Sexual selection and sexual size dimorphism: a meta-analysis of comparative studies
Additional analyses
Lennart Winkler1, Robert P
Freckleton2, Tamas Szekely3, 4 &
Tim Janicke1,5
1Applied Zoology,
Technical University Dresden 2Department of
Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS,
UK
3Milner Centre for Evolution, University of Bath,
Bath, UK84Department of Evolutionary Zoology and
Human Behaviour, University of Debrecen, Debrecen, Hungary
5Centre d’Écologie Fonctionnelle et Évolutive, UMR 5175,
CNRS, Université de Montpellier
Last updated: 2023-05-24
Checks: 7 0
Knit directory:
SSD_and_sexual_selection_2023/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20230430) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version c1d3311. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .Rhistory
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown (analysis/index2.Rmd) and HTML
(docs/index2.html) files. If you’ve configured a remote Git
repository (see ?wflow_git_remote), click on the hyperlinks
in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | c1d3311 | LennartWinkler | 2023-05-24 | wflow_publish(all = T) |
| html | 71d667c | LennartWinkler | 2023-05-03 | Build site. |
| Rmd | 378a58c | LennartWinkler | 2023-05-03 | wflow_publish(republish = TRUE, all = T) |
| html | 085f45f | LennartWinkler | 2023-05-03 | Build site. |
| Rmd | 9c4346c | LennartWinkler | 2023-05-03 | wflow_publish(republish = TRUE, all = T) |
| html | 9c4346c | LennartWinkler | 2023-05-03 | wflow_publish(republish = TRUE, all = T) |
| Rmd | 85c143e | LennartWinkler | 2023-05-03 | update |
| html | 8edd942 | LennartWinkler | 2023-05-01 | Build site. |
| Rmd | d131cd9 | LennartWinkler | 2023-05-01 | wflow_publish(republish = TRUE, all = T) |
| Rmd | 57ca562 | LennartWinkler | 2023-04-30 | update |
| html | 57ca562 | LennartWinkler | 2023-04-30 | update |
| Rmd | 7a0f67c | LennartWinkler | 2023-04-30 | Set up project |
Supplementary material reporting R code for the manuscript ‘Sexual selection and sexual size dimorphism: a meta-analysis of comparative studies’. Additional analyses excluding studies that did not correct for phylogenetic non-independence (see Supplement 2). # Load and prepare data Before we started the analyses, we loaded all necessary packages and data.
rm(list = ls()) # Clear work environment
# Load R-packages ####
list_of_packages=cbind('ape','matrixcalc','metafor','Matrix','MASS','pwr','psych','multcomp','data.table','ggplot2','RColorBrewer','MCMCglmm','ggdist','cowplot','PupillometryR','dplyr','wesanderson')
lapply(list_of_packages, require, character.only = TRUE)
# Load data set ####
MetaData <- read.csv("./data/Supplement4_SexSelSSD_V01.csv", sep=";", header=TRUE) # Load data set
#Remove studies that did not correct for phylogenetic non-independence
MetaData=MetaData[MetaData$PhyloControlled=='Yes',]
N_Studies <- length(summary(as.factor(MetaData$Study_ID))) # Number of included primary studies
Tree<- read.tree("./data/Supplement6_SexSelSSD_V01.txt") # Load phylogenetic tree
# Prune phylogenetic tree
MetaData_Class_Data <- unique(MetaData$Class)
Tree_Class<-drop.tip(Tree, Tree$tip.label[-na.omit(match(MetaData_Class_Data, Tree$tip.label))])
forcedC_Moderators <- as.matrix(forceSymmetric(vcv(Tree_Class, corr=TRUE)))
# Order moderator levels
MetaData$SexSel_Mode=as.factor(MetaData$SexSel_Mode)
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("post-copulatory"))
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("pre-copulatory"))
MetaData$SexSel_Sex=as.factor(MetaData$SexSel_Sex)
MetaData$SexSel_Sex=relevel(MetaData$SexSel_Sex,c("Male"))
# Set figure theme and colors
theme=theme(panel.border = element_blank(),
panel.background = element_blank(),
panel.grid.major = element_blank(),
panel.grid.minor = element_blank(),
legend.position = c(0.2,0.5),
legend.title = element_blank(),
legend.text = element_text(colour="black", size=12),
axis.line.x = element_line(colour = "black", size = 1),
axis.line.y = element_line(colour = "black", size = 1),
axis.text.x = element_text(face="plain", color="black", size=16, angle=0),
axis.text.y = element_text(face="plain", color="black", size=16, angle=0),
axis.title.x = element_text(size=16,face="plain", margin = margin(r=0,10,0,0)),
axis.title.y = element_text(size=16,face="plain", margin = margin(r=10,0,0,0)),
axis.ticks = element_line(size = 1),
axis.ticks.length = unit(.3, "cm"))
colpal=c("#4DAF4A","#377EB8","#E41A1C")
colpal2=brewer.pal(7, 'Dark2')
colpal3=wes_palette('FantasticFox1', 9, type = c("continuous"))
colpal4=c("grey50","grey65")
Meta_col=c('grey85','grey50','grey20','black')
# Global models ####
# Phylogenetic Model
Model_REML_Null = rma.mv(r ~ 1, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_Null)
# Non-phylogenetic Model
Model_cREML_Null = rma.mv(r ~ 1, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_Null)Global model Version 1
First, we tested if increasing sexual selection (estimated via diverse proxies; see Table S1) correlated with the degree of SSD. To this end we ran a global model on the absolute correlation coefficients extracted from primary studies (i.e. increasing sexual selection could correlate with a female- or male-bias in SSD, with all resulting correlation coefficients being positive). First, we ran a global model including the phylogeny:
# Set all correlation coeficients as positive
MetaData$absSSDcor=NA
MetaData$absSSDcor=abs(MetaData$r)
# Phylogenetic Model
Model_REML_Null = rma.mv(absSSDcor ~ 1, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_Null)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
10.3881 -20.7762 -12.7762 -3.4533 -12.2128
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0092 0.0958 45 no Study_ID no
sigma^2.2 0.0178 0.1334 77 no Index no
sigma^2.3 0.0132 0.1149 9 no Class yes
Test for Heterogeneity:
Q(df = 76) = 545.0814, p-val < .0001
Model Results:
estimate se zval pval ci.lb ci.ub
0.3032 0.0722 4.1998 <.0001 0.1617 0.4447 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Second, we ran a global model without the phylogeny:
# Non-phylogenetic Model
Model_cREML_Null = rma.mv(absSSDcor ~ 1, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_Null)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
8.0489 -16.0978 -10.0978 -3.1056 -9.7645
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0153 0.1236 45 no Study_ID
sigma^2.2 0.0198 0.1409 77 no Index
Test for Heterogeneity:
Q(df = 76) = 545.0814, p-val < .0001
Model Results:
estimate se zval pval ci.lb ci.ub
0.3547 0.0290 12.2423 <.0001 0.2979 0.4115 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Global models Version 2
Next, we addressed the question if increasing sexual selection correlated with an increasingly male-biased SSD. For this we ran a global model including an observation-level index and the study identifier as random termson correlation coefficients that were positive if increasing sexual selection correlated with an increasingly male-biased SSD, but negative if increasing sexual selection correlated with an increasingly female-biased SSD. First, we ran a global model including the phylogeny:
Model_REML_Null = rma.mv(r ~ 1, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_Null)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-19.6871 39.3743 47.3743 56.6972 47.9377
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0000 0.0000 45 no Study_ID no
sigma^2.2 0.0757 0.2751 77 no Index no
sigma^2.3 0.0237 0.1539 9 no Class yes
Test for Heterogeneity:
Q(df = 76) = 1182.7000, p-val < .0001
Model Results:
estimate se zval pval ci.lb ci.ub
0.2341 0.0963 2.4321 0.0150 0.0455 0.4228 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Second, we ran a global model without the phylogeny:
Model_cREML_Null = rma.mv(r ~ 1, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_Null)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-22.2286 44.4572 50.4572 57.4494 50.7905
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0143 0.1195 45 no Study_ID
sigma^2.2 0.0772 0.2778 77 no Index
Test for Heterogeneity:
Q(df = 76) = 1182.7000, p-val < .0001
Model Results:
estimate se zval pval ci.lb ci.ub
0.2393 0.0401 5.9736 <.0001 0.1608 0.3178 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Moderator tests for phylogenetic models
Next, we ran a series of models that test the effect of different moderators. Again we started with models including the phylogeny.
Sexual selection mode
The first model explores the effect of the sexual selection mode (i.e. pre-copulatory, post-copulatory or both):
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("pre-copulatory"))
Model_REML_by_SexSelMode = rma.mv(r ~ factor(SexSel_Mode), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelMode)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-7.6879 15.3757 27.3757 41.2001 28.6294
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0013 0.0361 45 no Study_ID no
sigma^2.2 0.0489 0.2212 77 no Index no
sigma^2.3 0.0231 0.1519 9 no Class yes
Test for Residual Heterogeneity:
QE(df = 74) = 944.4229, p-val < .0001
Test of Moderators (coefficients 2:3):
QM(df = 2) = 30.0173, p-val < .0001
Model Results:
estimate se zval pval ci.lb
intrcpt 0.2305 0.0954 2.4172 0.0156 0.0436
factor(SexSel_Mode)post-copulatory -0.4418 0.1035 -4.2669 <.0001 -0.6447
factor(SexSel_Mode)both 0.1330 0.0653 2.0381 0.0415 0.0051
ci.ub
intrcpt 0.4174 *
factor(SexSel_Mode)post-copulatory -0.2388 ***
factor(SexSel_Mode)both 0.2610 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("post-copulatory"))
Model_REML_by_SexSelMode2 = rma.mv(r ~ factor(SexSel_Mode), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelMode2)
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("both"))
Model_REML_by_SexSelMode3 = rma.mv(r ~ factor(SexSel_Mode), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelMode3)Finally, we computed FDR corrected p-values:
tab1=as.data.frame(round(p.adjust(c(0.0029, 0.1203, .0001), method = 'fdr'),digit=3),row.names=cbind("Pre-copulatory","Post-copulatory","Both"))
colnames(tab1)<-cbind('P-value')
tab1 P-value
Pre-copulatory 0.004
Post-copulatory 0.120
Both 0.000Plot sexual selection mode (Figure 2)
Here we plot the sexual selection mode moderator:
MetaData$SexSel_Mode=factor(MetaData$SexSel_Mode, levels = c("both","post-copulatory" ,"pre-copulatory"))
ggplot(MetaData, aes(x=SexSel_Mode, y=r, fill = SexSel_Mode, colour = SexSel_Mode)) +
geom_hline(yintercept=0, linetype="longdash", color = "black", linewidth=1)+
geom_flat_violin(position = position_nudge(x = 0.25, y = 0),adjust =1, trim = F,alpha=0.6)+
geom_point(position = position_jitter(width = .1), size = 2.5,alpha=0.6,stroke=0,shape=19)+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelMode$b[1,1], x=3.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelMode2$b[1,1], x=2.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelMode3$b[1,1], x=1.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelMode$ci.lb[1], x=3.25, xend= 3.25, yend= Model_REML_by_SexSelMode$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelMode2$ci.lb[1], x=2.25, xend= 2.25, yend= Model_REML_by_SexSelMode2$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelMode3$ci.lb[1], x=1.25, xend= 1.25, yend= Model_REML_by_SexSelMode3$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
ylab(expression(paste("Effect size (", italic("r"),')')))+xlab('Sexual selection mode')+coord_flip()+guides(fill = FALSE, colour = FALSE) +
scale_color_manual(values =colpal)+
scale_fill_manual(values =colpal)+
scale_x_discrete(labels=c("Both","Post-copulatory" ,"Pre-copulatory"),expand=c(.1,0))+
annotate("text", x=1, y=1.2, label= "n = 37",size=4.5) +
annotate("text", x=2, y=1.2, label= "n = 9",size=4.5) +
annotate("text", x=3, y=1.2, label= "n = 39",size=4.5) + theme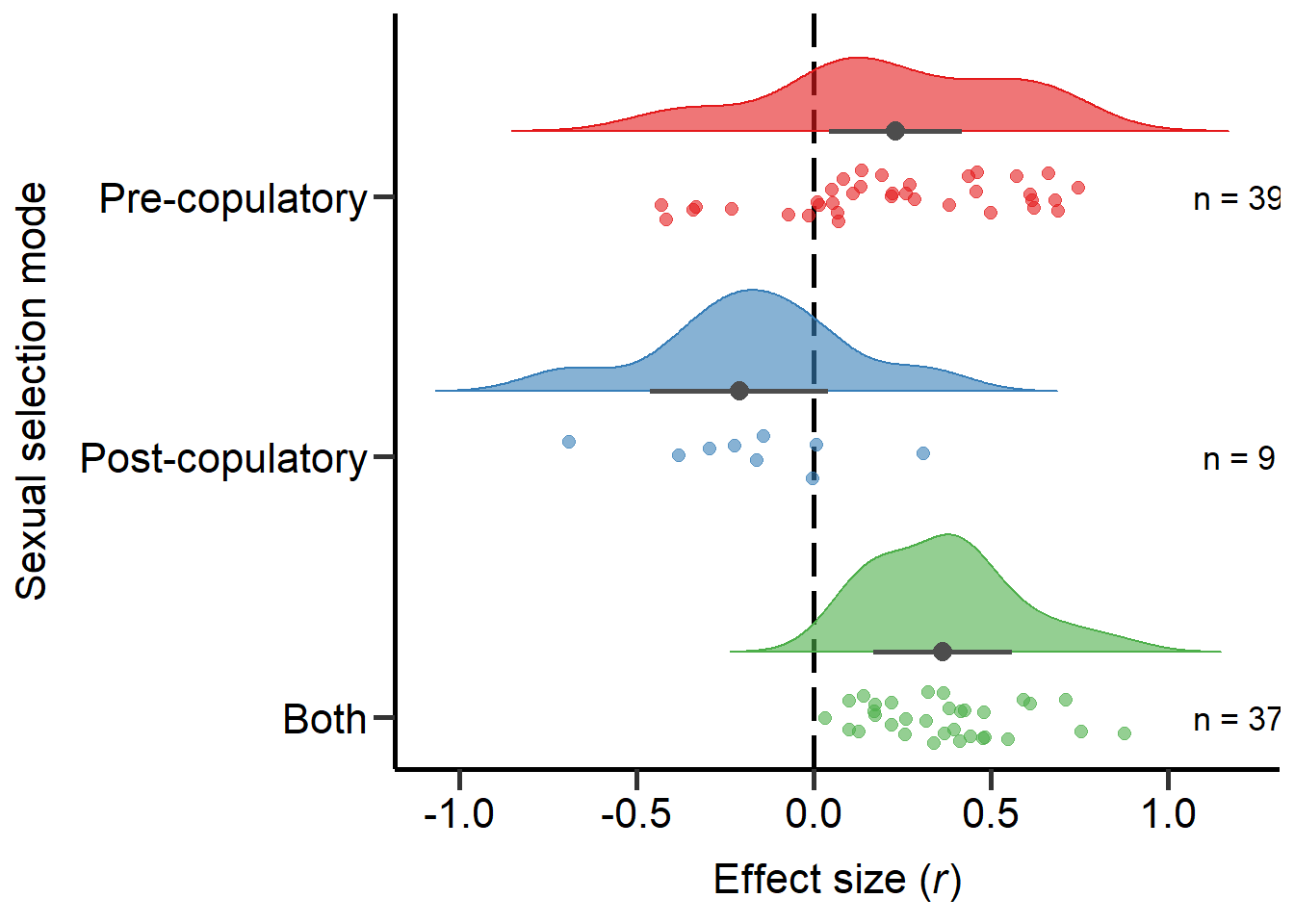
Figure 2: Raincloud plot of correlation coefficients between SSD and the modes of sexual selection proxies (i.e. pre-copulatory, post-copulatory or both) including sample sizes and estimates with 95%CI from phylogenetic model.
Sexual selection category
Next we explored the effect of the sexual selection category (i.e. density, mating system, operational sex ratio (OSR), post-mating competition, pre-mating competition, trait-based, other):
MetaData$SexSel_Category=as.factor(MetaData$SexSel_Category)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Postmating competition"))
Model_REML_by_SexSelCat = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelCat)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
1.5992 -3.1985 16.8015 39.2865 20.5304
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0099 0.0995 45 no Study_ID no
sigma^2.2 0.0280 0.1674 77 no Index no
sigma^2.3 0.0211 0.1452 9 no Class yes
Test for Residual Heterogeneity:
QE(df = 70) = 614.1683, p-val < .0001
Test of Moderators (coefficients 2:7):
QM(df = 6) = 64.7133, p-val < .0001
Model Results:
estimate se zval pval
intrcpt -0.2406 0.1219 -1.9737 0.0484
factor(SexSel_Category)Density 0.4303 0.1370 3.1415 0.0017
factor(SexSel_Category)Mating system 0.5912 0.1037 5.7025 <.0001
factor(SexSel_Category)OSR 0.8225 0.1502 5.4765 <.0001
factor(SexSel_Category)Other 0.5567 0.1141 4.8791 <.0001
factor(SexSel_Category)Premating competition 0.5294 0.1022 5.1790 <.0001
factor(SexSel_Category)Trait-based 0.1821 0.1149 1.5850 0.1130
ci.lb ci.ub
intrcpt -0.4795 -0.0017 *
factor(SexSel_Category)Density 0.1618 0.6988 **
factor(SexSel_Category)Mating system 0.3880 0.7944 ***
factor(SexSel_Category)OSR 0.5282 1.1169 ***
factor(SexSel_Category)Other 0.3331 0.7803 ***
factor(SexSel_Category)Premating competition 0.3290 0.7297 ***
factor(SexSel_Category)Trait-based -0.0431 0.4073
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Trait-based"))
Model_REML_by_SexSelCat2 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelCat2)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Density"))
Model_REML_by_SexSelCat3 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelCat3)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Premating competition"))
Model_REML_by_SexSelCat4 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelCat4)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Mating system"))
Model_REML_by_SexSelCat5 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelCat5)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("OSR"))
Model_REML_by_SexSelCat6 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelCat6)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Other"))
Model_REML_by_SexSelCat7 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SexSelCat7)Finally, we computed FDR corrected p-values:
tab2=as.data.frame(round(p.adjust(c(0.0705, 0.5745, 0.1057, 0.0002, .0001, .0001, 0.0011), method = 'fdr'),digit=3),row.names=cbind("Postmating competition","Trait-based","Density",'Premating competition',"Mating system","OSR","Other"))
colnames(tab2)<-cbind('P-value')
tab2 P-value
Postmating competition 0.099
Trait-based 0.575
Density 0.123
Premating competition 0.000
Mating system 0.000
OSR 0.000
Other 0.002Plot sexual selection category (Figure S1)
Here we plot the sexual selection category moderator:
MetaData$SexSel_Category=factor(MetaData$SexSel_Category, levels = rev(c( "Postmating competition","Trait-based" ,"Density","Premating competition" ,"Mating system" , "OSR", "Other")))
ggplot(MetaData, aes(x=SexSel_Category, y=r, fill = SexSel_Category, colour = SexSel_Category)) +
geom_hline(yintercept=0, linetype="longdash", color = "black", linewidth=1)+
geom_flat_violin(position = position_nudge(x = 0.25, y = 0),adjust =1, trim = F,alpha=0.6)+
geom_point(position = position_jitter(width = .1), size = 2.5,alpha=0.6,stroke=0,shape=19)+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat$b[1,1], x=7.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat2$b[1,1], x=6.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat3$b[1,1], x=5.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat4$b[1,1], x=4.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat5$b[1,1], x=3.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat6$b[1,1], x=2.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat7$b[1,1], x=1.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat$ci.lb[1], x=7.25, xend= 7.25, yend= Model_REML_by_SexSelCat$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat2$ci.lb[1], x=6.25, xend= 6.25, yend= Model_REML_by_SexSelCat2$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat3$ci.lb[1], x=5.25, xend= 5.25, yend= Model_REML_by_SexSelCat3$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat4$ci.lb[1], x=4.25, xend= 4.25, yend= Model_REML_by_SexSelCat4$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat5$ci.lb[1], x=3.25, xend= 3.25, yend= Model_REML_by_SexSelCat5$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat6$ci.lb[1], x=2.25, xend= 2.25, yend= Model_REML_by_SexSelCat6$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SexSelCat7$ci.lb[1], x=1.25, xend= 1.25, yend= Model_REML_by_SexSelCat7$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
ylab(expression(paste("Effect size (", italic("r"),')')))+xlab('Sexual selection category')+coord_flip()+guides(fill = FALSE, colour = FALSE) +
scale_color_manual(values =colpal2)+
scale_fill_manual(values =colpal2)+
scale_x_discrete(labels=rev(c( "Post-mating\ncompetition","Trait-based" ,"Density","Pre-mating\ncompetition" ,"Mating\nsystem" , "OSR", "Other")),expand=c(.1,0))+
annotate("text", x=7, y=1.2, label= "n = 9",size=4.5) +
annotate("text", x=6, y=1.2, label= "n = 12",size=4.5) +
annotate("text", x=5, y=1.2, label= "n = 5",size=4.5) +
annotate("text", x=4, y=1.2, label= "n = 18",size=4.5) +
annotate("text", x=3, y=1.2, label= "n = 23",size=4.5) +
annotate("text", x=2, y=1.2, label= "n = 6",size=4.5) +
annotate("text", x=1, y=1.2, label= "n = 12",size=4.5) + theme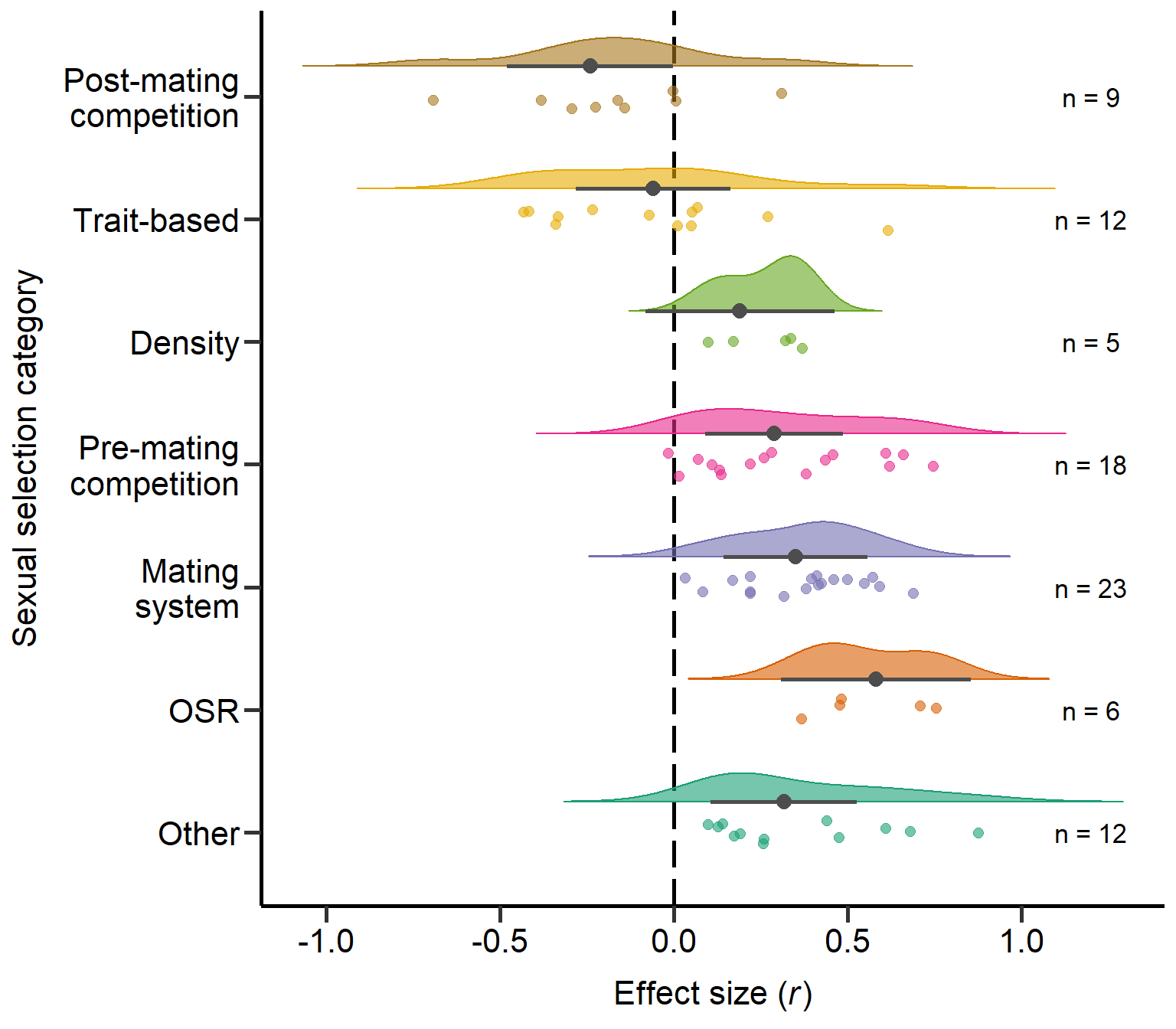
Figure S1: Raincloud plot of correlation coefficients between SSD and different sexual selection categories including sample sizes and estimates with 95%CI from phylogenetic model.
Type of SSD measure
Next we explored the effect of the type of SSD measure (i.e. body mass or size):
MetaData$SSD_Proxy=as.factor(MetaData$SSD_Proxy)
MetaData$SSD_Proxy=relevel(MetaData$SSD_Proxy,c("Body mass"))
Model_REML_by_SSDMeasure = rma.mv(r ~ SSD_Proxy, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SSDMeasure)
Multivariate Meta-Analysis Model (k = 75; method: REML)
logLik Deviance AIC BIC AICc
-19.3875 38.7751 48.7751 60.2274 49.6706
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0000 0.0000 44 no Study_ID no
sigma^2.2 0.0799 0.2826 75 no Index no
sigma^2.3 0.0120 0.1095 9 no Class yes
Test for Residual Heterogeneity:
QE(df = 73) = 1137.0026, p-val < .0001
Test of Moderators (coefficient 2):
QM(df = 1) = 2.4142, p-val = 0.1202
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 0.3354 0.1021 3.2857 0.0010 0.1353 0.5354 **
SSD_ProxyBody size -0.1372 0.0883 -1.5538 0.1202 -0.3102 0.0359
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$SSD_Proxy=relevel(MetaData$SSD_Proxy,c("Body size"))
Model_REML_by_SSDMeasure2 = rma.mv(r ~ SSD_Proxy, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_SSDMeasure2)Finally, we computed FDR corrected p-values:
tab3=as.data.frame(round(p.adjust(c(0.0004, 0.0008), method = 'fdr'),digit=3),row.names=cbind("Body mass","Body size"))
colnames(tab3)<-cbind('P-value')
tab3 P-value
Body mass 0.001
Body size 0.001Plot Type of SSD measure (Figure S2A)
Here we plot the type of SSD measure moderator:
MetaData_NAProxy=MetaData[!is.na(MetaData$SSD_Proxy),]
MetaData_NAProxy$SSD_Proxy=as.factor(MetaData_NAProxy$SSD_Proxy)
MetaData_NAProxy$SSD_Proxy=relevel(MetaData_NAProxy$SSD_Proxy,c("Body size"))
ggplot(MetaData_NAProxy, aes(x=SSD_Proxy, y=r, fill = SSD_Proxy, colour = SSD_Proxy)) +
geom_hline(yintercept=0, linetype="longdash", color = "black", linewidth=1)+
geom_flat_violin(position = position_nudge(x = 0.25, y = 0),adjust =1, trim = F,alpha=0.6)+
geom_point(position = position_jitter(width = .1), size = 2.5,alpha=0.6,stroke=0,shape=19)+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SSDMeasure$b[1,1], x=2.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_SSDMeasure2$b[1,1], x=1.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SSDMeasure$ci.lb[1], x=2.25, xend= 2.25, yend= Model_REML_by_SSDMeasure$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_SSDMeasure2$ci.lb[1], x=1.25, xend= 1.25, yend= Model_REML_by_SSDMeasure2$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
ylab(expression(paste("Effect size (", italic("r"),')')))+xlab('Type of SSD measure')+coord_flip()+guides(fill = FALSE, colour = FALSE) +
scale_color_manual(values =colpal4)+
scale_fill_manual(values =colpal4)+
scale_x_discrete(labels=rev(c("Body mass","Body size")),expand=c(.15,0))+
annotate("text", x=2, y=1.2, label= "n = 37",size=4.5) +
annotate("text", x=1, y=1.2, label= "n = 46",size=4.5) + theme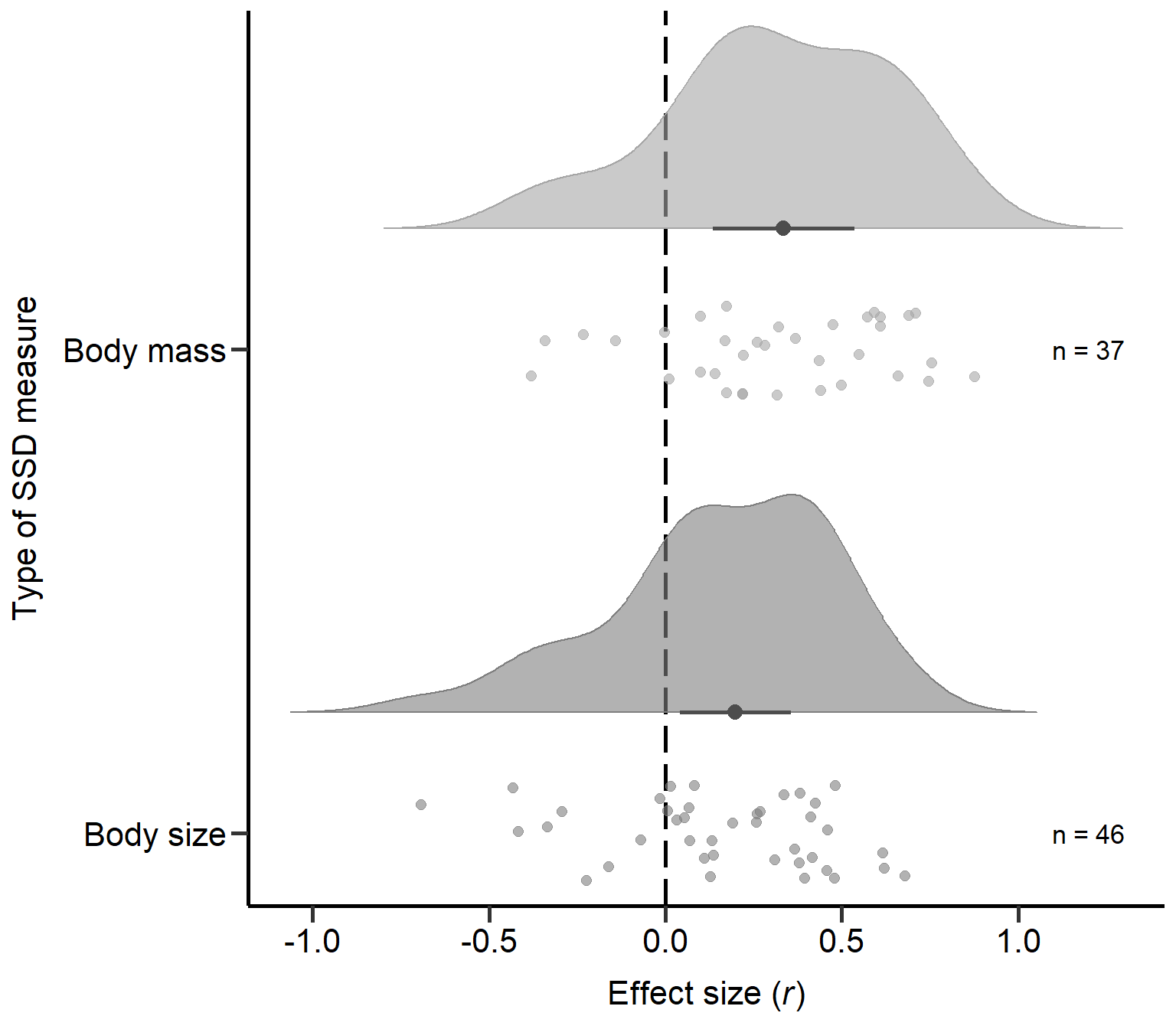
Figure S2A: Raincloud plot of correlation coefficients for different types of SSD measures (i.e. body mass or size) including sample sizes and estimates with 95% CI from phylogenetic model.
SSD measure controlled for body size?
Next we explored the effect if the primary study controlled the SSD for body size (i.e. uncontrolled or controlled):
MetaData$BodySizeControlled=as.factor(MetaData$BodySizeControlled)
MetaData$BodySizeControlled=relevel(MetaData$BodySizeControlled,c("No"))
Model_REML_by_BodySizeCont = rma.mv(r ~ BodySizeControlled, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_BodySizeCont)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-19.8069 39.6139 49.6139 61.2013 50.4835
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0000 0.0000 45 no Study_ID no
sigma^2.2 0.0768 0.2772 77 no Index no
sigma^2.3 0.0236 0.1537 9 no Class yes
Test for Residual Heterogeneity:
QE(df = 75) = 1181.8972, p-val < .0001
Test of Moderators (coefficient 2):
QM(df = 1) = 0.0691, p-val = 0.7927
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 0.2405 0.0995 2.4185 0.0156 0.0456 0.4355 *
BodySizeControlledYes -0.0266 0.1013 -0.2628 0.7927 -0.2252 0.1719
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$BodySizeControlled=relevel(MetaData$BodySizeControlled,c("Yes"))
Model_REML_by_BodySizeCont2 = rma.mv(r ~ BodySizeControlled, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML")
summary(Model_REML_by_BodySizeCont2)Finally, we computed FDR corrected p-values:
tab4=as.data.frame(round(p.adjust(c(0.0006, 0.0337), method = 'fdr'),digit=3),row.names=cbind("uncontrolled","controlled"))
colnames(tab4)<-cbind('P-value')
tab4 P-value
uncontrolled 0.001
controlled 0.034Plot: SSD measure controlled for body size? (Figure S2B)
Here we plot effect sizes if type of SSD measure controlled for body size:
MetaData$BodySizeControlled=as.factor(MetaData$BodySizeControlled)
MetaData$BodySizeControlled=relevel(MetaData$BodySizeControlled,c("Yes"))
ggplot(MetaData, aes(x=BodySizeControlled, y=r, fill = BodySizeControlled, colour = BodySizeControlled)) +
geom_hline(yintercept=0, linetype="longdash", color = "black", linewidth=1)+
geom_flat_violin(position = position_nudge(x = 0.25, y = 0),adjust =1, trim = F,alpha=0.6)+
geom_point(position = position_jitter(width = .1), size = 2.5,alpha=0.6,stroke=0,shape=19)+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_BodySizeCont$b[1,1], x=2.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_point(inherit.aes = F,mapping = aes(y=Model_REML_by_BodySizeCont2$b[1,1], x=1.25), size = 3.5,alpha=1,stroke=0,shape=19,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_BodySizeCont$ci.lb[1], x=2.25, xend= 2.25, yend= Model_REML_by_BodySizeCont$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
geom_segment(inherit.aes = F,mapping = aes(y=Model_REML_by_BodySizeCont2$ci.lb[1], x=1.25, xend= 1.25, yend= Model_REML_by_BodySizeCont2$ci.ub[1]), alpha=1,linewidth=1,color='grey30')+
ylab(expression(paste("Effect size (", italic("r"),')')))+xlab('SSD controlled for body size?')+coord_flip()+guides(fill = FALSE, colour = FALSE) +
scale_color_manual(values =colpal4)+
scale_fill_manual(values =colpal4)+
scale_x_discrete(labels=(c("Controlled","Uncontrolled")),expand=c(.15,0))+
annotate("text", x=2, y=1.2, label= "n = 69",size=4.5) +
annotate("text", x=1, y=1.2, label= "n = 16",size=4.5) + theme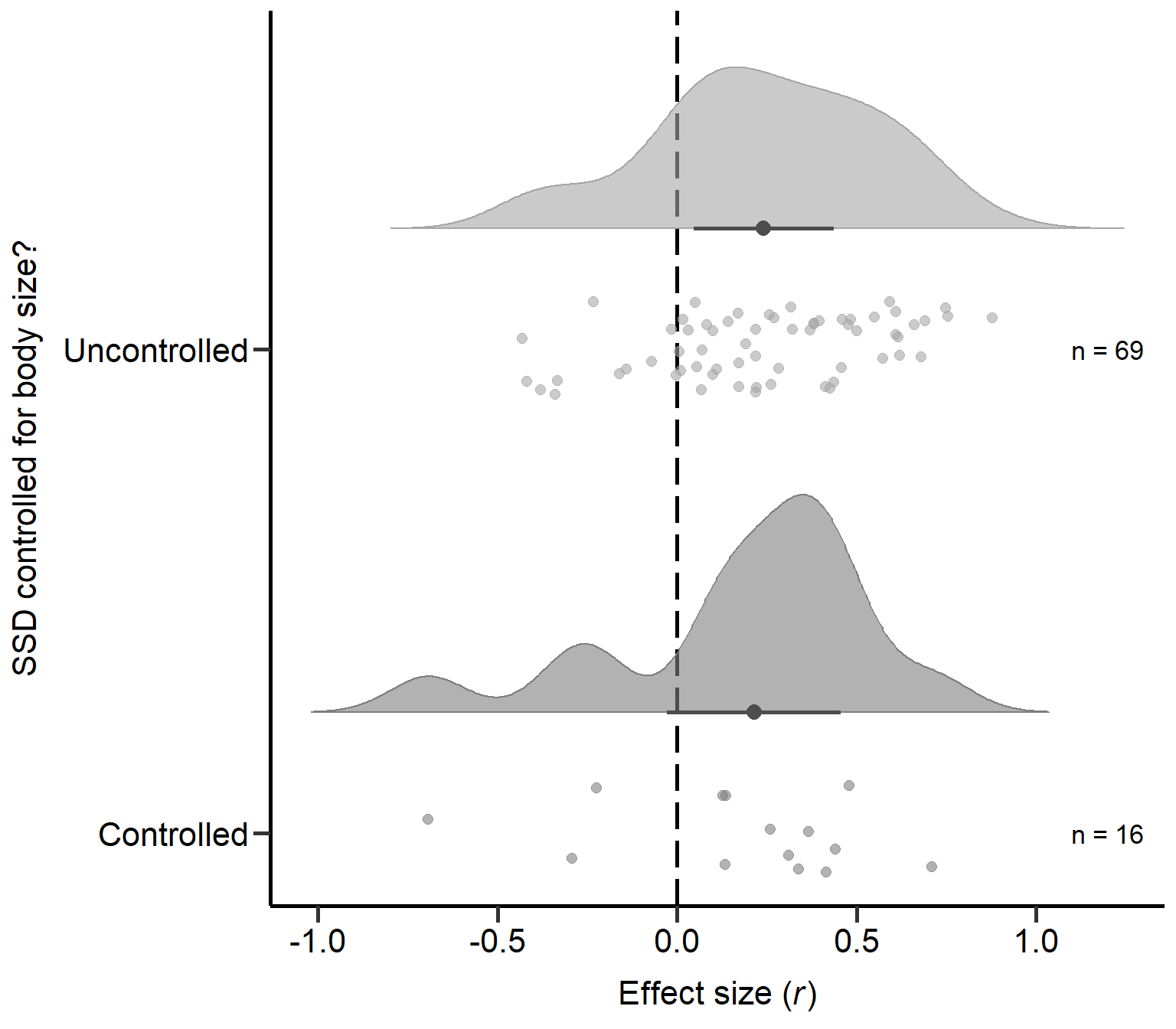
Figure S2B: Raincloud plot of correlation coefficients for primary studies controlling SSD for body size or mass (uncontrolled or controlled) including sample sizes and estimates with 95% CI from phylogenetic model.
Test for publication bias
To test for publication bias, we transformed r into z scores and ran multilevel mixed-effects models (restricted maximum likelihood) with z as the predictor and its standard error as the response with study ID and an observation level random effect. Models were weight by the mean standard error of z across all studies. While the variance in r depends on the effect size and the sample size, the variance in z is only dependent on the sample size. Hence, if z values correlate with the variance in z, this indicates that small studies were only published, if the effect was large, suggesting publication bias.
Model_REML_PublBias = rma.mv(z ~ SE_z, V=rep((mean(SE_z)*mean(SE_z))*N,length(SE_z)), data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML",control=list(rel.tol=1e-8))
summary(Model_REML_PublBias)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-91.3260 182.6520 192.6520 204.2394 193.5215
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0000 0.0000 45 no Study_ID no
sigma^2.2 0.0000 0.0000 77 no Index no
sigma^2.3 0.0000 0.0003 9 no Class yes
Test for Residual Heterogeneity:
QE(df = 75) = 14.1179, p-val = 1.0000
Test of Moderators (coefficient 2):
QM(df = 1) = 2.5184, p-val = 0.1125
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 0.0245 0.2601 0.0941 0.9250 -0.4853 0.5343
SE_z 1.5405 0.9708 1.5869 0.1125 -0.3621 3.4432
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Figure S2D
# Extract model predictions
model_p2 <- predict(Model_REML_PublBias)
ggplot(MetaData, aes(x=SE_z, y=z)) +
theme(panel.grid.major = element_blank(), panel.grid.minor = element_blank(),panel.background = element_blank(), axis.line = element_line(colour = "black"))+
theme(axis.text=element_text(size=13),
axis.title=element_text(size=14))+ theme(legend.position="none")+ylab(expression(paste("Effect size (", italic("z"),')')))+
geom_point(shape=16, size = 3,alpha=0.5)+xlab('Standard error')+
geom_hline(yintercept=0, linetype="dashed", color = "black", linewidth=1)+
geom_line( aes(y = model_p2$pred), size = 1)+
geom_ribbon( aes(ymin = model_p2$ci.lb, ymax = model_p2$ci.ub, color = 'black',linetype=NA), alpha = .15,show.legend = F, outline.type = "both") +
theme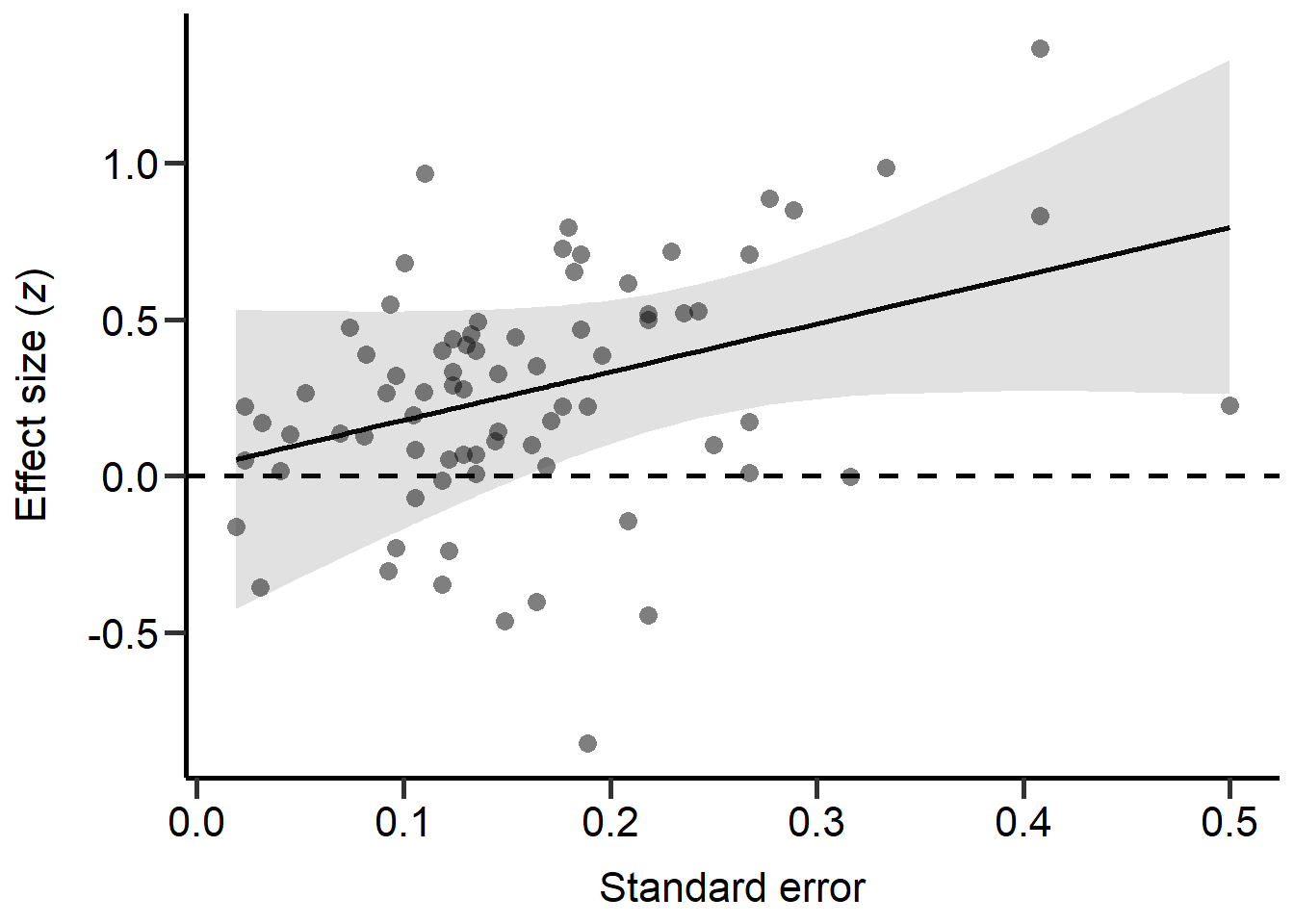
Figure S2D: Scatter plot of the standard error in z scores of each study against the z score of each primary study (transformed correlation coefficients). Dashed line marks a correlation coefficient of zero, black line represents predictions from REML model (controlling for phylogeny) and grey area represents 95% CI on model predictions.
Publication year
Next we explored the effect of the publication year of each study:
Model_REML_by_Year = rma.mv(r ~ Year, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML",control=list(rel.tol=1e-8))
summary(Model_REML_by_Year)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-19.5055 39.0110 49.0110 60.5985 49.8806
Variance Components:
estim sqrt nlvls fixed factor R
sigma^2.1 0.0000 0.0001 45 no Study_ID no
sigma^2.2 0.0754 0.2745 77 no Index no
sigma^2.3 0.0248 0.1575 9 no Class yes
Test for Residual Heterogeneity:
QE(df = 75) = 1060.4395, p-val < .0001
Test of Moderators (coefficient 2):
QM(df = 1) = 0.8325, p-val = 0.3616
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 7.7711 8.2609 0.9407 0.3469 -8.4200 23.9622
Year -0.0037 0.0041 -0.9124 0.3616 -0.0118 0.0043
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Plot publication year (Figure S2E)
Here we plot the publication year:
# Extract model predictions
Model_REML_by_Year = rma.mv(r ~ Year, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index, ~ 1 | Class), R = list(Class = forcedC_Moderators), method = "REML",control=list(rel.tol=1e-8))
model_p4 <- predict(Model_REML_by_Year)
ggplot(MetaData, aes(x=as.numeric(Year), y=r)) +
theme(panel.grid.major = element_blank(), panel.grid.minor = element_blank(),panel.background = element_blank(), axis.line = element_line(colour = "black"))+
theme(axis.text=element_text(size=13),
axis.title=element_text(size=14))+ theme(legend.position="none")+
geom_point(shape=16, size = 3,alpha=0.5)+xlab('Publication year')+ylab(expression(paste("Effect size (", italic("r"),')')))+
geom_hline(yintercept=0, linetype="dashed", color = "black", linewidth=1)+
geom_line( aes(y = model_p4$pred), size = 1)+
geom_ribbon( aes(ymin = model_p4$ci.lb, ymax = model_p4$ci.ub, color = 'black',linetype=NA), alpha = .15,show.legend = F, outline.type = "both") +
theme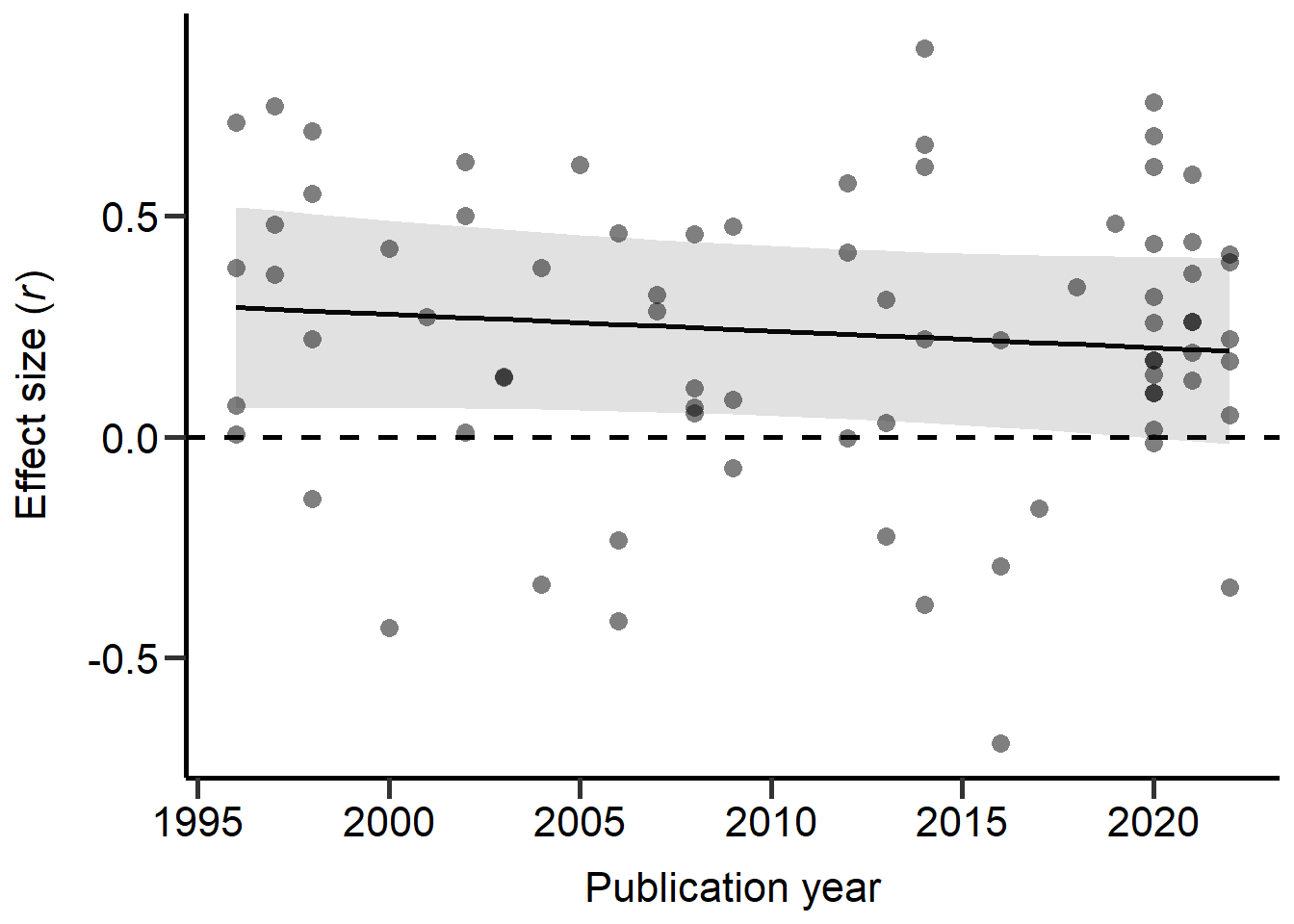
Figure S2E: Scatter plot of correlation coefficients against the publication year of each study. Dashed line marks a correlation coefficient of zero, black line represents predictions from REML model (controlling for phylogeny) and grey area represents 95% CI on model predictions.
Moderator tests for non-phylogenetic models
Here we ran all models without the phylogeny.
Sexual selection mode
The first model explores the effect of the sexual selection mode (i.e. pre-copulatory, post-copulatory or both):
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("pre-copulatory"))
Model_REML_by_cSexSelMode = rma.mv(r ~ factor(SexSel_Mode), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelMode)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-11.4194 22.8387 32.8387 44.3590 33.7211
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0094 0.0969 45 no Study_ID
sigma^2.2 0.0567 0.2381 77 no Index
Test for Residual Heterogeneity:
QE(df = 74) = 944.4229, p-val < .0001
Test of Moderators (coefficients 2:3):
QM(df = 2) = 25.9764, p-val < .0001
Model Results:
estimate se zval pval ci.lb
intrcpt 0.2187 0.0492 4.4484 <.0001 0.1223
factor(SexSel_Mode)both 0.1643 0.0710 2.3126 0.0207 0.0251
factor(SexSel_Mode)post-copulatory -0.4065 0.1105 -3.6780 0.0002 -0.6232
ci.ub
intrcpt 0.3150 ***
factor(SexSel_Mode)both 0.3036 *
factor(SexSel_Mode)post-copulatory -0.1899 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("post-copulatory"))
Model_REML_by_cSexSelMode2 = rma.mv(r ~ factor(SexSel_Mode), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelMode2)
MetaData$SexSel_Mode=relevel(MetaData$SexSel_Mode,c("both"))
Model_REML_by_cSexSelMode3 = rma.mv(r ~ factor(SexSel_Mode), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelMode3)Finally, we computed FDR corrected p-values:
tab1=as.data.frame(round(p.adjust(c(0.0001, 0.0756, .0001), method = 'fdr'),digit=3),row.names=cbind("Pre-copulatory","Post-copulatory","Both"))
colnames(tab1)<-cbind('P-value')
tab1 P-value
Pre-copulatory 0.000
Post-copulatory 0.076
Both 0.000Sexual selection category
Next we explored the effect of the sexual selection category (i.e. density, mating system, operational sex ratio (OSR), post-mating competition, pre-mating competition, trait-based, other):
MetaData$SexSel_Category=as.factor(MetaData$SexSel_Category)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Postmating competition"))
Model_REML_by_cSexSelCat = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelCat)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-0.3304 0.6607 18.6607 38.8972 21.6607
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0196 0.1399 45 no Study_ID
sigma^2.2 0.0290 0.1704 77 no Index
Test for Residual Heterogeneity:
QE(df = 70) = 614.1683, p-val < .0001
Test of Moderators (coefficients 2:7):
QM(df = 6) = 64.8410, p-val < .0001
Model Results:
estimate se zval pval
intrcpt -0.1780 0.0913 -1.9498 0.0512
factor(SexSel_Category)Other 0.5575 0.1200 4.6453 <.0001
factor(SexSel_Category)OSR 0.7963 0.1520 5.2401 <.0001
factor(SexSel_Category)Mating system 0.5724 0.1067 5.3629 <.0001
factor(SexSel_Category)Premating competition 0.5179 0.1091 4.7481 <.0001
factor(SexSel_Category)Density 0.4395 0.1440 3.0525 0.0023
factor(SexSel_Category)Trait-based 0.1278 0.1152 1.1092 0.2674
ci.lb ci.ub
intrcpt -0.3570 0.0009 .
factor(SexSel_Category)Other 0.3223 0.7927 ***
factor(SexSel_Category)OSR 0.4985 1.0942 ***
factor(SexSel_Category)Mating system 0.3632 0.7816 ***
factor(SexSel_Category)Premating competition 0.3041 0.7316 ***
factor(SexSel_Category)Density 0.1573 0.7217 **
factor(SexSel_Category)Trait-based -0.0980 0.3536
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Trait-based"))
Model_REML_by_cSexSelCat2 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelCat2)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Density"))
Model_REML_by_cSexSelCat3 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelCat3)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Premating competition"))
Model_REML_by_cSexSelCat4 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelCat4)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Mating system"))
Model_REML_by_cSexSelCat5 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelCat5)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("OSR"))
Model_REML_by_cSexSelCat6 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelCat6)
MetaData$SexSel_Category=relevel(MetaData$SexSel_Category,c("Other"))
Model_REML_by_cSexSelCat7 = rma.mv(r ~ factor(SexSel_Category), V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSexSelCat7)Finally, we computed FDR corrected p-values:
tab2=as.data.frame(round(p.adjust(c(0.0685, 0.5996, 0.0151, .0001, .0001, .0001, .0001), method = 'fdr'),digit=3),row.names=cbind("Postmating competition","Trait-based","Density",'Premating competition',"Mating system","OSR","Other"))
colnames(tab2)<-cbind('P-value')
tab2 P-value
Postmating competition 0.080
Trait-based 0.600
Density 0.021
Premating competition 0.000
Mating system 0.000
OSR 0.000
Other 0.000Phylogenetic classes
Next we explored the effect of the phylogenetic classes:
MetaData$Class=as.factor(MetaData$Class)
MetaData$Class=relevel(MetaData$Class,c("Nematoda"))
Model_cREML_by_Class = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-16.1287 32.2574 54.2574 78.6720 58.9717
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0000 0.0000 45 no Study_ID
sigma^2.2 0.0774 0.2782 77 no Index
Test for Residual Heterogeneity:
QE(df = 68) = 776.5810, p-val < .0001
Test of Moderators (coefficients 2:9):
QM(df = 8) = 17.6591, p-val = 0.0239
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 0.4222 0.2298 1.8376 0.0661 -0.0281 0.8726 .
ClassActinopterygii -0.2636 0.2605 -1.0118 0.3116 -0.7741 0.2470
ClassAmphibia -0.3092 0.2763 -1.1189 0.2632 -0.8507 0.2324
ClassAnimalia -0.1963 0.3107 -0.6318 0.5275 -0.8053 0.4127
ClassAves -0.3019 0.2390 -1.2629 0.2066 -0.7704 0.1666
ClassInsecta -0.1607 0.2684 -0.5987 0.5494 -0.6866 0.3653
ClassMammalia -0.0199 0.2370 -0.0841 0.9329 -0.4844 0.4445
ClassPisces -0.1639 0.3641 -0.4502 0.6526 -0.8776 0.5498
ClassReptilia -0.4246 0.2551 -1.6645 0.0960 -0.9246 0.0754 .
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$Class=relevel(MetaData$Class,c("Insecta"))
Model_cREML_by_Class2 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class2)
MetaData$Class=relevel(MetaData$Class,c("Aves"))
Model_cREML_by_Class3 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class3)
MetaData$Class=relevel(MetaData$Class,c("Reptilia"))
Model_cREML_by_Class4 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class4)
MetaData$Class=relevel(MetaData$Class,c("Mammalia"))
Model_cREML_by_Class5 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class5)
MetaData$Class=relevel(MetaData$Class,c("Amphibia"))
Model_cREML_by_Class6 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class6)
MetaData$Class=relevel(MetaData$Class,c("Actinopterygii"))
Model_cREML_by_Class7 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class7)
MetaData$Class=relevel(MetaData$Class,c("Pisces"))
Model_cREML_by_Class8 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class8)
MetaData$Class=relevel(MetaData$Class,c("Animalia"))
Model_cREML_by_Class9 = rma.mv(r ~ Class, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Class9)Finally, we computed FDR corrected p-values:
tab2=as.data.frame(round(p.adjust(c(0.2557 ,0.4534 , 0.3747 ,0.0048 ,0.0016 ,.0001 ,0.1202 , 0.3966 ,0.4405 ), method = 'fdr'),digit=3),row.names=cbind( "Nematoda","Insecta","Aves","Reptilia","Mammalia","Amphibia","Actinopterygii","Pisces","Animalia"))
colnames(tab2)<-cbind('P-value')
tab2 P-value
Nematoda 0.453
Insecta 0.453
Aves 0.453
Reptilia 0.014
Mammalia 0.007
Amphibia 0.001
Actinopterygii 0.270
Pisces 0.453
Animalia 0.453Plot phylogenetic classes (Figure 3)
Here we plot the phylogenetic classes:
MetaData$Class=as.factor(MetaData$Class)
MetaData$Class=factor(MetaData$Class, levels = (c("Animalia","Pisces" ,"Actinopterygii","Amphibia","Mammalia","Reptilia","Aves","Insecta","Nematoda")))
ggplot(MetaData, aes(x=Class, y=r, fill = Class, colour = Class)) +
geom_hline(yintercept=0, linetype="longdash", color = "black", linewidth=1)+
geom_point(position = position_jitter(width = .1), size = 2.5,alpha=0.8,stroke=0,shape=19)+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class$b[1,1], x=9.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[9])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class2$b[1,1], x=8.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[8])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class3$b[1,1], x=7.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[7])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class4$b[1,1], x=6.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[6])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class5$b[1,1], x=5.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[5])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class6$b[1,1], x=4.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[4])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class7$b[1,1], x=3.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[3])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class8$b[1,1], x=2.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[2])+
geom_point(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class9$b[1,1], x=1.35), size = 3.5,alpha=1,stroke=0,shape=19,color=colpal3[1])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class$ci.lb[1], x=9.35, xend= 9.35, yend= Model_cREML_by_Class$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[9])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class2$ci.lb[1], x=8.35, xend= 8.35, yend= Model_cREML_by_Class2$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[8])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class3$ci.lb[1], x=7.35, xend= 7.35, yend= Model_cREML_by_Class3$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[7])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class4$ci.lb[1], x=6.35, xend= 6.35, yend= Model_cREML_by_Class4$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[6])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class5$ci.lb[1], x=5.35, xend= 5.35, yend= Model_cREML_by_Class5$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[5])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class6$ci.lb[1], x=4.35, xend= 4.35, yend= Model_cREML_by_Class6$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[4])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class7$ci.lb[1], x=3.35, xend= 3.35, yend= Model_cREML_by_Class7$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[3])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class8$ci.lb[1], x=2.35, xend= 2.35, yend= Model_cREML_by_Class8$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[2])+
geom_segment(inherit.aes = F,mapping = aes(y=Model_cREML_by_Class9$ci.lb[1], x=1.35, xend= 1.35, yend= Model_cREML_by_Class9$ci.ub[1]), alpha=1,linewidth=1,color=colpal3[1])+
ylab(expression(paste("Effect size (", italic("r"),')')))+xlab('Class')+coord_flip()+guides(fill = FALSE, colour = FALSE) +
scale_color_manual(values =colpal3)+
scale_fill_manual(values =colpal3)+
scale_x_discrete(labels=(c("Animalia","Pisces" ,"Actinopterygii","Amphibia","Mammalia","Reptilia","Aves","Insecta","Nematoda")),expand=c(.1,0))+
theme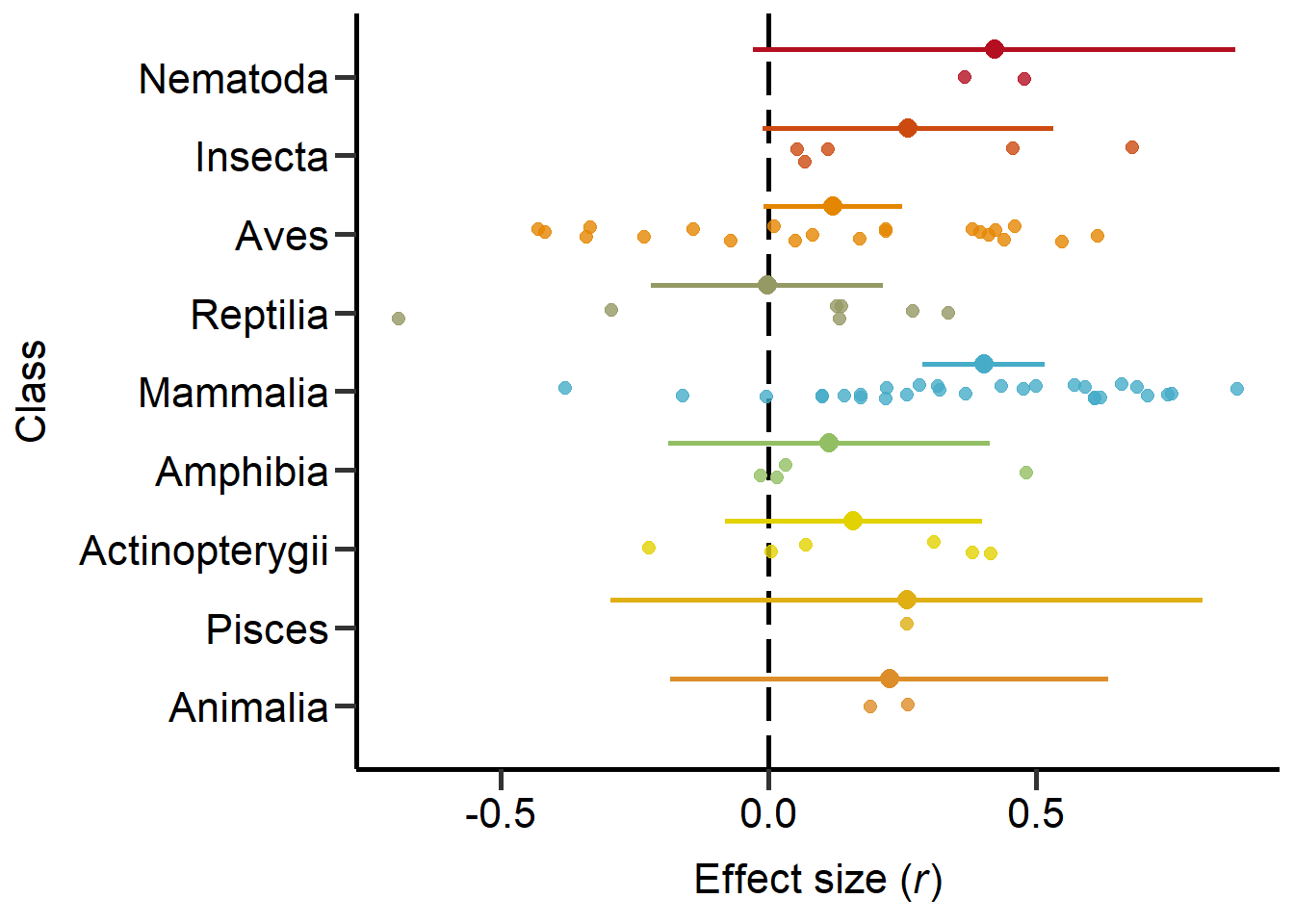
Figure 3: Phylogeny based on classes including sample sizes for the number of studies and number of effect sizes, respectively, and estimates with 95%CI from non-phylogenetic model.
Type of SSD measure
Next we explored the effect of the type of SSD measure (i.e. body mass or size):
MetaData$SSD_Proxy=as.factor(MetaData$SSD_Proxy)
MetaData$SSD_Proxy=relevel(MetaData$SSD_Proxy,c("Body mass"))
Model_REML_by_cSSDMeasure = rma.mv(r ~ SSD_Proxy, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSSDMeasure)
Multivariate Meta-Analysis Model (k = 75; method: REML)
logLik Deviance AIC BIC AICc
-20.0151 40.0302 48.0302 57.1920 48.6184
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0000 0.0000 44 no Study_ID
sigma^2.2 0.0856 0.2925 75 no Index
Test for Residual Heterogeneity:
QE(df = 73) = 1137.0026, p-val < .0001
Test of Moderators (coefficient 2):
QM(df = 1) = 6.0488, p-val = 0.0139
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 0.3367 0.0549 6.1291 <.0001 0.2290 0.4443 ***
SSD_ProxyBody size -0.1825 0.0742 -2.4594 0.0139 -0.3279 -0.0371 *
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$SSD_Proxy=relevel(MetaData$SSD_Proxy,c("Body size"))
Model_REML_by_cSSDMeasure2 = rma.mv(r ~ SSD_Proxy, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cSSDMeasure2)Finally, we computed FDR corrected p-values:
tab3=as.data.frame(round(p.adjust(c(.0001, .0001), method = 'fdr'),digit=3),row.names=cbind("Body mass","Body size"))
colnames(tab3)<-cbind('P-value')
tab3 P-value
Body mass 0
Body size 0SSD measure controlled for body size?
Next we explored the effect if the primary study controlled the SSD for body size (i.e. uncontrolled or controlled):
MetaData$BodySizeControlled=as.factor(MetaData$BodySizeControlled)
MetaData$BodySizeControlled=relevel(MetaData$BodySizeControlled,c("No"))
Model_REML_by_cBodySizeCont = rma.mv(r ~ BodySizeControlled, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cBodySizeCont)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-22.0756 44.1512 52.1512 61.4212 52.7227
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0149 0.1220 45 no Study_ID
sigma^2.2 0.0769 0.2773 77 no Index
Test for Residual Heterogeneity:
QE(df = 75) = 1181.8972, p-val < .0001
Test of Moderators (coefficient 2):
QM(df = 1) = 0.5960, p-val = 0.4401
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 0.2547 0.0447 5.6926 <.0001 0.1670 0.3424 ***
BodySizeControlledYes -0.0787 0.1020 -0.7720 0.4401 -0.2786 0.1212
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1We then re-leveled the model for post-hoc comparisons:
MetaData$BodySizeControlled=relevel(MetaData$BodySizeControlled,c("Yes"))
Model_REML_by_cBodySizeCont2 = rma.mv(r ~ BodySizeControlled, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_REML_by_cBodySizeCont2)Finally, we computed FDR corrected p-values:
tab4=as.data.frame(round(p.adjust(c(.0001, 0.0168), method = 'fdr'),digit=3),row.names=cbind("uncontrolled","controlled"))
colnames(tab4)<-cbind('P-value')
tab4 P-value
uncontrolled 0.000
controlled 0.017Test for publication bias
Model_cREML_PublBias = rma.mv(z ~ SE_z, V=rep(((mean(SE_z)*mean(SE_z))*N),length(SE_z)), data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_PublBias)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-91.3260 182.6520 190.6520 199.9219 191.2234
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0000 0.0000 45 no Study_ID
sigma^2.2 0.0000 0.0000 77 no Index
Test for Residual Heterogeneity:
QE(df = 75) = 14.1179, p-val = 1.0000
Test of Moderators (coefficient 2):
QM(df = 1) = 2.5184, p-val = 0.1125
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 0.0245 0.2601 0.0941 0.9250 -0.4853 0.5343
SE_z 1.5405 0.9708 1.5869 0.1125 -0.3621 3.4432
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Publication year
Next we explored the effect of the publication year of each study:
Model_cREML_by_Year = rma.mv(r ~ Year, V=Var_r, data = MetaData, random = c(~ 1 | Study_ID,~ 1 | Index), method = "REML")
summary(Model_cREML_by_Year)
Multivariate Meta-Analysis Model (k = 77; method: REML)
logLik Deviance AIC BIC AICc
-22.0838 44.1677 52.1677 61.4376 52.7391
Variance Components:
estim sqrt nlvls fixed factor
sigma^2.1 0.0193 0.1390 45 no Study_ID
sigma^2.2 0.0733 0.2707 77 no Index
Test for Residual Heterogeneity:
QE(df = 75) = 1060.4395, p-val < .0001
Test of Moderators (coefficient 2):
QM(df = 1) = 0.5199, p-val = 0.4709
Model Results:
estimate se zval pval ci.lb ci.ub
intrcpt 7.0575 9.4535 0.7466 0.4553 -11.4710 25.5860
Year -0.0034 0.0047 -0.7210 0.4709 -0.0126 0.0058
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1Forest plot
# Sort data by Class
MetaData$Class=as.factor(MetaData$Class)
MetaData$Class=factor(MetaData$Class, levels = c("Animalia","Pisces" ,"Actinopterygii","Amphibia","Mammalia","Reptilia","Aves","Insecta","Nematoda"))
MetaData_sorted <-MetaData[order(MetaData$Class),]
# Add global effect size to data
forestData=MetaData_sorted[,c(3,5,12,17,20,21)]
GlobalES=as.data.frame(cbind('Global effect size','','Global effect size',Model_REML_Null$b[1,1],Model_REML_Null$ci.lb[1],Model_REML_Null$ci.ub[1]))
colnames(GlobalES)=c('AuthorsAndYear','Class','SexSel_Mode','r','lCI','uCI')
forestData=rbind(GlobalES,forestData)
forestData[,c(4:6)]=lapply(forestData[,c(4:6)],as.numeric)
forestData$SexSel_Mode=as.factor(forestData$SexSel_Mode)
forestData$SexSel_Mode=factor(forestData$SexSel_Mode, levels = c("pre-copulatory","post-copulatory" ,"both","Global effect size"))Figure 1
ggplot(data=forestData, aes(y=1:nrow(forestData), x=r, xmin=lCI, xmax=uCI,color=SexSel_Mode)) +
geom_vline(xintercept=0, color='black', linetype='dashed', alpha=.5,linewidth=0.8) +
geom_point(size = ifelse(1:nrow(forestData) == c(1), 2.75, 1.8))+
geom_errorbarh(height=0,size = ifelse(1:nrow(forestData) == c(1), 1.25, 1))+
scale_y_continuous(breaks=seq(1, nrow(forestData), by=1), labels=forestData$AuthorsAndYear,limits=c(1,length(forestData$AuthorsAndYear)),expand=c(0.015,0.015)) +
labs(title='', x=expression(paste(italic("r "),'(95% CI)')), y = 'Study') +
theme_classic()+theme(axis.text.x=element_text(size=12),
axis.title=element_text(size=12))+ labs(color = c("Sexual selection mode"))+
scale_colour_manual(values=Meta_col, labels=c('Pre-copulatory','Post-copulatory',"Both","Global effect size"))+
guides(color = guide_legend(override.aes = list(size = .9)))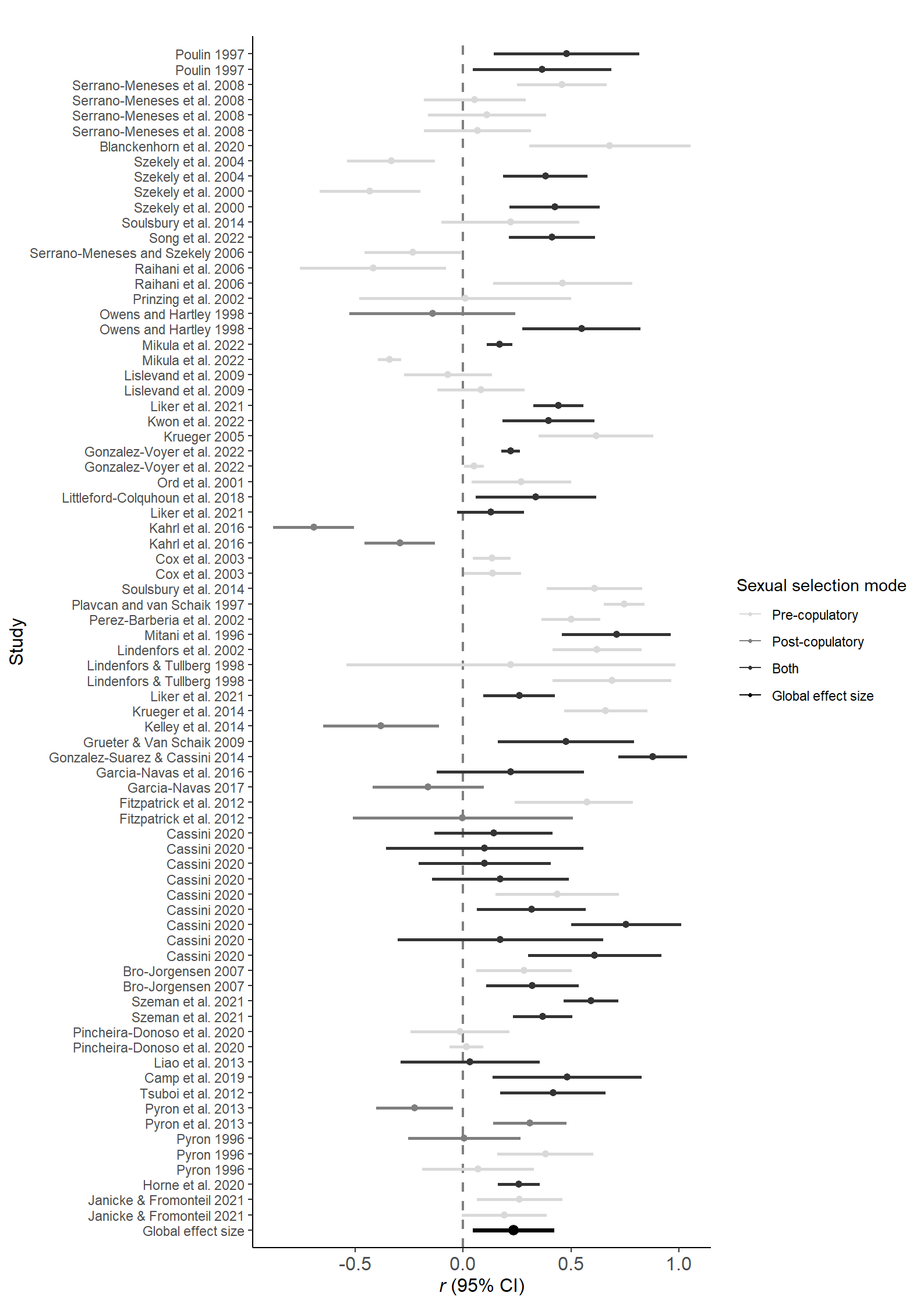
Figure 1: Forest plot including correlation coefficients (±95% CI), as well as the global effect size (in grey) with and without controlling for phylogeny. Different animal classes highlighted by background colors (see Figure 3 for class names) and sexual selection modes pre- (red), post-copulatory (blue) and both (green) by color of effect size.
sessionInfo()R version 4.2.3 (2023-03-15 ucrt)
Platform: x86_64-w64-mingw32/x64 (64-bit)
Running under: Windows 10 x64 (build 19045)
Matrix products: default
locale:
[1] LC_COLLATE=German_Germany.utf8 LC_CTYPE=German_Germany.utf8
[3] LC_MONETARY=German_Germany.utf8 LC_NUMERIC=C
[5] LC_TIME=German_Germany.utf8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] wesanderson_0.3.6 PupillometryR_0.0.4 rlang_1.1.0
[4] dplyr_1.1.1 cowplot_1.1.1 ggdist_3.3.0
[7] MCMCglmm_2.34 coda_0.19-4 RColorBrewer_1.1-3
[10] ggplot2_3.4.2 data.table_1.14.8 multcomp_1.4-23
[13] TH.data_1.1-2 survival_3.5-3 mvtnorm_1.1-3
[16] psych_2.3.3 pwr_1.3-0 MASS_7.3-58.2
[19] metafor_4.2-0 numDeriv_2016.8-1.1 metadat_1.2-0
[22] Matrix_1.5-3 matrixcalc_1.0-6 ape_5.7-1
[25] workflowr_1.7.0
loaded via a namespace (and not attached):
[1] httr_1.4.5 sass_0.4.5 jsonlite_1.8.4
[4] splines_4.2.3 bslib_0.4.2 getPass_0.2-2
[7] distributional_0.3.2 highr_0.10 tensorA_0.36.2
[10] yaml_2.3.7 pillar_1.9.0 lattice_0.20-45
[13] glue_1.6.2 digest_0.6.31 promises_1.2.0.1
[16] colorspace_2.1-0 sandwich_3.0-2 htmltools_0.5.5
[19] httpuv_1.6.9 pkgconfig_2.0.3 corpcor_1.6.10
[22] scales_1.2.1 processx_3.8.0 whisker_0.4.1
[25] later_1.3.0 cubature_2.0.4.6 git2r_0.31.0
[28] tibble_3.2.1 generics_0.1.3 farver_2.1.1
[31] cachem_1.0.7 withr_2.5.0 cli_3.6.1
[34] mnormt_2.1.1 magrittr_2.0.3 evaluate_0.20
[37] ps_1.7.3 fs_1.6.1 fansi_1.0.4
[40] nlme_3.1-162 tools_4.2.3 lifecycle_1.0.3
[43] stringr_1.5.0 munsell_0.5.0 callr_3.7.3
[46] compiler_4.2.3 jquerylib_0.1.4 grid_4.2.3
[49] rstudioapi_0.14 labeling_0.4.2 rmarkdown_2.21
[52] gtable_0.3.3 codetools_0.2-19 R6_2.5.1
[55] zoo_1.8-12 knitr_1.42 fastmap_1.1.1
[58] utf8_1.2.3 mathjaxr_1.6-0 rprojroot_2.0.3
[61] stringi_1.7.12 parallel_4.2.3 Rcpp_1.0.10
[64] vctrs_0.6.1 tidyselect_1.2.0 xfun_0.38