Acute CCl4 mouse model
Last updated: 2020-12-23
Checks: 7 0
Knit directory: meta-liver/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20201218) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version d4f78fa. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/human-diehl-nafld_cache/
Ignored: analysis/human-hampe13-nash_cache/
Ignored: analysis/human-hampe14-misc_cache/
Ignored: analysis/human-hoang-nafld_cache/
Ignored: analysis/human-ramnath-fibrosis_cache/
Ignored: analysis/meta-chronic-vs-acute_cache/
Ignored: analysis/meta-mouse-vs-human_cache/
Ignored: analysis/mouse-acute-apap_cache/
Ignored: analysis/mouse-acute-bdl_cache/
Ignored: analysis/mouse-acute-lps_cache/
Ignored: analysis/mouse-acute-ph_cache/
Ignored: analysis/mouse-acute-tunicamycin_cache/
Ignored: analysis/mouse-chronic-ccl4_cache/
Ignored: analysis/plot-acute-apap_cache/
Ignored: analysis/plot-acute-bdl_cache/
Ignored: analysis/plot-acute-ccl4_cache/
Ignored: analysis/plot-acute-ph_cache/
Ignored: analysis/plot-chronic-ccl4_cache/
Ignored: analysis/plot-chronic-vs-acute_cache/
Ignored: analysis/plot-precision-recall_cache/
Ignored: analysis/plot-study-overview_cache/
Ignored: code/.DS_Store
Ignored: code/README.html
Ignored: data/.DS_Store
Ignored: data/README.html
Ignored: data/annotation/
Ignored: data/human-diehl-nafld/
Ignored: data/human-hampe13-nash/
Ignored: data/human-hampe14-misc/
Ignored: data/human-hoang-nafld/
Ignored: data/human-ramnath-fibrosis/
Ignored: data/meta-chronic-vs-acute/
Ignored: data/meta-mouse-vs-human/
Ignored: data/mouse-acute-apap/
Ignored: data/mouse-acute-bdl/
Ignored: data/mouse-acute-ccl4/
Ignored: data/mouse-acute-lps/
Ignored: data/mouse-acute-ph/
Ignored: data/mouse-acute-tunicamycin/
Ignored: data/mouse-chronic-ccl4/
Ignored: external_software/.DS_Store
Ignored: external_software/README.html
Ignored: external_software/stem/.DS_Store
Ignored: figures/
Ignored: output/.DS_Store
Ignored: output/README.html
Ignored: output/human-diehl-nafld/
Ignored: output/human-hampe13-nash/
Ignored: output/human-hampe14-misc/
Ignored: output/human-hoang-nafld/
Ignored: output/human-ramnath-fibrosis/
Ignored: output/meta-chronic-vs-acute/
Ignored: output/meta-mouse-vs-human/
Ignored: output/mouse-acute-apap/
Ignored: output/mouse-acute-bdl/
Ignored: output/mouse-acute-ccl4/
Ignored: output/mouse-acute-lps/
Ignored: output/mouse-acute-ph/
Ignored: output/mouse-acute-tunicamycin/
Ignored: output/mouse-chronic-ccl4/
Ignored: renv/library/
Ignored: renv/staging/
Unstaged changes:
Modified: analysis/_site.yml
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/mouse-acute-ccl4.Rmd) and HTML (docs/mouse-acute-ccl4.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | d4f78fa | christianholland | 2020-12-23 | wflow_publish("analysis/*.Rmd", delete_cache = T) |
| html | 8a9b7bf | christianholland | 2020-12-20 | Build site. |
| html | e22a40b | christianholland | 2020-12-20 | Build site. |
| html | bea437a | christianholland | 2020-12-20 | Build site. |
| Rmd | c78e883 | christianholland | 2020-12-20 | stem characterization |
| html | af38450 | christianholland | 2020-12-19 | Build site. |
| Rmd | 78a25d5 | christianholland | 2020-12-19 | minor changes |
| html | c518c89 | christianholland | 2020-12-19 | Build site. |
| Rmd | 961f67d | christianholland | 2020-12-19 | added acute ccl4 study |
Introduction
Here we analysis a mouse model of CCl4 induced acute liver damage. The transcriptomic profiles were measured at 9 different time points ranging from 2 hours to 16 days.
Libraries and sources
These libraries and sources are used for this analysis.
library(mouse4302.db)
library(tidyverse)
library(tidylog)
library(here)
library(oligo)
library(annotate)
library(limma)
library(biobroom)
library(progeny)
library(dorothea)
library(janitor)
library(msigdf) # remotes::install_github("ToledoEM/msigdf@v7.1")
library(AachenColorPalette)
library(cowplot)
library(lemon)
library(patchwork)
options("tidylog.display" = list(print))
source(here("code/utils-microarray.R"))
source(here("code/utils-utils.R"))
source(here("code/utils-plots.R"))Definition of global variables that are used throughout this analysis.
# i/o
data_path <- "data/mouse-acute-ccl4"
output_path <- "output/mouse-acute-ccl4"
# graphical parameters
# fontsize
fz <- 9Data processing
Load .CEL files and quality control
The array quality is controlled based on the relative log expression values (RLE) and the normalized unscaled standard errors (NUSE).
# load cel files and check quality
platforms <- readRDS(here("data/annotation/platforms.rds"))
raw_eset <- list.celfiles(here(data_path), listGzipped = T, full.names = T) %>%
read.celfiles() %>%
ma_qc() # Discarding in total 1 arrays: 6h_m3_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_2hm1_16_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_2hm2_17_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_2hm3_18_(Mouse430_2)_2.CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_2hm4_19_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_2hm5_20_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_8hm1_21_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_8hm2_22_(Mouse430_2)_2.CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_8hm3_23_(Mouse430_2)_2.CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_8hm4_24_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_8hm5_25_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D16M1_51_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D16M2_52_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D16M3_53_(Mouse430_2)_2.CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D16M4_54_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D16M5_55_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D16M6_56_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d1m1_26_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d1m2_27_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d1m3_28_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d1m4_29_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d1m5_30_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d2m1_31_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d2m2_32_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d2m3_33_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d2m4_34_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d2m5_35_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d4m1_36_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d4m2_37_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d4m3_38_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d4m4_39_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d4m5_40_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d6m1_41_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d6m2_42_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d6m3_43_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d6m4_44_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_d6m5_45_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D8M1_46_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D8M2_47_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D8M3_48_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D8M4_49_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_D8M5_50_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_KM1_1_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_KM2_2_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_KM3_3_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_KM4_4_(Mouse430_2).CEL
#> Reading in : /Users/cholland/Projects/meta-liver/data/mouse-acute-ccl4/Patricio_KM5_5_(Mouse430_2).CELNormalization and probe annotation
Probe intensities are normalized with the rma() function. Probes are annotated with MGI symbols.
eset <- rma(raw_eset)
#> Background correcting
#> Normalizing
#> Calculating Expression
# annotate microarray probes with mgi symbols
expr <- ma_annotate(eset, platforms)
pattern <- str_c(c(
"Patricio_",
"_\\(Mouse430_2\\).CEL",
"_\\(Mouse430_2\\)_2.CEL"
), collapse = "|")
colnames(expr) <- str_c(
"sample", str_remove_all(colnames(expr), pattern),
sep = "_"
) %>%
str_to_lower()
# save normalized expression
saveRDS(expr, here(output_path, "normalized_expression.rds"))Build meta data
Meta information are parsed from the sample names.
# build meta data
meta <- colnames(expr) %>%
enframe(name = NULL, value = "sample") %>%
separate(sample, into = c("tmp", "key", "run"), remove = F) %>%
dplyr::select(-tmp) %>%
separate(key, into = c("group", "rep"), sep = "m", remove = T) %>%
mutate(time = case_when(
group == "k" ~ 0,
str_detect(group, "h") ~ parse_number(group),
str_detect(group, "d") ~ parse_number(group) * 24
)) %>%
mutate(time = ordered(time)) %>%
mutate(group = case_when(
group == "k" ~ "control",
str_detect(group, "h") ~ str_c("h", parse_number(group)),
TRUE ~ group
)) %>%
mutate(group = factor(group, levels = c(
"control", "h2", "h8", "d1", "d2",
"d4", "d6", "d8", "d16"
))) %>%
arrange(sample)
#> mutate: new variable 'time' (double) with 9 unique values and 0% NA
#> mutate: converted 'time' from double to ordered factor (0 new NA)
#> mutate: changed 15 values (33%) of 'group' (0 new NA)
#> mutate: converted 'group' from character to factor (0 new NA)
# save meta data
saveRDS(meta, here(output_path, "meta_data.rds"))Exploratory analysis
PCA of normalized data
PCA plot of normalized expression data contextualized based on the time point. Only the top 1000 most variable genes are used as features.
expr <- readRDS(here(output_path, "normalized_expression.rds"))
meta <- readRDS(here(output_path, "meta_data.rds"))
pca_result <- do_pca(expr, meta, top_n_var_genes = 1000)
#> left_join: added 4 columns (group, rep, run, time)
#> > rows only in x 0
#> > rows only in y ( 0)
#> > matched rows 46
#> > ====
#> > rows total 46
saveRDS(pca_result, here(output_path, "pca_result.rds"))
plot_pca(pca_result, feature = "time") +
my_theme()
Differential gene expression analysis
Running limma
Differential gene expression analysis via limma with the aim to identify the effect of CCl4 intoxication for the different time points.
# load expression and meta data
expr <- readRDS(here(output_path, "normalized_expression.rds"))
meta <- readRDS(here(output_path, "meta_data.rds"))
stopifnot(colnames(expr) == meta$sample)
# build design matrix
design <- model.matrix(~ 0 + group, data = meta)
rownames(design) <- meta$sample
colnames(design) <- levels(meta$group)
# define contrasts
contrasts <- makeContrasts(
# effect of ccl4 treatment
ccl_2h_vs_0h = h2 - control,
ccl_8h_vs_0h = h8 - control,
ccl_24h_vs_0h = d1 - control,
ccl_48h_vs_0h = d2 - control,
ccl_96h_vs_0h = d4 - control,
ccl_144h_vs_0h = d6 - control,
ccl_192h_vs_0h = d8 - control,
ccl_384h_vs_0h = d16 - control,
# consecutive time point comparison
consec_2h_vs_0h = h2 - control,
consec_8h_vs_2h = h8 - h2,
consec_24h_vs_8h = d1 - h8,
consec_48h_vs_24h = d2 - d1,
consec_96h_vs_48h = d4 - d2,
consec_144h_vs_96h = d6 - d4,
consec_192h_vs_144h = d8 - d6,
consec_384h_vs_192h = d16 - d8,
levels = design
)
limma_result <- run_limma(expr, design, contrasts) %>%
assign_deg()
#> select: renamed 3 variables (contrast, logFC, pval) and dropped one variable
#> group_by: one grouping variable (contrast)
#> mutate (grouped): new variable 'fdr' (double) with 151,248 unique values and 0% NA
#> ungroup: no grouping variables
#> mutate: new variable 'regulation' (character) with 3 unique values and 0% NA
#> mutate: converted 'regulation' from character to factor (0 new NA)
deg_df <- limma_result %>%
mutate(contrast = fct_inorder(contrast)) %>%
mutate(contrast_reference = case_when(
str_detect(contrast, "ccl") ~ "ccl4",
str_detect(contrast, "consec") ~ "consec"
))
#> mutate: changed 0 values (0%) of 'contrast' (0 new NA)
#> mutate: new variable 'contrast_reference' (character) with 2 unique values and 0% NA
saveRDS(deg_df, here(output_path, "limma_result.rds"))Volcano plots
Volcano plots visualizing the effect of CCl4 on gene expression.
df <- readRDS(here(output_path, "limma_result.rds"))
df %>%
filter(contrast_reference == "ccl4") %>%
plot_volcano() +
my_theme(grid = "y", fsize = fz)
#> filter: removed 164,072 rows (50%), 164,072 rows remaining
#> rename: renamed one variable (p)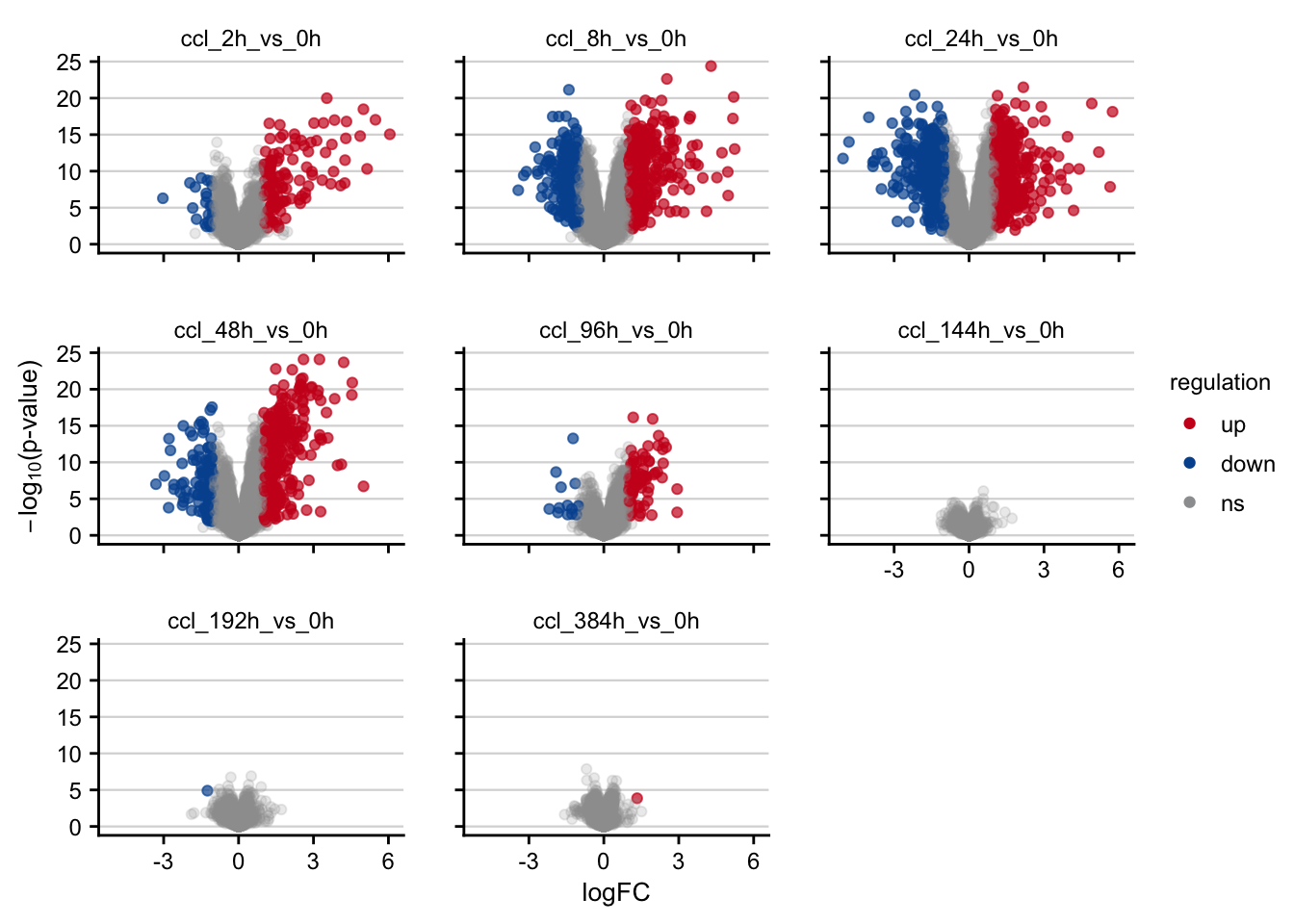
Time series clustering
Gene expression trajectories are clustered using the STEM software. The cluster algorithm is described here.
Prepare input
# prepare input for stem analysis
df <- readRDS(here(output_path, "limma_result.rds"))
stem_inputs <- df %>%
mutate(class = str_c("Hour ", parse_number(as.character(contrast)))) %>%
mutate(class = factor(class, levels = unique(.$class))) %>%
select(gene, class, logFC, contrast_reference)
#> mutate: new variable 'class' (character) with 8 unique values and 0% NA
#> mutate: converted 'class' from character to factor (0 new NA)
#> select: dropped 5 variables (contrast, statistic, pval, fdr, regulation)
stem_inputs %>%
filter(contrast_reference == "ccl4") %>%
select(-contrast_reference) %>%
pivot_wider(names_from = class, values_from = logFC) %>%
write_delim(here(output_path, "stem/input/ccl4.txt"), delim = "\t")
#> filter: removed 164,072 rows (50%), 164,072 rows remaining
#> select: dropped one variable (contrast_reference)
#> pivot_wider: reorganized (class, logFC) into (Hour 2, Hour 8, Hour 24, Hour 48, Hour 96, …) [was 164072x3, now 20509x9]Run STEM
STEM is implemented in Java. The .jar file is called from R. Only significant time series clusters are visualized.
# execute stem
stem_res <- run_stem(here(output_path, "stem"), clear_output = T)
#> distinct: no rows removed
#> mutate: new variable 'gene' (character) with 20,509 unique values and 0% NA
#> distinct: no rows removed
#> mutate: new variable 'key' (character) with one unique value and 0% NA
#> select: dropped one variable (spot)
#> gather: reorganized (x0, hour_2, hour_8, hour_24, hour_48, …) into (time, value) [was 2085x12, now 18765x5]
#> mutate: converted 'time' from character to double (0 new NA)
#> mutate: new variable 'key' (character) with one unique value and 0% NA
#> select: renamed 4 variables (profile, y_coords, size, p) and dropped 2 variables
#> inner_join: added 3 columns (y_coords, size, p)
#> > rows only in x ( 0)
#> > rows only in y ( 0)
#> > matched rows 18,765
#> > ========
#> > rows total 18,765
#> inner_join: added one column (symbol)
#> > rows only in x ( 0)
#> > rows only in y (18,424)
#> > matched rows 18,765
#> > ========
#> > rows total 18,765
#> transmute: dropped one variable (symbol)
#> changed 18,621 values (99%) of 'gene' (0 new NA)
saveRDS(stem_res, here(output_path, "stem_result.rds"))
stem_res %>%
filter(p <= 0.05) %>%
filter(key == "ccl4") %>%
distinct() %>%
plot_stem_profiles(model_profile = F, ncol = 2) +
labs(x = "Time in Hours", y = "logFC") +
my_theme(grid = "y", fsize = fz)
#> filter: removed 2,781 rows (15%), 15,984 rows remaining
#> filter: no rows removed
#> distinct: no rows removed
#> group_by: 4 grouping variables (key, profile, p, time)
#> ungroup: no grouping variables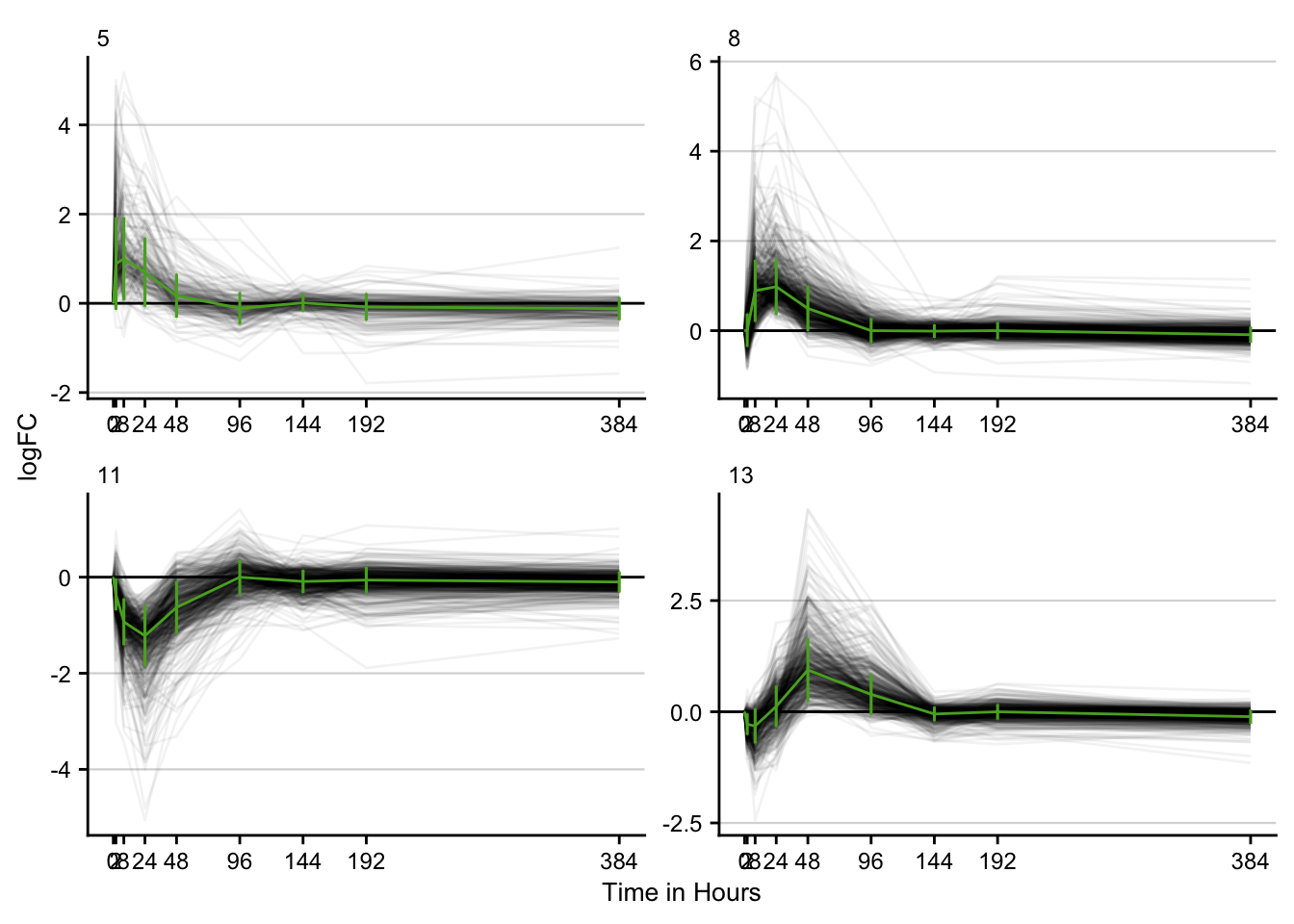
Cluster characterization
STEM clusters are characterized by GO terms, PROGENy’s pathways and DoRothEA’s TFs. As statistic over-representation analysis is used.
stem_res = readRDS(here(output_path, "stem_result.rds"))
signatures = stem_res %>%
filter(p <= 0.05) %>%
distinct(profile, gene, p_profile = p)
#> filter: removed 2,781 rows (15%), 15,984 rows remaining
#> distinct: removed 14,208 rows (89%), 1,776 rows remaining
genesets = load_genesets() %>%
filter(confidence %in% c(NA,"A", "B", "C"))
#> filter: removed 2,340,732 rows (80%), 597,560 rows remaining
#> select: renamed one variable (gene) and dropped 2 variables
#> mutate: new variable 'group' (character) with one unique value and 0% NA
#> gather: reorganized (Androgen, EGFR, Estrogen, Hypoxia, JAK-STAT, …) into (geneset, weight) [was 1299x15, now 18186x3]
#> filter: removed 16,785 rows (92%), 1,401 rows remaining
#> select: dropped one variable (weight)
#> mutate: new variable 'group' (character) with one unique value and 0% NA
#> select: renamed 2 variables (geneset, gene) and dropped one variable
#> mutate: new variable 'group' (character) with one unique value and 0% NA
#> filter: removed 396,818 rows (39%), 612,694 rows remaining
ora_res = signatures %>%
nest(sig = c(-profile)) %>%
dplyr::mutate(ora = sig %>% map(run_ora, sets = genesets, min_size = 10,
options = list(alternative = "greater"),
background_n = 20000)) %>%
select(-sig) %>%
unnest(ora)
#> add_count: new variable 'n' (integer) with 643 unique values and 0% NA
#> filter: removed 13,996 rows (2%), 598,698 rows remaining
#> select: dropped one variable (n)
#> mutate: new variable 'contingency_table' (list) with 1,618 unique values and 0% NA
#> mutate: new variable 'stat' (list) with 1,520 unique values and 0% NA
#> group_by: one grouping variable (group)
#> mutate (grouped): new variable 'fdr' (double) with 679 unique values and 0% NA
#> ungroup: no grouping variables
#> select: dropped 4 variables (set, conf.low, conf.high, method)
#> add_count: new variable 'n' (integer) with 643 unique values and 0% NA
#> filter: removed 13,996 rows (2%), 598,698 rows remaining
#> select: dropped one variable (n)
#> mutate: new variable 'contingency_table' (list) with 1,681 unique values and 0% NA
#> mutate: new variable 'stat' (list) with 1,575 unique values and 0% NA
#> group_by: one grouping variable (group)
#> mutate (grouped): new variable 'fdr' (double) with 977 unique values and 0% NA
#> ungroup: no grouping variables
#> select: dropped 4 variables (set, conf.low, conf.high, method)
#> add_count: new variable 'n' (integer) with 643 unique values and 0% NA
#> filter: removed 13,996 rows (2%), 598,698 rows remaining
#> select: dropped one variable (n)
#> mutate: new variable 'contingency_table' (list) with 1,379 unique values and 0% NA
#> mutate: new variable 'stat' (list) with 1,207 unique values and 0% NA
#> group_by: one grouping variable (group)
#> mutate (grouped): new variable 'fdr' (double) with 832 unique values and 0% NA
#> ungroup: no grouping variables
#> select: dropped 4 variables (set, conf.low, conf.high, method)
#> add_count: new variable 'n' (integer) with 643 unique values and 0% NA
#> filter: removed 13,996 rows (2%), 598,698 rows remaining
#> select: dropped one variable (n)
#> mutate: new variable 'contingency_table' (list) with 1,578 unique values and 0% NA
#> mutate: new variable 'stat' (list) with 1,403 unique values and 0% NA
#> group_by: one grouping variable (group)
#> mutate (grouped): new variable 'fdr' (double) with 412 unique values and 0% NA
#> ungroup: no grouping variables
#> select: dropped 4 variables (set, conf.low, conf.high, method)
#> select: dropped one variable (sig)
saveRDS(ora_res, here(output_path, "stem_characterization.rds"))Time spend to execute this analysis: 02:11 minutes.
sessionInfo()
#> R version 4.0.2 (2020-06-22)
#> Platform: x86_64-apple-darwin17.0 (64-bit)
#> Running under: macOS Mojave 10.14.5
#>
#> Matrix products: default
#> BLAS: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRblas.dylib
#> LAPACK: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRlapack.dylib
#>
#> locale:
#> [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
#>
#> attached base packages:
#> [1] parallel stats4 stats graphics grDevices datasets utils
#> [8] methods base
#>
#> other attached packages:
#> [1] pd.mouse430.2_3.12.0 DBI_1.1.0 RSQLite_2.2.1
#> [4] patchwork_1.1.1 lemon_0.4.5 cowplot_1.1.0
#> [7] AachenColorPalette_1.1.2 msigdf_7.1 janitor_2.0.1
#> [10] dorothea_1.0.1 progeny_1.10.0 biobroom_1.20.0
#> [13] broom_0.7.3 limma_3.44.3 annotate_1.66.0
#> [16] XML_3.99-0.5 oligo_1.52.1 Biostrings_2.56.0
#> [19] XVector_0.28.0 oligoClasses_1.50.4 here_1.0.1
#> [22] tidylog_1.0.2 forcats_0.5.0 stringr_1.4.0
#> [25] dplyr_1.0.2 purrr_0.3.4 readr_1.4.0
#> [28] tidyr_1.1.2 tibble_3.0.4 ggplot2_3.3.2
#> [31] tidyverse_1.3.0 mouse4302.db_3.2.3 org.Mm.eg.db_3.11.4
#> [34] AnnotationDbi_1.50.3 IRanges_2.22.2 S4Vectors_0.26.1
#> [37] Biobase_2.48.0 BiocGenerics_0.34.0 workflowr_1.6.2
#>
#> loaded via a namespace (and not attached):
#> [1] colorspace_2.0-0 ellipsis_0.3.1
#> [3] rprojroot_2.0.2 snakecase_0.11.0
#> [5] GenomicRanges_1.40.0 fs_1.5.0
#> [7] rstudioapi_0.13 farver_2.0.3
#> [9] affyio_1.58.0 ggrepel_0.9.0
#> [11] bit64_4.0.5 fansi_0.4.1
#> [13] lubridate_1.7.9.2 xml2_1.3.2
#> [15] codetools_0.2-16 splines_4.0.2
#> [17] knitr_1.30 jsonlite_1.7.2
#> [19] bcellViper_1.24.0 dbplyr_2.0.0
#> [21] BiocManager_1.30.10 compiler_4.0.2
#> [23] httr_1.4.2 backports_1.2.1
#> [25] assertthat_0.2.1 Matrix_1.2-18
#> [27] cli_2.2.0 later_1.1.0.1
#> [29] htmltools_0.5.0 tools_4.0.2
#> [31] gtable_0.3.0 glue_1.4.2
#> [33] GenomeInfoDbData_1.2.3 affxparser_1.60.0
#> [35] Rcpp_1.0.5 cellranger_1.1.0
#> [37] vctrs_0.3.6 preprocessCore_1.50.0
#> [39] iterators_1.0.13 xfun_0.19
#> [41] rvest_0.3.6 lifecycle_0.2.0
#> [43] renv_0.12.3 zlibbioc_1.34.0
#> [45] scales_1.1.1 clisymbols_1.2.0
#> [47] hms_0.5.3 promises_1.1.1
#> [49] SummarizedExperiment_1.18.2 yaml_2.2.1
#> [51] gridExtra_2.3 memoise_1.1.0
#> [53] stringi_1.5.3 foreach_1.5.1
#> [55] GenomeInfoDb_1.24.2 rlang_0.4.9
#> [57] pkgconfig_2.0.3 bitops_1.0-6
#> [59] matrixStats_0.57.0 evaluate_0.14
#> [61] lattice_0.20-41 labeling_0.4.2
#> [63] bit_4.0.4 tidyselect_1.1.0
#> [65] plyr_1.8.6 magrittr_2.0.1
#> [67] R6_2.5.0 generics_0.1.0
#> [69] DelayedArray_0.14.1 pillar_1.4.7
#> [71] haven_2.3.1 whisker_0.4
#> [73] withr_2.3.0 RCurl_1.98-1.2
#> [75] modelr_0.1.8 crayon_1.3.4
#> [77] rmarkdown_2.6 grid_4.0.2
#> [79] readxl_1.3.1 blob_1.2.1
#> [81] git2r_0.27.1 reprex_0.3.0
#> [83] digest_0.6.27 xtable_1.8-4
#> [85] ff_4.0.4 httpuv_1.5.4
#> [87] munsell_0.5.0 viridisLite_0.3.0