SCZ - Brain Nucleus accumbens basal ganglia
sheng Qian
2021-2-6
Last updated: 2022-03-05
Checks: 5 2
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 4a5db1c. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .ipynb_checkpoints/
Ignored: data/AF/
Untracked files:
Untracked: Rplot.png
Untracked: analysis/.ipynb_checkpoints/
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_analysis_S.sbatch
Untracked: code/run_SCZ_analysis_S.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_SCZ_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: code/wflow_build.R
Untracked: code/wflow_build.sbatch
Untracked: data/.ipynb_checkpoints/
Untracked: data/BMI/
Untracked: data/SCZ/
Untracked: data/SCZ_S/
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Unstaged changes:
Modified: analysis/BMI_Brain_Putamen_basal_ganglia.Rmd
Modified: analysis/SCZ_Brain_Amygdala.Rmd
Modified: analysis/SCZ_Brain_Anterior_cingulate_cortex_BA24.Rmd
Modified: analysis/SCZ_Brain_Caudate_basal_ganglia.Rmd
Modified: analysis/SCZ_Brain_Cerebellar_Hemisphere.Rmd
Modified: analysis/SCZ_Brain_Cerebellum.Rmd
Modified: analysis/SCZ_Brain_Cortex.Rmd
Modified: analysis/SCZ_Brain_Frontal_Cortex_BA9.Rmd
Modified: analysis/SCZ_Brain_Hippocampus.Rmd
Modified: analysis/SCZ_Brain_Hypothalamus.Rmd
Modified: analysis/SCZ_Brain_Nucleus_accumbens_basal_ganglia.Rmd
Modified: analysis/SCZ_Brain_Spinal_cord_cervical_c-1.Rmd
Modified: analysis/SCZ_Brain_Substantia_nigra.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_Brain_Nucleus_accumbens_basal_ganglia.Rmd) and HTML (docs/SCZ_Brain_Nucleus_accumbens_basal_ganglia.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 4a5db1c | sq-96 | 2022-03-03 | update |
| html | 4a5db1c | sq-96 | 2022-03-03 | update |
| html | 75a1466 | sq-96 | 2022-02-27 | Build site. |
| Rmd | 1c69dd2 | sq-96 | 2022-02-27 | update |
| html | ff6403a | sq-96 | 2022-02-27 | Build site. |
| Rmd | 3dd5b4c | sq-96 | 2022-02-27 | update |
Weight QC
#number of imputed weights
nrow(qclist_all)[1] 11518#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1135 816 688 449 575 581 558 432 426 464 694 651 223 386 374 531
17 18 19 20 21 22
679 185 885 358 134 294 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 9119#proportion of imputed weights without missing variants
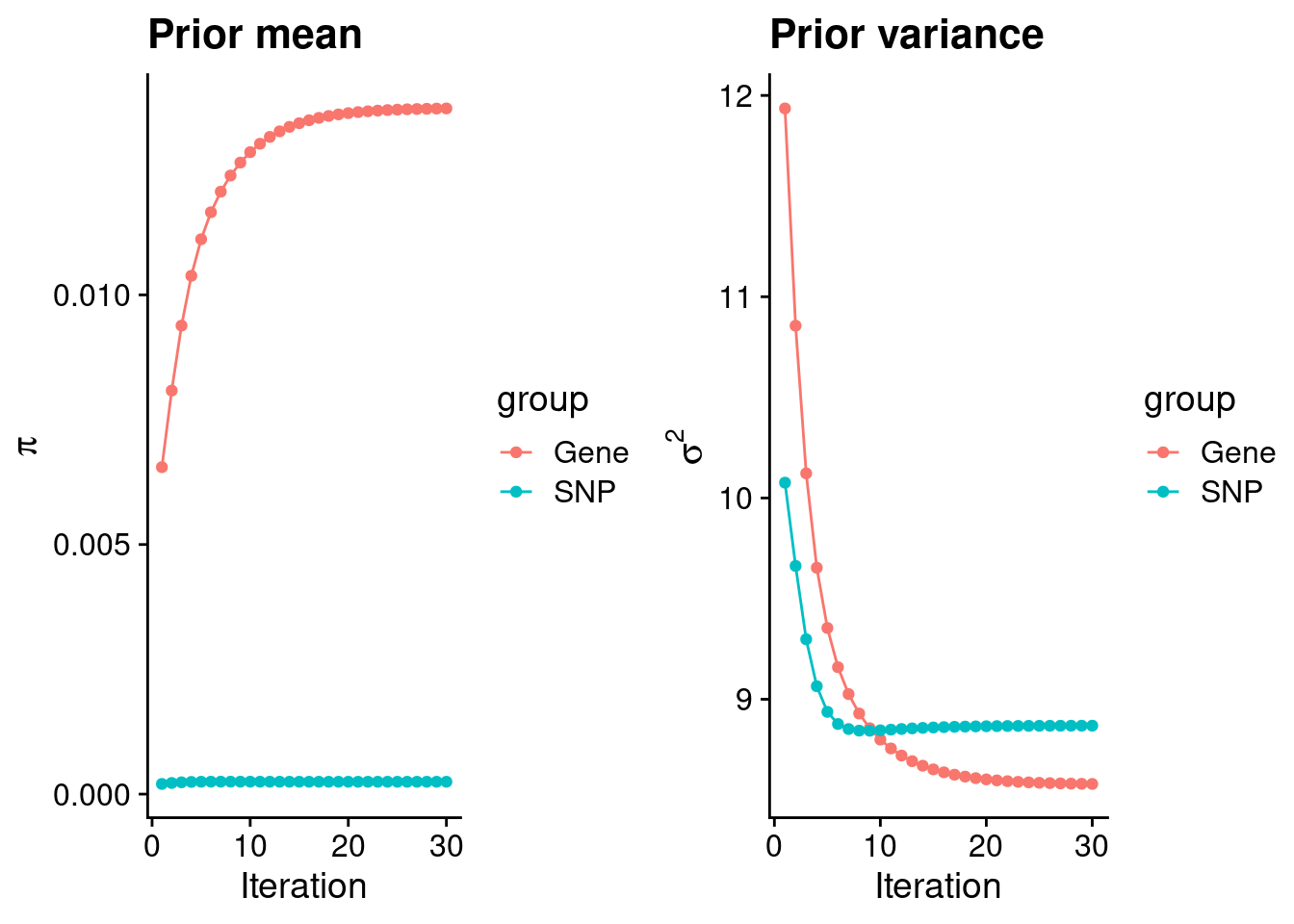
mean(qclist_all$nmiss==0)[1] 0.7917Check convergence of parameters
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0137343 0.0002475 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
8.579 8.869 #report sample size
print(sample_size)[1] 82315#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 11518 7573890#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.01649 0.20198 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.08618 1.45805Genes with highest PIPs
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
genename region_tag susie_pip mu2 PVE z num_eqtl
4131 SPECC1 17_16 0.9939 30.61 0.0003696 5.625 2
11067 ZNF823 19_10 0.9819 28.88 0.0003445 5.483 2
5491 FURIN 15_42 0.9804 44.25 0.0005271 -7.000 1
13453 RP11-230C9.4 6_102 0.9661 23.94 0.0002810 -4.872 2
11808 NPTXR 22_15 0.9254 21.85 0.0002456 4.512 2
13743 CWC25 17_23 0.8673 20.73 0.0002185 -4.015 3
6509 TMEM56 1_58 0.8341 20.15 0.0002041 -3.918 1
3067 SF3B1 2_117 0.8259 42.69 0.0004284 6.725 1
11955 LINC00390 13_17 0.8053 20.17 0.0001973 -4.220 1
6535 TADA1 1_82 0.7967 21.93 0.0002123 -4.168 2
6291 ARFGAP2 11_29 0.7860 23.76 0.0002269 4.740 1
10921 PCBP2 12_33 0.7827 20.28 0.0001928 4.202 1
12231 AC073283.4 2_30 0.7761 20.60 0.0001942 -3.892 2
10150 ACOT1 14_34 0.7648 21.86 0.0002031 4.044 2
2658 VPS29 12_67 0.7276 24.06 0.0002126 -4.937 2
9374 COX8A 11_35 0.7272 24.38 0.0002154 -4.750 1
4719 SOX5 12_17 0.6951 20.31 0.0001715 4.309 1
107 ELAC2 17_11 0.6793 22.42 0.0001850 4.227 1
3206 MAP7D1 1_22 0.6731 23.08 0.0001887 4.907 1
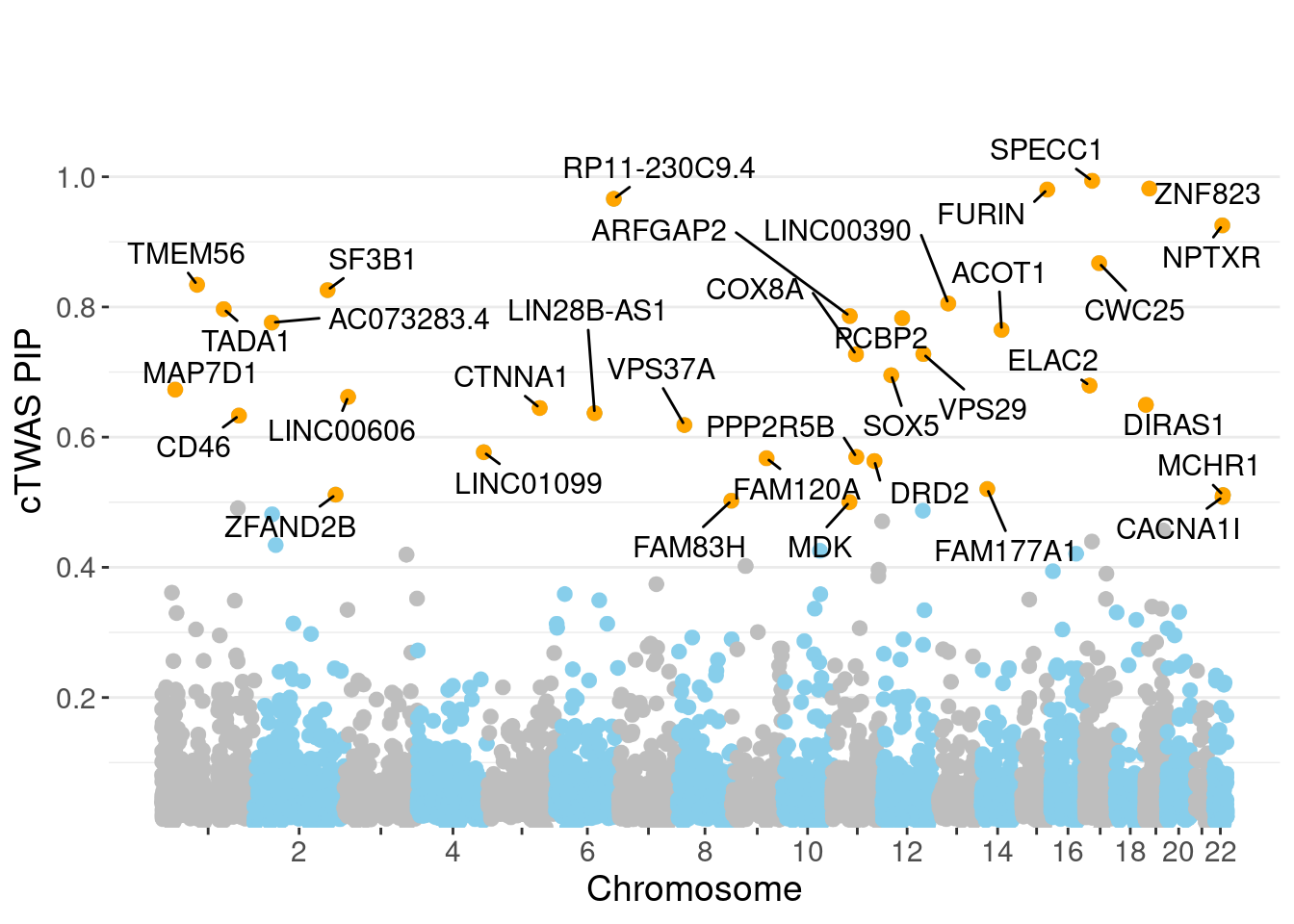
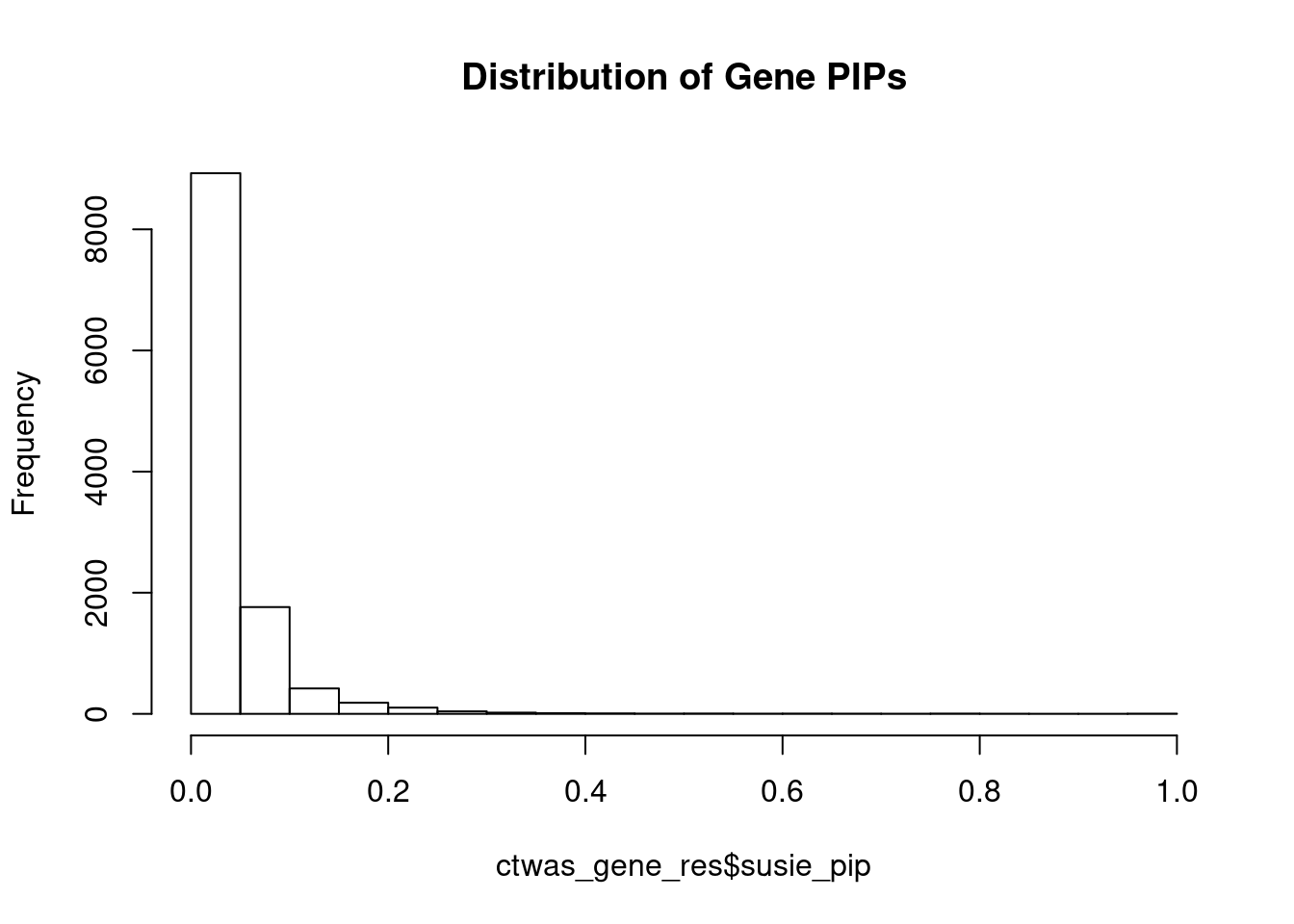
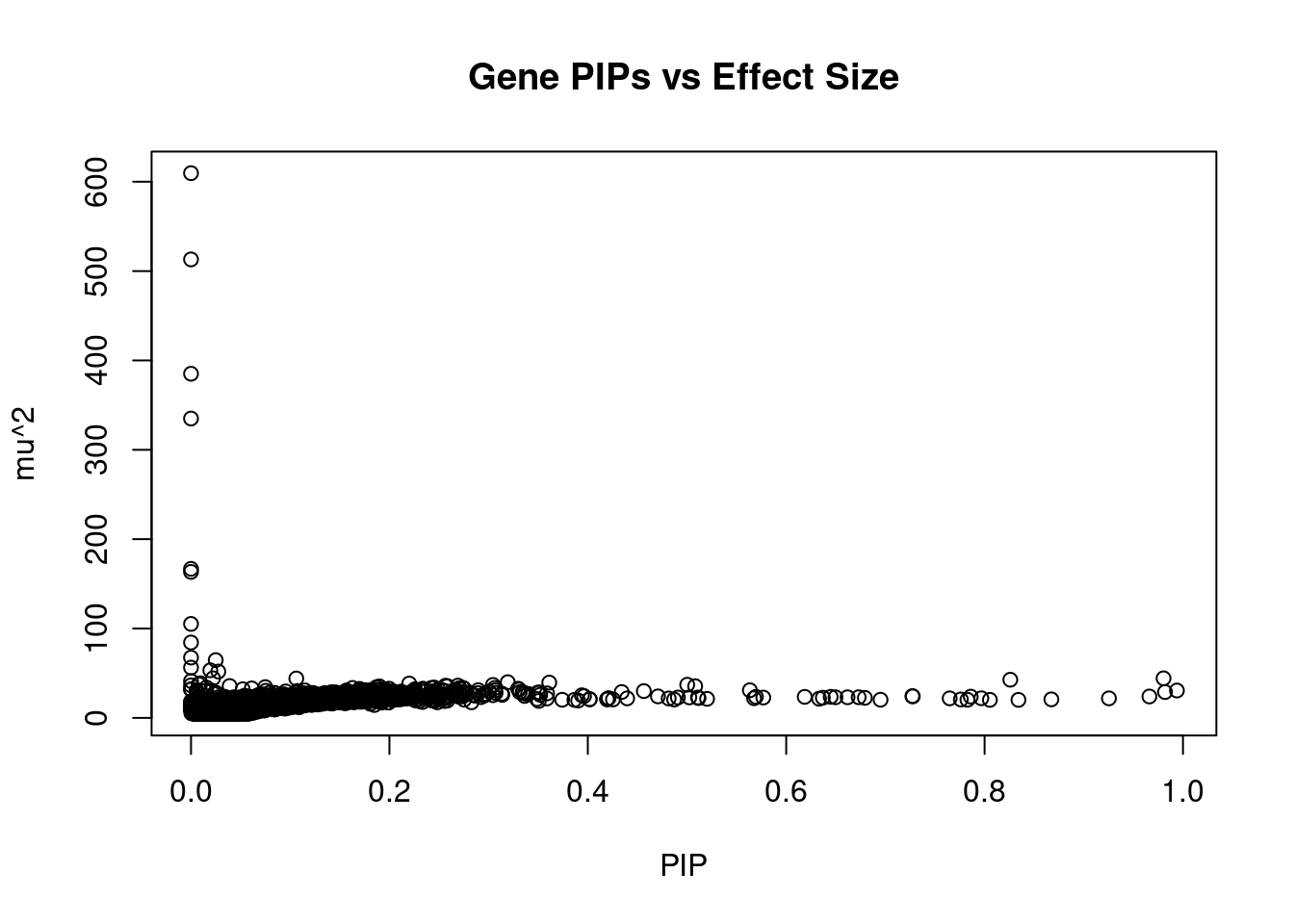
11957 LINC00606 3_8 0.6618 23.04 0.0001853 -3.964 1Genes with largest effect sizes
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
genename region_tag susie_pip mu2 PVE z num_eqtl
9682 HLA-DQB1 6_26 1.221e-13 609.57 9.044e-16 4.3395 1
12152 HLA-DQB2 6_26 1.634e-13 513.04 1.019e-15 -3.5679 1
12325 HLA-DQA2 6_26 1.289e-13 385.09 6.030e-16 0.2164 1
11395 MSH5 6_26 4.995e-13 334.97 2.033e-15 8.1001 2
10748 HLA-DRB1 6_26 9.848e-14 166.78 1.995e-16 -1.8363 1
9862 ACBD4 17_27 0.000e+00 163.38 0.000e+00 1.6939 2
10865 HLA-DQA1 6_26 7.232e-13 105.14 9.237e-16 -0.7786 1
11647 CLIC1 6_26 4.017e-13 84.36 4.117e-16 -0.4634 1
10450 HEXIM1 17_27 0.000e+00 67.49 0.000e+00 -2.8451 1
10415 BTN3A2 6_20 2.490e-02 64.62 1.955e-05 9.0374 3
2422 GOSR2 17_27 0.000e+00 56.26 0.000e+00 -3.4300 2
10915 ZSCAN26 6_22 1.946e-02 53.24 1.259e-05 8.6508 3
9788 HIST1H2BC 6_20 2.752e-02 51.86 1.734e-05 -8.0277 1
2790 TRIM38 6_20 2.223e-02 44.29 1.196e-05 -7.4660 2
5491 FURIN 15_42 9.804e-01 44.25 5.271e-04 -7.0004 1
10558 ZSCAN23 6_22 1.061e-01 44.07 5.682e-05 -6.7082 2
3067 SF3B1 2_117 8.259e-01 42.69 4.284e-04 6.7253 1
4135 CDHR3 7_65 0.000e+00 41.22 0.000e+00 2.9707 2
13283 LINC01415 18_30 3.193e-01 39.97 1.550e-04 -5.3243 1
10418 TMEM222 1_19 3.612e-01 39.49 1.733e-04 3.9022 1Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_eqtl
5491 FURIN 15_42 0.9804 44.25 0.0005271 -7.000 1
3067 SF3B1 2_117 0.8259 42.69 0.0004284 6.725 1
4131 SPECC1 17_16 0.9939 30.61 0.0003696 5.625 2
11067 ZNF823 19_10 0.9819 28.88 0.0003445 5.483 2
13453 RP11-230C9.4 6_102 0.9661 23.94 0.0002810 -4.872 2
11808 NPTXR 22_15 0.9254 21.85 0.0002456 4.512 2
6291 ARFGAP2 11_29 0.7860 23.76 0.0002269 4.740 1
2602 MDK 11_28 0.5002 37.08 0.0002253 -6.357 1
1571 CACNA1I 22_16 0.5082 35.45 0.0002189 5.841 1
13743 CWC25 17_23 0.8673 20.73 0.0002185 -4.015 3
9374 COX8A 11_35 0.7272 24.38 0.0002154 -4.750 1
2658 VPS29 12_67 0.7276 24.06 0.0002126 -4.937 2
6535 TADA1 1_82 0.7967 21.93 0.0002123 -4.168 2
6304 DRD2 11_67 0.5634 30.91 0.0002115 -5.938 2
6509 TMEM56 1_58 0.8341 20.15 0.0002041 -3.918 1
10150 ACOT1 14_34 0.7648 21.86 0.0002031 4.044 2
11955 LINC00390 13_17 0.8053 20.17 0.0001973 -4.220 1
12231 AC073283.4 2_30 0.7761 20.60 0.0001942 -3.892 2
10921 PCBP2 12_33 0.7827 20.28 0.0001928 4.202 1
3206 MAP7D1 1_22 0.6731 23.08 0.0001887 4.907 1Genes with largest z scores
genename region_tag susie_pip mu2 PVE z num_eqtl
10415 BTN3A2 6_20 2.490e-02 64.62 1.955e-05 9.037 3
10915 ZSCAN26 6_22 1.946e-02 53.24 1.259e-05 8.651 3
11395 MSH5 6_26 4.995e-13 334.97 2.033e-15 8.100 2
9788 HIST1H2BC 6_20 2.752e-02 51.86 1.734e-05 -8.028 1
6275 CNNM2 10_66 6.121e-02 32.97 2.452e-05 -7.547 2
2790 TRIM38 6_20 2.223e-02 44.29 1.196e-05 -7.466 2
5491 FURIN 15_42 9.804e-01 44.25 5.271e-04 -7.000 1
3067 SF3B1 2_117 8.259e-01 42.69 4.284e-04 6.725 1
7442 TYW5 2_118 3.896e-02 35.64 1.686e-05 -6.718 2
10558 ZSCAN23 6_22 1.061e-01 44.07 5.682e-05 -6.708 2
9922 ARL6IP4 12_75 9.368e-03 38.77 4.413e-06 6.491 1
6404 ABCB9 12_75 7.758e-03 37.56 3.540e-06 6.404 1
2602 MDK 11_28 5.002e-01 37.08 2.253e-04 -6.357 1
9771 HARBI1 11_28 1.872e-01 34.44 7.832e-05 6.169 1
11548 DNAJC19 3_111 2.691e-01 36.11 1.180e-04 6.158 1
8554 INO80E 16_24 3.044e-01 36.89 1.364e-04 6.121 2
6304 DRD2 11_67 5.634e-01 30.91 2.115e-04 -5.938 2
7634 GNL3 3_36 1.699e-01 32.47 6.702e-05 5.899 2
1571 CACNA1I 22_16 5.082e-01 35.45 2.189e-04 5.841 1
11136 ZKSCAN8 6_22 1.558e-02 33.88 6.415e-06 5.837 1Comparing z scores and PIPs
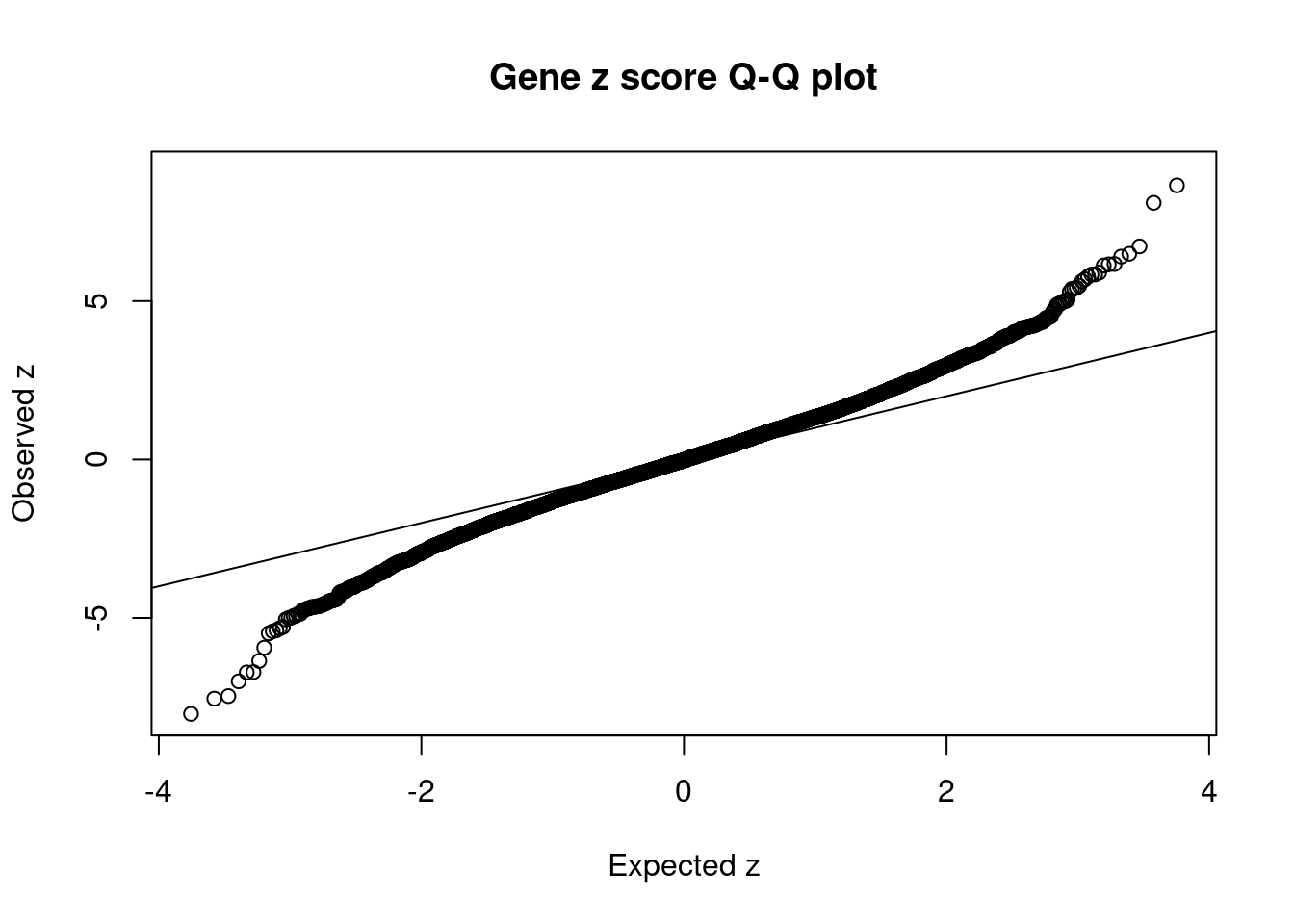
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
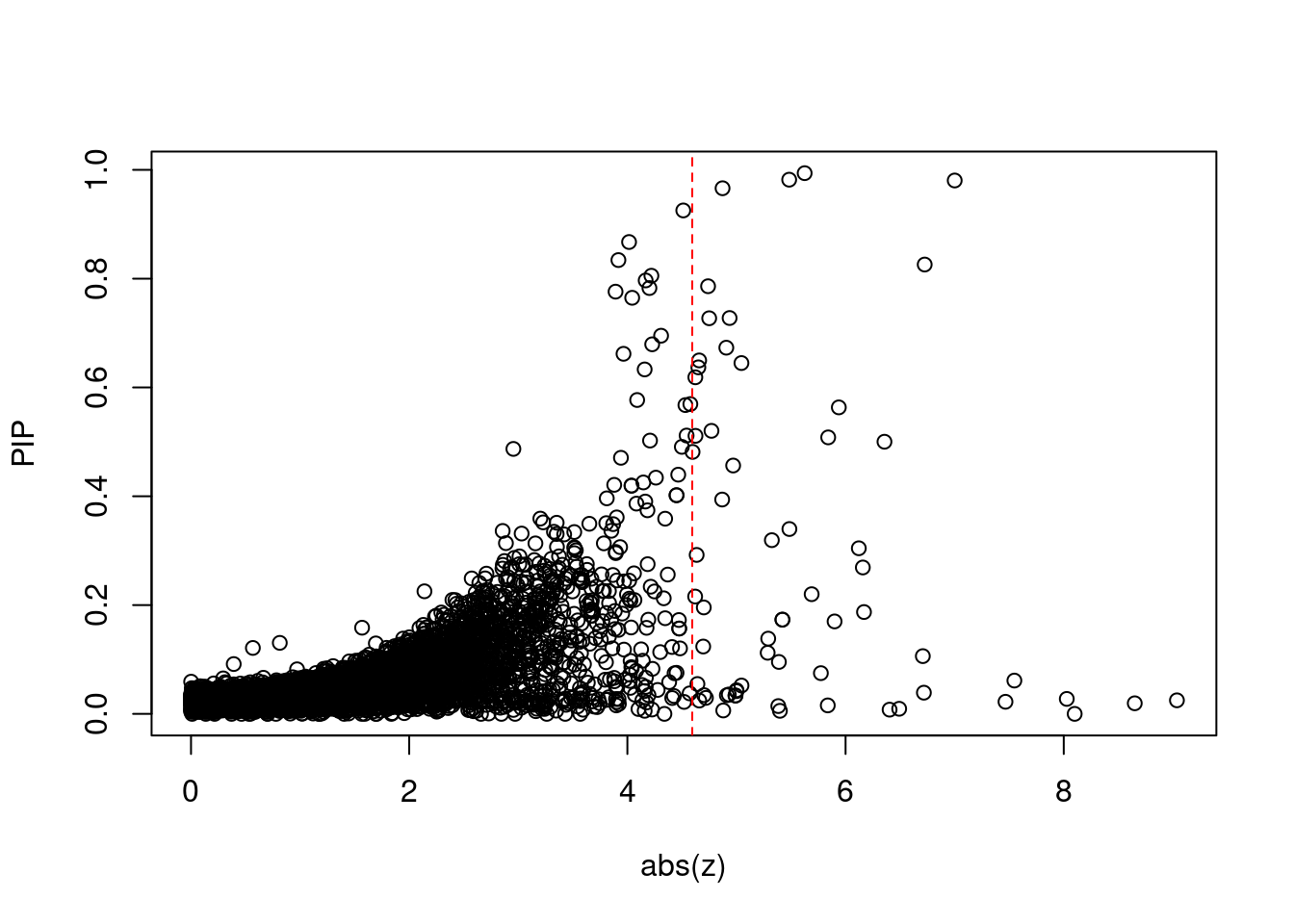
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
[1] 0.00547GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 35Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"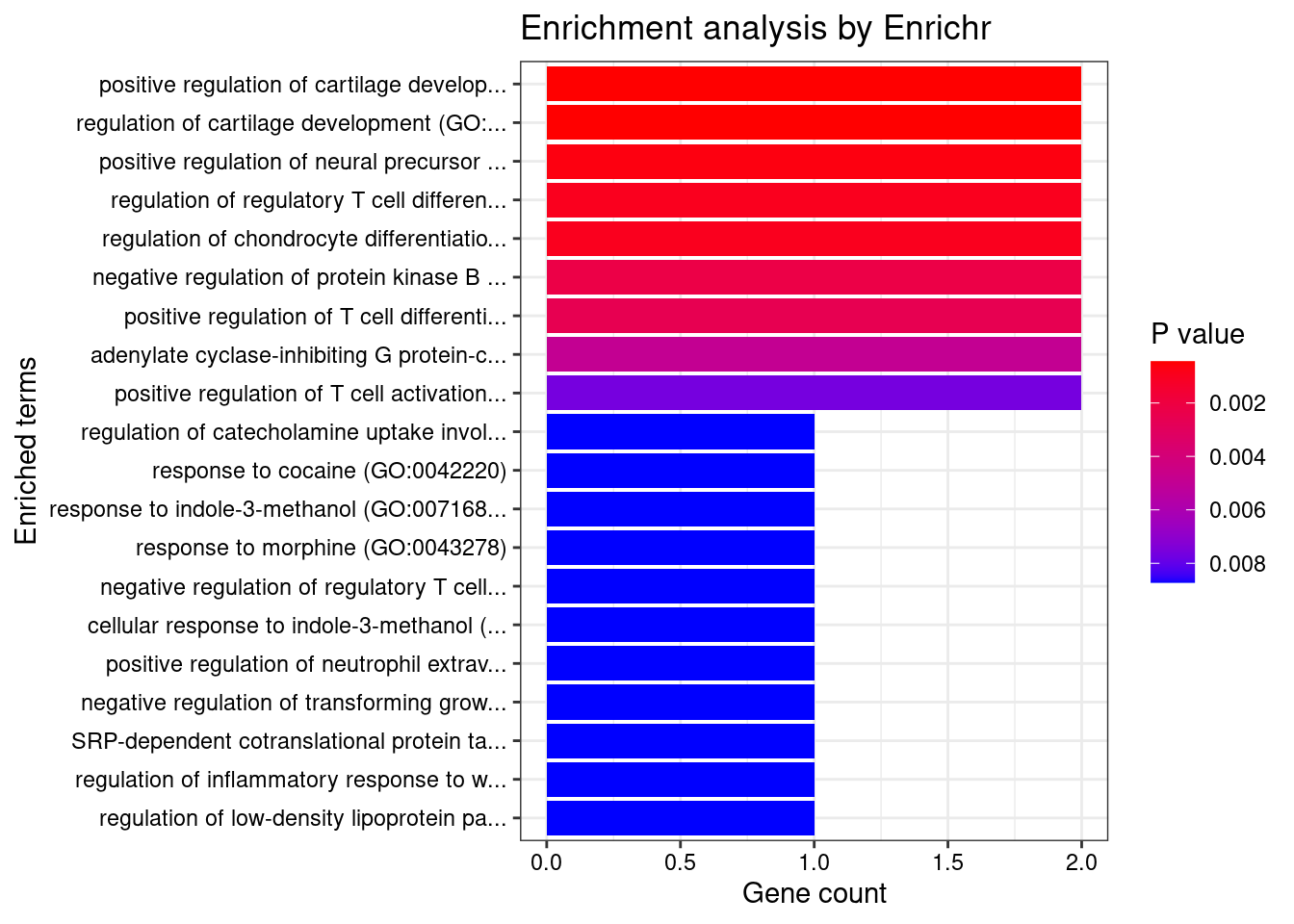
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Cellular_Component_2021"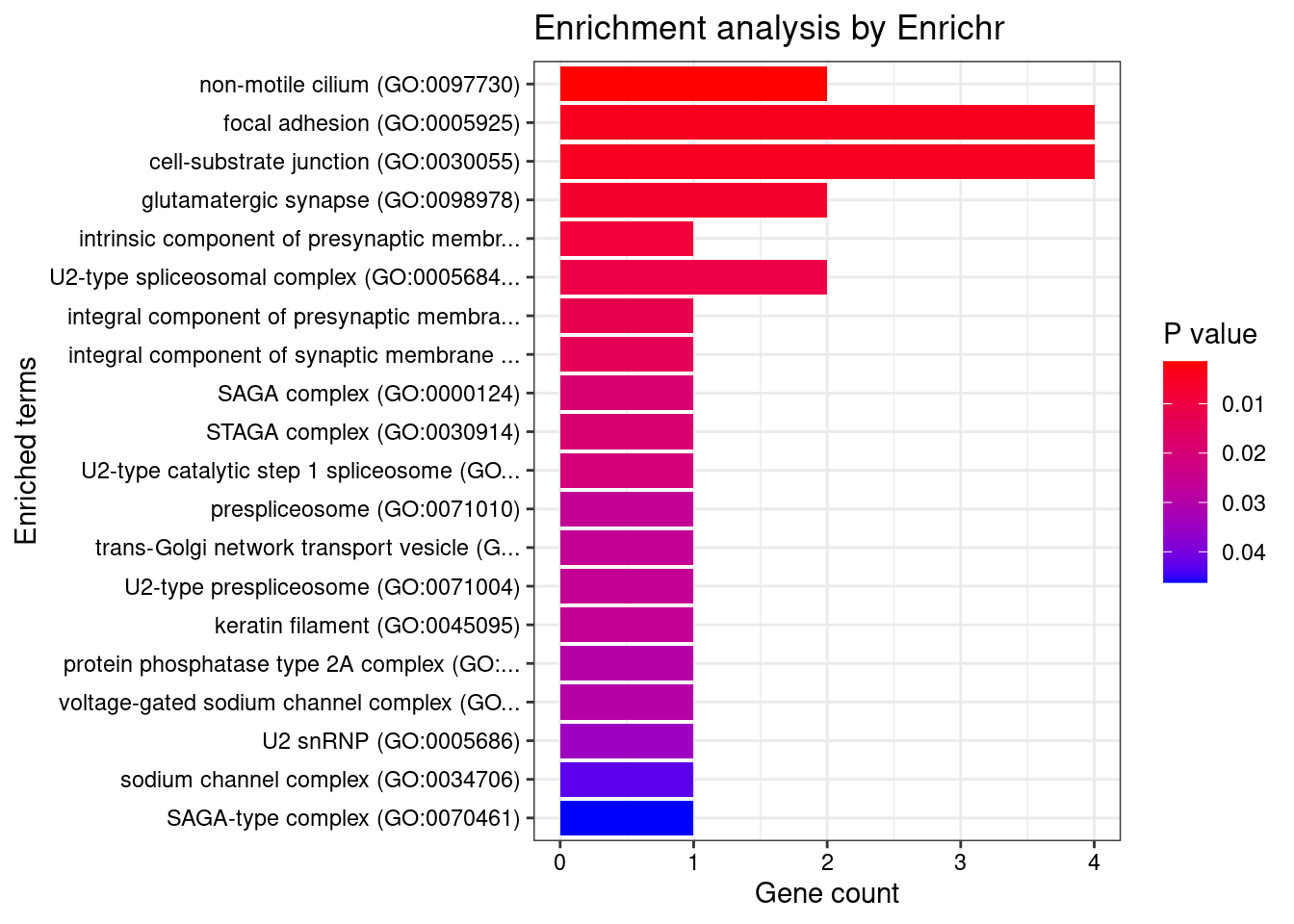
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"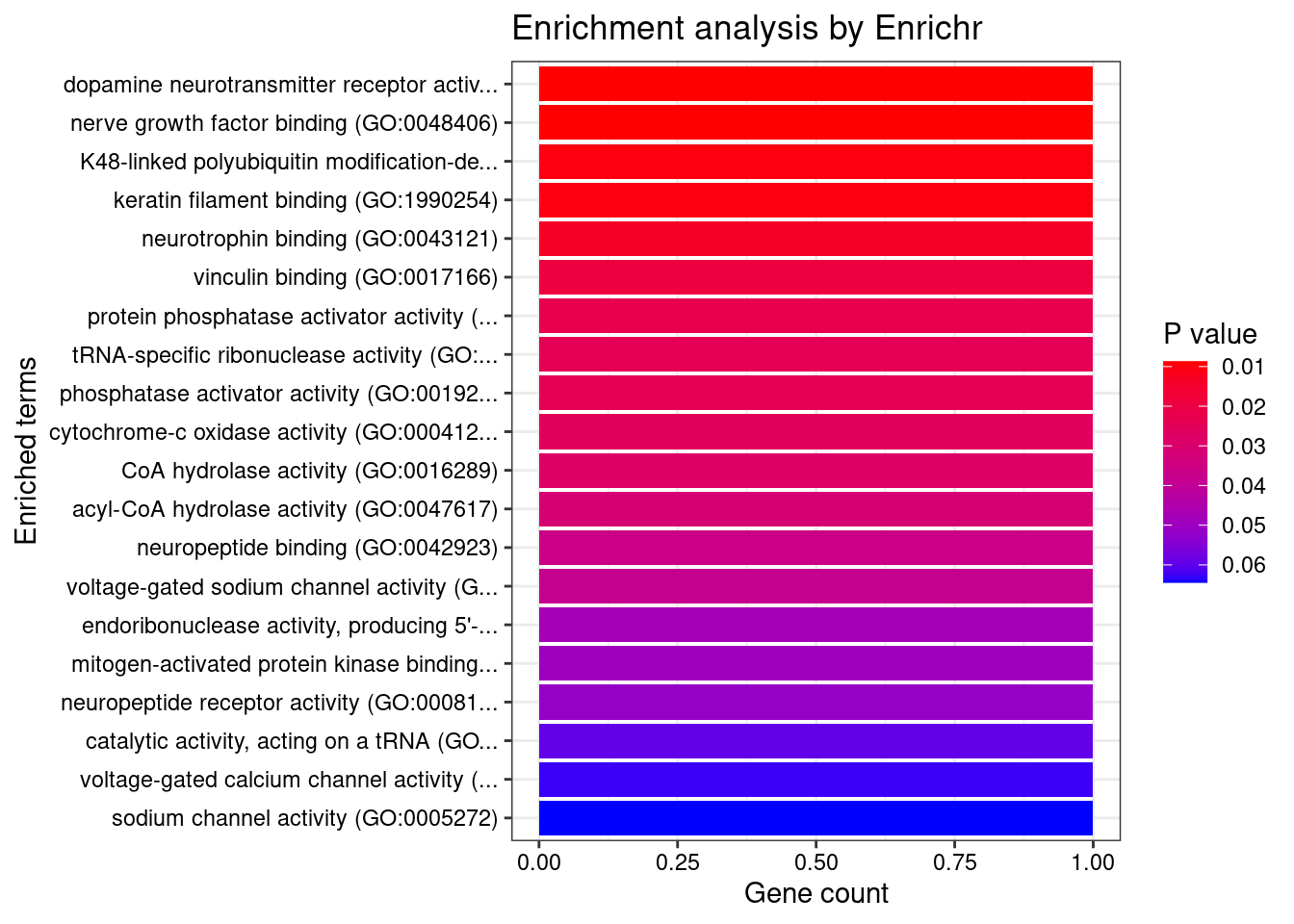
| Version | Author | Date |
|---|---|---|
| ff6403a | sq-96 | 2022-02-27 |
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio BgRatio
5 Anxiety Disorders 0.02052 2/14 44/9703
50 Measles 0.02052 1/14 1/9703
51 Memory Disorders 0.02052 2/14 43/9703
92 Memory impairment 0.02052 2/14 44/9703
120 Anxiety States, Neurotic 0.02052 2/14 44/9703
148 Age-Related Memory Disorders 0.02052 2/14 43/9703
149 Memory Disorder, Semantic 0.02052 2/14 43/9703
150 Memory Disorder, Spatial 0.02052 2/14 43/9703
151 Memory Loss 0.02052 2/14 43/9703
169 Anxiety neurosis (finding) 0.02052 2/14 44/9703WebGestalt enrichment analysis for genes with PIP>0.5
Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLSensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 130#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 59#significance threshold for TWAS
print(sig_thresh)[1] 4.594#number of ctwas genes
length(ctwas_genes)[1] 9#number of TWAS genes
length(twas_genes)[1] 63#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_eqtl
6509 TMEM56 1_58 0.8341 20.15 0.0002041 -3.918 1
11955 LINC00390 13_17 0.8053 20.17 0.0001973 -4.220 1
13743 CWC25 17_23 0.8673 20.73 0.0002185 -4.015 3
11808 NPTXR 22_15 0.9254 21.85 0.0002456 4.512 2#sensitivity / recall
print(sensitivity) ctwas TWAS
0.02308 0.07692 #specificity
print(specificity) ctwas TWAS
0.9995 0.9954 #precision / PPV
print(precision) ctwas TWAS
0.3333 0.1587 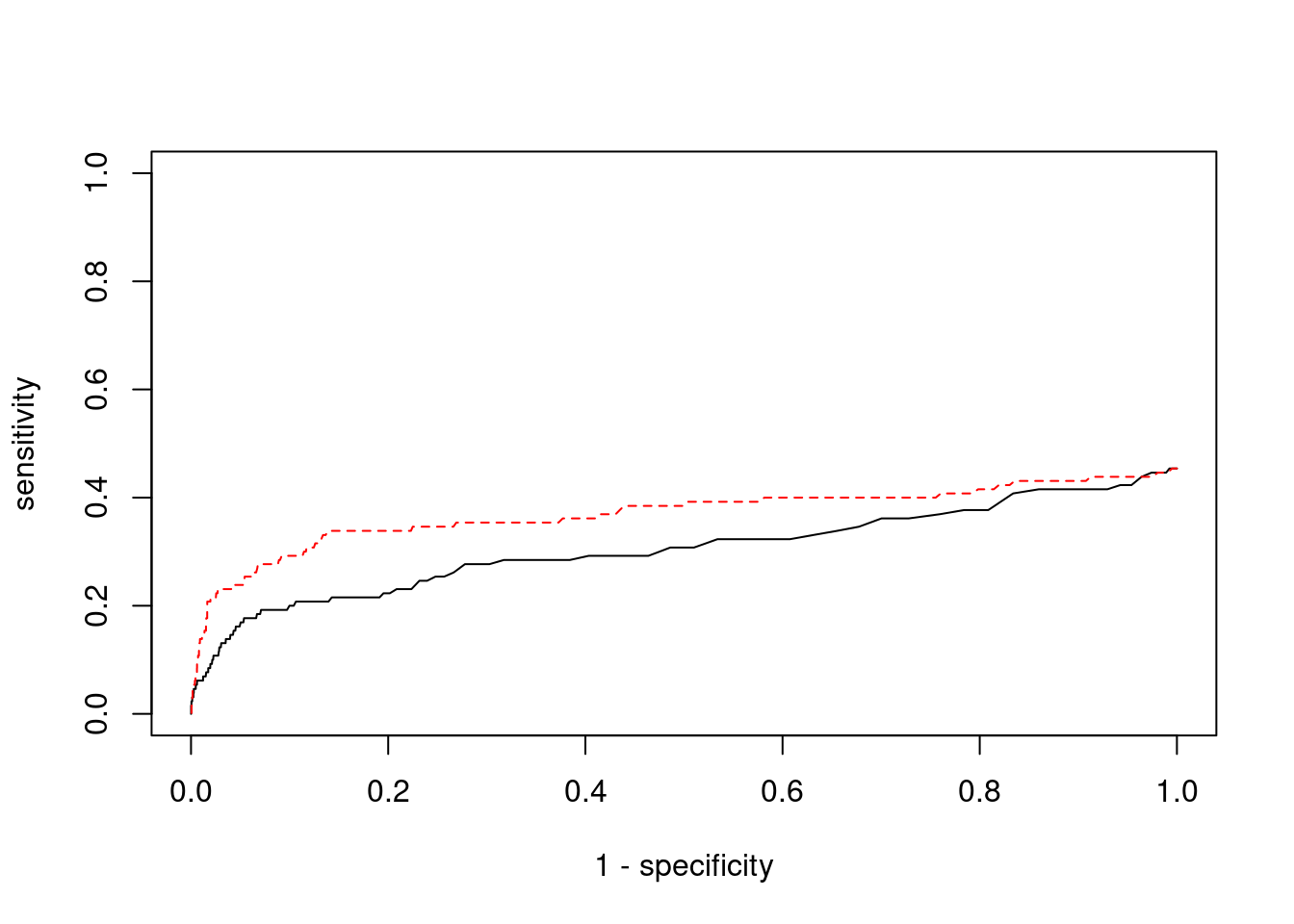
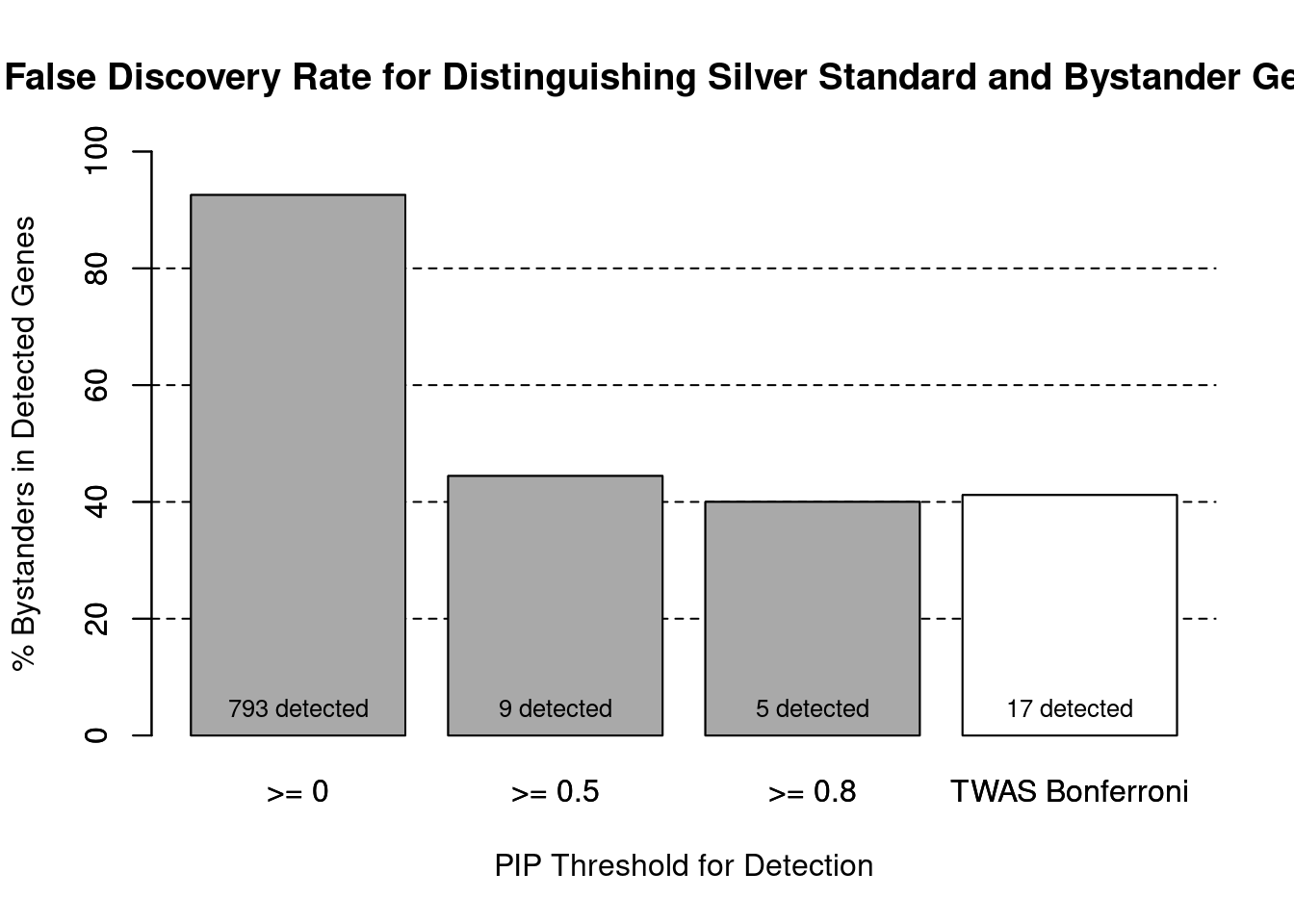
cTWAS is more precise than TWAS in distinguishing silver standard and bystander genes
#number of genes in known annotations (with imputed expression)
print(length(known_annotations))[1] 59#number of bystander genes (with imputed expression)
print(length(unrelated_genes))[1] 734#subset results to genes in known annotations or bystanders
ctwas_gene_res_subset <- ctwas_gene_res[ctwas_gene_res$genename %in% c(known_annotations, unrelated_genes),]
#assign ctwas and TWAS genes
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>0.8]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>sig_thresh]
#significance threshold for TWAS
print(sig_thresh)[1] 4.594#number of ctwas genes (in known annotations or bystanders)
length(ctwas_genes)[1] 5#number of TWAS genes (in known annotations or bystanders)
length(twas_genes)[1] 17#sensitivity / recall
sensitivity ctwas TWAS
0.05085 0.16949 #specificity / (1 - False Positive Rate)
specificity ctwas TWAS
0.9973 0.9905 #precision / PPV / (1 - False Discovery Rate)
precision ctwas TWAS
0.6000 0.5882 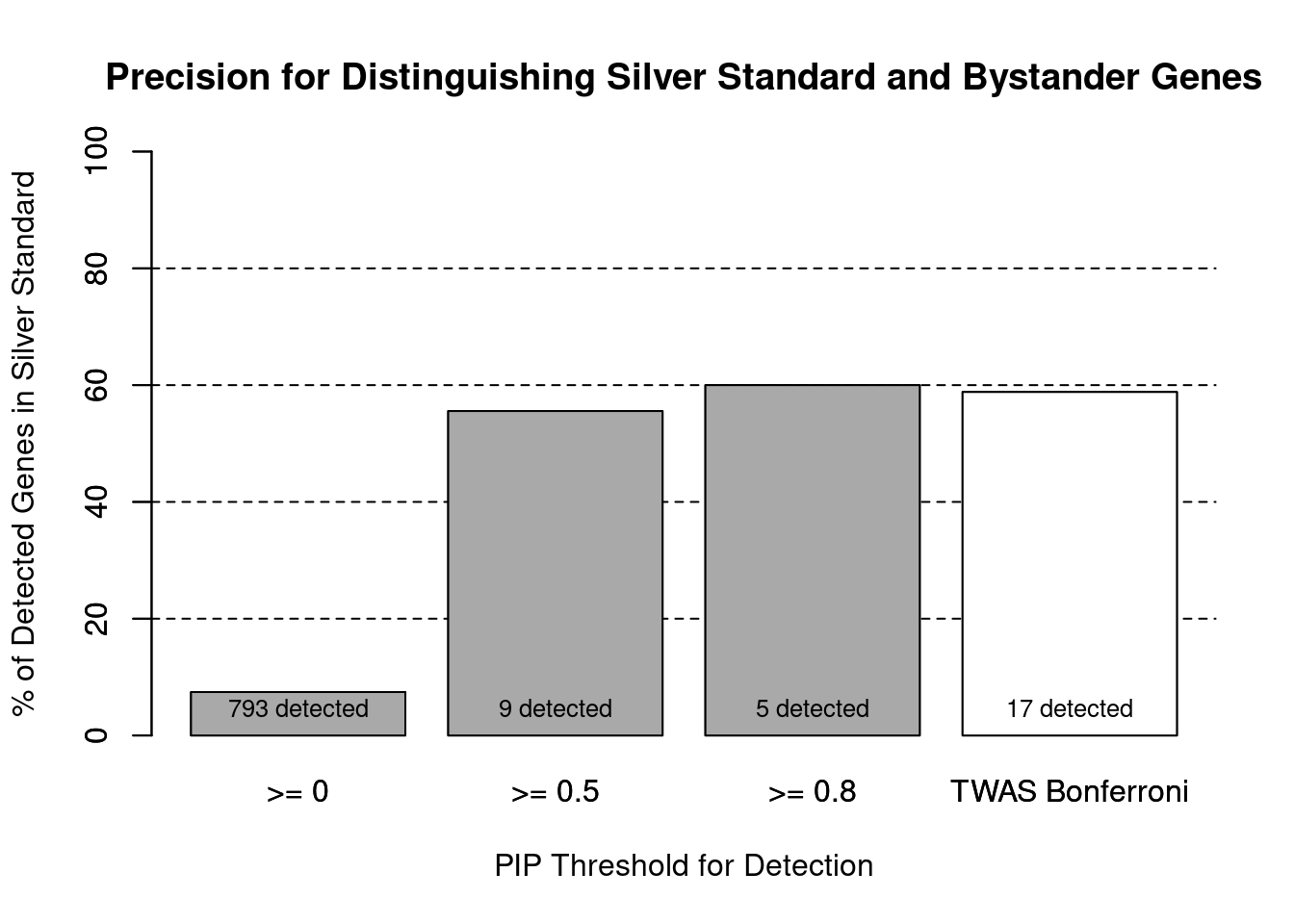
| Version | Author | Date |
|---|---|---|
| 4a5db1c | sq-96 | 2022-03-03 |
| Version | Author | Date |
|---|---|---|
| 4a5db1c | sq-96 | 2022-03-03 |
pip_range <- (0:1000)/1000
sensitivity <- rep(NA, length(pip_range))
specificity <- rep(NA, length(pip_range))
for (index in 1:length(pip_range)){
pip <- pip_range[index]
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>=pip]
sensitivity[index] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
}
plot(1-specificity, sensitivity, type="l", xlim=c(0,1), ylim=c(0,1), main="", xlab="1 - Specificity", ylab="Sensitivity")
title(expression("ROC Curve for cTWAS (black) and TWAS (" * phantom("red") * ")"))
title(expression(phantom("ROC Curve for cTWAS (black) and TWAS (") * "red" * phantom(")")), col.main="red")
sig_thresh_range <- seq(from=0, to=max(abs(ctwas_gene_res_subset$z)), length.out=length(pip_range))
for (index in 1:length(sig_thresh_range)){
sig_thresh_plot <- sig_thresh_range[index]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>=sig_thresh_plot]
sensitivity[index] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
}
lines(1-specificity, sensitivity, xlim=c(0,1), ylim=c(0,1), col="red", lty=1)
abline(a=0,b=1,lty=3)
#add previously computed points from the analysis
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>0.8]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>sig_thresh]
points(1-specificity_plot["ctwas"], sensitivity_plot["ctwas"], pch=21, bg="black")
points(1-specificity_plot["TWAS"], sensitivity_plot["TWAS"], pch=21, bg="red")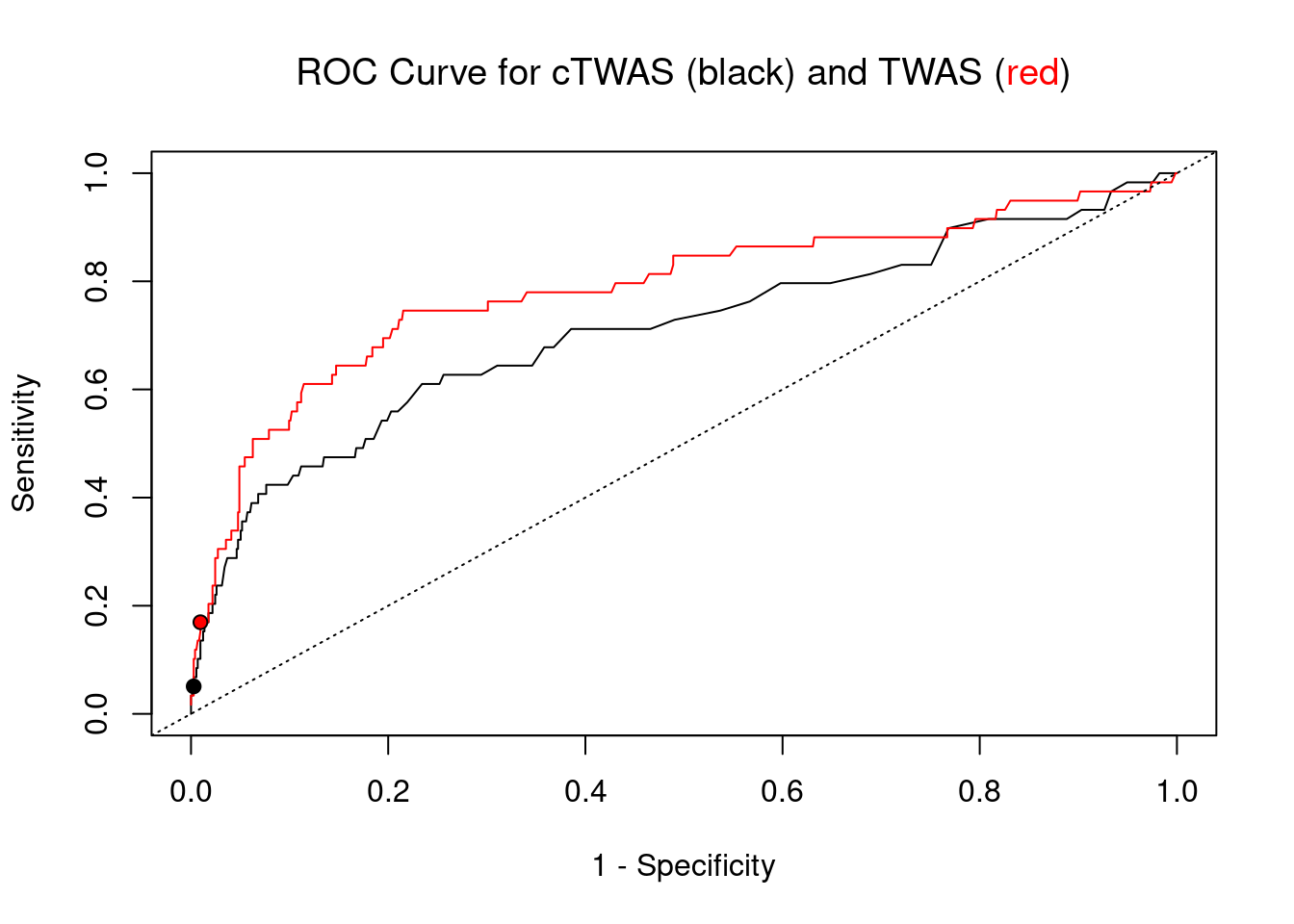
| Version | Author | Date |
|---|---|---|
| 4a5db1c | sq-96 | 2022-03-03 |
Undetected silver standard genes have low TWAS z-scores or stronger signal from nearby variants
#table of outcomes for silver standard genes
-sort(-table(silver_standard_case))silver_standard_case
Not Imputed Insignificant z-score Nearby SNP(s)
71 49 7
Detected (PIP > 0.8)
3 #show inconclusive genes
silver_standard_case[silver_standard_case=="Inconclusive"]named character(0)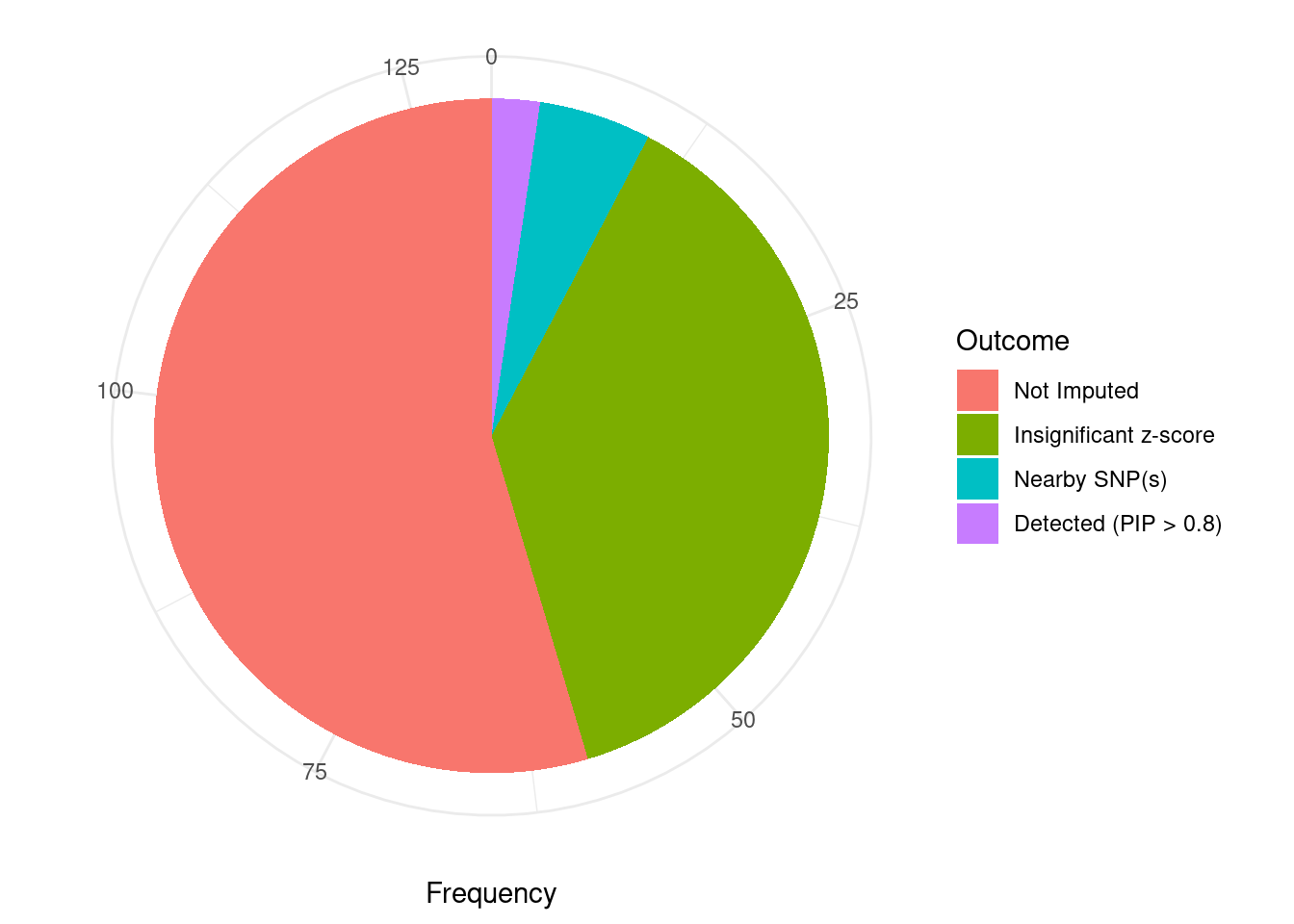
| Version | Author | Date |
|---|---|---|
| 4a5db1c | sq-96 | 2022-03-03 |
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.3.1 forcats_0.5.1 stringr_1.4.0 dplyr_1.0.7
[5] purrr_0.3.4 readr_2.1.1 tidyr_1.1.4 tidyverse_1.3.1
[9] tibble_3.1.6 WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0
[13] cowplot_1.0.0 ggplot2_3.3.5 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] fs_1.5.2 lubridate_1.8.0 bit64_4.0.5 doParallel_1.0.17
[5] httr_1.4.2 rprojroot_2.0.2 tools_3.6.1 backports_1.4.1
[9] doRNG_1.8.2 utf8_1.2.2 R6_2.5.1 vipor_0.4.5
[13] DBI_1.1.2 colorspace_2.0-2 withr_2.4.3 ggrastr_1.0.1
[17] tidyselect_1.1.1 processx_3.5.2 bit_4.0.4 curl_4.3.2
[21] compiler_3.6.1 git2r_0.26.1 rvest_1.0.2 cli_3.1.0
[25] Cairo_1.5-12.2 xml2_1.3.3 labeling_0.4.2 scales_1.1.1
[29] callr_3.7.0 apcluster_1.4.8 digest_0.6.29 rmarkdown_2.11
[33] svglite_1.2.2 pkgconfig_2.0.3 htmltools_0.5.2 dbplyr_2.1.1
[37] fastmap_1.1.0 highr_0.9 rlang_1.0.1 rstudioapi_0.13
[41] RSQLite_2.2.8 jquerylib_0.1.4 farver_2.1.0 generics_0.1.1
[45] jsonlite_1.7.2 vroom_1.5.7 magrittr_2.0.2 Matrix_1.2-18
[49] ggbeeswarm_0.6.0 Rcpp_1.0.8 munsell_0.5.0 fansi_1.0.2
[53] gdtools_0.1.9 lifecycle_1.0.1 stringi_1.7.6 whisker_0.3-2
[57] yaml_2.2.1 plyr_1.8.6 grid_3.6.1 blob_1.2.2
[61] ggrepel_0.9.1 parallel_3.6.1 promises_1.0.1 crayon_1.5.0
[65] lattice_0.20-38 haven_2.4.3 hms_1.1.1 knitr_1.36
[69] ps_1.6.0 pillar_1.6.4 igraph_1.2.10 rjson_0.2.20
[73] rngtools_1.5.2 reshape2_1.4.4 codetools_0.2-16 reprex_2.0.1
[77] glue_1.6.2 evaluate_0.14 getPass_0.2-2 modelr_0.1.8
[81] data.table_1.14.2 vctrs_0.3.8 tzdb_0.2.0 httpuv_1.5.1
[85] foreach_1.5.2 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[89] cachem_1.0.6 xfun_0.29 broom_0.7.10 later_0.8.0
[93] iterators_1.0.14 beeswarm_0.2.3 memoise_2.0.1 ellipsis_0.3.2