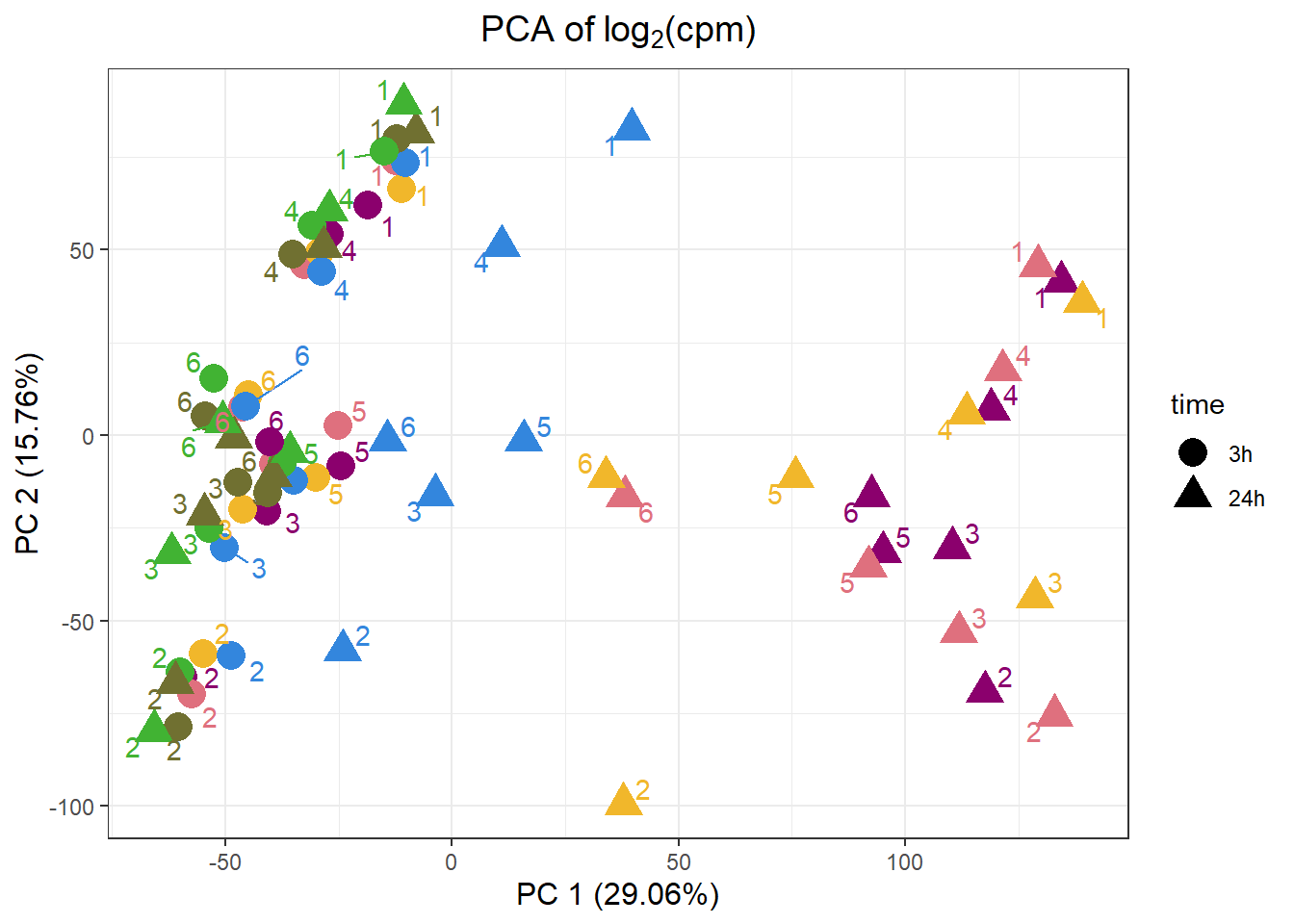
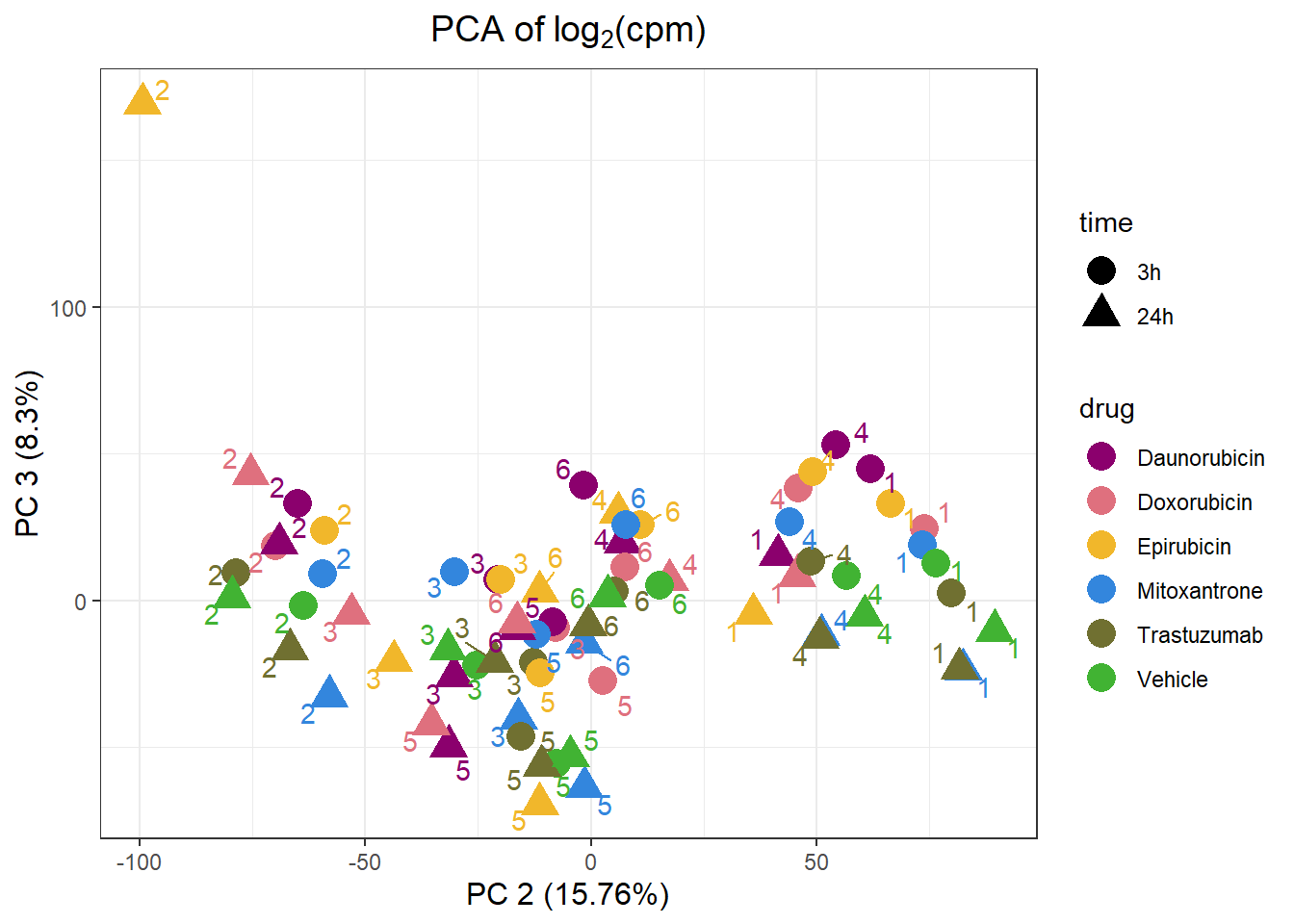
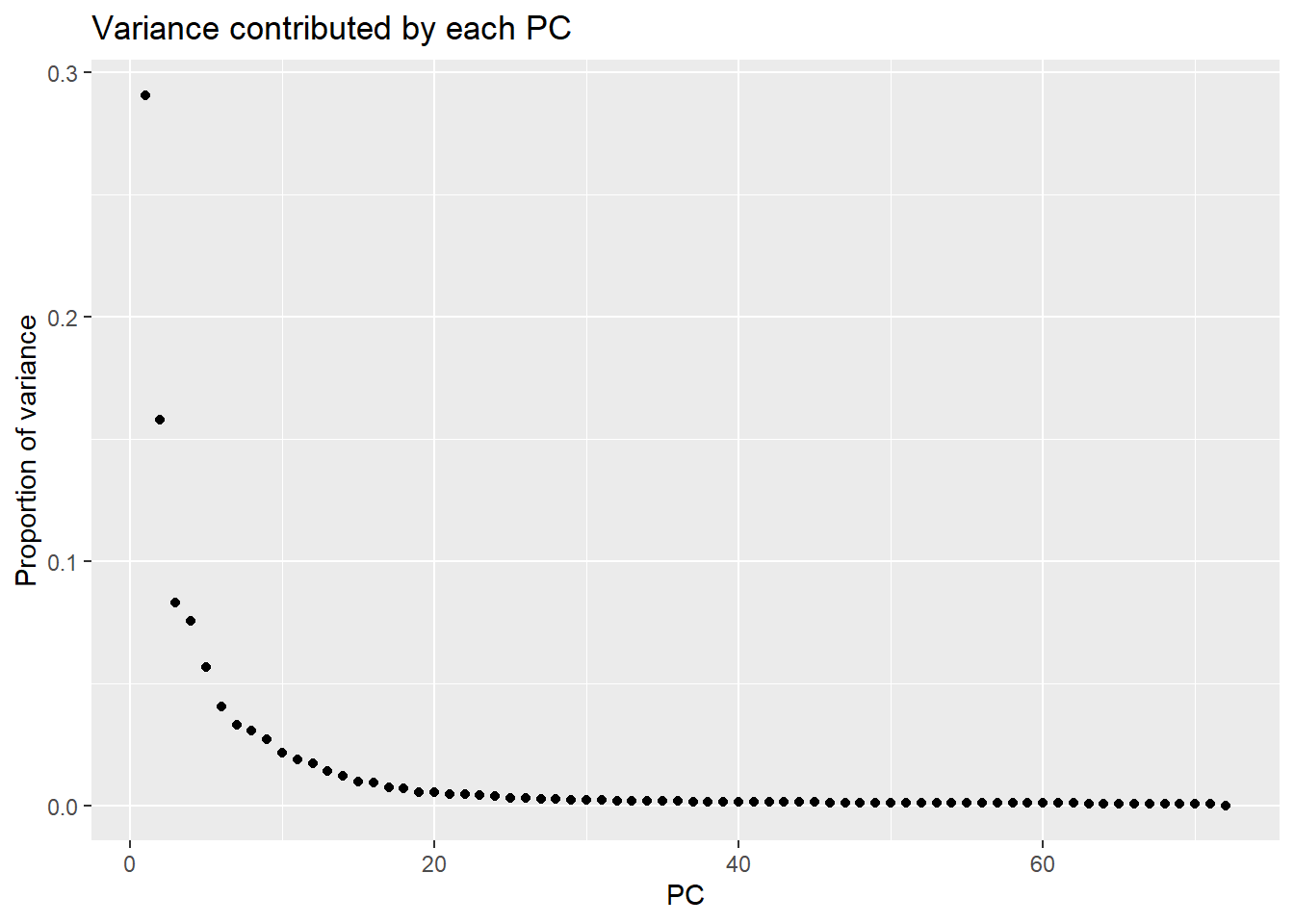
Importance of components:
PC1 PC2 PC3 PC4 PC5 PC6
Standard deviation 63.9774 47.1102 34.19844 32.56486 28.23410 23.90465
Proportion of Variance 0.2906 0.1576 0.08303 0.07529 0.05659 0.04057
Cumulative Proportion 0.2906 0.4481 0.53117 0.60645 0.66304 0.70361
PC7 PC8 PC9 PC10 PC11 PC12
Standard deviation 21.54760 20.74762 19.52266 17.46914 16.25667 15.49063
Proportion of Variance 0.03296 0.03056 0.02706 0.02166 0.01876 0.01704
Cumulative Proportion 0.73657 0.76713 0.79419 0.81586 0.83462 0.85165
PC13 PC14 PC15 PC16 PC17 PC18
Standard deviation 14.02995 13.01851 11.78389 11.40612 10.15585 9.76592
Proportion of Variance 0.01397 0.01203 0.00986 0.00924 0.00732 0.00677
Cumulative Proportion 0.86563 0.87766 0.88752 0.89675 0.90408 0.91085
PC19 PC20 PC21 PC22 PC23 PC24 PC25
Standard deviation 8.79138 8.66072 8.09173 7.8730 7.46626 7.09040 6.61801
Proportion of Variance 0.00549 0.00533 0.00465 0.0044 0.00396 0.00357 0.00311
Cumulative Proportion 0.91633 0.92166 0.92631 0.9307 0.93466 0.93823 0.94134
PC26 PC27 PC28 PC29 PC30 PC31 PC32
Standard deviation 6.31712 6.05845 5.86317 5.72293 5.54398 5.49527 5.27437
Proportion of Variance 0.00283 0.00261 0.00244 0.00233 0.00218 0.00214 0.00197
Cumulative Proportion 0.94418 0.94678 0.94922 0.95155 0.95373 0.95587 0.95785
PC33 PC34 PC35 PC36 PC37 PC38 PC39
Standard deviation 5.18844 5.11708 4.86120 4.83415 4.71966 4.52799 4.50677
Proportion of Variance 0.00191 0.00186 0.00168 0.00166 0.00158 0.00146 0.00144
Cumulative Proportion 0.95976 0.96162 0.96330 0.96495 0.96654 0.96799 0.96943
PC40 PC41 PC42 PC43 PC44 PC45 PC46
Standard deviation 4.39472 4.34878 4.30170 4.23851 4.15240 4.12290 4.05711
Proportion of Variance 0.00137 0.00134 0.00131 0.00128 0.00122 0.00121 0.00117
Cumulative Proportion 0.97080 0.97215 0.97346 0.97474 0.97596 0.97717 0.97834
PC47 PC48 PC49 PC50 PC51 PC52 PC53
Standard deviation 4.01633 3.9421 3.90038 3.83199 3.81069 3.80580 3.76258
Proportion of Variance 0.00115 0.0011 0.00108 0.00104 0.00103 0.00103 0.00101
Cumulative Proportion 0.97948 0.9806 0.98166 0.98271 0.98374 0.98477 0.98577
PC54 PC55 PC56 PC57 PC58 PC59 PC60
Standard deviation 3.71747 3.69618 3.64557 3.59456 3.5595 3.51279 3.46169
Proportion of Variance 0.00098 0.00097 0.00094 0.00092 0.0009 0.00088 0.00085
Cumulative Proportion 0.98675 0.98772 0.98866 0.98958 0.9905 0.99136 0.99221
PC61 PC62 PC63 PC64 PC65 PC66 PC67
Standard deviation 3.45216 3.40460 3.34569 3.27664 3.21965 3.1371 3.12182
Proportion of Variance 0.00085 0.00082 0.00079 0.00076 0.00074 0.0007 0.00069
Cumulative Proportion 0.99305 0.99388 0.99467 0.99543 0.99617 0.9969 0.99756
PC68 PC69 PC70 PC71 PC72
Standard deviation 3.09899 3.05675 2.88458 2.6632 8.662e-14
Proportion of Variance 0.00068 0.00066 0.00059 0.0005 0.000e+00
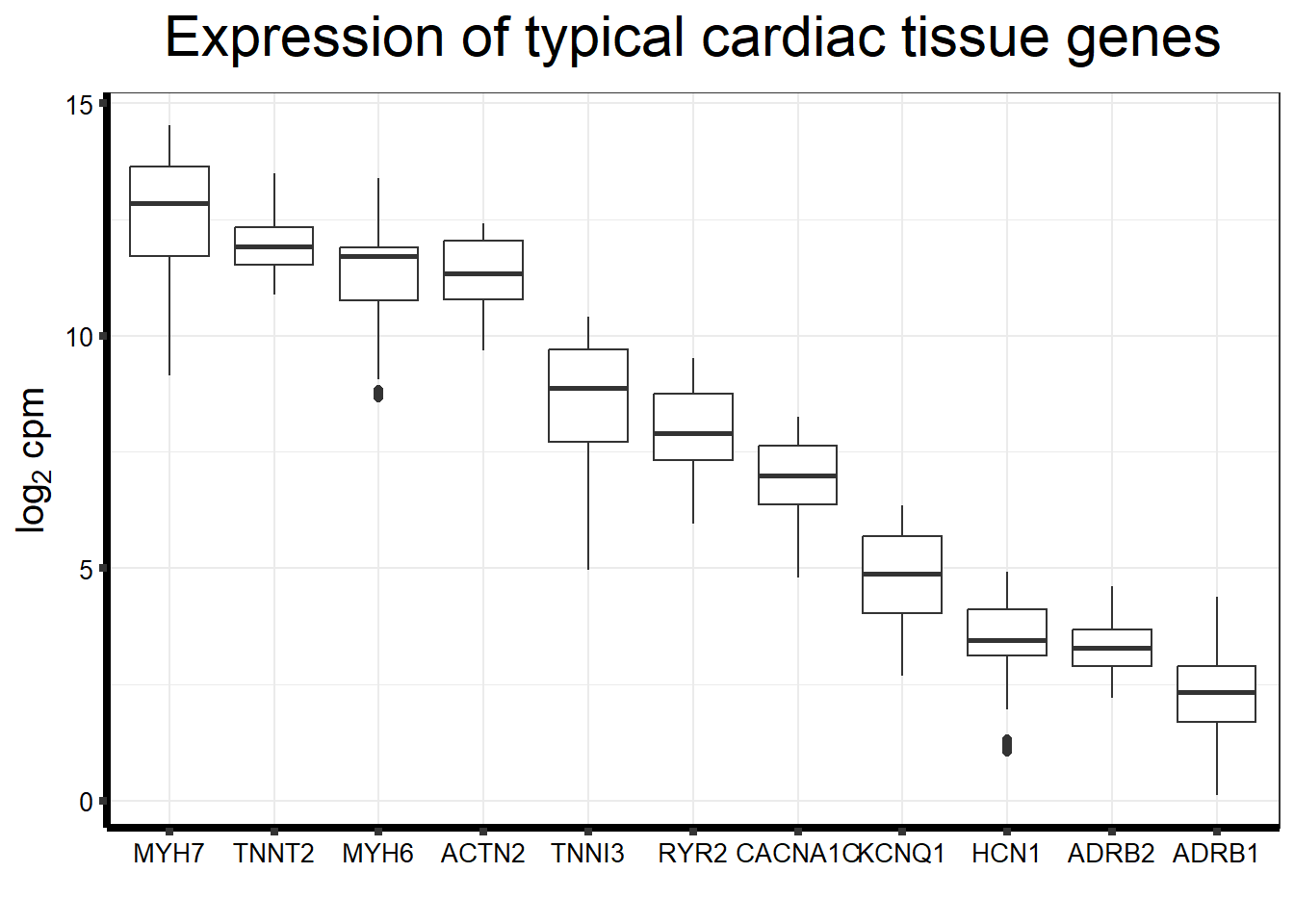
Cumulative Proportion 0.99824 0.99891 0.99950 1.0000 1.000e+00Typical genes expressed in iPSC-CMS
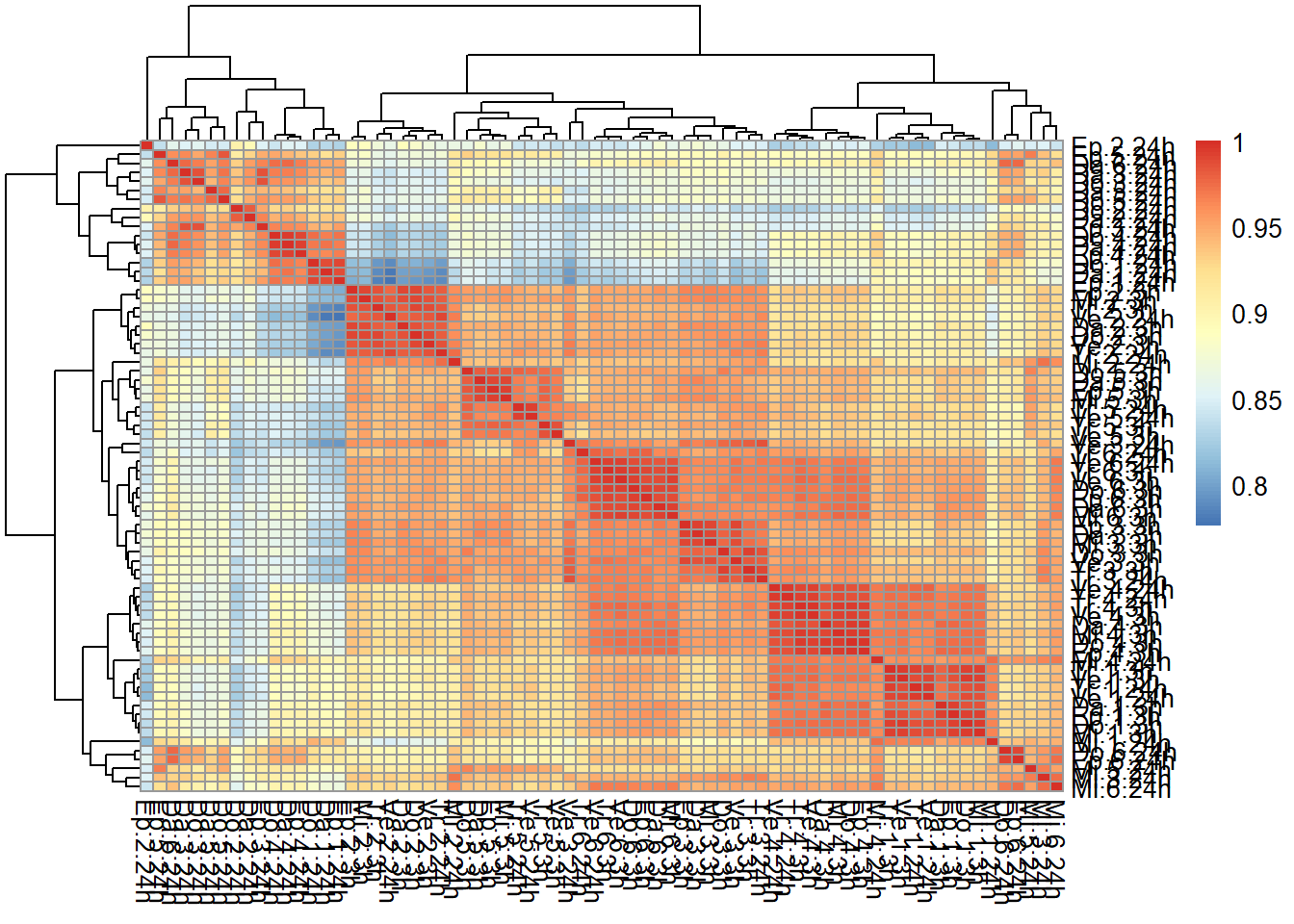
correlation heatmap of counts matrix
now to get the counts set for DEG!!
DEG analysis
summary
V.DA V.DX V.EP V.MT V.TR V.DA24 V.DX24 V.EP24 V.MT24 V.TR24
Down 108 3 29 22 0 3542 3341 3111 430 0
NotSig 13569 14067 13876 14014 14086 7062 7431 7751 12964 14086
Up 409 16 181 50 0 3482 3314 3224 692 0cormotif analysis
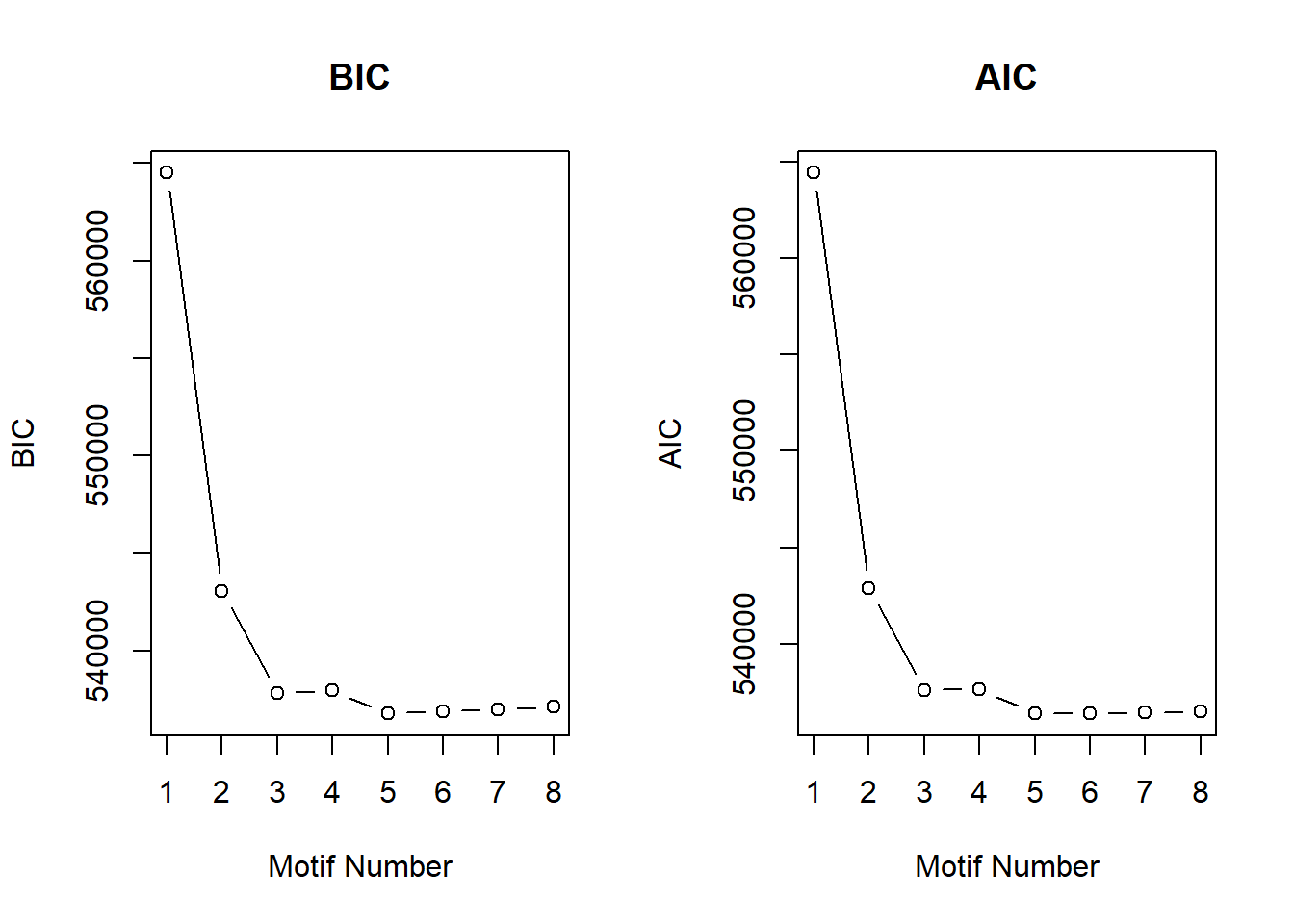

[1] 14086 10Response sets look similar to previous results. This data is based on the filtered count matrix (using rowmeans>0 of cpm(log=true)). Classification of patterns appear to be:
motif 1- No Response set: 7490 (gene list made by filtering each column by posterior probability of <0.5) motif 2- Time-independent Top2Bi response cluster: 116
motif 3- Early Top2Bi response cluster: 424
motif 4- All BC drug response set (only 1!)
motif 5- Late Top2Bi response cluster:1615 **these are based on the most recent plots (motif numbers have changed a little)
R version 4.2.2 (2022-10-31 ucrt)
Platform: x86_64-w64-mingw32/x64 (64-bit)
Running under: Windows 10 x64 (build 19044)
Matrix products: default
locale:
[1] LC_COLLATE=English_United States.utf8
[2] LC_CTYPE=English_United States.utf8
[3] LC_MONETARY=English_United States.utf8
[4] LC_NUMERIC=C
[5] LC_TIME=English_United States.utf8
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] Cormotif_1.42.0
[2] affy_1.74.0
[3] Hmisc_4.8-0
[4] Formula_1.2-5
[5] survival_3.5-3
[6] corrplot_0.92
[7] ggrepel_0.9.3
[8] cowplot_1.1.1
[9] Homo.sapiens_1.3.1
[10] TxDb.Hsapiens.UCSC.hg19.knownGene_3.2.2
[11] org.Hs.eg.db_3.15.0
[12] GO.db_3.15.0
[13] OrganismDbi_1.38.1
[14] GenomicFeatures_1.48.4
[15] GenomicRanges_1.48.0
[16] GenomeInfoDb_1.32.4
[17] AnnotationDbi_1.58.0
[18] IRanges_2.30.1
[19] S4Vectors_0.34.0
[20] Biobase_2.56.0
[21] biomaRt_2.52.0
[22] scales_1.2.1
[23] lubridate_1.9.2
[24] forcats_1.0.0
[25] stringr_1.5.0
[26] dplyr_1.1.0
[27] purrr_1.0.1
[28] readr_2.1.4
[29] tidyr_1.3.0
[30] tibble_3.1.8
[31] tidyverse_2.0.0
[32] AnnotationHub_3.4.0
[33] BiocFileCache_2.4.0
[34] dbplyr_2.3.1
[35] BiocGenerics_0.42.0
[36] reshape2_1.4.4
[37] gridExtra_2.3
[38] mixOmics_6.20.0
[39] ggplot2_3.4.1
[40] lattice_0.20-45
[41] MASS_7.3-58.2
[42] RColorBrewer_1.1-3
[43] edgeR_3.38.4
[44] limma_3.52.4
[45] workflowr_1.7.0
loaded via a namespace (and not attached):
[1] utf8_1.2.3 tidyselect_1.2.0
[3] RSQLite_2.3.0 htmlwidgets_1.6.1
[5] grid_4.2.2 BiocParallel_1.30.4
[7] munsell_0.5.0 codetools_0.2-19
[9] preprocessCore_1.58.0 interp_1.1-3
[11] statmod_1.5.0 withr_2.5.0
[13] colorspace_2.1-0 filelock_1.0.2
[15] highr_0.10 knitr_1.42
[17] rstudioapi_0.14 MatrixGenerics_1.8.1
[19] labeling_0.4.2 git2r_0.31.0
[21] GenomeInfoDbData_1.2.8 bit64_4.0.5
[23] farver_2.1.1 pheatmap_1.0.12
[25] rprojroot_2.0.3 vctrs_0.5.2
[27] generics_0.1.3 xfun_0.37
[29] timechange_0.2.0 R6_2.5.1
[31] locfit_1.5-9.7 bitops_1.0-7
[33] cachem_1.0.7 DelayedArray_0.22.0
[35] promises_1.2.0.1 BiocIO_1.6.0
[37] nnet_7.3-18 gtable_0.3.1
[39] processx_3.8.0 rlang_1.0.6
[41] splines_4.2.2 rtracklayer_1.56.1
[43] checkmate_2.1.0 BiocManager_1.30.20
[45] yaml_2.3.7 backports_1.4.1
[47] httpuv_1.6.9 RBGL_1.72.0
[49] tools_4.2.2 affyio_1.66.0
[51] ellipsis_0.3.2 jquerylib_0.1.4
[53] Rcpp_1.0.10 plyr_1.8.8
[55] base64enc_0.1-3 progress_1.2.2
[57] zlibbioc_1.42.0 RCurl_1.98-1.10
[59] ps_1.7.2 prettyunits_1.1.1
[61] rpart_4.1.19 deldir_1.0-6
[63] SummarizedExperiment_1.26.1 cluster_2.1.4
[65] fs_1.6.1 magrittr_2.0.3
[67] data.table_1.14.8 RSpectra_0.16-1
[69] whisker_0.4.1 matrixStats_0.63.0
[71] hms_1.1.2 mime_0.12
[73] evaluate_0.20 xtable_1.8-4
[75] XML_3.99-0.13 jpeg_0.1-10
[77] compiler_4.2.2 ellipse_0.4.3
[79] crayon_1.5.2 htmltools_0.5.4
[81] corpcor_1.6.10 later_1.3.0
[83] tzdb_0.3.0 DBI_1.1.3
[85] rappdirs_0.3.3 Matrix_1.5-3
[87] cli_3.6.0 parallel_4.2.2
[89] igraph_1.4.1 pkgconfig_2.0.3
[91] getPass_0.2-2 GenomicAlignments_1.32.1
[93] foreign_0.8-84 xml2_1.3.3
[95] rARPACK_0.11-0 bslib_0.4.2
[97] XVector_0.36.0 callr_3.7.3
[99] digest_0.6.31 graph_1.74.0
[101] Biostrings_2.64.1 rmarkdown_2.20
[103] htmlTable_2.4.1 restfulr_0.0.15
[105] curl_5.0.0 shiny_1.7.4
[107] Rsamtools_2.12.0 rjson_0.2.21
[109] lifecycle_1.0.3 jsonlite_1.8.4
[111] fansi_1.0.4 pillar_1.8.1
[113] KEGGREST_1.36.3 fastmap_1.1.1
[115] httr_1.4.5 interactiveDisplayBase_1.34.0
[117] glue_1.6.2 png_0.1-8
[119] BiocVersion_3.15.2 bit_4.0.5
[121] stringi_1.7.12 sass_0.4.5
[123] blob_1.2.3 latticeExtra_0.6-30
[125] memoise_2.0.1