SCZ 2018 - Brain_Cerebellum
sheng Qian
2021-2-6
Last updated: 2022-05-19
Checks: 5 2
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 7d08c9b. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .ipynb_checkpoints/
Untracked files:
Untracked: G_list.RData
Untracked: Rplot.png
Untracked: SCZ_annotation.xlsx
Untracked: analysis/.ipynb_checkpoints/
Untracked: analysis/ttt.Rmd
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_2014_EUR_out/
Untracked: code/SCZ_2018_S_out/
Untracked: code/SCZ_2018_out/
Untracked: code/SCZ_2020_Single_out/
Untracked: code/SCZ_2020_out/
Untracked: code/SCZ_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/process_scz_2018_snps.R
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2014_EUR_analysis.sbatch
Untracked: code/run_SCZ_2014_EUR_analysis.sh
Untracked: code/run_SCZ_2014_EUR_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_analysis.sbatch
Untracked: code/run_SCZ_2018_analysis.sh
Untracked: code/run_SCZ_2018_analysis_S.sbatch
Untracked: code/run_SCZ_2018_analysis_S.sh
Untracked: code/run_SCZ_2018_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2020_Single_analysis.sbatch
Untracked: code/run_SCZ_2020_Single_analysis.sh
Untracked: code/run_SCZ_2020_Single_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2020_analysis.sbatch
Untracked: code/run_SCZ_2020_analysis.sh
Untracked: code/run_SCZ_2020_ctwas_rss_LDR.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_analysis_S.sbatch
Untracked: code/run_SCZ_analysis_S.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_SCZ_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: code/wflow_build.R
Untracked: code/wflow_build.sbatch
Untracked: data/.ipynb_checkpoints/
Untracked: data/GO_Terms/
Untracked: data/PGC3_SCZ_wave3_public.v2.tsv
Untracked: data/SCZ/
Untracked: data/SCZ_2014_EUR/
Untracked: data/SCZ_2018/
Untracked: data/SCZ_2018_S/
Untracked: data/SCZ_2020/
Untracked: data/SCZ_S/
Untracked: data/Supplementary Table 15 - MAGMA.xlsx
Untracked: data/Supplementary Table 20 - Prioritised Genes.xlsx
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/scz_2018.RDS
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Untracked: top_genes_32.txt
Untracked: top_genes_37.txt
Untracked: top_genes_43.txt
Untracked: top_genes_81.txt
Untracked: z_snp_pos_SCZ.RData
Untracked: z_snp_pos_SCZ_2014_EUR.RData
Untracked: z_snp_pos_SCZ_2018.RData
Untracked: z_snp_pos_SCZ_2020.RData
Unstaged changes:
Deleted: analysis/BMI_S_results.Rmd
Modified: analysis/SCZ_2018_Brain_Amygdala_S.Rmd
Modified: analysis/SCZ_2018_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/SCZ_2018_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellum_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cortex_S.Rmd
Modified: analysis/SCZ_2018_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hippocampus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hypothalamus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/SCZ_2018_Brain_Substantia_nigra_S.Rmd
Modified: analysis/SCZ_Annotation_Analysis.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_2018_Brain_Cerebellum_S.Rmd) and HTML (docs/SCZ_2018_Brain_Cerebellum_S.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 7d08c9b | sq-96 | 2022-05-18 | update |
| html | 7d08c9b | sq-96 | 2022-05-18 | update |
| Rmd | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 011327d | sq-96 | 2022-05-12 | update |
| Rmd | 6c6abbd | sq-96 | 2022-05-12 | update |
library(reticulate)
use_python("/scratch/midway2/shengqian/miniconda3/envs/PythonForR/bin/python",required=T)Weight QC
#number of imputed weights
nrow(qclist_all)[1] 27353#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
2535 1830 1661 982 1135 1371 1536 916 1175 1171 1678 1471 543 971 987 1200
17 18 19 20 21 22
1981 337 2002 917 48 906 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 23734#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8677INFO:numexpr.utils:Note: NumExpr detected 56 cores but "NUMEXPR_MAX_THREADS" not set, so enforcing safe limit of 8.finish
Attaching package: 'dplyr'The following objects are masked from 'package:stats':
filter, lagThe following objects are masked from 'package:base':
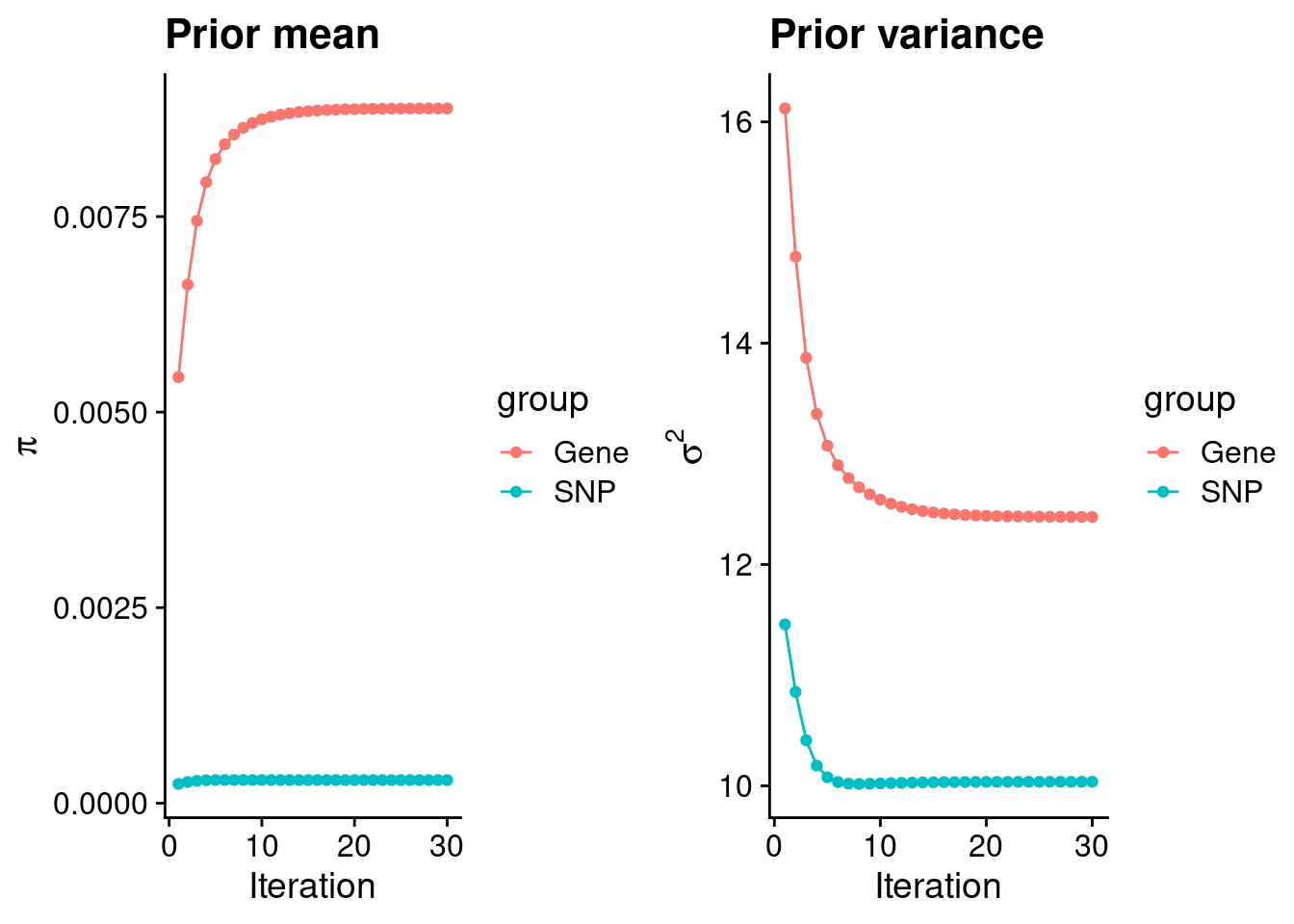
intersect, setdiff, setequal, unionCheck convergence of parameters
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
gene snp
0.0088825 0.0002955 gene snp
12.43 10.04 [1] 105318[1] 8002 6309950 gene snp
0.008389 0.177721 [1] 0.02987 1.06067Genes with highest PIPs
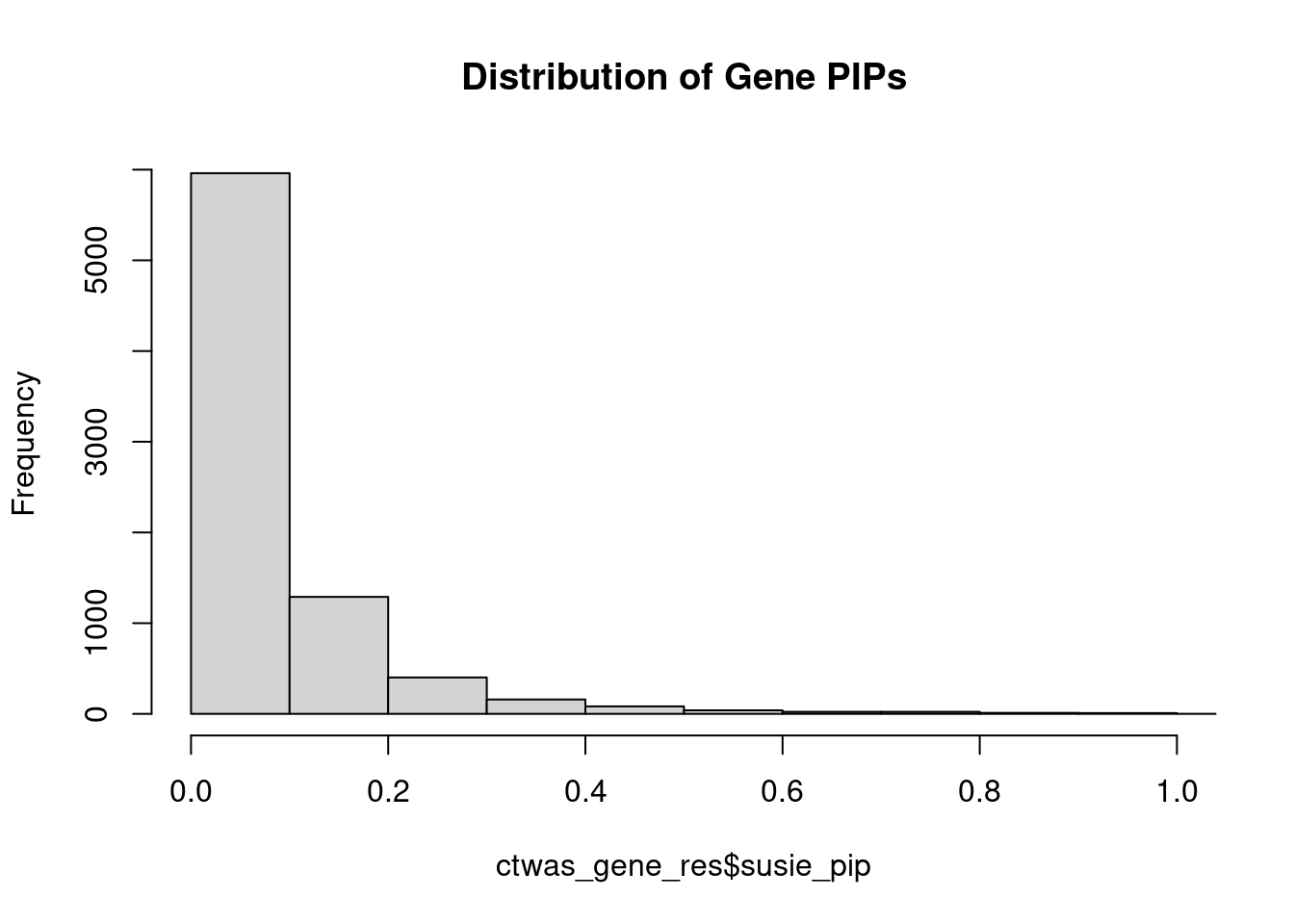
genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
3723 LRP8 1_33 1.2179 33.07 0.0003523 -4.820 10 11
5397 R3HDM2 12_36 1.1443 44.06 0.0004924 6.634 10 12
3563 LAMA5 20_36 1.1434 23.61 0.0002517 4.603 24 38
7577 WDR27 6_111 1.0487 17.72 0.0001014 -2.341 29 37
2782 GIGYF1 7_62 0.9748 26.79 0.0002375 -5.266 5 5
854 BUB1B-PAK6 15_14 0.9723 30.33 0.0002604 -5.588 4 5
299 AKT3 1_128 0.9654 35.61 0.0003005 6.350 6 7
4166 MRPS33 7_87 0.9654 20.31 0.0001744 -4.304 6 6
7712 ZDHHC20 13_2 0.9572 24.94 0.0002118 -4.784 3 4
4518 NPIPA1 16_15 0.9556 24.97 0.0002096 4.689 3 3
3632 LINC00320 21_6 0.9542 29.24 0.0002419 -5.336 3 3
6119 SF3B1 2_117 0.9478 45.88 0.0003746 7.053 5 5
1668 CRTAP 3_24 0.9010 19.87 0.0001503 3.929 3 3
1179 CCDC57 17_47 0.8904 20.00 0.0001041 3.022 36 46
7231 TSNARE1 8_93 0.8894 34.70 0.0002087 6.287 10 12
469 APOPT1 14_54 0.8857 43.21 0.0003125 7.429 6 7
765 BDNF 11_19 0.8820 23.81 0.0001695 4.348 3 3
5383 PYROXD2 10_62 0.8732 20.71 0.0001347 -3.755 12 14
4756 PATJ 1_39 0.8686 23.29 0.0001371 -2.798 16 19
360 ANAPC7 12_67 0.8369 37.61 0.0002240 6.385 7 7
640 ATP2B2 3_8 0.8241 26.05 0.0001568 4.229 7 8
698 B3GAT1 11_84 0.8157 23.68 0.0001377 4.324 6 9
4586 NTRK3 15_41 0.8046 24.66 0.0001392 4.457 2 2
1126 CBWD1 9_1 0.8033 20.46 0.0001186 4.060 3 4Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_intron
468 APOM 6_26 0.3686 623.03 0.0008033 11.590 3
849 BTN3A1 6_20 0.7393 146.39 0.0006649 13.091 8
5397 R3HDM2 12_36 1.1443 44.06 0.0004924 6.634 10
6119 SF3B1 2_117 0.9478 45.88 0.0003746 7.053 5
3723 LRP8 1_33 1.2179 33.07 0.0003523 -4.820 10
469 APOPT1 14_54 0.8857 43.21 0.0003125 7.429 6
1318 CENPM 22_17 0.7509 57.80 0.0003094 -6.506 1
299 AKT3 1_128 0.9654 35.61 0.0003005 6.350 6
854 BUB1B-PAK6 15_14 0.9723 30.33 0.0002604 -5.588 4
3563 LAMA5 20_36 1.1434 23.61 0.0002517 4.603 24
3632 LINC00320 21_6 0.9542 29.24 0.0002419 -5.336 3
2782 GIGYF1 7_62 0.9748 26.79 0.0002375 -5.266 5
7547 VWA7 6_26 0.1940 627.25 0.0002242 11.553 1
360 ANAPC7 12_67 0.8369 37.61 0.0002240 6.385 7
7712 ZDHHC20 13_2 0.9572 24.94 0.0002118 -4.784 3
4518 NPIPA1 16_15 0.9556 24.97 0.0002096 4.689 3
7231 TSNARE1 8_93 0.8894 34.70 0.0002087 6.287 10
7160 TRANK1 3_27 0.7490 39.04 0.0001917 -6.365 8
1449 CLCN3 4_110 0.7913 29.64 0.0001762 5.470 1
4166 MRPS33 7_87 0.9654 20.31 0.0001744 -4.304 6
num_sqtl
468 3
849 8
5397 12
6119 5
3723 11
469 7
1318 1
299 7
854 5
3563 38
3632 3
2782 5
7547 1
360 7
7712 4
4518 3
7231 12
7160 8
1449 2
4166 6Comparing z scores and PIPs
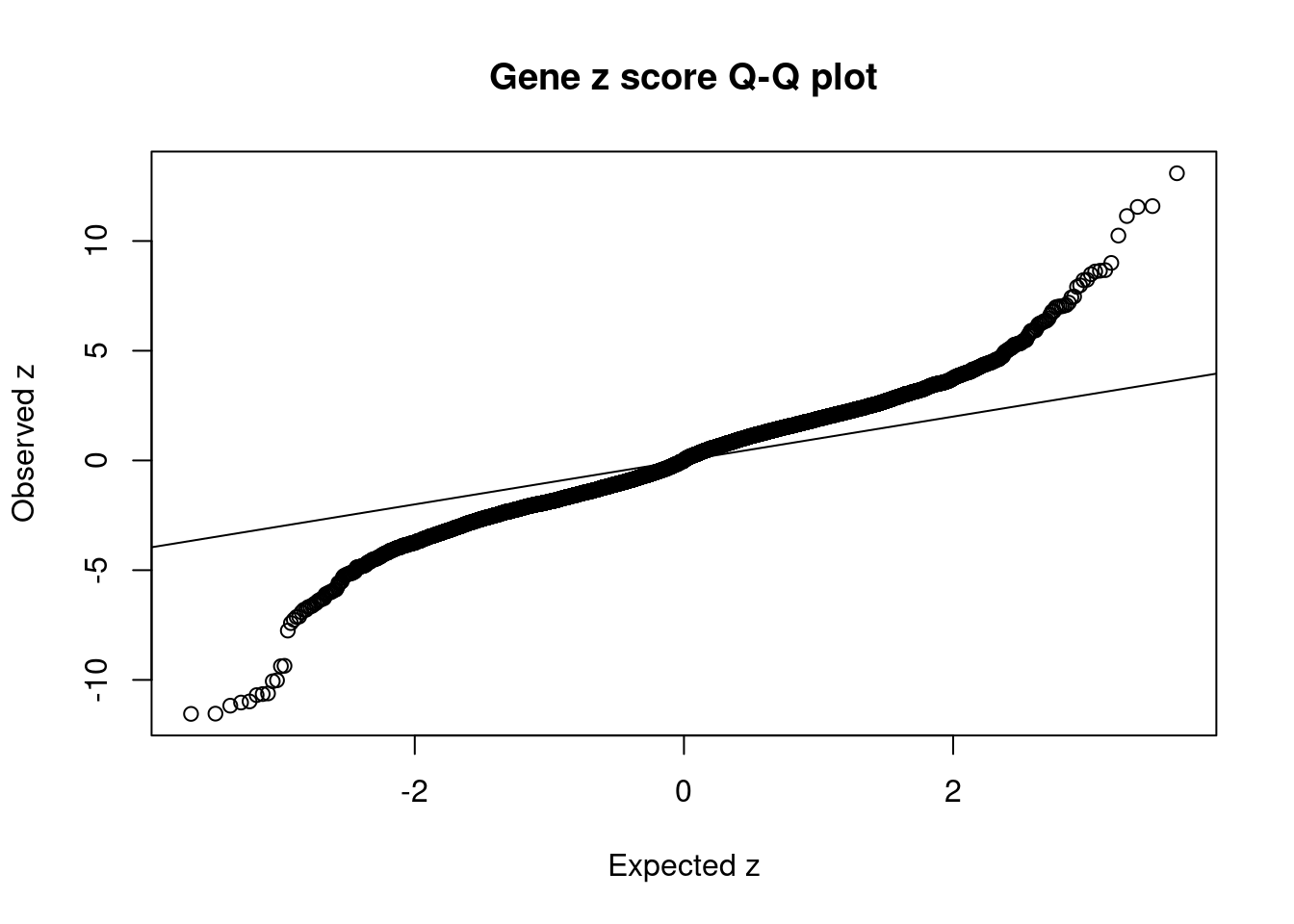
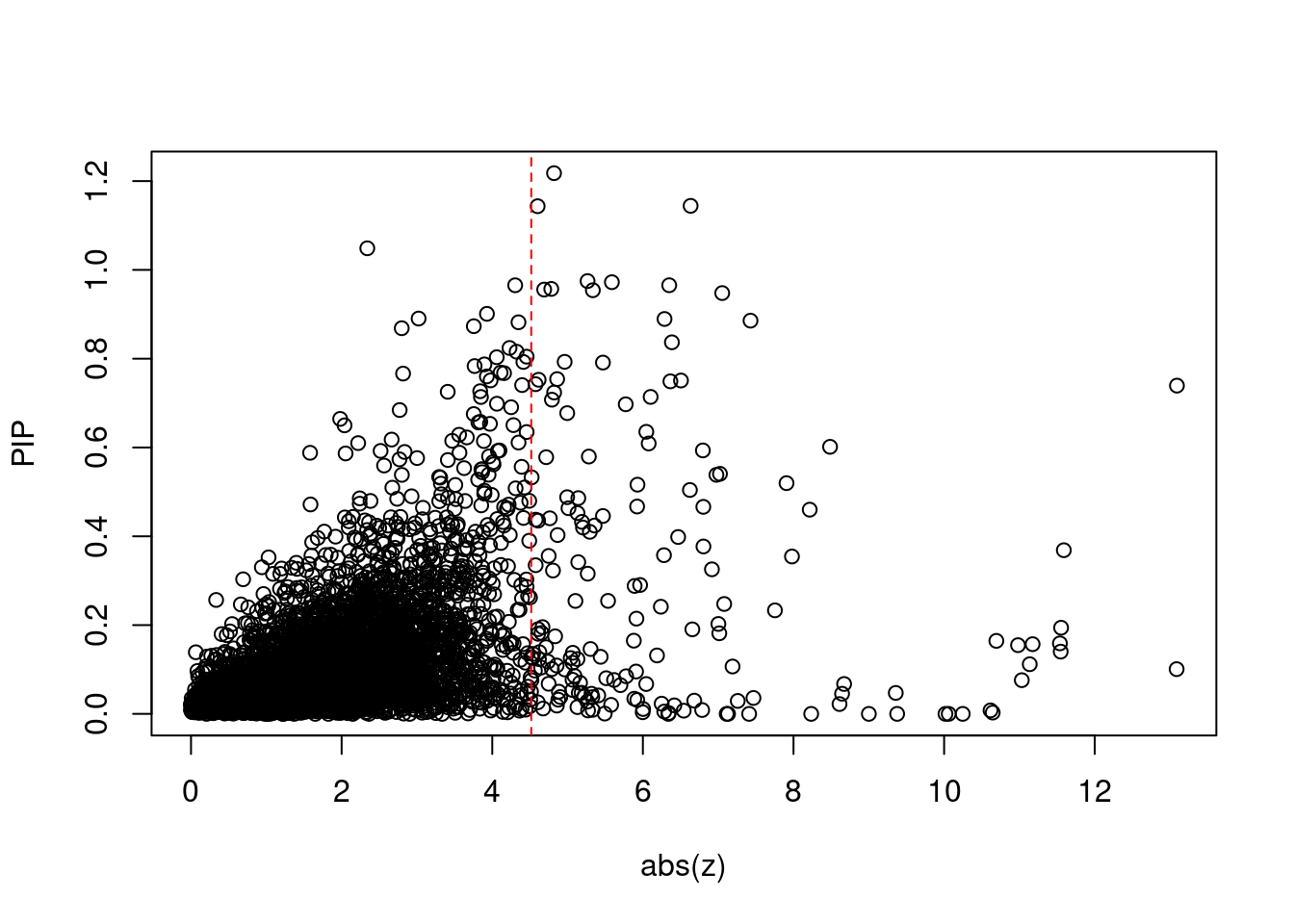
[1] 0.02124 genename region_tag susie_pip mu2 PVE z num_intron
849 BTN3A1 6_20 7.393e-01 146.39 6.649e-04 13.091 8
4876 PGBD1 6_22 1.007e-01 160.95 7.079e-06 13.087 5
468 APOM 6_26 3.686e-01 623.03 8.033e-04 11.590 3
7547 VWA7 6_26 1.940e-01 627.25 2.242e-04 11.553 1
7489 VARS 6_26 1.402e-01 623.95 1.165e-04 -11.548 2
4179 MSH5 6_26 1.588e-01 627.91 1.503e-04 -11.538 3
1888 DDR1 6_25 1.570e-01 105.86 2.456e-05 -11.175 3
7490 VARS2 6_25 1.118e-01 104.74 1.206e-05 11.137 2
979 C6orf136 6_25 7.591e-02 87.21 4.771e-06 -11.031 2
2623 FLOT1 6_25 1.547e-01 87.22 1.952e-05 -10.981 7
850 BTN3A2 6_20 1.644e-01 94.96 1.183e-05 -10.694 3
2841 GNL1 6_25 2.920e-03 78.25 6.334e-09 -10.645 1
7183 TRIM39 6_25 7.839e-03 82.27 4.800e-08 -10.616 1
716 BAG6 6_26 1.491e-09 498.08 1.051e-20 10.247 6
5195 PPT2 6_26 7.799e-12 464.25 2.681e-25 -10.061 10
5261 PRRT1 6_26 2.706e-12 462.51 3.216e-26 -10.018 1
2909 GPSM3 6_26 8.360e-14 414.68 2.752e-29 -9.377 1
1203 CCHCR1 6_25 4.718e-02 69.57 6.124e-07 -9.358 17
7105 TNXB 6_26 1.527e-13 452.13 1.000e-28 9.001 6
5832 RP5-874C20.8 6_22 6.731e-02 53.73 1.605e-06 8.672 6
num_sqtl
849 8
4876 6
468 3
7547 1
7489 2
4179 3
1888 3
7490 2
979 2
2623 7
850 5
2841 1
7183 1
716 7
5195 12
5261 1
2909 1
1203 30
7105 7
5832 6GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 109Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"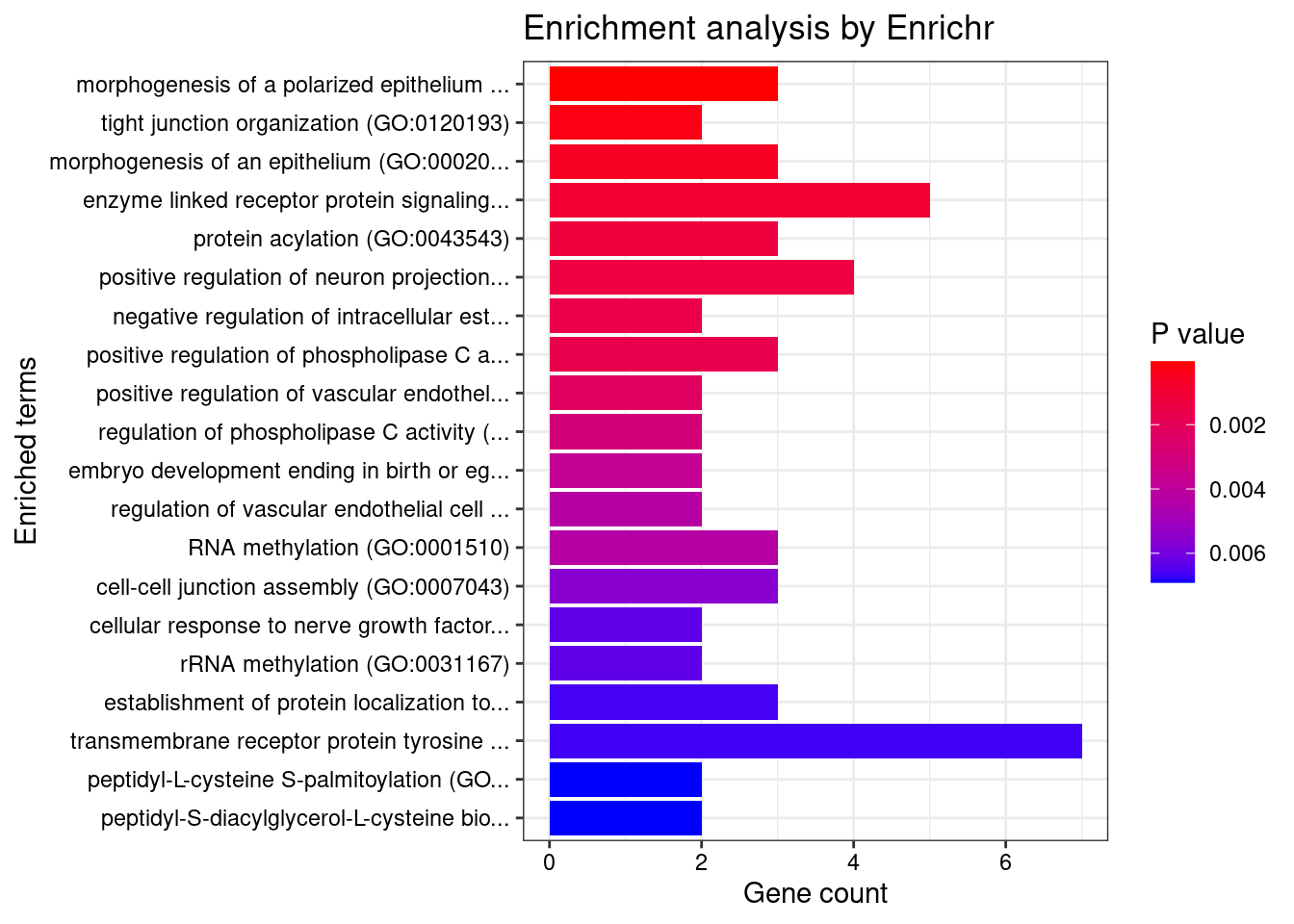
Term Overlap Adjusted.P.value
1 morphogenesis of a polarized epithelium (GO:0001738) 3/12 0.02988
Genes
1 AHI1;LAMA5;ACTG1
[1] "GO_Cellular_Component_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio BgRatio
62 Glioma 0.04106 4/58 87/9703
90 Measles 0.04106 1/58 1/9703
128 Schizophrenia 0.04106 12/58 883/9703
156 Electroencephalogram abnormal 0.04106 1/58 1/9703
160 Polydactyly 0.04106 4/58 117/9703
196 Short upturned nose 0.04106 1/58 1/9703
199 mixed gliomas 0.04106 4/58 70/9703
218 Hypoglycemia, leucine-induced 0.04106 1/58 1/9703
276 Interfrontal craniofaciosynostosis 0.04106 1/58 1/9703
277 Osteoglophonic dwarfism 0.04106 1/58 1/9703WebGestalt enrichment analysis for genes with PIP>0.5
Warning: replacing previous import 'lifecycle::last_warnings' by
'rlang::last_warnings' when loading 'hms'Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Warning: ggrepel: 66 unlabeled data points (too many overlaps). Consider
increasing max.overlaps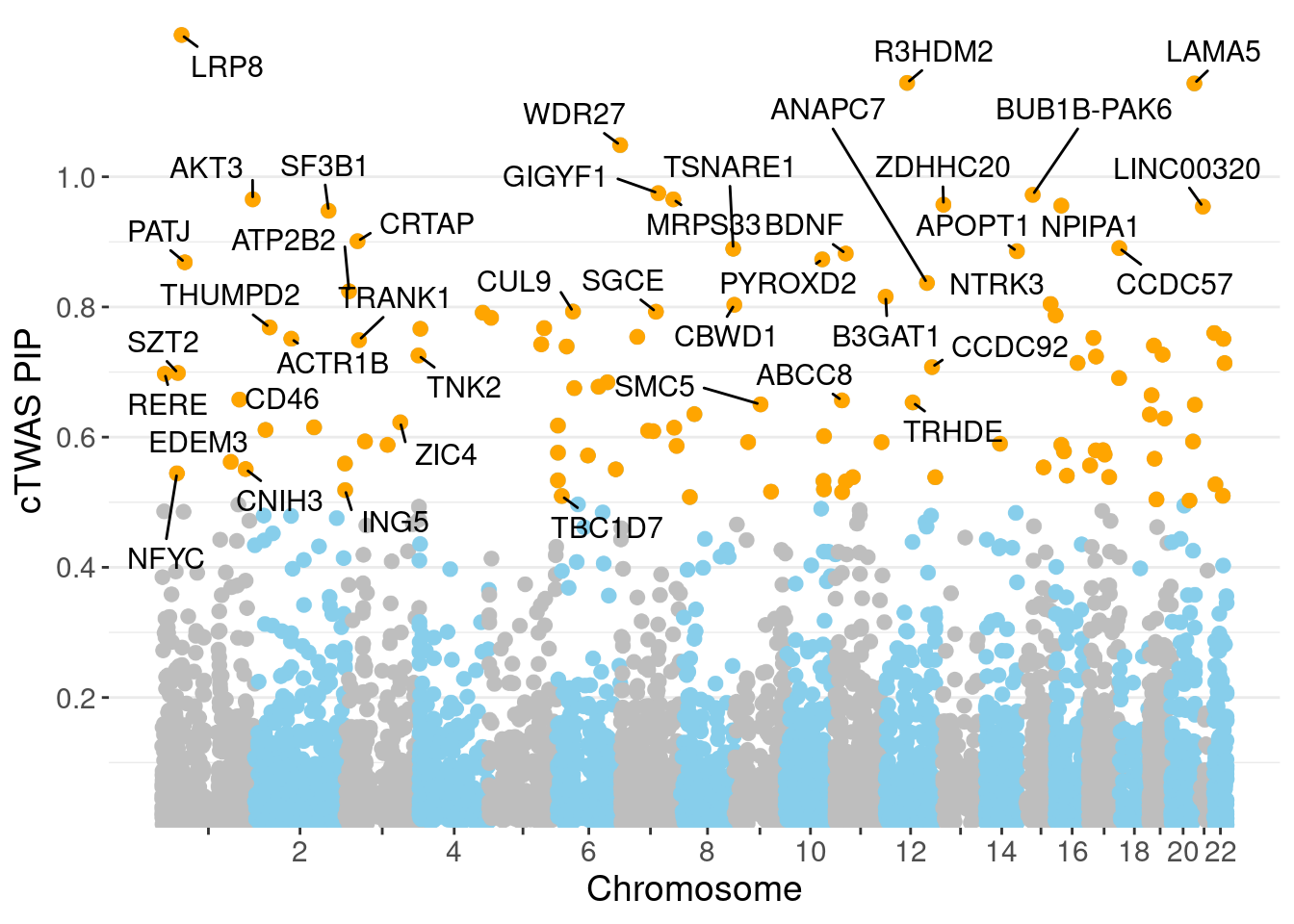
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 130#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 59#significance threshold for TWAS
print(sig_thresh)[1] 4.518#number of ctwas genes
length(ctwas_genes)[1] 24#number of TWAS genes
length(twas_genes)[1] 170#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
640 ATP2B2 3_8 0.8241 26.05 0.0001568 4.229 7 8
698 B3GAT1 11_84 0.8157 23.68 0.0001377 4.324 6 9
765 BDNF 11_19 0.8820 23.81 0.0001695 4.348 3 3
1126 CBWD1 9_1 0.8033 20.46 0.0001186 4.060 3 4
1179 CCDC57 17_47 0.8904 20.00 0.0001041 3.022 36 46
1668 CRTAP 3_24 0.9010 19.87 0.0001503 3.929 3 3
4166 MRPS33 7_87 0.9654 20.31 0.0001744 -4.304 6 6
4586 NTRK3 15_41 0.8046 24.66 0.0001392 4.457 2 2
4756 PATJ 1_39 0.8686 23.29 0.0001371 -2.798 16 19
5383 PYROXD2 10_62 0.8732 20.71 0.0001347 -3.755 12 14
7577 WDR27 6_111 1.0487 17.72 0.0001014 -2.341 29 37#sensitivity / recall
print(sensitivity) ctwas TWAS
0.03846 0.17692 #specificity
print(specificity) ctwas TWAS
0.9976 0.9815 #precision / PPV
print(precision) ctwas TWAS
0.2083 0.1353
sessionInfo()R version 4.1.0 (2021-05-18)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.3.13-el7-x86_64/lib/libopenblas_haswellp-r0.3.13.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.4.0 forcats_0.5.1 stringr_1.4.0 purrr_0.3.4
[5] readr_1.4.0 tidyr_1.1.3 tidyverse_1.3.1 tibble_3.1.7
[9] WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0 cowplot_1.1.1
[13] ggplot2_3.3.5 dplyr_1.0.7 reticulate_1.25 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] fs_1.5.0 lubridate_1.7.10 doParallel_1.0.16 httr_1.4.2
[5] rprojroot_2.0.2 tools_4.1.0 backports_1.2.1 doRNG_1.8.2
[9] bslib_0.2.5.1 utf8_1.2.1 R6_2.5.0 vipor_0.4.5
[13] DBI_1.1.1 colorspace_2.0-2 withr_2.4.2 ggrastr_1.0.1
[17] tidyselect_1.1.1 processx_3.5.2 curl_4.3.2 compiler_4.1.0
[21] git2r_0.28.0 rvest_1.0.0 cli_3.0.0 Cairo_1.5-15
[25] xml2_1.3.2 labeling_0.4.2 sass_0.4.0 scales_1.1.1
[29] callr_3.7.0 systemfonts_1.0.4 apcluster_1.4.9 digest_0.6.27
[33] rmarkdown_2.9 svglite_2.0.0 pkgconfig_2.0.3 htmltools_0.5.1.1
[37] dbplyr_2.1.1 highr_0.9 rlang_1.0.2 rstudioapi_0.13
[41] jquerylib_0.1.4 farver_2.1.0 generics_0.1.0 jsonlite_1.7.2
[45] magrittr_2.0.1 Matrix_1.3-3 ggbeeswarm_0.6.0 Rcpp_1.0.7
[49] munsell_0.5.0 fansi_0.5.0 lifecycle_1.0.0 stringi_1.6.2
[53] whisker_0.4 yaml_2.2.1 plyr_1.8.6 grid_4.1.0
[57] ggrepel_0.9.1 parallel_4.1.0 promises_1.2.0.1 crayon_1.4.1
[61] lattice_0.20-44 haven_2.4.1 hms_1.1.0 knitr_1.33
[65] ps_1.6.0 pillar_1.7.0 igraph_1.2.6 rjson_0.2.20
[69] rngtools_1.5 reshape2_1.4.4 codetools_0.2-18 reprex_2.0.0
[73] glue_1.4.2 evaluate_0.14 getPass_0.2-2 modelr_0.1.8
[77] data.table_1.14.0 png_0.1-7 vctrs_0.3.8 httpuv_1.6.1
[81] foreach_1.5.1 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] xfun_0.24 broom_0.7.8 later_1.2.0 iterators_1.0.13
[89] beeswarm_0.4.0 ellipsis_0.3.2 here_1.0.1