SCZ 2018 - Brain_Substantia_nigra
sheng Qian
2021-2-6
Last updated: 2022-05-19
Checks: 5 2
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 7d08c9b. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .ipynb_checkpoints/
Untracked files:
Untracked: G_list.RData
Untracked: Rplot.png
Untracked: SCZ_annotation.xlsx
Untracked: analysis/.ipynb_checkpoints/
Untracked: analysis/ttt.Rmd
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_2014_EUR_out/
Untracked: code/SCZ_2018_S_out/
Untracked: code/SCZ_2018_out/
Untracked: code/SCZ_2020_Single_out/
Untracked: code/SCZ_2020_out/
Untracked: code/SCZ_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/process_scz_2018_snps.R
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2014_EUR_analysis.sbatch
Untracked: code/run_SCZ_2014_EUR_analysis.sh
Untracked: code/run_SCZ_2014_EUR_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_analysis.sbatch
Untracked: code/run_SCZ_2018_analysis.sh
Untracked: code/run_SCZ_2018_analysis_S.sbatch
Untracked: code/run_SCZ_2018_analysis_S.sh
Untracked: code/run_SCZ_2018_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2020_Single_analysis.sbatch
Untracked: code/run_SCZ_2020_Single_analysis.sh
Untracked: code/run_SCZ_2020_Single_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2020_analysis.sbatch
Untracked: code/run_SCZ_2020_analysis.sh
Untracked: code/run_SCZ_2020_ctwas_rss_LDR.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_analysis_S.sbatch
Untracked: code/run_SCZ_analysis_S.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_SCZ_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: code/wflow_build.R
Untracked: code/wflow_build.sbatch
Untracked: data/.ipynb_checkpoints/
Untracked: data/GO_Terms/
Untracked: data/PGC3_SCZ_wave3_public.v2.tsv
Untracked: data/SCZ/
Untracked: data/SCZ_2014_EUR/
Untracked: data/SCZ_2018/
Untracked: data/SCZ_2018_S/
Untracked: data/SCZ_2020/
Untracked: data/SCZ_S/
Untracked: data/Supplementary Table 15 - MAGMA.xlsx
Untracked: data/Supplementary Table 20 - Prioritised Genes.xlsx
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/scz_2018.RDS
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Untracked: top_genes_32.txt
Untracked: top_genes_37.txt
Untracked: top_genes_43.txt
Untracked: top_genes_81.txt
Untracked: z_snp_pos_SCZ.RData
Untracked: z_snp_pos_SCZ_2014_EUR.RData
Untracked: z_snp_pos_SCZ_2018.RData
Untracked: z_snp_pos_SCZ_2020.RData
Unstaged changes:
Deleted: analysis/BMI_S_results.Rmd
Modified: analysis/SCZ_2018_Brain_Amygdala_S.Rmd
Modified: analysis/SCZ_2018_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/SCZ_2018_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellum_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cortex_S.Rmd
Modified: analysis/SCZ_2018_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hippocampus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hypothalamus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/SCZ_2018_Brain_Substantia_nigra_S.Rmd
Modified: analysis/SCZ_Annotation_Analysis.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_2018_Brain_Substantia_nigra_S.Rmd) and HTML (docs/SCZ_2018_Brain_Substantia_nigra_S.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 7d08c9b | sq-96 | 2022-05-18 | update |
| html | 7d08c9b | sq-96 | 2022-05-18 | update |
| Rmd | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 011327d | sq-96 | 2022-05-12 | update |
| Rmd | 6c6abbd | sq-96 | 2022-05-12 | update |
library(reticulate)
use_python("/scratch/midway2/shengqian/miniconda3/envs/PythonForR/bin/python",required=T)Weight QC
#number of imputed weights
nrow(qclist_all)[1] 15170#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1400 1082 880 623 588 770 877 489 649 714 913 791 301 549 531 626
17 18 19 20 21 22
1040 214 1116 542 34 441 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 13605#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8968INFO:numexpr.utils:Note: NumExpr detected 56 cores but "NUMEXPR_MAX_THREADS" not set, so enforcing safe limit of 8.finish
Attaching package: 'dplyr'The following objects are masked from 'package:stats':
filter, lagThe following objects are masked from 'package:base':
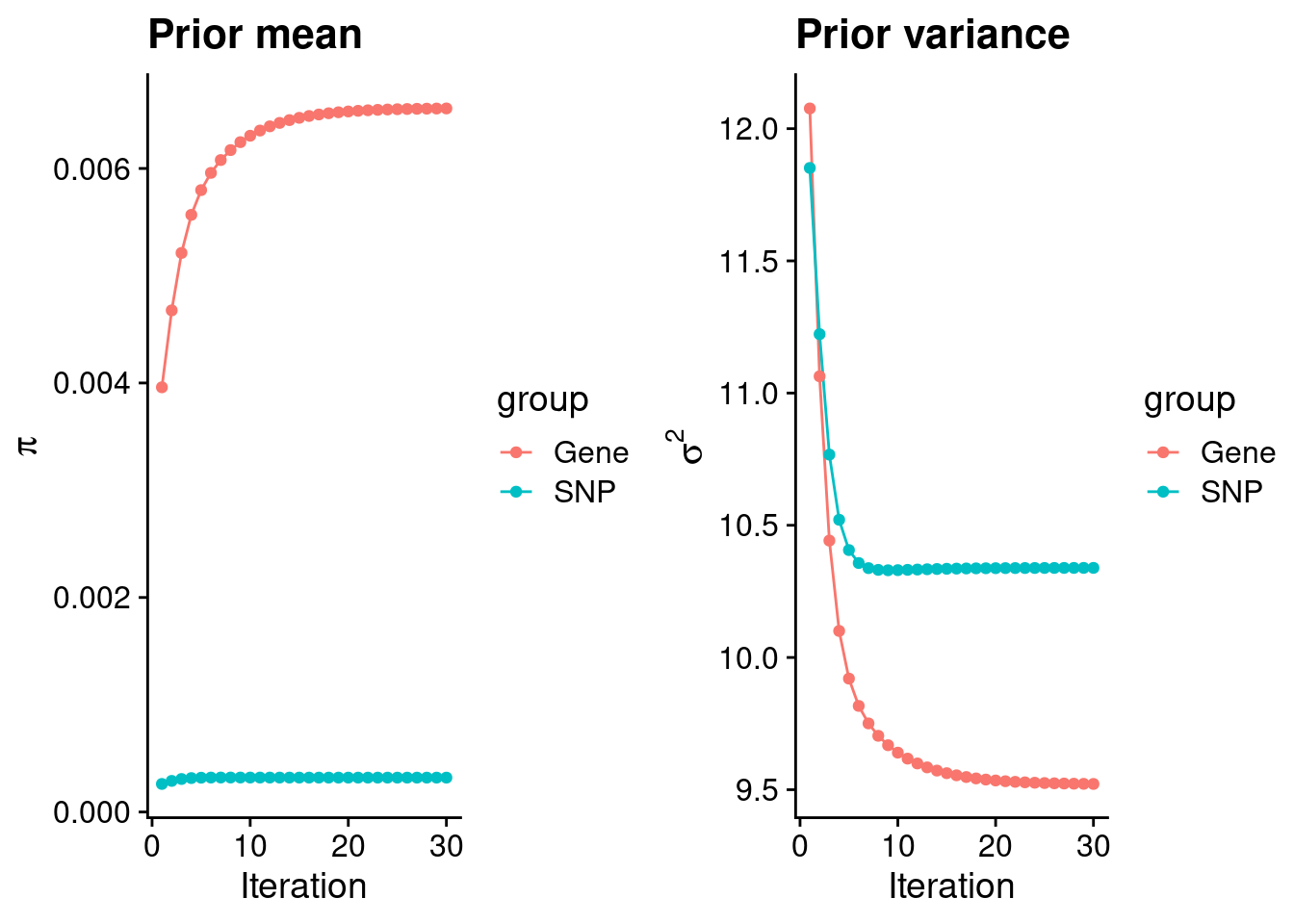
intersect, setdiff, setequal, unionCheck convergence of parameters
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
gene snp
0.0065597 0.0003205 gene snp
9.522 10.339 [1] 105318[1] 6278 6309950 gene snp
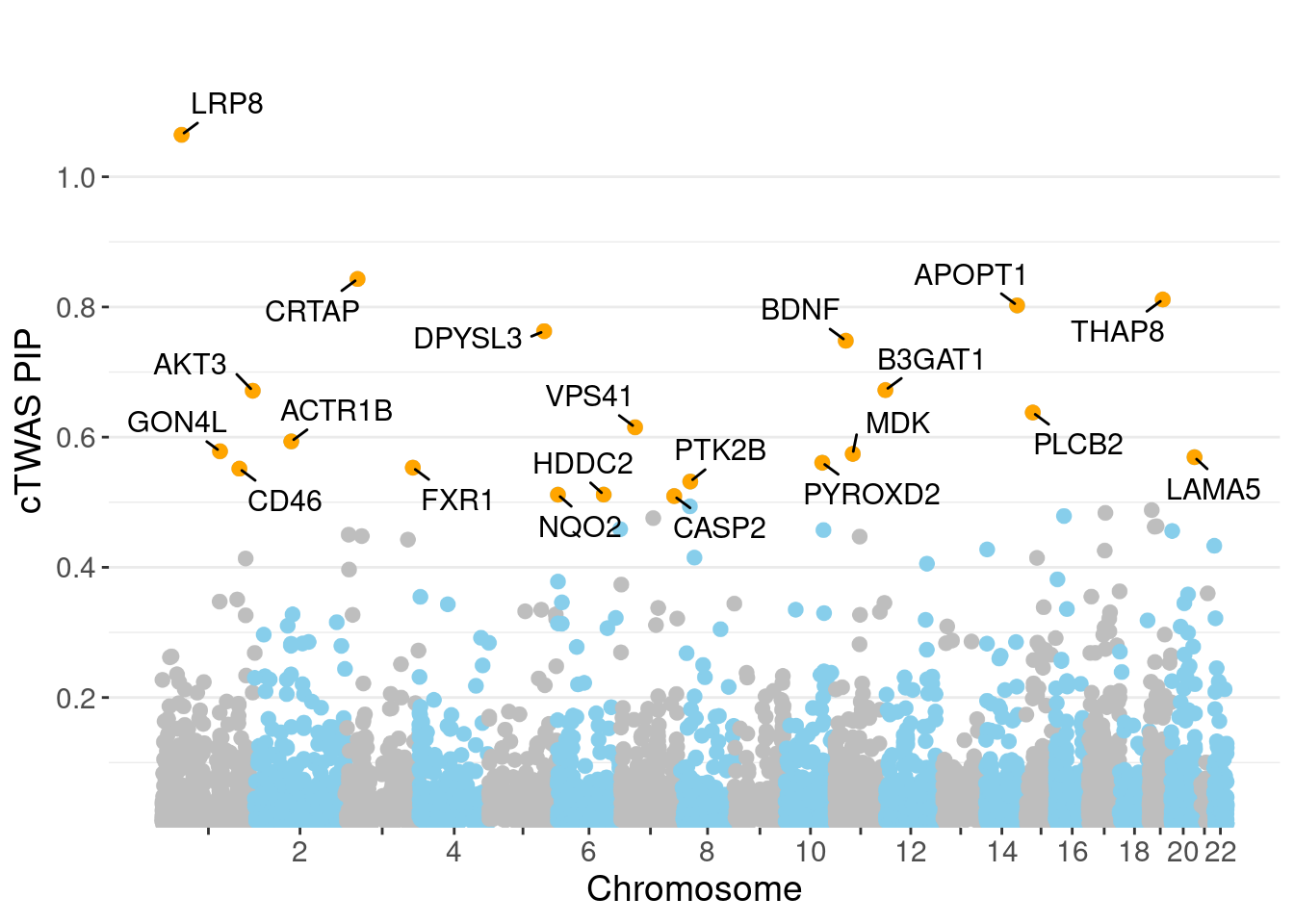
0.003723 0.198505 [1] 0.008784 1.088938Genes with highest PIPs
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
3022 LRP8 1_33 1.0645 32.61 2.549e-04 -4.624 6 6
1361 CRTAP 3_24 0.8430 20.01 1.343e-04 3.929 2 2
5465 THAP8 19_25 0.8114 20.21 1.261e-04 3.847 2 2
380 APOPT1 14_54 0.8024 44.24 2.642e-04 7.429 6 9
1685 DPYSL3 5_86 0.7628 23.36 1.291e-04 4.157 1 1
619 BDNF 11_19 0.7481 22.63 1.203e-04 4.348 1 1
564 B3GAT1 11_84 0.6722 31.39 1.272e-04 -4.516 6 10
241 AKT3 1_128 0.6713 34.01 1.356e-04 -6.291 6 6
4024 PLCB2 15_14 0.6378 24.42 8.300e-05 4.470 3 4
5949 VPS41 7_28 0.6151 25.12 8.874e-05 -4.509 2 2
129 ACTR1B 2_57 0.5934 22.34 7.367e-05 3.978 3 3
2308 GON4L 1_76 0.5783 27.63 8.773e-05 4.084 1 1
3192 MDK 11_28 0.5743 45.88 1.437e-04 7.159 1 1
2881 LAMA5 20_36 0.5692 32.47 8.011e-05 3.967 10 15
4326 PYROXD2 10_62 0.5608 33.32 7.427e-05 -3.718 10 11
2166 FXR1 3_111 0.5532 42.91 1.221e-04 -6.873 4 4
1022 CD46 1_105 0.5515 18.45 5.268e-05 -3.654 6 6
4280 PTK2B 8_27 0.5318 26.09 6.953e-05 4.730 2 3
2446 HDDC2 6_84 0.5117 19.46 3.262e-05 2.383 15 20
3670 NQO2 6_3 0.5116 25.24 4.071e-05 3.051 16 24Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
380 APOPT1 14_54 0.8024 44.24 2.642e-04 7.429 6 9
3022 LRP8 1_33 1.0645 32.61 2.549e-04 -4.624 6 6
3192 MDK 11_28 0.5743 45.88 1.437e-04 7.159 1 1
241 AKT3 1_128 0.6713 34.01 1.356e-04 -6.291 6 6
1361 CRTAP 3_24 0.8430 20.01 1.343e-04 3.929 2 2
1685 DPYSL3 5_86 0.7628 23.36 1.291e-04 4.157 1 1
564 B3GAT1 11_84 0.6722 31.39 1.272e-04 -4.516 6 10
5465 THAP8 19_25 0.8114 20.21 1.261e-04 3.847 2 2
2166 FXR1 3_111 0.5532 42.91 1.221e-04 -6.873 4 4
619 BDNF 11_19 0.7481 22.63 1.203e-04 4.348 1 1
2203 GATAD2A 19_15 0.4632 45.09 9.073e-05 -6.640 4 4
5949 VPS41 7_28 0.6151 25.12 8.874e-05 -4.509 2 2
2308 GON4L 1_76 0.5783 27.63 8.773e-05 4.084 1 1
4024 PLCB2 15_14 0.6378 24.42 8.300e-05 4.470 3 4
2881 LAMA5 20_36 0.5692 32.47 8.011e-05 3.967 10 15
4326 PYROXD2 10_62 0.5608 33.32 7.427e-05 -3.718 10 11
129 ACTR1B 2_57 0.5934 22.34 7.367e-05 3.978 3 3
4280 PTK2B 8_27 0.5318 26.09 6.953e-05 4.730 2 3
5406 TCAIM 3_31 0.4480 35.10 6.170e-05 4.053 5 5
3090 MAD1L1 7_3 0.3735 62.14 5.662e-05 8.215 4 6Comparing z scores and PIPs
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
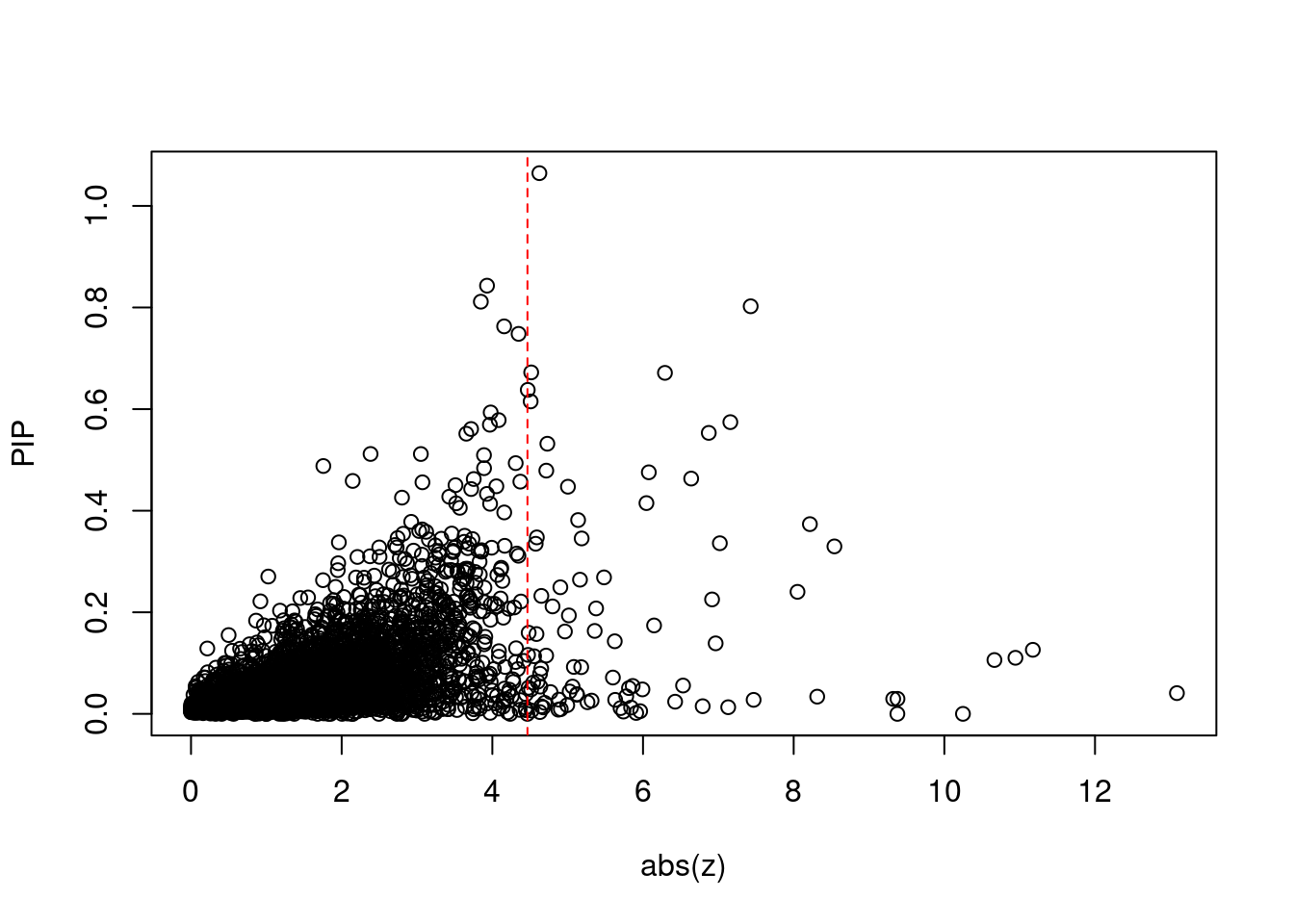
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
[1] 0.01434 genename region_tag susie_pip mu2 PVE z num_intron
3948 PGBD1 6_22 4.087e-02 155.68 9.882e-07 -13.087 2
1537 DDR1 6_25 1.261e-01 100.79 1.477e-05 11.175 3
2118 FLOT1 6_24 1.104e-01 77.49 8.920e-06 -10.944 5
692 BTN3A2 6_20 1.060e-01 88.48 4.796e-06 -10.665 4
574 BAG6 6_26 3.796e-05 160.21 1.643e-12 -10.247 5
984 CCHCR1 6_25 2.958e-02 62.23 3.774e-07 -9.378 5
2347 GPSM3 6_26 1.690e-06 118.03 3.202e-15 -9.377 1
6126 ZKSCAN3 6_22 2.955e-02 55.20 1.934e-07 -9.321 3
3706 NT5C2 10_66 3.297e-01 46.96 4.551e-05 -8.541 8
4651 RP5-874C20.8 6_22 3.391e-02 45.29 3.198e-07 -8.313 4
3090 MAD1L1 7_3 3.735e-01 62.14 5.662e-05 8.215 4
463 AS3MT 10_66 2.402e-01 44.47 2.402e-05 8.051 3
6270 ZSCAN16 6_22 2.759e-02 52.38 2.415e-07 7.468 3
380 APOPT1 14_54 8.024e-01 44.24 2.642e-04 7.429 6
3192 MDK 11_28 5.743e-01 45.88 1.437e-04 7.159 1
209 AIF1 6_26 1.311e-02 59.25 9.670e-08 -7.131 5
5564 TMEM219 16_24 3.359e-01 45.65 4.891e-05 -7.020 1
1581 DGKZ 11_28 1.388e-01 43.55 7.970e-06 -6.964 1
2643 INO80E 16_24 2.253e-01 44.02 1.928e-05 -6.917 4
2166 FXR1 3_111 5.532e-01 42.91 1.221e-04 -6.873 4
num_sqtl
3948 2
1537 3
2118 5
692 4
574 7
984 8
2347 1
6126 3
3706 11
4651 5
3090 6
463 3
6270 3
380 9
3192 1
209 5
5564 1
1581 2
2643 5
2166 4GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 21Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"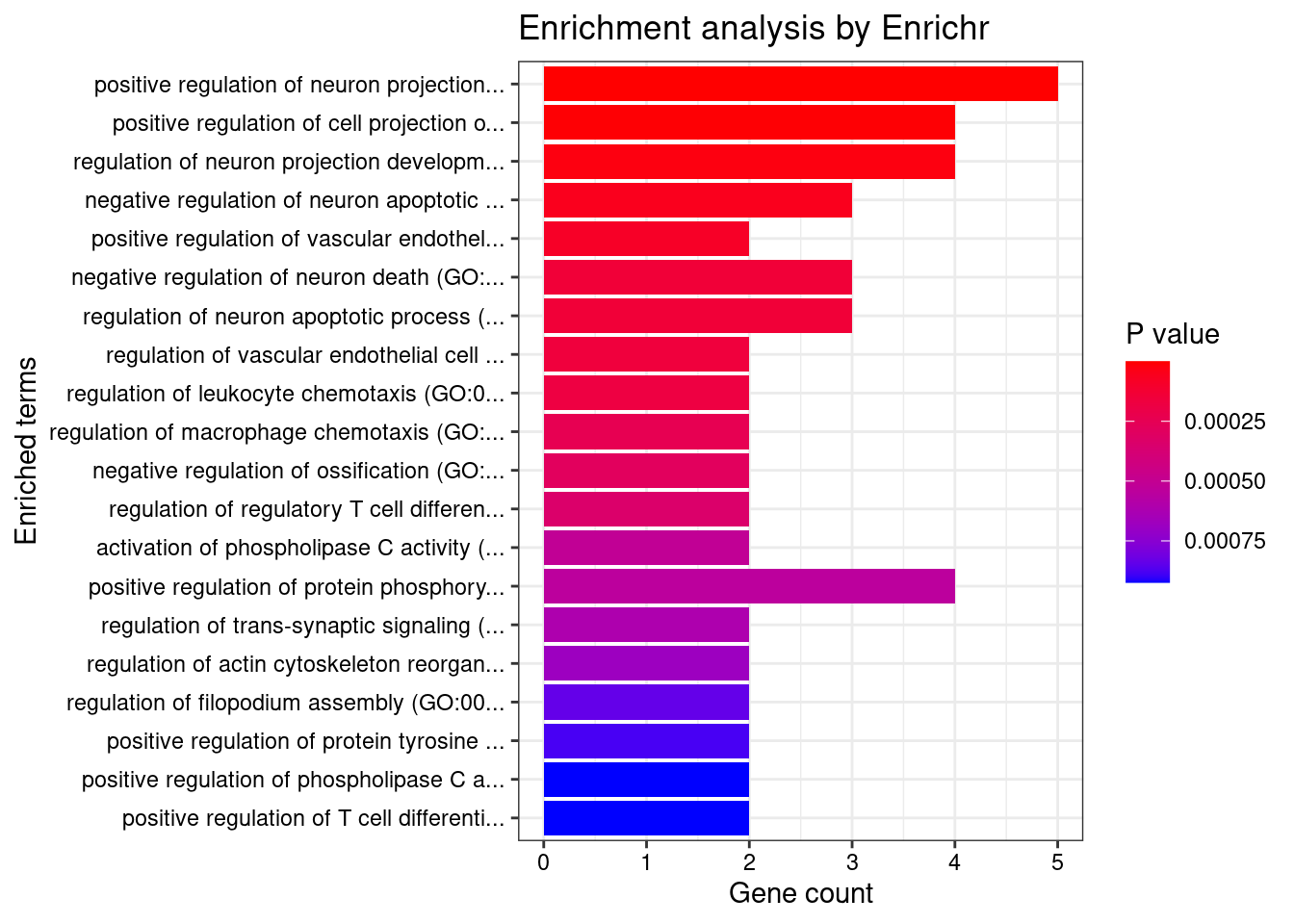
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
Term
1 positive regulation of neuron projection development (GO:0010976)
2 positive regulation of cell projection organization (GO:0031346)
3 regulation of neuron projection development (GO:0010975)
4 negative regulation of neuron apoptotic process (GO:0043524)
5 positive regulation of vascular endothelial cell proliferation (GO:1905564)
6 negative regulation of neuron death (GO:1901215)
7 regulation of neuron apoptotic process (GO:0043523)
8 regulation of vascular endothelial cell proliferation (GO:1905562)
9 regulation of leukocyte chemotaxis (GO:0002688)
10 regulation of macrophage chemotaxis (GO:0010758)
11 negative regulation of ossification (GO:0030279)
12 regulation of regulatory T cell differentiation (GO:0045589)
13 activation of phospholipase C activity (GO:0007202)
14 positive regulation of protein phosphorylation (GO:0001934)
15 regulation of trans-synaptic signaling (GO:0099177)
16 regulation of actin cytoskeleton reorganization (GO:2000249)
17 regulation of filopodium assembly (GO:0051489)
18 positive regulation of protein tyrosine kinase activity (GO:0061098)
19 positive regulation of phospholipase C activity (GO:0010863)
20 positive regulation of T cell differentiation (GO:0045582)
21 negative regulation of apoptotic process (GO:0043066)
22 apoptotic process (GO:0006915)
23 positive regulation of cell-substrate adhesion (GO:0010811)
24 integrin-mediated signaling pathway (GO:0007229)
25 positive regulation of T cell activation (GO:0050870)
26 positive regulation of endothelial cell proliferation (GO:0001938)
Overlap Adjusted.P.value Genes
1 5/88 1.185e-05 BDNF;MDK;DPYSL3;PTK2B;LRP8
2 4/117 1.291e-03 BDNF;MDK;DPYSL3;PTK2B
3 4/165 3.346e-03 BDNF;MDK;DPYSL3;PTK2B
4 3/71 5.705e-03 BDNF;MDK;PTK2B
5 2/13 6.816e-03 MDK;AKT3
6 3/98 8.270e-03 BDNF;MDK;PTK2B
7 3/98 8.270e-03 BDNF;MDK;PTK2B
8 2/18 8.270e-03 MDK;AKT3
9 2/19 8.270e-03 MDK;PTK2B
10 2/22 1.004e-02 MDK;PTK2B
11 2/24 1.089e-02 MDK;PTK2B
12 2/26 1.174e-02 MDK;CD46
13 2/32 1.625e-02 BDNF;PLCB2
14 4/371 1.625e-02 FXR1;BDNF;PTK2B;LRP8
15 2/35 1.709e-02 BDNF;LRP8
16 2/37 1.791e-02 MDK;PTK2B
17 2/41 1.936e-02 FXR1;DPYSL3
18 2/42 1.936e-02 BDNF;LRP8
19 2/43 1.936e-02 BDNF;PLCB2
20 2/43 1.936e-02 MDK;CD46
21 4/485 2.938e-02 BDNF;MDK;CASP2;PTK2B
22 3/231 3.303e-02 FXR1;CASP2;PTK2B
23 2/70 4.425e-02 MDK;PTK2B
24 2/75 4.663e-02 LAMA5;PTK2B
25 2/75 4.663e-02 MDK;CD46
26 2/77 4.722e-02 MDK;AKT3
[1] "GO_Cellular_Component_2021"
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio BgRatio
3 Alcoholic Intoxication, Chronic 0.01997 4/13 268/9703
34 Profound Mental Retardation 0.01997 3/13 139/9703
41 Measles 0.01997 1/13 1/9703
44 Memory Disorders 0.01997 2/13 43/9703
45 Mental Retardation, Psychosocial 0.01997 3/13 139/9703
77 Memory impairment 0.01997 2/13 44/9703
134 Age-Related Memory Disorders 0.01997 2/13 43/9703
135 Memory Disorder, Semantic 0.01997 2/13 43/9703
136 Memory Disorder, Spatial 0.01997 2/13 43/9703
137 Memory Loss 0.01997 2/13 43/9703WebGestalt enrichment analysis for genes with PIP>0.5
Warning: replacing previous import 'lifecycle::last_warnings' by
'rlang::last_warnings' when loading 'hms'Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLSensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 130#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 42#significance threshold for TWAS
print(sig_thresh)[1] 4.466#number of ctwas genes
length(ctwas_genes)[1] 4#number of TWAS genes
length(twas_genes)[1] 90#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
1361 CRTAP 3_24 0.8430 20.01 0.0001343 3.929 2 2
5465 THAP8 19_25 0.8114 20.21 0.0001261 3.847 2 2#sensitivity / recall
print(sensitivity) ctwas TWAS
0.007692 0.076923 #specificity
print(specificity) ctwas TWAS
0.9995 0.9872 #precision / PPV
print(precision) ctwas TWAS
0.2500 0.1111
sessionInfo()R version 4.1.0 (2021-05-18)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.3.13-el7-x86_64/lib/libopenblas_haswellp-r0.3.13.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.4.0 forcats_0.5.1 stringr_1.4.0 purrr_0.3.4
[5] readr_1.4.0 tidyr_1.1.3 tidyverse_1.3.1 tibble_3.1.7
[9] WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0 cowplot_1.1.1
[13] ggplot2_3.3.5 dplyr_1.0.7 reticulate_1.25 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] fs_1.5.0 lubridate_1.7.10 doParallel_1.0.16 httr_1.4.2
[5] rprojroot_2.0.2 tools_4.1.0 backports_1.2.1 doRNG_1.8.2
[9] bslib_0.2.5.1 utf8_1.2.1 R6_2.5.0 vipor_0.4.5
[13] DBI_1.1.1 colorspace_2.0-2 withr_2.4.2 ggrastr_1.0.1
[17] tidyselect_1.1.1 processx_3.5.2 curl_4.3.2 compiler_4.1.0
[21] git2r_0.28.0 rvest_1.0.0 cli_3.0.0 Cairo_1.5-15
[25] xml2_1.3.2 labeling_0.4.2 sass_0.4.0 scales_1.1.1
[29] callr_3.7.0 systemfonts_1.0.4 apcluster_1.4.9 digest_0.6.27
[33] rmarkdown_2.9 svglite_2.0.0 pkgconfig_2.0.3 htmltools_0.5.1.1
[37] dbplyr_2.1.1 highr_0.9 rlang_1.0.2 rstudioapi_0.13
[41] jquerylib_0.1.4 farver_2.1.0 generics_0.1.0 jsonlite_1.7.2
[45] magrittr_2.0.1 Matrix_1.3-3 ggbeeswarm_0.6.0 Rcpp_1.0.7
[49] munsell_0.5.0 fansi_0.5.0 lifecycle_1.0.0 stringi_1.6.2
[53] whisker_0.4 yaml_2.2.1 plyr_1.8.6 grid_4.1.0
[57] ggrepel_0.9.1 parallel_4.1.0 promises_1.2.0.1 crayon_1.4.1
[61] lattice_0.20-44 haven_2.4.1 hms_1.1.0 knitr_1.33
[65] ps_1.6.0 pillar_1.7.0 igraph_1.2.6 rjson_0.2.20
[69] rngtools_1.5 reshape2_1.4.4 codetools_0.2-18 reprex_2.0.0
[73] glue_1.4.2 evaluate_0.14 getPass_0.2-2 modelr_0.1.8
[77] data.table_1.14.0 png_0.1-7 vctrs_0.3.8 httpuv_1.6.1
[81] foreach_1.5.1 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] xfun_0.24 broom_0.7.8 later_1.2.0 iterators_1.0.13
[89] beeswarm_0.4.0 ellipsis_0.3.2 here_1.0.1