SCZ 2018 - Brain_Nucleus_accumbens_basal_ganglia
sheng Qian
2021-2-6
Last updated: 2022-05-19
Checks: 5 2
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version bcaadf3. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .ipynb_checkpoints/
Untracked files:
Untracked: G_list.RData
Untracked: Rplot.png
Untracked: SCZ_annotation.xlsx
Untracked: analysis/.ipynb_checkpoints/
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_2014_EUR_out/
Untracked: code/SCZ_2018_S_out/
Untracked: code/SCZ_2018_out/
Untracked: code/SCZ_2020_Single_out/
Untracked: code/SCZ_2020_out/
Untracked: code/SCZ_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/process_scz_2018_snps.R
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2014_EUR_analysis.sbatch
Untracked: code/run_SCZ_2014_EUR_analysis.sh
Untracked: code/run_SCZ_2014_EUR_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_analysis.sbatch
Untracked: code/run_SCZ_2018_analysis.sh
Untracked: code/run_SCZ_2018_analysis_S.sbatch
Untracked: code/run_SCZ_2018_analysis_S.sh
Untracked: code/run_SCZ_2018_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2020_Single_analysis.sbatch
Untracked: code/run_SCZ_2020_Single_analysis.sh
Untracked: code/run_SCZ_2020_Single_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2020_analysis.sbatch
Untracked: code/run_SCZ_2020_analysis.sh
Untracked: code/run_SCZ_2020_ctwas_rss_LDR.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_analysis_S.sbatch
Untracked: code/run_SCZ_analysis_S.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_SCZ_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: code/wflow_build.R
Untracked: code/wflow_build.sbatch
Untracked: data/.ipynb_checkpoints/
Untracked: data/GO_Terms/
Untracked: data/PGC3_SCZ_wave3_public.v2.tsv
Untracked: data/SCZ/
Untracked: data/SCZ_2014_EUR/
Untracked: data/SCZ_2018/
Untracked: data/SCZ_2018_S/
Untracked: data/SCZ_2020/
Untracked: data/SCZ_S/
Untracked: data/Supplementary Table 15 - MAGMA.xlsx
Untracked: data/Supplementary Table 20 - Prioritised Genes.xlsx
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/scz_2018.RDS
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Untracked: top_genes_32.txt
Untracked: top_genes_37.txt
Untracked: top_genes_43.txt
Untracked: top_genes_54.txt
Untracked: top_genes_81.txt
Untracked: z_snp_pos_SCZ.RData
Untracked: z_snp_pos_SCZ_2014_EUR.RData
Untracked: z_snp_pos_SCZ_2018.RData
Untracked: z_snp_pos_SCZ_2020.RData
Unstaged changes:
Deleted: analysis/BMI_S_results.Rmd
Modified: analysis/SCZ_2018_Brain_Amygdala_S.Rmd
Modified: analysis/SCZ_2018_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/SCZ_2018_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellum_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cortex_S.Rmd
Modified: analysis/SCZ_2018_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hippocampus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hypothalamus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/SCZ_2018_Brain_Substantia_nigra_S.Rmd
Modified: analysis/ttt.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_2018_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd) and HTML (docs/SCZ_2018_Brain_Nucleus_accumbens_basal_ganglia_S.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | bcaadf3 | sq-96 | 2022-05-19 | update |
| html | bcaadf3 | sq-96 | 2022-05-19 | update |
| Rmd | be614ed | sq-96 | 2022-05-19 | update |
| html | be614ed | sq-96 | 2022-05-19 | update |
| Rmd | 7d08c9b | sq-96 | 2022-05-18 | update |
| html | 7d08c9b | sq-96 | 2022-05-18 | update |
| Rmd | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 011327d | sq-96 | 2022-05-12 | update |
| Rmd | 6c6abbd | sq-96 | 2022-05-12 | update |
library(reticulate)
use_python("/scratch/midway2/shengqian/miniconda3/envs/PythonForR/bin/python",required=T)Weight QC
#number of imputed weights
nrow(qclist_all)[1] 21642#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1929 1532 1343 876 885 1118 1258 748 873 1021 1288 1205 442 765 754 863
17 18 19 20 21 22
1460 296 1534 750 40 662 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 18965#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8763INFO:numexpr.utils:Note: NumExpr detected 56 cores but "NUMEXPR_MAX_THREADS" not set, so enforcing safe limit of 8.finish
Attaching package: 'dplyr'The following objects are masked from 'package:stats':
filter, lagThe following objects are masked from 'package:base':
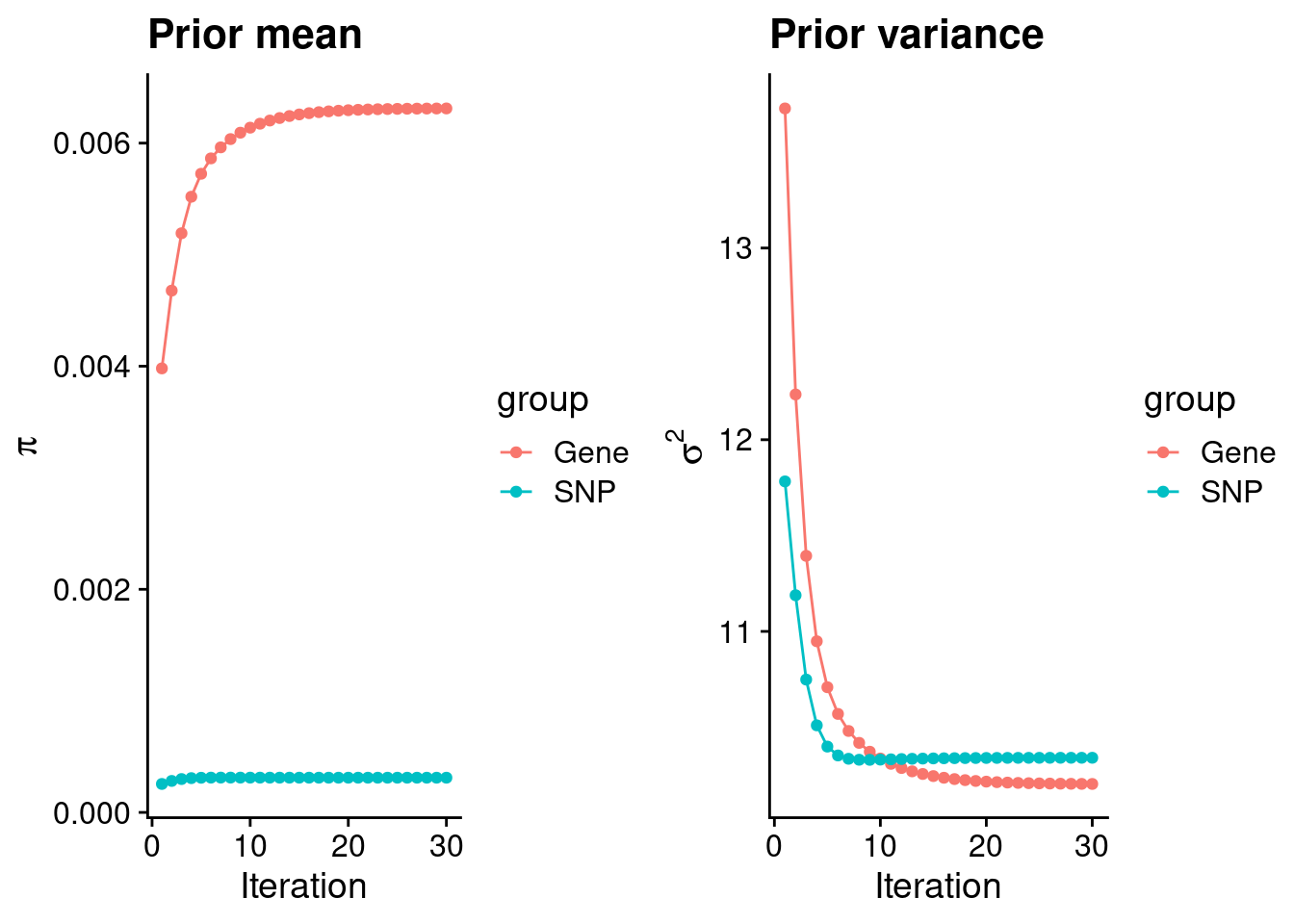
intersect, setdiff, setequal, unionCheck convergence of parameters
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
gene snp
0.0063107 0.0003111 gene snp
10.20 10.34 [1] 105318[1] 7622 6309950 gene snp
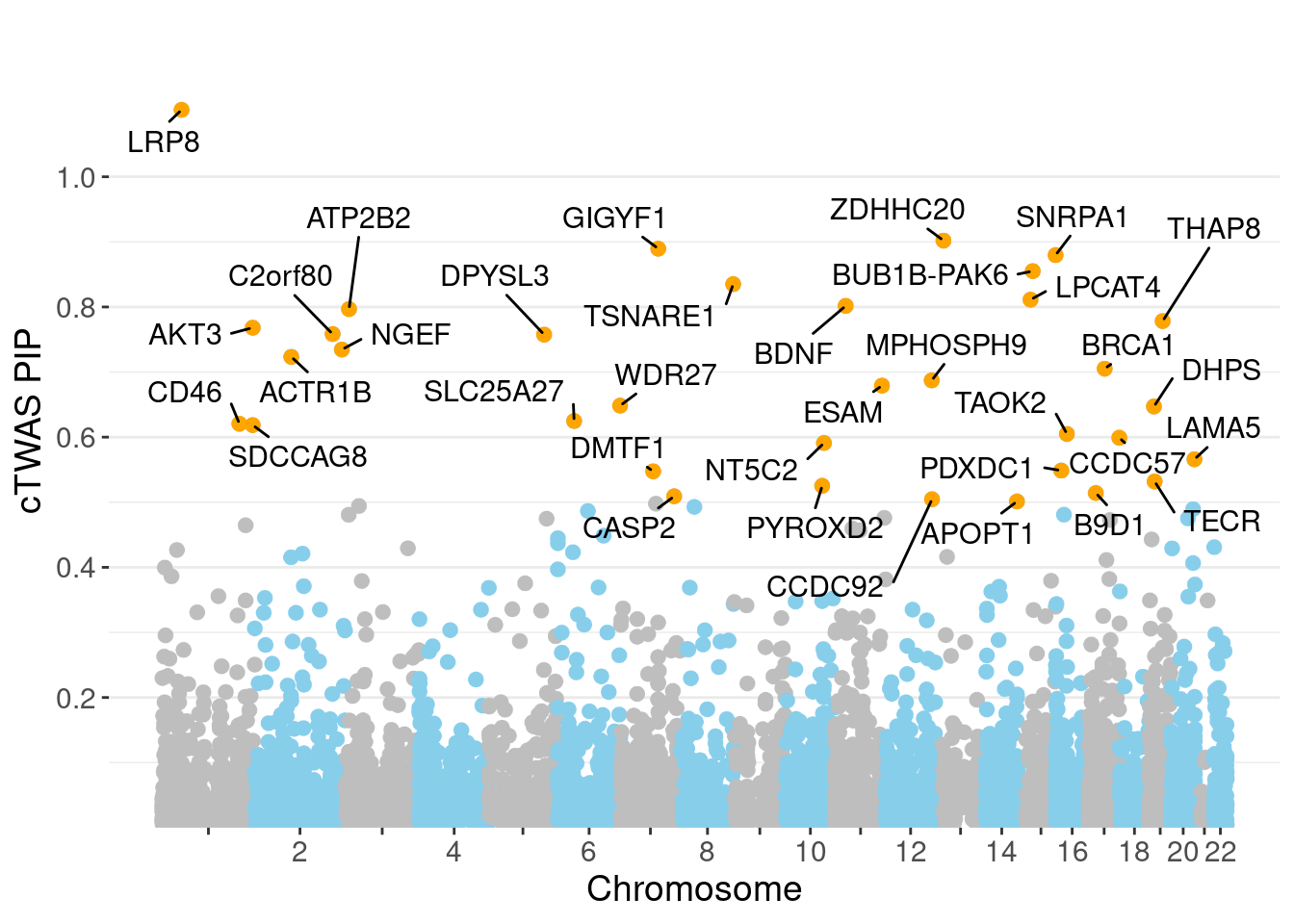
0.00466 0.19277 [1] 0.01395 1.10685Genes with highest PIPs
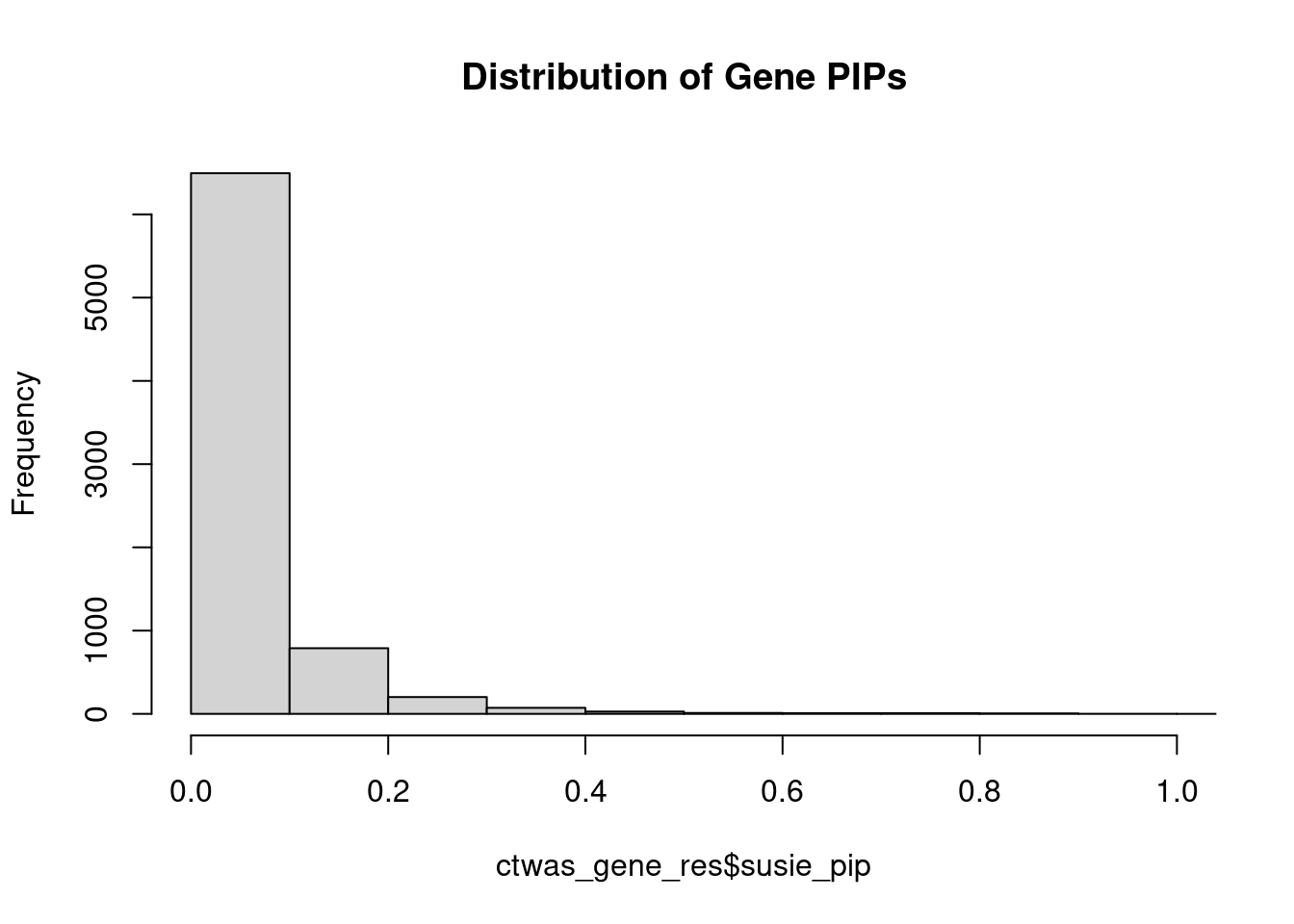
genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
3585 LRP8 1_33 1.1028 32.85 2.908e-04 -4.820 5 5
7379 ZDHHC20 13_2 0.9020 24.49 1.796e-04 -4.784 5 6
2652 GIGYF1 7_62 0.8896 26.12 1.951e-04 -5.266 4 4
6179 SNRPA1 15_50 0.8796 20.85 1.460e-04 -4.098 4 6
811 BUB1B-PAK6 15_14 0.8551 29.84 2.051e-04 5.588 2 2
6923 TSNARE1 8_93 0.8350 27.20 1.779e-04 5.555 11 11
3570 LPCAT4 15_10 0.8112 26.23 1.599e-04 4.892 3 5
731 BDNF 11_19 0.8017 23.22 1.365e-04 4.348 3 4
613 ATP2B2 3_8 0.7968 26.02 1.436e-04 4.229 5 6
6602 THAP8 19_25 0.7784 20.83 1.195e-04 3.847 2 2
292 AKT3 1_128 0.7682 34.93 1.847e-04 -6.350 7 8
924 C2orf80 2_123 0.7584 24.25 9.972e-05 3.053 12 13
1985 DPYSL3 5_86 0.7575 23.63 1.287e-04 4.157 1 1
4279 NGEF 2_137 0.7346 30.69 1.537e-04 7.036 3 3
159 ACTR1B 2_57 0.7234 20.56 1.003e-04 -3.978 5 5
775 BRCA1 17_25 0.7052 31.00 8.244e-05 -3.837 20 22
3953 MPHOSPH9 12_75 0.6872 60.79 2.709e-04 -8.201 2 4
2244 ESAM 11_77 0.6792 35.97 1.325e-04 5.889 2 2
7239 WDR27 6_111 0.6484 16.96 3.988e-05 2.235 21 33
1856 DHPS 19_10 0.6472 25.49 1.014e-04 -4.396 1 1Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_intron
3585 LRP8 1_33 1.1028 32.85 0.0002908 -4.820 5
3953 MPHOSPH9 12_75 0.6872 60.79 0.0002709 -8.201 2
811 BUB1B-PAK6 15_14 0.8551 29.84 0.0002051 5.588 2
459 APOM 6_26 0.1818 627.31 0.0001967 11.590 3
2652 GIGYF1 7_62 0.8896 26.12 0.0001951 -5.266 4
292 AKT3 1_128 0.7682 34.93 0.0001847 -6.350 7
7379 ZDHHC20 13_2 0.9020 24.49 0.0001796 -4.784 5
6923 TSNARE1 8_93 0.8350 27.20 0.0001779 5.555 11
3570 LPCAT4 15_10 0.8112 26.23 0.0001599 4.892 3
3622 LSM2 6_26 0.1619 635.43 0.0001581 -11.599 1
6484 TAOK2 16_24 0.6049 46.28 0.0001540 7.024 5
4279 NGEF 2_137 0.7346 30.69 0.0001537 7.036 3
7159 VARS 6_26 0.1563 629.91 0.0001462 -11.620 1
6179 SNRPA1 15_50 0.8796 20.85 0.0001460 -4.098 4
4405 NT5C2 10_66 0.5910 46.04 0.0001450 -8.511 11
613 ATP2B2 3_8 0.7968 26.02 0.0001436 4.229 5
731 BDNF 11_19 0.8017 23.22 0.0001365 4.348 3
2244 ESAM 11_77 0.6792 35.97 0.0001325 5.889 2
1985 DPYSL3 5_86 0.7575 23.63 0.0001287 4.157 1
6602 THAP8 19_25 0.7784 20.83 0.0001195 3.847 2
num_sqtl
3585 5
3953 4
811 2
459 3
2652 4
292 8
7379 6
6923 11
3570 5
3622 1
6484 6
4279 3
7159 1
6179 6
4405 15
613 6
731 4
2244 2
1985 1
6602 2Comparing z scores and PIPs
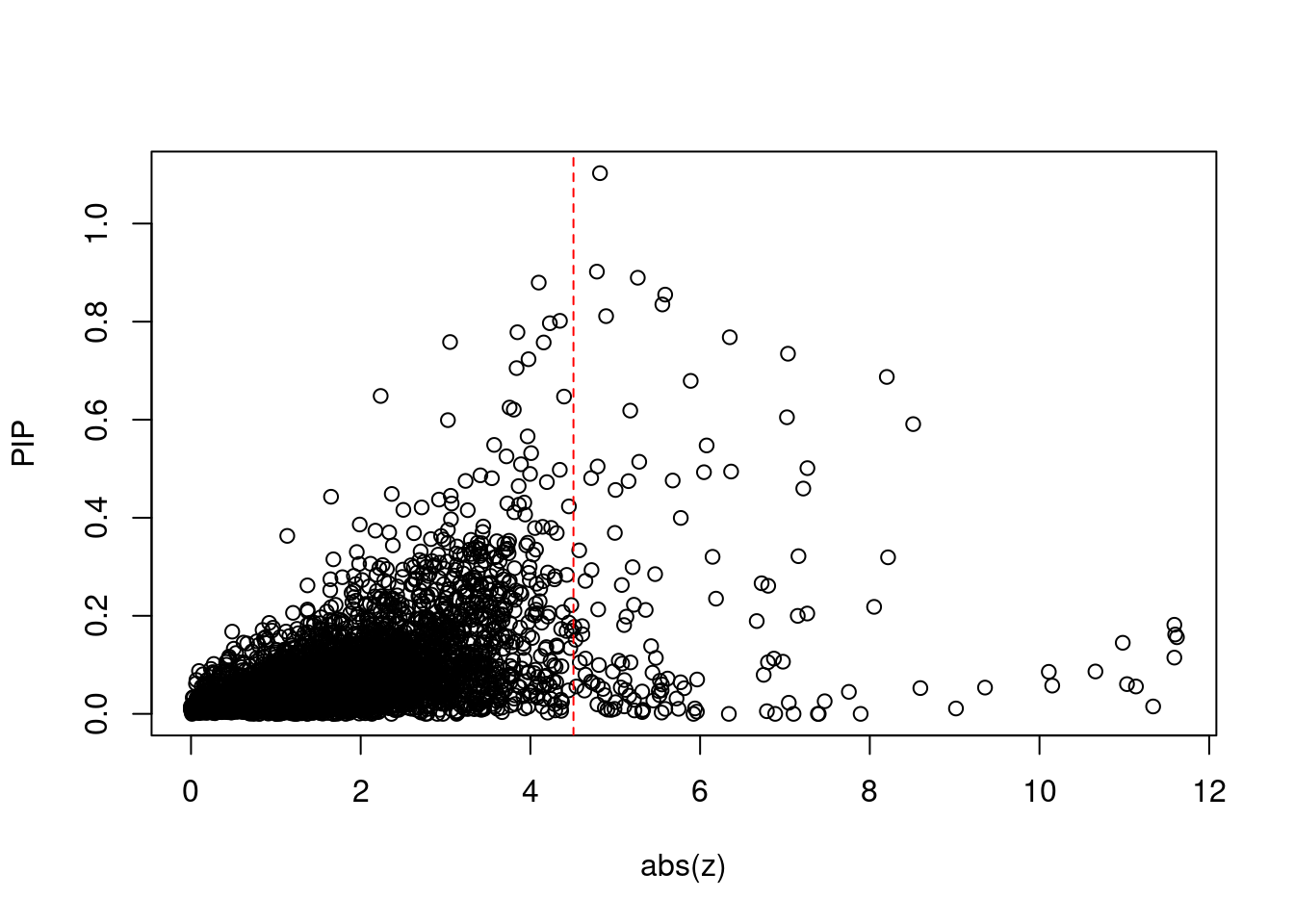
[1] 0.01719 genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
7159 VARS 6_26 0.15633 629.91 1.462e-04 -11.620 1 1
3622 LSM2 6_26 0.16187 635.43 1.581e-04 -11.599 1 1
459 APOM 6_26 0.18177 627.31 1.967e-04 11.590 3 3
682 BAG6 6_26 0.11475 627.31 7.843e-05 -11.590 6 6
1732 CYP21A2 6_26 0.01511 659.18 1.430e-06 -11.340 1 1
7160 VARS2 6_25 0.05583 101.38 3.000e-06 -11.137 1 1
941 C6orf136 6_24 0.06069 79.63 2.785e-06 -11.031 2 2
2501 FLOT1 6_24 0.14500 78.29 1.560e-05 10.981 6 7
808 BTN3A2 6_20 0.08647 90.16 2.598e-06 -10.659 3 3
2949 HLA-B 6_25 0.05761 76.72 9.724e-07 10.150 11 21
805 BTN2A1 6_20 0.08574 82.29 3.455e-06 10.110 5 6
1153 CCHCR1 6_25 0.05360 62.58 9.944e-07 -9.358 10 14
1799 DDR1 6_25 0.01101 67.83 7.808e-08 9.016 1 1
2950 HLA-DMA 6_27 0.05258 65.16 9.905e-07 8.596 4 7
4405 NT5C2 10_66 0.59099 46.04 1.450e-04 -8.511 11 15
3664 MAD1L1 7_3 0.31932 63.77 4.772e-05 -8.215 3 3
3953 MPHOSPH9 12_75 0.68723 60.79 2.709e-04 -8.201 2 4
554 AS3MT 10_66 0.21828 44.51 1.947e-05 8.051 6 7
4030 MSH5 6_26 0.00000 236.73 0.000e+00 -7.892 3 3
841 C12orf65 12_75 0.04490 54.18 9.788e-07 -7.754 2 2GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 35Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"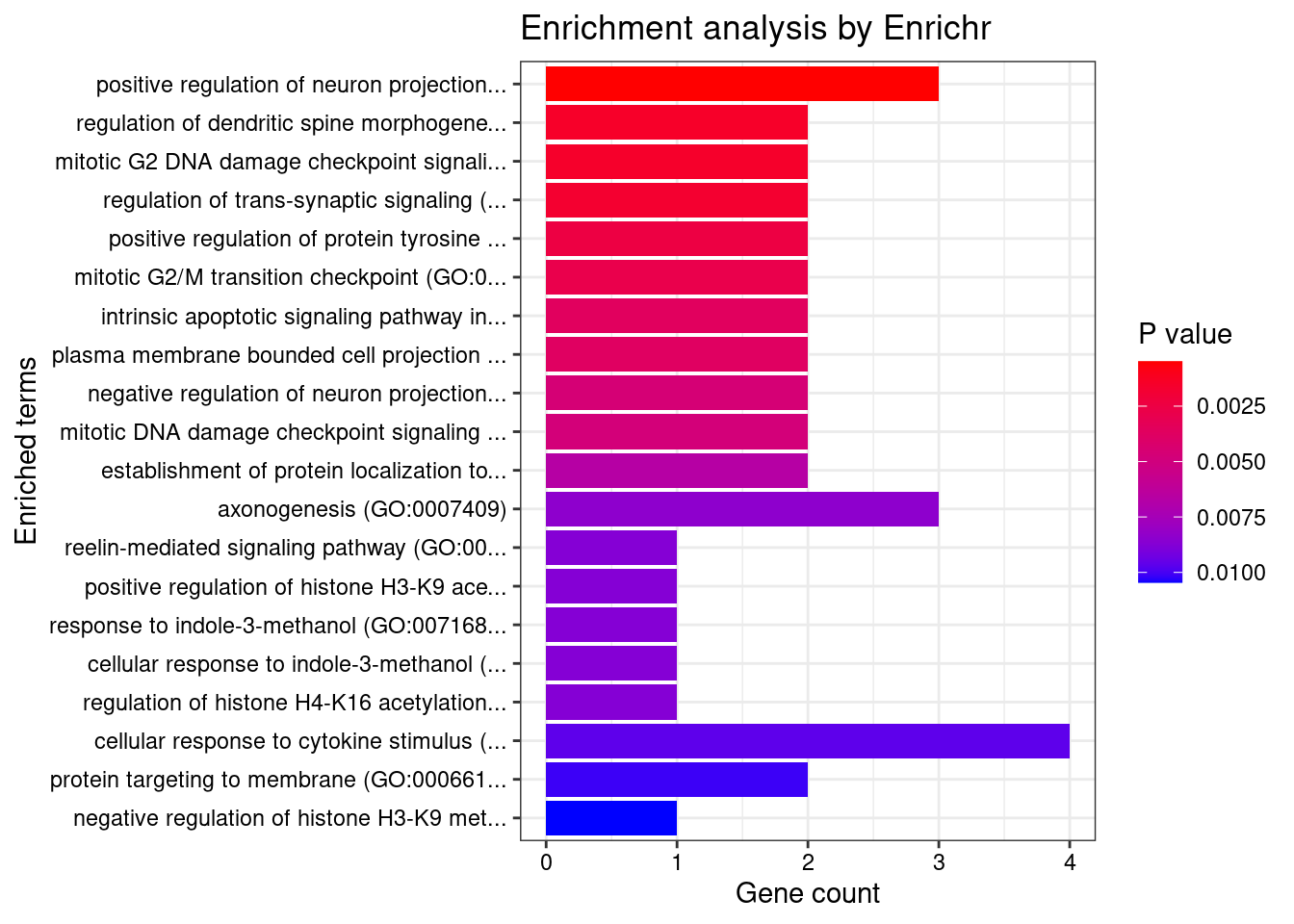
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Cellular_Component_2021"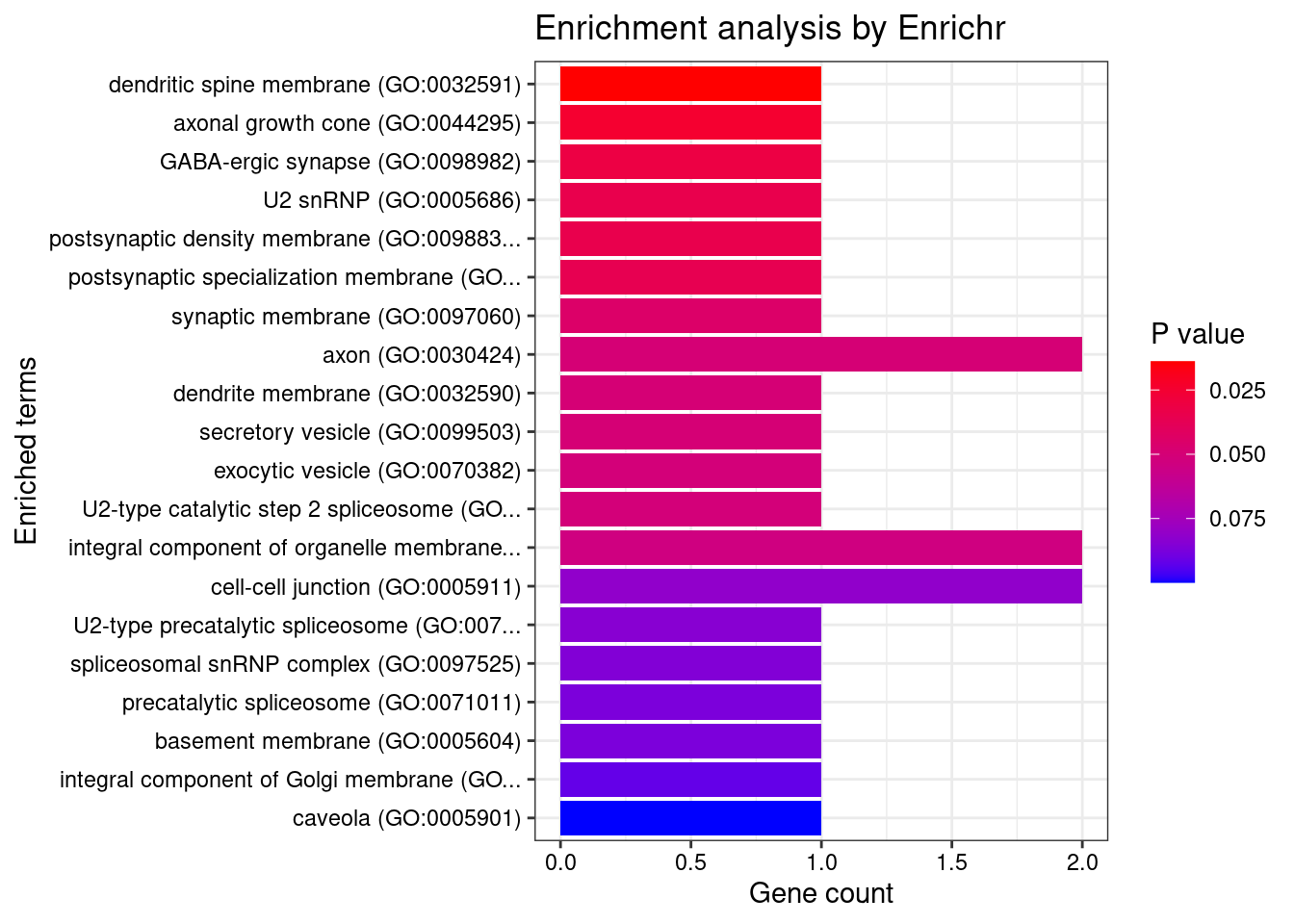
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"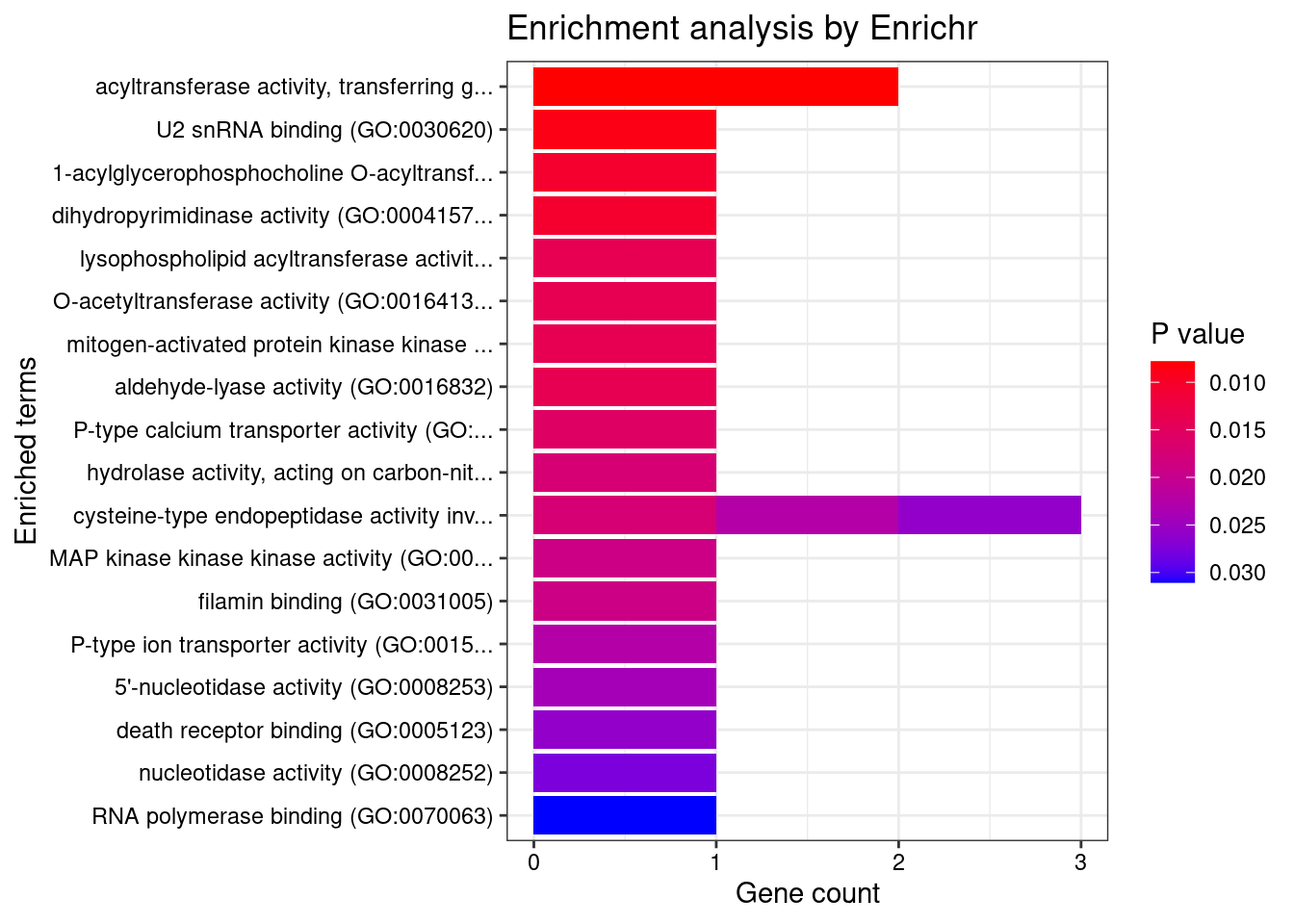
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio
48 Measles 0.02256 1/18
69 Schizophrenia 0.02256 7/18
85 Electroencephalogram abnormal 0.02256 1/18
178 Sporadic Breast Carcinoma 0.02256 1/18
181 Primary peritoneal carcinoma 0.02256 1/18
190 BREAST-OVARIAN CANCER, FAMILIAL, SUSCEPTIBILITY TO, 1 0.02256 1/18
191 BREAST CANCER, FAMILIAL, SUSCEPTIBILITY TO, 1 0.02256 1/18
192 OVARIAN CANCER, FAMILIAL, SUSCEPTIBILITY TO, 1 0.02256 1/18
193 HEMOLYTIC UREMIC SYNDROME, ATYPICAL, SUSCEPTIBILITY TO, 2 0.02256 1/18
198 SENIOR-LOKEN SYNDROME 7 0.02256 1/18
BgRatio
48 1/9703
69 883/9703
85 1/9703
178 1/9703
181 1/9703
190 1/9703
191 1/9703
192 1/9703
193 1/9703
198 1/9703WebGestalt enrichment analysis for genes with PIP>0.5
Warning: replacing previous import 'lifecycle::last_warnings' by
'rlang::last_warnings' when loading 'hms'Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 130#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 51#significance threshold for TWAS
print(sig_thresh)[1] 4.507#number of ctwas genes
length(ctwas_genes)[1] 8#number of TWAS genes
length(twas_genes)[1] 131#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
731 BDNF 11_19 0.8017 23.22 0.0001365 4.348 3 4
6179 SNRPA1 15_50 0.8796 20.85 0.0001460 -4.098 4 6#sensitivity / recall
print(sensitivity) ctwas TWAS
0.007692 0.100000 #specificity
print(specificity) ctwas TWAS
0.9991 0.9844 #precision / PPV
print(precision) ctwas TWAS
0.12500 0.09924
sessionInfo()R version 4.1.0 (2021-05-18)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.3.13-el7-x86_64/lib/libopenblas_haswellp-r0.3.13.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.4.0 forcats_0.5.1 stringr_1.4.0 purrr_0.3.4
[5] readr_1.4.0 tidyr_1.1.3 tidyverse_1.3.1 tibble_3.1.7
[9] WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0 cowplot_1.1.1
[13] ggplot2_3.3.5 dplyr_1.0.7 reticulate_1.25 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] fs_1.5.0 lubridate_1.7.10 doParallel_1.0.16 httr_1.4.2
[5] rprojroot_2.0.2 tools_4.1.0 backports_1.2.1 doRNG_1.8.2
[9] bslib_0.2.5.1 utf8_1.2.1 R6_2.5.0 vipor_0.4.5
[13] DBI_1.1.1 colorspace_2.0-2 withr_2.4.2 ggrastr_1.0.1
[17] tidyselect_1.1.1 processx_3.5.2 curl_4.3.2 compiler_4.1.0
[21] git2r_0.28.0 rvest_1.0.0 cli_3.0.0 Cairo_1.5-15
[25] xml2_1.3.2 labeling_0.4.2 sass_0.4.0 scales_1.1.1
[29] callr_3.7.0 systemfonts_1.0.4 apcluster_1.4.9 digest_0.6.27
[33] rmarkdown_2.9 svglite_2.0.0 pkgconfig_2.0.3 htmltools_0.5.1.1
[37] dbplyr_2.1.1 highr_0.9 rlang_1.0.2 rstudioapi_0.13
[41] jquerylib_0.1.4 farver_2.1.0 generics_0.1.0 jsonlite_1.7.2
[45] magrittr_2.0.1 Matrix_1.3-3 ggbeeswarm_0.6.0 Rcpp_1.0.7
[49] munsell_0.5.0 fansi_0.5.0 lifecycle_1.0.0 stringi_1.6.2
[53] whisker_0.4 yaml_2.2.1 plyr_1.8.6 grid_4.1.0
[57] ggrepel_0.9.1 parallel_4.1.0 promises_1.2.0.1 crayon_1.4.1
[61] lattice_0.20-44 haven_2.4.1 hms_1.1.0 knitr_1.33
[65] ps_1.6.0 pillar_1.7.0 igraph_1.2.6 rjson_0.2.20
[69] rngtools_1.5 reshape2_1.4.4 codetools_0.2-18 reprex_2.0.0
[73] glue_1.4.2 evaluate_0.14 getPass_0.2-2 modelr_0.1.8
[77] data.table_1.14.0 png_0.1-7 vctrs_0.3.8 httpuv_1.6.1
[81] foreach_1.5.1 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] xfun_0.24 broom_0.7.8 later_1.2.0 iterators_1.0.13
[89] beeswarm_0.4.0 ellipsis_0.3.2 here_1.0.1