Analysis of Experiment 2
Last updated: 2020-05-02
Checks: 6 1
Knit directory: social_immunity/
This reproducible R Markdown analysis was created with workflowr (version 1.6.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20191017) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/experiment2.Rmd) and HTML (docs/experiment2.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| html | fa8c179 | lukeholman | 2020-05-02 | Build site. |
| Rmd | f188968 | lukeholman | 2020-05-02 | tweak colours |
| html | f188968 | lukeholman | 2020-05-02 | tweak colours |
| html | 2227713 | lukeholman | 2020-05-02 | Build site. |
| Rmd | f97baee | lukeholman | 2020-05-02 | Lots of formatting changes |
| html | f97baee | lukeholman | 2020-05-02 | Lots of formatting changes |
| html | 1c9a1c3 | lukeholman | 2020-05-02 | Build site. |
| Rmd | 3d21d6a | lukeholman | 2020-05-02 | wflow_publish("*", republish = T) |
| html | 3d21d6a | lukeholman | 2020-05-02 | wflow_publish("*", republish = T) |
| html | 93c487a | lukeholman | 2020-04-30 | Build site. |
| html | 5c45197 | lukeholman | 2020-04-30 | Build site. |
| html | 4bd75dc | lukeholman | 2020-04-30 | Build site. |
| Rmd | 12953af | lukeholman | 2020-04-30 | test new theme |
| html | 12953af | lukeholman | 2020-04-30 | test new theme |
| html | d6437a5 | lukeholman | 2020-04-25 | Build site. |
| html | e58e720 | lukeholman | 2020-04-25 | Build site. |
| html | 71b6160 | lukeholman | 2020-04-25 | Build site. |
| Rmd | 76a317d | lukeholman | 2020-04-25 | tweaks |
| html | 2235ae4 | lukeholman | 2020-04-25 | Build site. |
| Rmd | 99649a7 | lukeholman | 2020-04-25 | tweaks |
| html | 99649a7 | lukeholman | 2020-04-25 | tweaks |
| html | 0ede6e3 | lukeholman | 2020-04-24 | Build site. |
| Rmd | a1f8dc2 | lukeholman | 2020-04-24 | tweaks |
| html | 8c3b471 | lukeholman | 2020-04-21 | Build site. |
| Rmd | 1ce9e19 | lukeholman | 2020-04-21 | First commit 2020 |
| html | 1ce9e19 | lukeholman | 2020-04-21 | First commit 2020 |
| Rmd | aae65cf | lukeholman | 2019-10-17 | First commit |
| html | aae65cf | lukeholman | 2019-10-17 | First commit |
Load data and R packages
# All but 1 of these packages can be easily installed from CRAN.
# However it was harder to install the showtext package. On Mac, I did this:
# installed 'homebrew' using Terminal: ruby -e "$(curl -fsSL https://raw.githubusercontent.com/Homebrew/install/master/install)"
# installed 'libpng' using Terminal: brew install libpng
# installed 'showtext' in R using: devtools::install_github("yixuan/showtext")
library(showtext)
library(brms)
library(bayesplot)
library(tidyverse)
library(gridExtra)
library(kableExtra)
library(bayestestR)
library(tidybayes)
library(cowplot)
source("code/helper_functions.R")
# set up nice font for figure
nice_font <- "Lora"
font_add_google(name = nice_font, family = nice_font, regular.wt = 400, bold.wt = 700)
showtext_auto()
exp2_treatments <- c("Ringers", "LPS")
durations <- read_csv("data/data_collection_sheets/experiment_durations.csv") %>%
filter(experiment == 2) %>% select(-experiment)
outcome_tally <- read_csv(file = "data/clean_data/experiment_2_outcome_tally.csv") %>%
mutate(
outcome = str_replace_all(outcome, "Stayed inside the hive", "Stayed inside"),
outcome = str_replace_all(outcome, "Left of own volition", "Left voluntarily"),
outcome = factor(outcome, levels = c("Stayed inside", "Left voluntarily", "Forced out")),
treatment = str_replace_all(treatment, "Ringer CHC", "Ringers"),
treatment = str_replace_all(treatment, "LPS CHC", "LPS"),
treatment = factor(treatment, levels = exp2_treatments))
# Re-formatted version of the same data, where each row is an individual bee. We need this format to run the brms model.
data_for_categorical_model <- outcome_tally %>%
mutate(id = 1:n()) %>%
split(.$id) %>%
map(function(x){
if(x$n[1] == 0) return(NULL)
data.frame(
treatment = x$treatment[1],
hive = x$hive[1],
colour = x$colour[1],
outcome = rep(x$outcome[1], x$n))
}) %>% do.call("rbind", .) %>% as_tibble() %>%
arrange(hive, treatment) %>%
mutate(outcome_numeric = as.numeric(outcome),
hive = as.character(hive),
treatment = factor(treatment, levels = exp2_treatments)) %>%
left_join(durations, by = "hive") %>%
mutate(hive = C(factor(hive), sum)) # use "sum coding" for hive, since there is no obvious reference levelInspect the raw data
Click the three tabs to see each table.
Sample sizes by treatment
sample_sizes <- data_for_categorical_model %>%
group_by(treatment) %>%
summarise(n = n())
sample_sizes %>%
kable() %>% kable_styling(full_width = FALSE)| treatment | n |
|---|---|
| Ringers | 294 |
| LPS | 291 |
Sample sizes by treatment and hive
data_for_categorical_model %>%
group_by(hive, treatment) %>%
summarise(n = n()) %>%
spread(treatment, n) %>%
kable() %>% kable_styling(full_width = FALSE)| hive | Ringers | LPS |
|---|---|---|
| Arts | 70 | 68 |
| Garden | 75 | 75 |
| Skylab | 99 | 100 |
| Zoology | 50 | 48 |
Oberved outcomes
outcome_tally %>%
select(-colour) %>%
spread(outcome, n) %>%
kable(digits = 3) %>% kable_styling(full_width = FALSE) | hive | treatment | Stayed inside | Left voluntarily | Forced out |
|---|---|---|---|---|
| Arts | Ringers | 64 | 5 | 1 |
| Arts | LPS | 56 | 5 | 7 |
| Garden | Ringers | 73 | 2 | 0 |
| Garden | LPS | 70 | 2 | 3 |
| Skylab | Ringers | 97 | 1 | 1 |
| Skylab | LPS | 93 | 2 | 5 |
| Zoology | Ringers | 42 | 2 | 6 |
| Zoology | LPS | 38 | 4 | 6 |
Plot showing raw means and 95% CI of the mean
pd <- position_dodge(.3)
outcome_tally %>%
group_by(treatment, outcome) %>%
summarise(n = sum(n)) %>% mutate() %>%
group_by(treatment) %>%
mutate(total_n = sum(n),
percent = 100 * n / sum(n),
SE = sqrt(total_n * (percent/100) * (1-(percent/100)))) %>%
ungroup() %>%
mutate(lowerCI = map_dbl(1:n(), ~ 100 * binom.test(n[.x], total_n[.x])$conf.int[1]),
upperCI = map_dbl(1:n(), ~ 100 * binom.test(n[.x], total_n[.x])$conf.int[2])) %>%
filter(outcome != "Stayed inside") %>%
ggplot(aes(treatment, percent, fill = outcome)) +
geom_errorbar(aes(ymin=lowerCI, ymax=upperCI), position = pd, width = 0) +
geom_point(stat = "identity", position = pd, colour = "grey15", pch = 21, size = 4) +
scale_fill_brewer(palette = "Pastel1", name = "Outcome", direction = -1) +
xlab("Treatment") + ylab("% bees (\u00B1 95% CIs)") +
theme_bw(20) +
theme(text = element_text(family = nice_font),
legend.position = "top") +
coord_flip()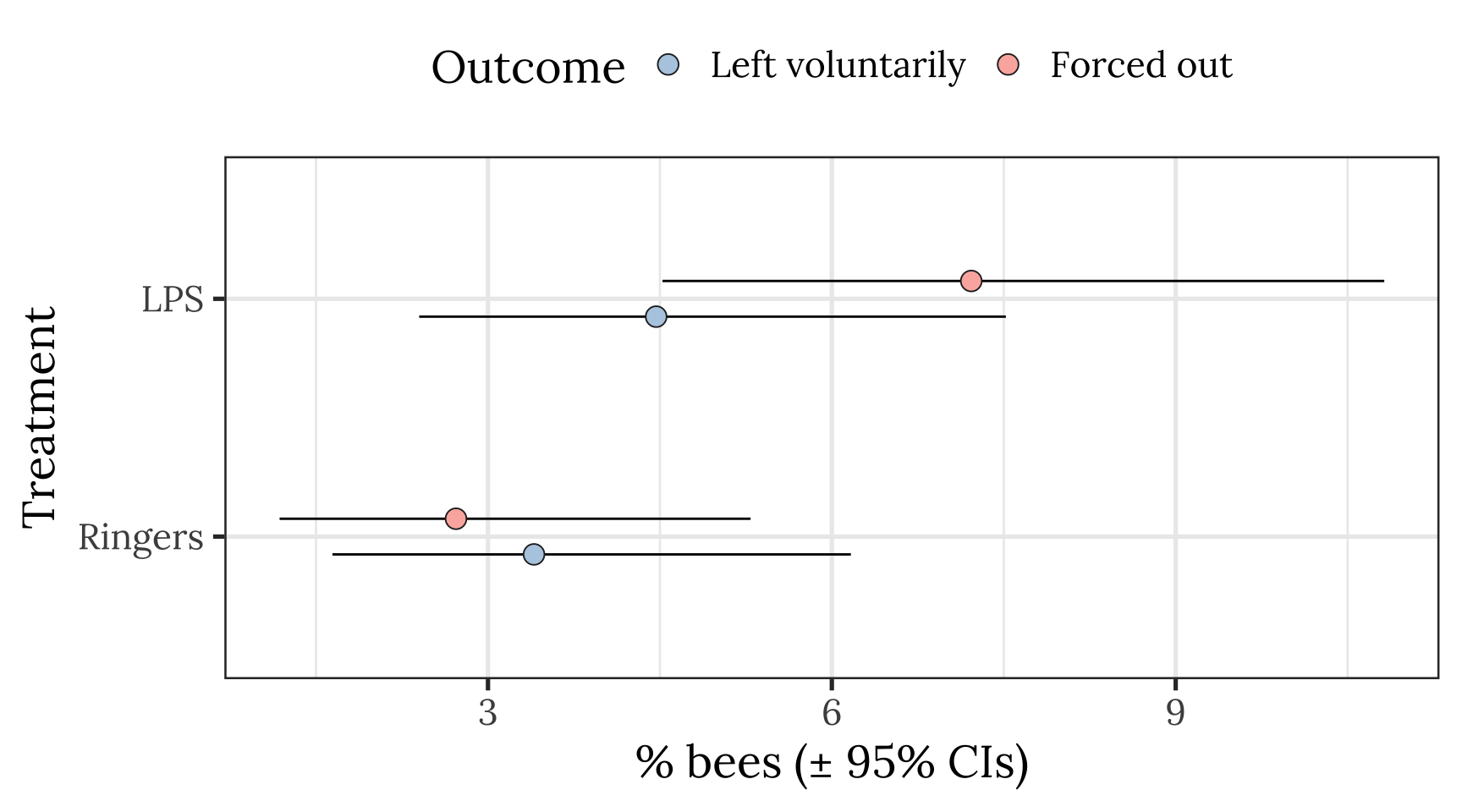
| Version | Author | Date |
|---|---|---|
| f97baee | lukeholman | 2020-05-02 |
Multinomial model of outcome
Run the models
Fit three different multinomial logisitic models, with 3 possible outcomes describing what happened to each bee introduced to the hive: stayed inside, left voluntarily, or forced out by the other workers.
if(!file.exists("output/exp2_model.rds")){
exp2_model_v1 <- brm(
outcome_numeric ~ treatment * hive + observation_time_minutes,
data = data_for_categorical_model,
prior = c(set_prior("normal(0, 3)", class = "b", dpar = "mu2"),
set_prior("normal(0, 3)", class = "b", dpar = "mu3")),
family = "categorical", save_all_pars = TRUE, sample_prior = TRUE,
chains = 4, cores = 1, iter = 5000, seed = 1)
exp2_model_v2 <- brm(
outcome_numeric ~ treatment + hive + observation_time_minutes,
data = data_for_categorical_model,
prior = c(set_prior("normal(0, 3)", class = "b", dpar = "mu2"),
set_prior("normal(0, 3)", class = "b", dpar = "mu3")),
family = "categorical", save_all_pars = TRUE, sample_prior = TRUE,
chains = 4, cores = 1, iter = 5000, seed = 1)
exp2_model_v3 <- brm(
outcome_numeric ~ hive + observation_time_minutes,
data = data_for_categorical_model,
prior = c(set_prior("normal(0, 3)", class = "b", dpar = "mu2"),
set_prior("normal(0, 3)", class = "b", dpar = "mu3")),
family = "categorical", save_all_pars = TRUE, sample_prior = TRUE,
chains = 4, cores = 1, iter = 5000, seed = 1)
posterior_model_probabilities <- tibble(
Model = c("treatment * hive + observation_time_minutes",
"treatment + hive + observation_time_minutes",
"hive + observation_time_minutes"),
post_prob = as.numeric(post_prob(exp2_model_v1,
exp2_model_v2,
exp2_model_v3))) %>%
arrange(-post_prob)
saveRDS(exp2_model_v2, "output/exp2_model.rds") # save the top model, treatment + hive
saveRDS(posterior_model_probabilities, "output/exp2_post_prob.rds")
}
exp2_model <- readRDS("output/exp2_model.rds")
posterior_model_probabilities <- readRDS("output/exp2_post_prob.rds")Posterior model probabilites
posterior_model_probabilities %>%
kable(digits = 3) %>% kable_styling()| Model | post_prob |
|---|---|
| hive + observation_time_minutes | 0.530 |
| treatment + hive + observation_time_minutes | 0.466 |
| treatment * hive + observation_time_minutes | 0.003 |
Fixed effects from the top model
Raw output of the treatment + hive model
summary(exp2_model) Family: categorical
Links: mu2 = logit; mu3 = logit
Formula: outcome_numeric ~ treatment + hive + observation_time_minutes
Data: data_for_categorical_model (Number of observations: 585)
Samples: 4 chains, each with iter = 5000; warmup = 2500; thin = 1;
total post-warmup samples = 10000
Population-Level Effects:
Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
mu2_Intercept -6.37 6.67 -19.69 6.68 1.00 3993 5078
mu3_Intercept -5.18 6.71 -18.32 7.89 1.00 4124 5202
mu2_treatmentLPS 0.38 0.44 -0.48 1.25 1.00 9937 7148
mu2_hive1 0.11 1.52 -2.87 3.08 1.00 4029 5114
mu2_hive2 -0.18 0.68 -1.51 1.16 1.00 4964 6483
mu2_hive3 0.08 2.53 -4.84 5.11 1.00 4134 5283
mu2_observation_time_minutes 0.03 0.07 -0.10 0.16 1.00 3993 5015
mu3_treatmentLPS 1.10 0.43 0.29 1.99 1.00 8715 6616
mu3_hive1 -0.03 1.54 -3.06 2.97 1.00 4022 5092
mu3_hive2 -0.85 0.71 -2.27 0.49 1.00 4813 6207
mu3_hive3 0.05 2.55 -4.89 5.09 1.00 4203 5413
mu3_observation_time_minutes 0.01 0.07 -0.12 0.15 1.00 4104 5308
Samples were drawn using sampling(NUTS). For each parameter, Bulk_ESS
and Tail_ESS are effective sample size measures, and Rhat is the potential
scale reduction factor on split chains (at convergence, Rhat = 1).Formatted brms output for Table S3
The code chunk below wrangles the raw output of the summary() function for brms models into a more readable table of results, and also adds ‘Bayesian p-values’ (i.e. the posterior probability that the true effect size has the same sign as the reported effect).
Table S3: Table summarising the posterior estimates of each fixed effect in the best-fitting model of Experiment 2. This was a multinomial model with three possible outcomes (stay inside, leave voluntarily, be forced out), and so there are two parameter estimates for the intercept and for each predictor in the model. ‘Treatment’ is a fixed factor with two levels, and the effect of LPS shown here is expressed relative to the ‘Ringers’ treatment. ‘Hive’ was a fixed factor with four levels; unlike for treatment, we modelled hive using deviation coding, such that the intercept term represents the mean across all hives (in the Ringers treatment), and the three hive terms represent the deviation from this mean for three of the four hives. Lastly, observation duration was a continuous predictor expressed to the nearest minute. The \(p\) column gives the posterior probability that the true effect size is opposite in sign to what is reported in the Estimate column, similarly to a \(p\)-value.
tableS3 <- get_fixed_effects_with_p_values(exp2_model) %>%
mutate(mu = map_chr(str_extract_all(Parameter, "mu[:digit:]"), ~ .x[1]),
Parameter = str_remove_all(Parameter, "mu[:digit:]_"),
Parameter = str_replace_all(Parameter, "treatment", "Treatment: "),
Parameter = str_replace_all(Parameter, "observation_time_minutes", "Observation duration (minutes)")) %>%
arrange(mu) %>%
select(-mu, -Rhat, -Bulk_ESS, -Tail_ESS)
names(tableS3)[3:5] <- c("Est. Error", "Lower 95% CI", "Upper 95% CI")
saveRDS(tableS3, file = "figures/tableS3.rds")
tableS3 %>%
kable(digits = 3) %>%
kable_styling(full_width = FALSE) %>%
pack_rows("% bees leaving voluntarily", 1, 6) %>%
pack_rows("% bees forced out", 7, 12)| Parameter | Estimate | Est. Error | Lower 95% CI | Upper 95% CI | PP | |
|---|---|---|---|---|---|---|
| % bees leaving voluntarily | ||||||
| Intercept | -6.368 | 6.669 | -19.689 | 6.679 | 0.168 | |
| Treatment: LPS | 0.377 | 0.439 | -0.480 | 1.245 | 0.197 | |
| hive1 | 0.111 | 1.522 | -2.868 | 3.075 | 0.466 | |
| hive2 | -0.183 | 0.679 | -1.513 | 1.160 | 0.390 | |
| hive3 | 0.082 | 2.531 | -4.835 | 5.113 | 0.491 | |
| Observation duration (minutes) | 0.029 | 0.068 | -0.103 | 0.165 | 0.337 | |
| % bees forced out | ||||||
| Intercept | -5.179 | 6.708 | -18.321 | 7.894 | 0.222 | |
| Treatment: LPS | 1.105 | 0.433 | 0.291 | 1.989 | 0.004 | ** |
| hive1 | -0.030 | 1.544 | -3.059 | 2.971 | 0.490 | |
| hive2 | -0.847 | 0.705 | -2.266 | 0.490 | 0.112 | |
| hive3 | 0.049 | 2.554 | -4.892 | 5.089 | 0.493 | |
| Observation duration (minutes) | 0.015 | 0.069 | -0.118 | 0.149 | 0.414 | |
Plotting estimates from the model
Derive prediction from the posterior
get_posterior_preds <- function(hive){
new <- expand.grid(treatment = levels(data_for_categorical_model$treatment),
hive = "Zoology",
observation_time_minutes = 120)
preds <- fitted(exp2_model, newdata = new, summary = FALSE)
dimnames(preds) <- list(NULL, paste(new$treatment, new$hive, sep = "~"), NULL)
rbind(
as.data.frame(preds[,, 1]) %>% mutate(outcome = "Stayed inside", posterior_sample = 1:n()),
as.data.frame(preds[,, 2]) %>% mutate(outcome = "Left voluntarily", posterior_sample = 1:n()),
as.data.frame(preds[,, 3]) %>% mutate(outcome = "Forced out", posterior_sample = 1:n())) %>%
gather(treatment, prop, contains("~")) %>%
mutate(treatment = strsplit(treatment, split = "~"),
hive = map_chr(treatment, ~ .x[2]),
treatment = map_chr(treatment, ~ .x[1]),
treatment = factor(treatment, c("Ringers", "LPS")),
outcome = factor(outcome, c("Stayed inside", "Left voluntarily", "Forced out"))) %>%
arrange(treatment, outcome) %>% as_tibble() %>% select(-hive)
}
# plotting data for panel A: one specific hive
plotting_data <- get_posterior_preds(hive = "Zoology")
# stats data: for comparing means across all hives
stats_data <- get_posterior_preds(hive = NA)Make Figure 2
cols <- c("#E69F00", "#009E73", "#0072B2")
panel_c_colour <- "#CC79A7"
dot_plot <- plotting_data %>%
left_join(sample_sizes, by = "treatment") %>%
arrange(treatment) %>%
mutate(treatment = factor(paste(treatment, "\n(n = ", n, ")", sep = ""),
unique(paste(treatment, "\n(n = ", n, ")", sep = "")))) %>%
ggplot(aes(100 * prop, treatment)) +
stat_dotsh(quantiles = 100, fill = "grey40", colour = "grey40") +
stat_pointintervalh(aes(colour = outcome, fill = outcome),
.width = c(0.5, 0.95),
position = position_nudge(y = -0.07), point_colour = "grey26", pch = 21, stroke = 0.4) +
scale_colour_manual(values = cols) +
scale_fill_manual(values = cols) +
facet_wrap( ~ outcome, scales = "free_x") +
xlab("% bees (posterior estimate)") + ylab("Treatment") +
theme_bw() +
coord_cartesian(ylim=c(1.4, 2.2)) +
theme(
text = element_text(family = nice_font),
strip.background = element_rect(fill = "#eff0f1"),
panel.grid.major.y = element_blank(),
legend.position = "none"
)
get_log_odds <- function(trt1, trt2){ # positive effect = odds of this outcome are higher for trt2 than trt1 (put control as trt1)
log((trt2 / (1 - trt2) / (trt1 / (1 - trt1))))
}
LOR <- plotting_data %>%
spread(treatment, prop) %>%
mutate(LOR = get_log_odds(Ringers, LPS)) %>%
select(posterior_sample, outcome, LOR)
LOR_plot <- LOR %>%
ggplot(aes(LOR, outcome, colour = outcome)) +
geom_vline(xintercept = 0, linetype = 2) +
geom_vline(xintercept = log(2), linetype = 2, colour = "pink") +
geom_vline(xintercept = -log(2), linetype = 2, colour = "pink") +
stat_pointintervalh(aes(colour = outcome, fill = outcome),
position = position_dodge(0.4),
.width = c(0.5, 0.95),
point_colour = "grey26", pch = 21, stroke = 0.4) +
scale_colour_manual(values = cols) +
scale_fill_manual(values = cols) +
xlab("Effect size of LPS\n(log odds ratio)") + ylab("Mode of exit") +
theme_bw() +
theme(
text = element_text(family = nice_font),
panel.grid.major.y = element_blank(),
legend.position = "none"
)
diff_in_forced_out_plot <- plotting_data %>%
spread(outcome, prop) %>%
mutate(prop_leavers_that_were_forced_out = `Forced out` / (`Forced out` + `Left voluntarily`)) %>%
select(posterior_sample, treatment, prop_leavers_that_were_forced_out) %>%
spread(treatment, prop_leavers_that_were_forced_out) %>%
mutate(difference_prop_forced_out_LOR = get_log_odds(Ringers, LPS)) %>%
ggplot(aes(difference_prop_forced_out_LOR, y =1)) +
geom_vline(xintercept = 0, linetype = 2) +
stat_dotsh(quantiles = 100, fill = "grey40", colour = "grey40") +
stat_pointintervalh(
colour = panel_c_colour, fill = panel_c_colour,
.width = c(0.5, 0.95),
position = position_nudge(y = -0.1),
point_colour = "grey26", pch = 21, stroke = 0.4) +
coord_cartesian(ylim=c(0.86, 2)) +
xlab("Effect of LPS on proportion\nbees leaving by force\n(log odds ratio)") +
ylab("Posterior density") +
theme_bw() +
theme(
text = element_text(family = nice_font),
axis.text.y = element_blank(),
axis.ticks.y = element_blank(),
panel.grid.major.y = element_blank(),
panel.grid.minor.y = element_blank(),
legend.position = "none"
)
#
# diff_in_forced_out_plot <- plotting_data %>%
# spread(outcome, prop) %>%
# mutate(prop_leavers_that_were_forced_out = `Forced out` / (`Forced out` + `Left voluntarily`)) %>%
# select(posterior_sample, treatment, prop_leavers_that_were_forced_out) %>%
# spread(treatment, prop_leavers_that_were_forced_out) %>%
# mutate(difference_prop_forced_out_LOR = get_log_odds(Ringers, LPS)) %>%
# ggplot(aes(difference_prop_forced_out_LOR, y =1)) +
# geom_vline(xintercept = 0, linetype = 2) +
# stat_dotsh(quantiles = 100, fill = "grey40", colour = "grey40") +
# stat_pointintervalh(
# colour = "#0CE3AC", fill = "#0CE3AC",
# .width = c(0.5, 0.95),
# position = position_nudge(y = -0.1),
# point_colour = "grey26", pch = 21, stroke = 0.4) +
# xlab("Effect of LPS on proportion\nbees leaving by force\n(log odds ratio)") +
# ylab("Posterior density") +
# theme_bw() +
# theme(
# text = element_text(family = nice_font),
# panel.grid.major.y = element_blank(),
# legend.position = "none"
# )
bottom_row <- cowplot::plot_grid(LOR_plot, diff_in_forced_out_plot,
labels = c("B", "C"),
nrow = 1, align = 'hv', axis = 'l', rel_heights = c(1.6, 1))
top_row <- cowplot::plot_grid(dot_plot, labels = "A")
p <- cowplot::plot_grid(top_row, bottom_row,
nrow = 2, align = 'v', axis = 'l', rel_heights = c(1.4, 1))
ggsave(plot = p, filename = "figures/fig2.pdf", height = 6, width = 6)
p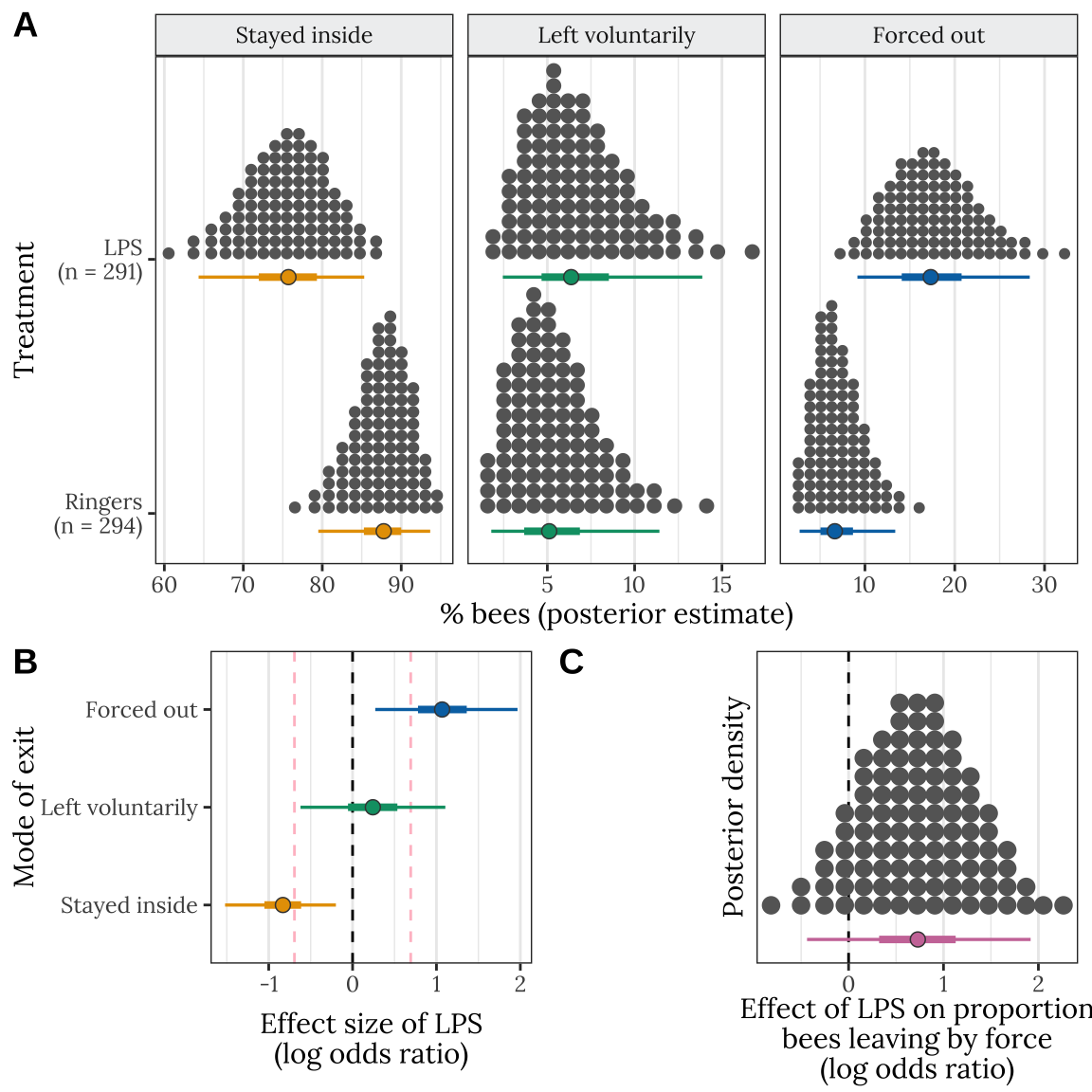
Figure 2: LEGEND HERE
Hypothesis testing and effect sizes
Posterior effect size estimates
This section calculates the effect size and 95% CIs that are shown in Figure 2B (and creates Table S4).
Table S4: This table gives statistics associated with each of the contrasts plotted in Figure 2B. Each pair of rows gives the absolute (i.e. the difference in % bees) and standardised effect size (as log odds ratio; LOR) for the LPS treatment, relative to the Ringers treatment, for one of the three possible outcomes (stayed inside, left voluntarily, or forced out). A LOR of \(|log(x)|\) indicates that the outcome is \(x\) times more frequent in one treatment compared to the other, e.g. \(log(2) = 0.69\) indicates a two-fold difference in frequency. The \(PP\) column gives the posterior probability that the true effect size has the same sign as is shown in the Estimate column; this metric has a similar interpretation to a one-tailed \(p\) value in frequentist statistics.
my_summary <- function(df, columns, outcome) {
lapply(columns, function(x){
p <- 1 - (df %>% pull(!! x) %>%
bayestestR::p_direction() %>% as.numeric())
df %>% pull(!! x) %>% posterior_summary() %>% as_tibble() %>%
mutate(PP = p, Outcome = outcome, Metric = x) %>%
select(Outcome, Metric, everything())
}) %>% do.call("rbind", .)
}
stats_table <- rbind(
plotting_data %>%
filter(outcome == "Stayed inside") %>%
spread(treatment, prop) %>%
mutate(`Absolute difference in % bees staying inside` = 100 * (LPS - Ringers),
`Log odds ratio` = get_log_odds(Ringers, LPS)) %>%
my_summary(c("Absolute difference in % bees staying inside",
"Log odds ratio"),
outcome = "Stayed inside") %>%
mutate(PP = c(" ", format(round(PP[2], 4), nsmall = 4))),
plotting_data %>%
filter(outcome == "Left voluntarily") %>%
spread(treatment, prop) %>%
mutate(`Absolute difference in % bees leaving voluntarily` = 100 * (LPS - Ringers),
`Log odds ratio` = get_log_odds(Ringers, LPS)) %>%
my_summary(c("Absolute difference in % bees leaving voluntarily",
"Log odds ratio"),
outcome = "Left voluntarily") %>%
mutate(PP = c(" ", format(round(PP[2], 4), nsmall = 4))),
plotting_data %>%
filter(outcome == "Forced out") %>%
spread(treatment, prop) %>%
mutate(`Absolute difference in % bees forced out` = 100 * (LPS - Ringers),
`Log odds ratio` = get_log_odds(Ringers, LPS)) %>%
my_summary(c("Absolute difference in % bees forced out",
"Log odds ratio"),
outcome = "Forced out") %>%
mutate(PP = c(" ", format(round(PP[2], 4), nsmall = 4)))
) %>%
mutate(` ` = ifelse(PP < 0.05, "\\*", ""),
` ` = replace(` `, PP < 0.01, "**"),
` ` = replace(` `, PP < 0.001, "***"),
` ` = replace(` `, PP == " ", ""))
stats_table[c(2,4,6), 1] <- " "
stats_table %>%
select(-Outcome) %>%
kable(digits = 3) %>% kable_styling(full_width = FALSE) %>%
row_spec(c(0,2,4,6), extra_css = "border-bottom: solid;") %>%
pack_rows("% bees staying inside", 1, 2) %>%
pack_rows("% bees leaving voluntarily", 3, 4) %>%
pack_rows("% bees forced out", 5, 6)| Metric | Estimate | Est.Error | Q2.5 | Q97.5 | PP | |
|---|---|---|---|---|---|---|
| % bees staying inside | ||||||
| Absolute difference in % bees staying inside | -11.949 | 4.942 | -22.208 | -2.753 | ||
| Log odds ratio | -0.836 | 0.331 | -1.520 | -0.199 | 0.0048 | ** |
| % bees leaving voluntarily | ||||||
| Absolute difference in % bees leaving voluntarily | 1.335 | 2.623 | -3.720 | 7.086 | ||
| Log odds ratio | 0.242 | 0.440 | -0.621 | 1.106 | 0.2881 | |
| % bees forced out | ||||||
| Absolute difference in % bees forced out | 10.614 | 4.627 | 2.538 | 20.464 | ||
| Log odds ratio | 1.079 | 0.433 | 0.270 | 1.967 | 0.0047 | ** |
Evaluating evidence for the null hypothesis
Here, we derive the result present in prose in the Results, where we calculated the posterior probability that the true effect size lies in the range \(-log(2) < LOR < log(2)\).
LOR_forced <- LOR %>%
filter(outcome == "Forced out") %>%
pull(LOR)
LOR_left <- LOR %>%
filter(outcome == "Left voluntarily") %>%
pull(LOR)
ROPE_calculation <- function(scale, LOR_data){
round(sum(LOR_data > -log(scale) & LOR_data < log(scale)) / length(LOR_data), 3)
}
cat(
paste(
paste("Probability that |LOR|<2 for % bees leaving voluntarily:", ROPE_calculation(2, LOR_left)), "\n",
paste("Probability that |LOR|<2 for % bees forced out:", ROPE_calculation(2, LOR_forced)), sep = "")
)Probability that |LOR|<2 for % bees leaving voluntarily: 0.832
Probability that |LOR|<2 for % bees forced out: 0.187Stats associated with Figure 2C
Here, we derive the result presented in prose in the Results, regarding the effect of LPS on the proportion of bees that left the hive by force.
difference_prop_forced_out_LOR <- plotting_data %>%
spread(outcome, prop) %>%
mutate(prop_leavers_that_were_forced_out =
`Forced out` / (`Forced out` + `Left voluntarily`)) %>%
select(posterior_sample,
treatment,
prop_leavers_that_were_forced_out) %>%
spread(treatment,
prop_leavers_that_were_forced_out) %>%
mutate(difference_prop_forced_out_LOR = get_log_odds(Ringers, LPS)) %>%
pull(difference_prop_forced_out_LOR)
hypothesis(difference_prop_forced_out_LOR,
"x > 0", alpha = 0.05)$hypothesis Hypothesis Estimate Est.Error CI.Lower CI.Upper Evid.Ratio Post.Prob Star
1 (x) > 0 0.7281842 0.5984938 -0.2336917 1.712823 8.082652 0.8899
sessionInfo()R version 3.6.3 (2020-02-29)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS Catalina 10.15.4
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRlapack.dylib
locale:
[1] en_AU.UTF-8/en_AU.UTF-8/en_AU.UTF-8/C/en_AU.UTF-8/en_AU.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] cowplot_1.0.0 tidybayes_2.0.1 bayestestR_0.5.1 kableExtra_1.1.0 gridExtra_2.3 forcats_0.5.0
[7] stringr_1.4.0 dplyr_0.8.5 purrr_0.3.3 readr_1.3.1 tidyr_1.0.2 tibble_3.0.0
[13] ggplot2_3.3.0 tidyverse_1.3.0 bayesplot_1.7.1 brms_2.12.0 Rcpp_1.0.4.6 showtext_0.7-1
[19] showtextdb_2.0 sysfonts_0.8
loaded via a namespace (and not attached):
[1] colorspace_1.4-1 ellipsis_0.3.0 ggridges_0.5.2 rsconnect_0.8.16 rprojroot_1.3-2
[6] markdown_1.1 base64enc_0.1-3 fs_1.4.1 rstudioapi_0.11 farver_2.0.3
[11] rstan_2.19.3 svUnit_0.7-12 DT_0.13 fansi_0.4.1 mvtnorm_1.1-0
[16] lubridate_1.7.8 xml2_1.3.1 bridgesampling_1.0-0 knitr_1.28 shinythemes_1.1.2
[21] jsonlite_1.6.1 workflowr_1.6.1 broom_0.5.4 dbplyr_1.4.2 shiny_1.4.0.2
[26] compiler_3.6.3 httr_1.4.1 backports_1.1.6 assertthat_0.2.1 Matrix_1.2-18
[31] fastmap_1.0.1 cli_2.0.2 later_1.0.0 htmltools_0.4.0.9003 prettyunits_1.1.1
[36] tools_3.6.3 igraph_1.2.5 coda_0.19-3 gtable_0.3.0 glue_1.4.0
[41] reshape2_1.4.4 cellranger_1.1.0 vctrs_0.2.4 nlme_3.1-147 crosstalk_1.1.0.1
[46] insight_0.8.1 xfun_0.13 ps_1.3.0 rvest_0.3.5 mime_0.9
[51] miniUI_0.1.1.1 lifecycle_0.2.0 gtools_3.8.2 zoo_1.8-7 scales_1.1.0
[56] colourpicker_1.0 hms_0.5.3 promises_1.1.0 Brobdingnag_1.2-6 parallel_3.6.3
[61] inline_0.3.15 RColorBrewer_1.1-2 shinystan_2.5.0 curl_4.3 yaml_2.2.1
[66] loo_2.2.0 StanHeaders_2.19.2 stringi_1.4.6 highr_0.8 dygraphs_1.1.1.6
[71] pkgbuild_1.0.6 rlang_0.4.5 pkgconfig_2.0.3 matrixStats_0.56.0 evaluate_0.14
[76] lattice_0.20-41 labeling_0.3 rstantools_2.0.0 htmlwidgets_1.5.1 processx_3.4.2
[81] tidyselect_1.0.0 plyr_1.8.6 magrittr_1.5 R6_2.4.1 generics_0.0.2
[86] DBI_1.1.0 pillar_1.4.3 haven_2.2.0 whisker_0.4 withr_2.1.2
[91] xts_0.12-0 abind_1.4-5 modelr_0.1.5 crayon_1.3.4 arrayhelpers_1.1-0
[96] rmarkdown_2.1.3 grid_3.6.3 readxl_1.3.1 callr_3.4.3 git2r_0.26.1
[101] threejs_0.3.3 reprex_0.3.0 digest_0.6.25 webshot_0.5.2 xtable_1.8-4
[106] httpuv_1.5.2 stats4_3.6.3 munsell_0.5.0 viridisLite_0.3.0 shinyjs_1.1