Evaluating classifier performance on Diplodia sapinea
Patrick Schratz, Friedrich-Schiller-University Jena
Last updated: 2019-05-24
Checks: 6 0
Knit directory: 2018-model-comparison/
This reproducible R Markdown analysis was created with workflowr (version 1.3.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20190523) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rproj.user/
Ignored: .drake/
Ignored: analysis/figure/
Ignored: code/07-paper/._files
Ignored: data/
Ignored: log/
Ignored: packrat/lib-R/
Ignored: packrat/lib-ext/
Ignored: packrat/lib/
Ignored: rosm.cache/
Ignored: tests/testthat/
Untracked files:
Untracked: analysis/bibliography.bib
Untracked: packrat/src/here/
Unstaged changes:
Modified: _drake.R
Modified: analysis/about.Rmd
Modified: analysis/benchmark-pathogens.Rmd
Modified: analysis/index.Rmd
Modified: code/06-reports.R
Modified: code/99-packages.R
Modified: inst/rsync-jupiter.sh
Modified: packrat/packrat.lock
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | ac7224e | pat-s | 2019-05-24 | wflow_publish(knitr_in(“analysis/benchmark-diplodia.Rmd”), view = |
| Rmd | 6af4181 | pat-s | 2019-05-23 | update reports |
| Rmd | 769718b | pat-s | 2019-05-23 | Start workflowr project. |
1 Introduction
This document shows the predictive performances for the possible infection risk of trees in the Basque Country by the pathogen Diplodia sapinea.
The following algorithms were benchmarked:
- Boosted Regression Trees (BRT)
- Generalized Additive Model (GAM)
- Generalized Linear Model (GLM)
- k-Nearest Neighbor (KNN)
- Random Forests (RF)
- Support Vector Machine (SVM)
- Extreme Gradient Boosting (XGBOOST)
2 Resampling Strategies
The abbreviations of the tabbed resampling strategies follow the scheme:
<outer resampling> / <inner resampling>
For example, setting “Spatial-Spatial” means that in both levels a “spatial cross-validation” (Brenning (2012)) has been applied.
The inner resampling refers to the hyperparameter tuning level of the nested cross-validation that was applied.
3 Results structure
The structure of the following results presentation is as follows:
- Table view of all performances for each resampling setting
- Boxplot comparison for each pathogen and algorithm
- Aggregated performances for each pathogen and algorithm
4 Performance results
4.1 Spatial-Spatial
task.id learner.id brier.test.mean timetrain.test.mean
1 diplodia classif.svm.tuned 0.2000721 194.6988
2 diplodia classif.gam.tuned 0.1899124 230.0244
3 diplodia classif.kknn.tuned 0.1877918 109.7034
4 diplodia classif.ranger.tuned 0.1594762 240.8807
5 diplodia classif.gbm.tuned 0.1590360 1820.59904.1.1 Boxplot comparison

4.1.2 Aggregated performances

4.2 Spatial-Non-Spatial
task.id learner.id brier.test.mean timetrain.test.mean
1 diplodia classif.svm.tuned 0.2125015 389.3593
2 diplodia classif.gam.tuned 0.1922458 294.0095
3 diplodia classif.kknn.tuned 0.1882630 121.3932
4 diplodia classif.gbm.tuned 0.1637896 2095.5327
5 diplodia classif.ranger.tuned 0.1528584 445.12064.2.1 Boxplot comparison

4.2.2 Aggregated performances

4.3 Non-Spatial-Non-Spatial
task.id learner.id brier.test.mean timetrain.test.mean
1 diplodia classif.gam.tuned 0.1395204 290.8944
2 diplodia classif.svm.tuned 0.1229645 406.3464
3 diplodia classif.kknn.tuned 0.1201311 121.3484
4 diplodia classif.gbm.tuned 0.1118638 1999.3005
5 diplodia classif.ranger.tuned 0.0995260 440.40304.3.1 Boxplot comparison

4.3.2 Aggregated performances
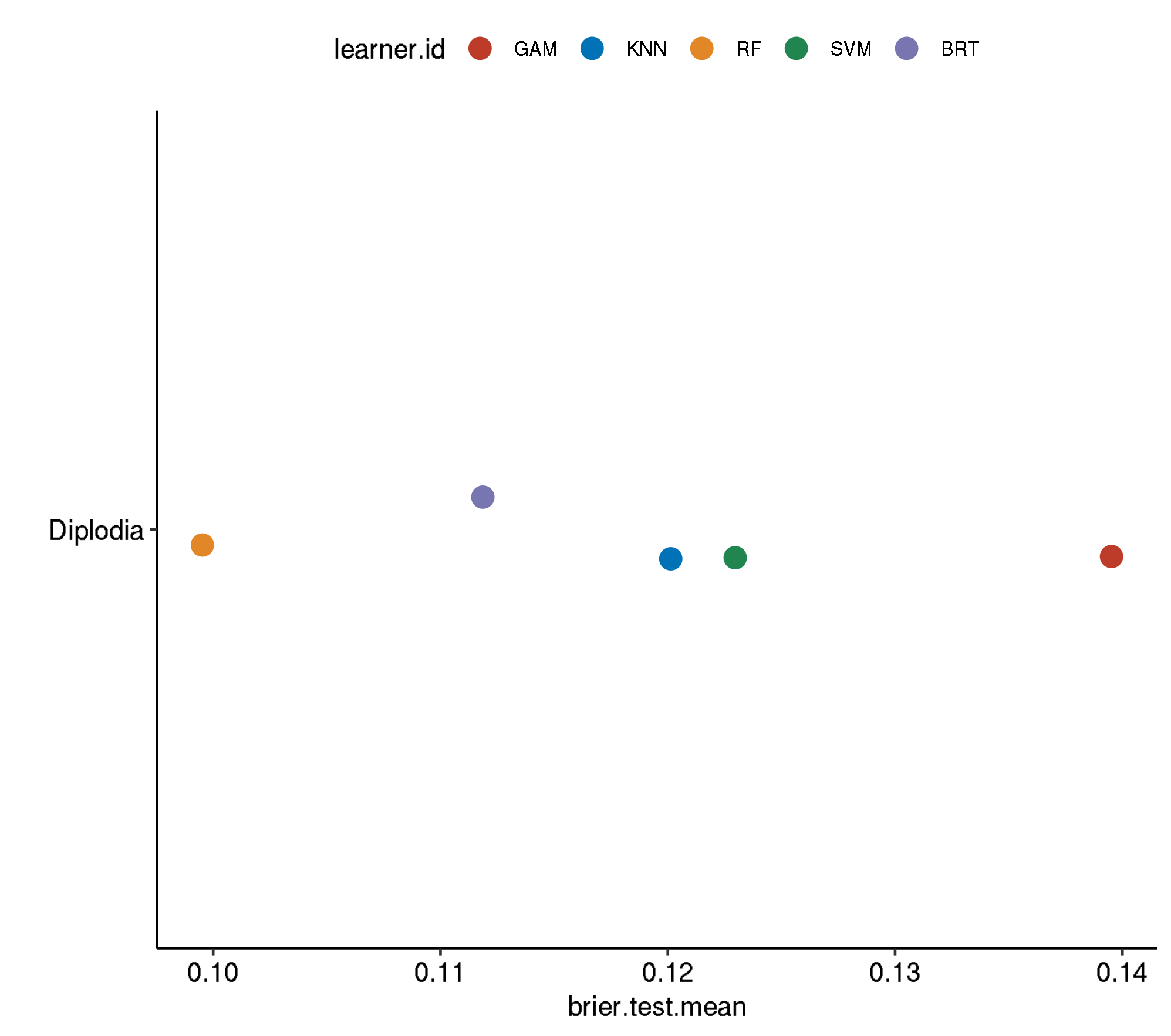
4.4 Non-Spatial-No Tuning
task.id learner.id brier.test.mean timetrain.test.mean
1 diplodia classif.svm 0.1603230 0.638496
2 diplodia classif.gam 0.1467264 0.615932
3 diplodia classif.kknn 0.1195669 0.000772
4 diplodia classif.binomial 0.1186126 0.021954
5 diplodia classif.gbm 0.1137512 0.060954
6 diplodia classif.ranger 0.1017046 0.6675564.4.1 Boxplot comparison

4.4.2 Aggregated performances

4.5 Spatial-No Tuning
task.id learner.id brier.test.mean timetrain.test.mean
1 diplodia classif.gam 0.2505253 0.569212
2 diplodia classif.svm 0.2009484 0.627202
3 diplodia classif.binomial 0.1703007 0.022436
4 diplodia classif.kknn 0.1689237 0.001134
5 diplodia classif.gbm 0.1598117 0.062854
6 diplodia classif.ranger 0.1499009 0.6519484.5.1 Boxplot comparison
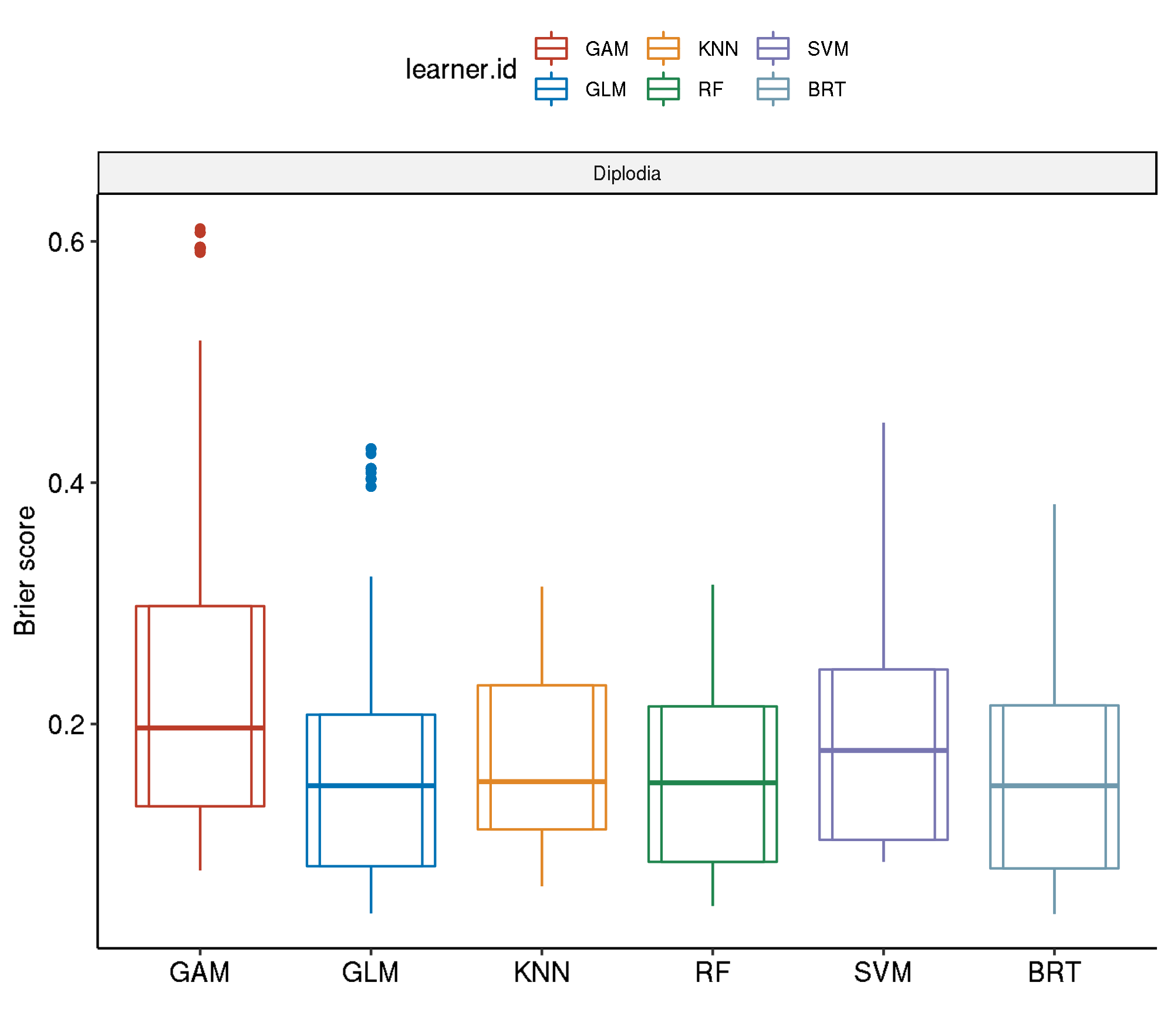
4.5.2 Aggregated performances

5 Boxplot comparison of all algorithm/tuning settings
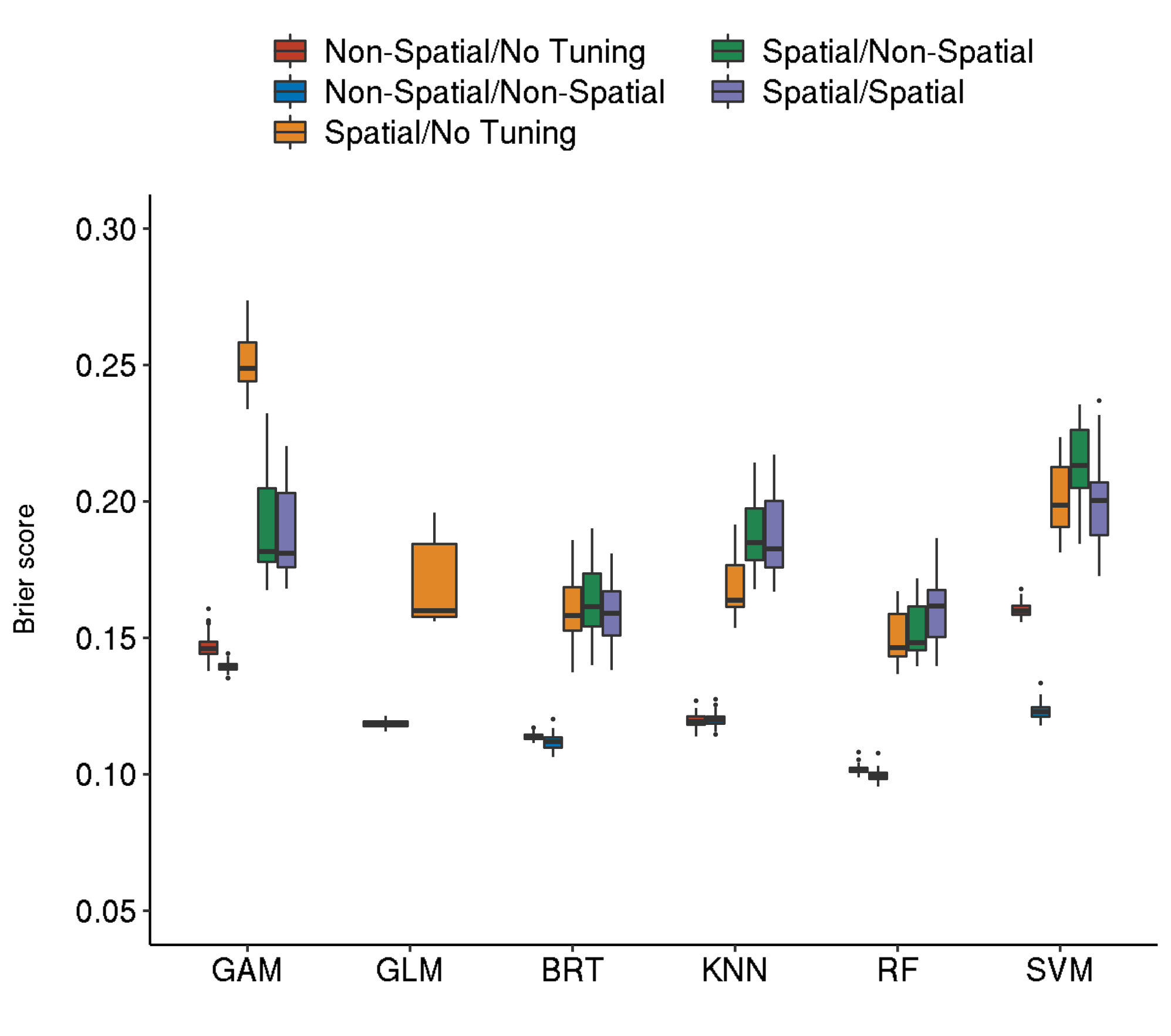
Warning! The custom fig.path you set was ignored by workflowr.
6 References
Brenning, A. 2012. “Spatial Cross-Validation and Bootstrap for the Assessment of Prediction Rules in Remote Sensing: The R Package Sperrorest.” In 2012 Ieee International Geoscience and Remote Sensing Symposium, 5372–5. https://doi.org/10.1109/IGARSS.2012.6352393.
R version 3.5.1 (2018-07-02)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: CentOS Linux 7 (Core)
Matrix products: default
BLAS/LAPACK: /opt/spack/opt/spack/linux-centos7-x86_64/gcc-7.3.0/openblas-0.3.5-zncvk4jccaqyfl4z3vszaboeps6hyzta/lib/libopenblas_zen-r0.3.5.so
locale:
[1] LC_CTYPE=en_GB.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_GB.UTF-8 LC_COLLATE=en_GB.UTF-8
[5] LC_MONETARY=en_GB.UTF-8 LC_MESSAGES=en_GB.UTF-8
[7] LC_PAPER=en_GB.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_GB.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] tidyselect_0.2.5 workflowr_1.3.0 here_0.1
[4] kableExtra_1.1.0 ggExtra_0.8 ggrepel_0.8.0
[7] reporttools_1.1.2 xtable_1.8-3 cowplot_0.9.3
[10] hrbrthemes_0.6.0 ggpubr_0.2 future.callr_0.4.0
[13] furrr_0.1.0.9002 future_1.11.1.1 ggsci_2.9
[16] clustermq_0.8.6 ggspatial_1.0.3 ggplot2_3.0.0
[19] rgenoud_5.8-3.0 fs_1.2.6 curl_3.2
[22] R.utils_2.7.0 R.oo_1.22.0 R.methodsS3_1.7.1
[25] GSIF_0.5-5 stringr_1.3.1 RSAGA_1.3.0
[28] plyr_1.8.4 shapefiles_0.7 foreign_0.8-71
[31] gstat_1.1-6 glue_1.3.0 rasterVis_0.45
[34] latticeExtra_0.6-28 RColorBrewer_1.1-2 lattice_0.20-35
[37] raster_2.8-19 viridis_0.5.1 viridisLite_0.3.0
[40] rgdal_1.4-3 sp_1.3-1 tibble_2.0.1
[43] forcats_0.3.0 lwgeom_0.1-6 dplyr_0.8.0.1
[46] sf_0.7-4 parallelMap_1.3 purrr_0.2.5
[49] mlrMBO_1.1.2 smoof_1.5.1 checkmate_1.8.5
[52] BBmisc_1.11 magrittr_1.5 mlr_2.13.9000
[55] ParamHelpers_1.11 drake_7.2.0
loaded via a namespace (and not attached):
[1] backports_1.1.2 Hmisc_4.2-0 fastmatch_1.1-0
[4] igraph_1.2.2 lazyeval_0.2.1 splines_3.5.1
[7] storr_1.2.1 listenv_0.7.0 digest_0.6.15
[10] htmltools_0.3.6 base64url_1.4 cluster_2.0.7-1
[13] readr_1.3.1 globals_0.12.4 extrafont_0.17
[16] xts_0.11-0 extrafontdb_1.0 colorspace_1.3-2
[19] rvest_0.3.2 pixmap_0.4-11 xfun_0.7
[22] callr_3.1.0 crayon_1.3.4 jsonlite_1.5
[25] hexbin_1.27.2 survival_2.42-3 zoo_1.8-3
[28] gtable_0.2.0 webshot_0.5.1 Rttf2pt1_1.3.7
[31] scales_1.0.0 DBI_1.0.0 miniUI_0.1.1.1
[34] Rcpp_1.0.0 plotrix_3.7-4 spData_0.2.9.0
[37] htmlTable_1.12 units_0.6-2 Formula_1.2-3
[40] intervals_0.15.1 dismo_1.1-4 htmlwidgets_1.3
[43] httr_1.3.1 FNN_1.1 aqp_1.17
[46] acepack_1.4.1 pkgconfig_2.0.2 reshape_0.8.8
[49] XML_3.98-1.16 nnet_7.3-12 RJSONIO_1.3-1.1
[52] labeling_0.3 later_0.7.5 rlang_0.3.1
[55] munsell_0.5.0 tools_3.5.1 evaluate_0.13
[58] yaml_2.2.0 processx_3.2.1 knitr_1.23
[61] mime_0.5 whisker_0.3-2 xml2_1.2.0
[64] compiler_3.5.1 rstudioapi_0.10 plotly_4.8.0
[67] e1071_1.7-0 spacetime_1.2-2 lhs_0.16
[70] stringi_1.2.4 ps_1.2.1 gdtools_0.1.7
[73] plot3D_1.1.1 Matrix_1.2-14 classInt_0.2-3
[76] pillar_1.3.1 plotKML_0.5-9 data.table_1.11.8
[79] httpuv_1.4.5 colorRamps_2.3 R6_2.2.2
[82] promises_1.0.1 gridExtra_2.3 codetools_0.2-15
[85] MASS_7.3-50 assertthat_0.2.0 rprojroot_1.3-2
[88] withr_2.1.2 hms_0.4.2 parallel_3.5.1
[91] grid_3.5.1 rpart_4.1-13 tidyr_0.8.2
[94] class_7.3-14 rmarkdown_1.12 misc3d_0.8-4
[97] mco_1.0-15.1 git2r_0.23.0 shiny_1.2.0
[100] base64enc_0.1-3