Analysis of Experiment 1
Last updated: 2020-04-24
Checks: 7 0
Knit directory: social_immunity/
This reproducible R Markdown analysis was created with workflowr (version 1.6.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20191017) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | a1f8dc2 | lukeholman | 2020-04-24 | tweaks |
| html | a1f8dc2 | lukeholman | 2020-04-24 | tweaks |
| html | 8c3b471 | lukeholman | 2020-04-21 | Build site. |
| Rmd | 1ce9e19 | lukeholman | 2020-04-21 | First commit 2020 |
| html | 1ce9e19 | lukeholman | 2020-04-21 | First commit 2020 |
| Rmd | aae65cf | lukeholman | 2019-10-17 | First commit |
| html | aae65cf | lukeholman | 2019-10-17 | First commit |
Load data and R packages
# All but 1 of these packages can be easily installed from CRAN.
# However it was harder to install the showtext package. On Mac, I did this:
# installed 'homebrew' using Terminal: ruby -e "$(curl -fsSL https://raw.githubusercontent.com/Homebrew/install/master/install)"
# installed 'libpng' using Terminal: brew install libpng
# installed 'showtext' in R using: devtools::install_github("yixuan/showtext")
library(showtext)
library(brms)
library(bayesplot)
library(tidyverse)
library(gridExtra)
library(kableExtra)
library(bayestestR)
library(tidybayes)
library(cowplot)
source("code/helper_functions.R")
# set up nice font for figure
nice_font <- "PT Serif"
font_add_google(name = nice_font, family = nice_font, regular.wt = 400, bold.wt = 700)
showtext_auto()
exp1_treatments <- c("Intact control", "Ringers", "Heat-treated LPS", "LPS")
durations <- read_csv("data/data_collection_sheets/experiment_durations.csv") %>%
filter(experiment == 1) %>% select(-experiment)
outcome_tally <- read_csv(file = "data/clean_data/experiment_1_outcome_tally.csv") %>%
mutate(outcome = replace(outcome, outcome == "Left of own volition", "Left voluntarily")) %>%
mutate(outcome = factor(outcome, levels = c("Stayed inside the hive", "Left voluntarily", "Forced out")),
treatment = factor(treatment, levels = exp1_treatments))
# Re-formatted version of the same data, where each row is an individual bee. We need this format to run the brms model.
data_for_categorical_model <- outcome_tally %>%
mutate(id = 1:n()) %>%
split(.$id) %>%
map(function(x){
if(x$n[1] == 0) return(NULL)
data.frame(
treatment = x$treatment[1],
hive = x$hive[1],
colour = x$colour[1],
outcome = rep(x$outcome[1], x$n))
}) %>% do.call("rbind", .) %>% as_tibble() %>%
arrange(hive, treatment) %>%
mutate(outcome_numeric = as.numeric(outcome),
hive = as.character(hive),
treatment = factor(treatment, levels = exp1_treatments)) %>%
left_join(durations, by = "hive") %>%
mutate(hive = C(factor(hive), sum)) # use sum coding for the factor levels of "hive"Inspect the raw data
Sample sizes by treatment
data_for_categorical_model %>%
group_by(treatment) %>%
summarise(n = n()) %>%
kable() %>% kable_styling(full_width = FALSE)| treatment | n |
|---|---|
| Intact control | 321 |
| Ringers | 153 |
| Heat-treated LPS | 186 |
| LPS | 182 |
Sample sizes by treatment and hive
data_for_categorical_model %>%
group_by(hive, treatment) %>%
summarise(n = n()) %>%
kable() %>% kable_styling(full_width = FALSE)| hive | treatment | n |
|---|---|---|
| Garden | Intact control | 77 |
| Garden | Ringers | 41 |
| Garden | Heat-treated LPS | 34 |
| Garden | LPS | 37 |
| SkyLab | Intact control | 105 |
| SkyLab | Heat-treated LPS | 58 |
| SkyLab | LPS | 43 |
| Zoology | Intact control | 106 |
| Zoology | Ringers | 80 |
| Zoology | Heat-treated LPS | 67 |
| Zoology | LPS | 67 |
| Zoology_2 | Intact control | 33 |
| Zoology_2 | Ringers | 32 |
| Zoology_2 | Heat-treated LPS | 27 |
| Zoology_2 | LPS | 35 |
Inspect the results
outcome_tally %>%
select(-colour) %>%
kable(digits = 3) %>% kable_styling(full_width = FALSE) %>%
scroll_box(height = "380px")| hive | treatment | outcome | n |
|---|---|---|---|
| Garden | Heat-treated LPS | Stayed inside the hive | 34 |
| Garden | Heat-treated LPS | Left voluntarily | 0 |
| Garden | Heat-treated LPS | Forced out | 0 |
| Garden | Intact control | Stayed inside the hive | 75 |
| Garden | Intact control | Left voluntarily | 0 |
| Garden | Intact control | Forced out | 2 |
| Garden | LPS | Stayed inside the hive | 37 |
| Garden | LPS | Left voluntarily | 0 |
| Garden | LPS | Forced out | 0 |
| Garden | Ringers | Stayed inside the hive | 41 |
| Garden | Ringers | Left voluntarily | 0 |
| Garden | Ringers | Forced out | 0 |
| SkyLab | Heat-treated LPS | Stayed inside the hive | 47 |
| SkyLab | Heat-treated LPS | Left voluntarily | 6 |
| SkyLab | Heat-treated LPS | Forced out | 5 |
| SkyLab | Intact control | Stayed inside the hive | 102 |
| SkyLab | Intact control | Left voluntarily | 3 |
| SkyLab | Intact control | Forced out | 0 |
| SkyLab | LPS | Stayed inside the hive | 35 |
| SkyLab | LPS | Left voluntarily | 6 |
| SkyLab | LPS | Forced out | 2 |
| Zoology | Heat-treated LPS | Stayed inside the hive | 59 |
| Zoology | Heat-treated LPS | Left voluntarily | 1 |
| Zoology | Heat-treated LPS | Forced out | 7 |
| Zoology | Intact control | Stayed inside the hive | 105 |
| Zoology | Intact control | Left voluntarily | 0 |
| Zoology | Intact control | Forced out | 1 |
| Zoology | LPS | Stayed inside the hive | 60 |
| Zoology | LPS | Left voluntarily | 2 |
| Zoology | LPS | Forced out | 5 |
| Zoology | Ringers | Stayed inside the hive | 74 |
| Zoology | Ringers | Left voluntarily | 1 |
| Zoology | Ringers | Forced out | 5 |
| Zoology_2 | Heat-treated LPS | Stayed inside the hive | 16 |
| Zoology_2 | Heat-treated LPS | Left voluntarily | 1 |
| Zoology_2 | Heat-treated LPS | Forced out | 10 |
| Zoology_2 | Intact control | Stayed inside the hive | 24 |
| Zoology_2 | Intact control | Left voluntarily | 1 |
| Zoology_2 | Intact control | Forced out | 8 |
| Zoology_2 | LPS | Stayed inside the hive | 21 |
| Zoology_2 | LPS | Left voluntarily | 2 |
| Zoology_2 | LPS | Forced out | 12 |
| Zoology_2 | Ringers | Stayed inside the hive | 23 |
| Zoology_2 | Ringers | Left voluntarily | 1 |
| Zoology_2 | Ringers | Forced out | 8 |
Means and 95% CIs of the raw data
all_hives <- outcome_tally %>%
group_by(treatment, outcome) %>%
summarise(n = sum(n)) %>%
ungroup() %>% mutate(hive = "All hives")
pd <- position_dodge(.3)
outcome_tally %>%
group_by(treatment, outcome) %>%
summarise(n = sum(n)) %>% mutate() %>%
group_by(treatment) %>%
mutate(total_n = sum(n),
percent = 100 * n / sum(n),
SE = sqrt(total_n * (percent/100) * (1-(percent/100)))) %>%
ungroup() %>%
mutate(lowerCI = map_dbl(1:n(), ~ 100 * binom.test(n[.x], total_n[.x])$conf.int[1]),
upperCI = map_dbl(1:n(), ~ 100 * binom.test(n[.x], total_n[.x])$conf.int[2])) %>%
filter(outcome != "Stayed inside the hive") %>%
ggplot(aes(treatment, percent, fill = outcome)) +
geom_errorbar(aes(ymin=lowerCI, ymax=upperCI), position = pd, width = 0) +
geom_point(stat = "identity", position = pd, colour = "grey15", pch = 21, size = 4) +
scale_fill_brewer(palette = "Pastel1", name = "Outcome", direction = -1) +
xlab("Treatment") + ylab("% bees (+95% CIs)") +
theme(legend.position = "top") +
coord_flip()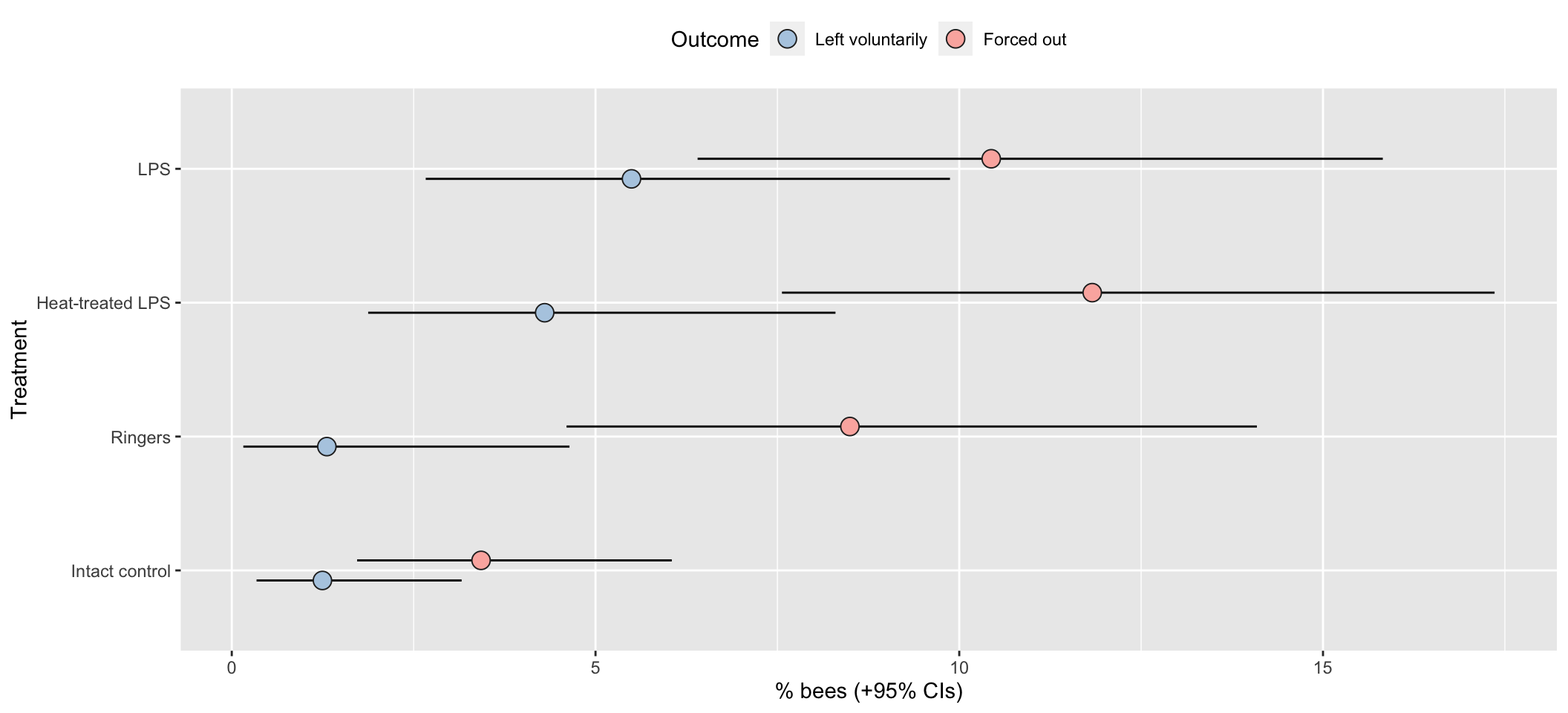
| Version | Author | Date |
|---|---|---|
| 1ce9e19 | lukeholman | 2020-04-21 |
Multinomial model of outcome
Run the models
Fit a multinomial logisitic model, with 3 possible outcomes describing what happened to each bee introduced to the hive: stayed inside, left of its own volition, or forced out by the other workers. To assess the effects of our predictor variables, we compare 5 models with different fixed factors, ranking them by posterior model probability.
if(!file.exists("output/exp1_model.rds")){
exp1_model_v1 <- brm(
outcome_numeric ~ treatment * hive + observation_time_minutes,
data = data_for_categorical_model,
prior = c(set_prior("normal(0, 3)", class = "b", dpar = "mu2"),
set_prior("normal(0, 3)", class = "b", dpar = "mu3")),
family = "categorical", save_all_pars = TRUE, sample_prior = TRUE,
chains = 4, cores = 1, iter = 5000, seed = 1)
exp1_model_v2 <- brm(
outcome_numeric ~ treatment + hive + observation_time_minutes,
data = data_for_categorical_model,
prior = c(set_prior("normal(0, 3)", class = "b", dpar = "mu2"),
set_prior("normal(0, 3)", class = "b", dpar = "mu3")),
family = "categorical", save_all_pars = TRUE, sample_prior = TRUE,
chains = 4, cores = 1, iter = 5000, seed = 1)
exp1_model_v3 <- brm(
outcome_numeric ~ hive + observation_time_minutes,
data = data_for_categorical_model,
prior = c(set_prior("normal(0, 3)", class = "b", dpar = "mu2"),
set_prior("normal(0, 3)", class = "b", dpar = "mu3")),
family = "categorical", save_all_pars = TRUE, sample_prior = TRUE,
chains = 4, cores = 1, iter = 5000, seed = 1)
posterior_model_probabilities <- tibble(
Model = c("treatment * hive + observation_time_minutes",
"treatment + hive + observation_time_minutes",
"hive + observation_time_minutes"),
post_prob = as.numeric(post_prob(exp1_model_v1,
exp1_model_v2,
exp1_model_v3))) %>%
arrange(-post_prob)
saveRDS(exp1_model_v2, "output/exp1_model.rds") # save the top model, treatment + hive
saveRDS(posterior_model_probabilities, "output/exp1_post_prob.rds")
}
exp1_model <- readRDS("output/exp1_model.rds")
posterior_model_probabilities <- readRDS("output/exp1_post_prob.rds")Fixed effects from the full model
The code chunk below wrangles the raw output of the summary() functions for brms models into a more readable table of results, and also adds ‘Bayesian p-values’ (i.e. the posterior probability that the true effect size has the same sign as the reported effect).
tableS1 <- get_fixed_effects_with_p_values(exp1_model) %>%
mutate(mu = map_chr(str_extract_all(Parameter, "mu[:digit:]"), ~ .x[1]),
Parameter = str_remove_all(Parameter, "mu[:digit:]_"),
Parameter = str_replace_all(Parameter, "treatment", "Treatment: "),
Parameter = str_replace_all(Parameter, "HeatMtreatedLPS", "Heat-treated LPS"),
Parameter = str_replace_all(Parameter, "observation_time_minutes", "Observation duration (minutes)")) %>%
arrange(mu) %>%
select(-mu, -Rhat, -Bulk_ESS, -Tail_ESS)
names(tableS1)[3:5] <- c("Est. Error", "Lower 95% CI", "Upper 95% CI")
saveRDS(tableS1, file = "figures/tableS1.rds")
tableS1 %>%
kable(digits = 3) %>%
kable_styling(full_width = FALSE) %>%
pack_rows("% bees leaving voluntarily", 1, 8) %>%
pack_rows("% bees forced out", 9, 16)| Parameter | Estimate | Est. Error | Lower 95% CI | Upper 95% CI | p | |
|---|---|---|---|---|---|---|
| % bees leaving voluntarily | ||||||
| Intercept | -14.866 | 9.431 | -35.346 | 2.072 | 0.045 | * |
| Treatment: Ringers | 0.658 | 0.970 | -1.395 | 2.433 | 0.232 | |
| Treatment: Heat-treated LPS | 1.298 | 0.612 | 0.126 | 2.530 | 0.015 | * |
| Treatment: LPS | 1.681 | 0.602 | 0.532 | 2.902 | 0.002 | ** |
| hive1 | -1.514 | 2.617 | -6.596 | 3.576 | 0.281 | |
| hive2 | 3.127 | 1.480 | 0.836 | 6.624 | 0.001 | ** |
| hive3 | -1.520 | 1.602 | -4.785 | 1.564 | 0.172 | |
| Observation duration (minutes) | 0.091 | 0.090 | -0.073 | 0.284 | 0.148 | |
| % bees forced out | ||||||
| Intercept | -7.403 | 6.705 | -20.821 | 5.738 | 0.134 | |
| Treatment: Ringers | 0.552 | 0.453 | -0.341 | 1.445 | 0.114 | |
| Treatment: Heat-treated LPS | 1.298 | 0.406 | 0.520 | 2.097 | 0.000 | *** |
| Treatment: LPS | 0.965 | 0.419 | 0.161 | 1.797 | 0.011 | * |
| hive1 | -0.394 | 2.523 | -5.315 | 4.675 | 0.437 | |
| hive2 | -0.115 | 0.654 | -1.392 | 1.160 | 0.431 | |
| hive3 | -0.762 | 1.518 | -3.826 | 2.200 | 0.309 | |
| Observation duration (minutes) | 0.038 | 0.068 | -0.095 | 0.176 | 0.288 | |
Table S1: Table summarising the posterior estimates of each fixed effect in the best-fitting model of Experiment 1. This was a multinomial model with three possible outcomes (stay inside, leave voluntarily, be forced out), and so there are two parameter estimates for the intercept and for each predictor in the model. ‘Treatment’ is a fixed factor with four levels, and the effects shown here are expressed relative to the ‘Intact control’ group. ‘Hive’ was also a fixed factor with four levels; unlike for treatment, we modelled hive using deviation coding, such that the intercept term represents the mean across all hives (in the intact control treatment), and the three hive terms represent the deviation from this mean for three of the four hives. Lastly, observation duration was a continuous variable expressed to the nearest minute. The \(p\) column gives the posterior probability that the true effect size is opposite in sign to what is reported in the Estimate column, similarly to a \(p\)-value.
Plotting estimates from the model
Derive prediction from the posterior
new <- expand.grid(treatment = levels(data_for_categorical_model$treatment),
hive = NA,
observation_time_minutes = 120)
preds <- fitted(exp1_model, newdata = new, summary = FALSE)
dimnames(preds) <- list(NULL, new[,1], NULL)
plotting_data <- rbind(
as.data.frame(preds[,, 1]) %>% mutate(outcome = "Stayed inside the hive", posterior_sample = 1:n()),
as.data.frame(preds[,, 2]) %>% mutate(outcome = "Left voluntarily", posterior_sample = 1:n()),
as.data.frame(preds[,, 3]) %>% mutate(outcome = "Forced out", posterior_sample = 1:n())) %>%
gather(treatment, prop, `Intact control`, Ringers, `Heat-treated LPS`, LPS) %>%
mutate(outcome = factor(outcome, c("Stayed inside the hive", "Left voluntarily", "Forced out"))) %>%
as_tibble() %>% arrange(treatment, outcome)
stats_data <- plotting_data
plotting_data$treatment <- factor(plotting_data$treatment, c("Intact control", "Ringers", "Heat-treated LPS", "LPS"))Make Figure 1
cols <- c("#34558b", "#4ec5a5", "#ffaf12")
dot_plot <- plotting_data %>%
mutate(outcome = str_replace_all(outcome, "Stayed inside the hive", "Stayed inside"),
outcome = factor(outcome, c("Stayed inside", "Left voluntarily", "Forced out"))) %>%
ggplot(aes(100 * prop, treatment)) +
stat_dotsh(quantiles = 100, fill = "grey40", colour = "grey40") + # aes(fill = treatment),
stat_pointintervalh(aes(colour = outcome, fill = outcome),
.width = c(0.5, 0.95),
position = position_nudge(y = -0.07),
point_colour = "grey26", pch = 21, stroke = 0.4) +
scale_colour_manual(values = cols) +
scale_fill_manual(values = cols) +
facet_wrap( ~ outcome, scales = "free_x") +
xlab("% bees (posterior estimate)") + ylab("Treatment") +
theme_bw() +
coord_cartesian(ylim=c(1.4, 4)) +
theme(
text = element_text(family = nice_font),
strip.background = element_rect(fill = "#eff0f1"),
panel.grid.major.y = element_blank(),
legend.position = "none"
)
# positive effect = odds of this outcome are higher for trt2 than trt1 (put control as trt1)
get_log_odds <- function(trt1, trt2){
log((trt2 / (1 - trt2) / (trt1 / (1 - trt1))))
}
LOR <- stats_data %>%
spread(treatment, prop) %>%
mutate(LOR_intact_Ringers = get_log_odds(`Intact control`, Ringers),
LOR_intact_heat = get_log_odds(`Intact control`, `Heat-treated LPS`),
LOR_intact_LPS = get_log_odds(`Intact control`, LPS),
LOR_Ringers_heat = get_log_odds(Ringers, `Heat-treated LPS`),
LOR_Ringers_LPS = get_log_odds(Ringers, LPS),
LOR_heat_LPS = get_log_odds(`Heat-treated LPS`, LPS)) %>%
select(posterior_sample, outcome, starts_with("LOR")) %>%
gather(LOR, comparison, starts_with("LOR")) %>%
mutate(LOR = str_remove_all(LOR, "LOR_"),
LOR = str_replace_all(LOR, "heat_LPS", "LPS\n(Heat-treated LPS)"),
LOR = str_replace_all(LOR, "Ringers_LPS", "LPS\n(Ringers)"),
LOR = str_replace_all(LOR, "Ringers_heat", "Heat-treated LPS\n(Ringers)"),
LOR = str_replace_all(LOR, "intact_Ringers", "Ringers\n(Intact control)"),
LOR = str_replace_all(LOR, "intact_heat", "Heat-treated LPS\n(Intact control)"),
LOR = str_replace_all(LOR, "intact_LPS", "LPS\n(Intact control)"))
levs <- LOR$LOR %>% unique() %>% sort()
LOR$LOR <- factor(LOR$LOR, rev(levs[c(3,5,4,2,1,6)]))
LOR_plot <- LOR %>%
mutate(outcome = str_replace_all(outcome, "Stayed inside the hive", "Stayed inside"),
outcome = factor(outcome, levels = rev(c("Forced out", "Left voluntarily", "Stayed inside")))) %>%
ggplot(aes(comparison, y = LOR, colour = outcome)) +
geom_vline(xintercept = 0, linetype = 2) +
geom_vline(xintercept = 0.6931472, linetype = 2, colour = "pink") +
geom_vline(xintercept = -0.6931472, linetype = 2, colour = "pink") +
stat_pointintervalh(aes(colour = outcome, fill = outcome),
.width = c(0.5, 0.95),
position = position_dodge(0.5),
point_colour = "grey26", pch = 21, stroke = 0.4) +
scale_colour_manual(values = cols) +
scale_fill_manual(values = cols) +
xlab("Effect size (posterior log odds ratio)") + ylab("Treatment (reference)") +
theme_bw() +
theme(
text = element_text(family = nice_font),
panel.grid.major.y = element_blank(),
legend.position = "none"
)
p <- cowplot::plot_grid(plotlist = list(dot_plot, LOR_plot), labels = c("A", "B"),
nrow = 2, align = 'v', axis = 'l', rel_heights = c(1.4, 1))
ggsave(plot = p, filename = "figures/fig1.pdf", height = 7, width = 4.7)
p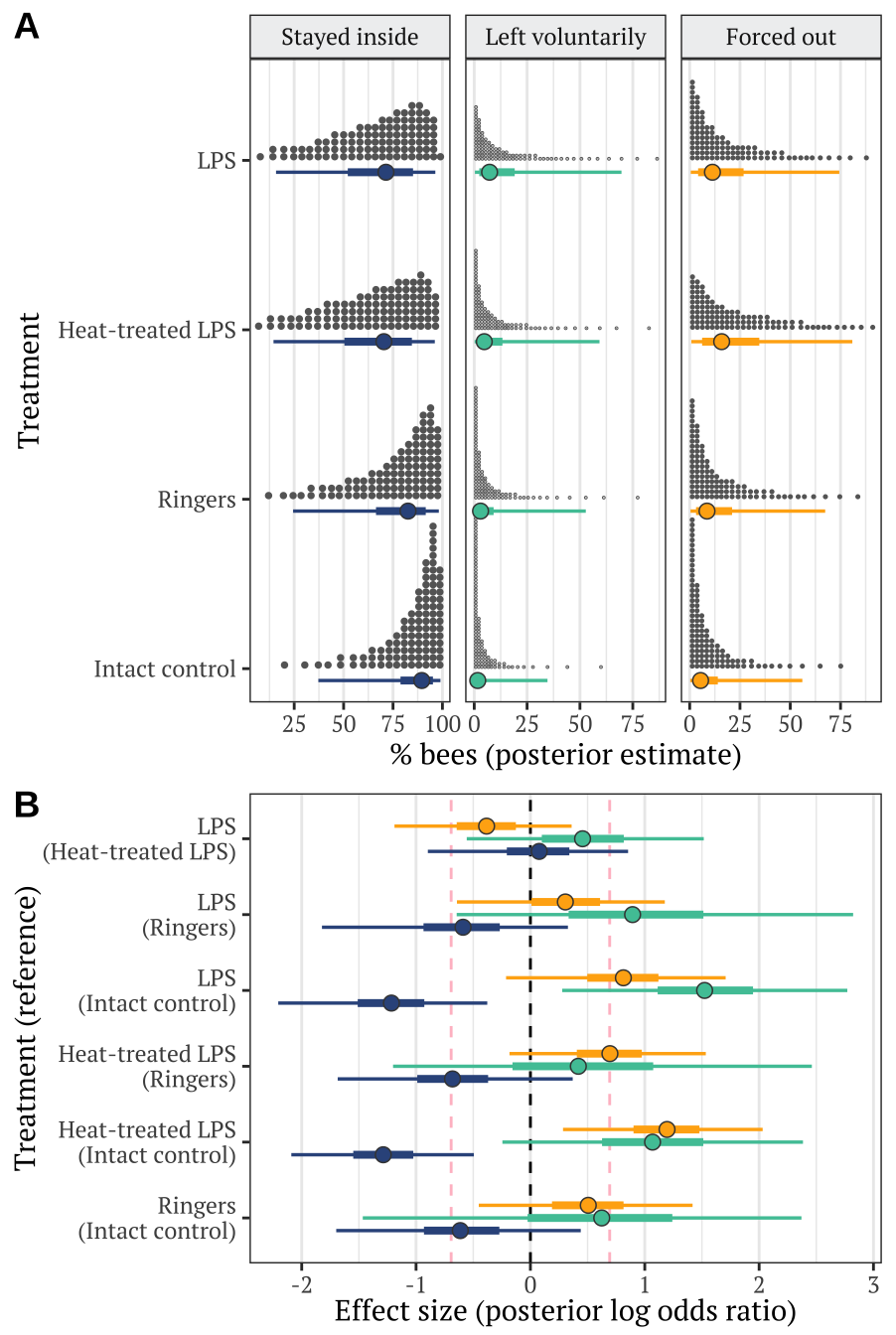
| Version | Author | Date |
|---|---|---|
| 1ce9e19 | lukeholman | 2020-04-21 |
Figure 1: Panel A shows the posterior estimate of the mean % bees staying inside the hive (left), leaving voluntarily (middle), or being forced out (right), for each of the four treatments. The quantile dot plot shows 100 approximately equally likely estimates of the true % bees, and the horizontal bars show the median and the 50% and 95% credible intervals of the posterior distribution. Panel B gives the posterior estimates of the effect size of each treatment, relative to one of the other treatments (the name of which appears in parentheses), and expressed as a log odds ratio (LOR). Positive LOR indicates that the % bees showing this particular outcome is higher in the treatment than the control; for example, more bees left voluntarily (green) or were forced out (orange) in the LPS treatment than in the intact control. The dashed lines mark \(LOR = 0\), indicating no effect, and \(LOR = \pm log(2)\), i.e. the point at which the odds are twice as high in one treatment as the other. Statistical results associated with this figure are described in Tables S1-S2.
Hypothesis testing and effect sizes
This section calculates the posterior difference in treatment group means, in order to perform some null hypothesis testing, calculate effect size (as a log odds ratio), and calculate the 95% credible intervals on the effect size.
The following code chunks perform planned contrasts between pairs of treatments that we consider important to the biological hypotheses under test. For example the contrast between the LPS treatment and the Ringers treatment provides information about the effect of immmune stimulation, while the Ringers - Intact Control contrast provides information about the effect of wounding in the absence of LPS.
Calculate contrasts: % bees staying inside the hive
# Helper function to summarise a posterior, including calculating
# p_direction, i.e. the posterior probability that the effect size has the stated direction,
# which has a similar interpretation to a one-tailed p-value
my_summary <- function(df, columns) {
lapply(columns, function(x){
p <- 1 - (df %>% pull(!! x) %>%
bayestestR::p_direction() %>% as.numeric())
df %>% pull(!! x) %>% posterior_summary() %>% as_tibble() %>%
mutate(p=p) %>% mutate(Metric = x) %>% select(Metric, everything()) %>%
mutate(` ` = ifelse(p < 0.1, "~", ""),
` ` = replace(` `, p < 0.05, "\\*"),
` ` = replace(` `, p < 0.01, "**"),
` ` = replace(` `, p < 0.001, "***"),
` ` = replace(` `, p == " ", ""))
}) %>% do.call("rbind", .)
}
# Helper to make one unit of the big stats table
make_stats_table <- function(
dat, groupA, groupB, comparison,
metric = "Absolute difference in % bees exiting the hive"){
output <- dat %>%
spread(treatment, prop) %>%
mutate(
metric_here = 100 * (!! enquo(groupA) - !! enquo(groupB)),
`Log odds ratio` = get_log_odds(!! enquo(groupB), !! enquo(groupA))) %>%
my_summary(c("metric_here", "Log odds ratio")) %>%
mutate(p = c(" ", format(round(p[2], 4), nsmall = 4)),
` ` = c(" ", ` `[2]),
Comparison = comparison) %>%
select(Comparison, everything()) %>%
mutate(Metric = replace(Metric, Metric == "metric_here", metric))
names(output)[names(output) == "metric_here"] <- metric
output
}
stayed_inside_stats_table <- rbind(
stats_data %>%
filter(outcome == "Stayed inside the hive") %>%
make_stats_table(`Heat-treated LPS`, `LPS`, "LPS (Heat-treated LPS)"),
stats_data %>%
filter(outcome == "Stayed inside the hive") %>%
make_stats_table(`Ringers`, `LPS`, "LPS (Ringers)"),
stats_data %>%
filter(outcome == "Stayed inside the hive") %>%
make_stats_table(`Intact control`, `LPS`, "LPS (Intact control)"),
stats_data %>%
filter(outcome == "Stayed inside the hive") %>%
make_stats_table(`Ringers`, `Heat-treated LPS`, "Heat-treated LPS (Ringers)"),
stats_data %>%
filter(outcome == "Stayed inside the hive") %>%
make_stats_table(`Intact control`, `Heat-treated LPS`, "Heat-treated LPS (Intact control)"),
stats_data %>%
filter(outcome == "Stayed inside the hive") %>%
make_stats_table(`Intact control`, `Ringers`, "Ringers (Intact control)")
) %>% as_tibble()
stayed_inside_stats_table[c(2,4,6,8,10,12), 1] <- " "% bees that left voluntarily
voluntary_stats_table <- rbind(
stats_data %>%
filter(outcome == "Left voluntarily") %>%
make_stats_table(`Heat-treated LPS`, `LPS`,
"LPS (Heat-treated LPS)",
metric = "Absolute difference in % bees that left voluntarily"),
stats_data %>%
filter(outcome == "Left voluntarily") %>%
make_stats_table(`Intact control`, `LPS`,
"LPS (Intact control)",
metric = "Absolute difference in % bees that left voluntarily"),
stats_data %>%
filter(outcome == "Left voluntarily") %>%
make_stats_table(`Ringers`, `LPS`,
"LPS (Ringers)",
metric = "Absolute difference in % bees that left voluntarily"),
stats_data %>%
filter(outcome == "Left voluntarily") %>%
make_stats_table(`Ringers`, `Heat-treated LPS`,
"Heat-treated LPS (Ringers)",
metric = "Absolute difference in % bees that left voluntarily"),
stats_data %>%
filter(outcome == "Left voluntarily") %>%
make_stats_table(`Intact control`, `Heat-treated LPS`,
"Heat-treated LPS (Intact control)",
metric = "Absolute difference in % bees that left voluntarily"),
stats_data %>%
filter(outcome == "Left voluntarily") %>%
make_stats_table(`Intact control`, `Ringers`,
"Ringers (Intact control)",
metric = "Absolute difference in % bees that left voluntarily")
) %>% as_tibble()
voluntary_stats_table[c(2,4,6,8,10,12), 1] <- " "% bees that were forced out
forced_out_stats_table <- rbind(
stats_data %>%
filter(outcome == "Forced out") %>%
make_stats_table(`Heat-treated LPS`, `LPS`,
"LPS (Heat-treated LPS)",
metric = "Absolute difference in % bees that were forced out"),
stats_data %>%
filter(outcome == "Forced out") %>%
make_stats_table(`Intact control`, `LPS`,
"LPS (Intact control)",
metric = "Absolute difference in % bees that were forced out"),
stats_data %>%
filter(outcome == "Forced out") %>%
make_stats_table(`Ringers`, `LPS`,
"LPS (Ringers)",
metric = "Absolute difference in % bees that were forced out"),
stats_data %>%
filter(outcome == "Forced out") %>%
make_stats_table(`Ringers`, `Heat-treated LPS`,
"Heat-treated LPS (Ringers)",
metric = "Absolute difference in % bees that were forced out"),
stats_data %>%
filter(outcome == "Forced out") %>%
make_stats_table(`Intact control`, `Heat-treated LPS`,
"Heat-treated LPS (Intact control)",
metric = "Absolute difference in % bees that were forced out"),
stats_data %>%
filter(outcome == "Left voluntarily") %>%
make_stats_table(`Intact control`, `Ringers`, "Ringers (Intact control)",
metric = "Absolute difference in % bees that were forced out")
) %>% as_tibble()
forced_out_stats_table[c(2,4,6,8,10,12), 1] <- " "Table S2: This table gives statistics associated with each of the contrasts plotted in Figure 1B. Each pair of rows gives the absolute and standardised effect size (as log odds ratio; LOR) for the focal treatment, relative to the treatment shown in parentheses, for one of the three possible outcomes (stayed inside, left voluntarily, or forced out). A LOR of \(|log(x)|\) indicates that the outcome is \(x\) times more frequent in one treatment compared to the other, e.g. \(|log(2) = 0.69|\) indicates a two-fold difference in frequency. The \(p\) column gives the posterior probability that the true effect size has the same sign as is shown in the Estimate column; this metric has a similar interpretation to a one-tailed \(p\) value in frequentist statistics.
bind_rows(
stayed_inside_stats_table,
voluntary_stats_table,
forced_out_stats_table) %>%
kable(digits = 2) %>% kable_styling(full_width = FALSE) %>%
row_spec(seq(2,36,by=2), extra_css = "border-bottom: solid;") %>%
pack_rows("% bees staying inside", 1, 12) %>%
pack_rows("% bees leaving voluntarily", 13, 24) %>%
pack_rows("% bees forced out", 25, 36)| Comparison | Metric | Estimate | Est.Error | Q2.5 | Q97.5 | p | |
|---|---|---|---|---|---|---|---|
| % bees staying inside | |||||||
| LPS (Heat-treated LPS) | Absolute difference in % bees exiting the hive | -0.98 | 8.09 | -17.18 | 17.01 | ||
| Log odds ratio | -0.05 | 0.43 | -0.85 | 0.90 | 0.4255 | ||
| LPS (Ringers) | Absolute difference in % bees exiting the hive | 9.54 | 9.87 | -5.35 | 33.80 | ||
| Log odds ratio | 0.62 | 0.54 | -0.33 | 1.82 | 0.1047 | ||
| LPS (Intact control) | Absolute difference in % bees exiting the hive | 17.27 | 10.86 | 1.89 | 42.03 | ||
| Log odds ratio | 1.23 | 0.46 | 0.38 | 2.21 | 0.0021 | ** | |
| Heat-treated LPS (Ringers) | Absolute difference in % bees exiting the hive | 10.52 | 9.66 | -5.76 | 31.87 | ||
| Log odds ratio | 0.68 | 0.51 | -0.37 | 1.69 | 0.0840 | ~ | |
| Heat-treated LPS (Intact control) | Absolute difference in % bees exiting the hive | 18.25 | 10.72 | 2.13 | 40.41 | ||
| Log odds ratio | 1.29 | 0.40 | 0.49 | 2.09 | 0.0021 | ** | |
| Ringers (Intact control) | Absolute difference in % bees exiting the hive | 7.73 | 8.93 | -4.96 | 29.75 | ||
| Log odds ratio | 0.61 | 0.54 | -0.44 | 1.70 | 0.1158 | ||
| % bees leaving voluntarily | |||||||
| LPS (Heat-treated LPS) | Absolute difference in % bees that left voluntarily | -3.64 | 6.63 | -22.30 | 5.35 | ||
| Log odds ratio | -0.46 | 0.53 | -1.52 | 0.56 | 0.1919 | ||
| LPS (Intact control) | Absolute difference in % bees that left voluntarily | -9.51 | 11.02 | -40.61 | -0.13 | ||
| Log odds ratio | -1.53 | 0.63 | -2.77 | -0.28 | 0.0091 | ** | |
| LPS (Ringers) | Absolute difference in % bees that left voluntarily | -6.27 | 10.01 | -35.00 | 5.24 | ||
| Log odds ratio | -0.95 | 0.90 | -2.82 | 0.64 | 0.1396 | ||
| Heat-treated LPS (Ringers) | Absolute difference in % bees that left voluntarily | -2.64 | 8.40 | -25.56 | 11.88 | ||
| Log odds ratio | -0.49 | 0.94 | -2.46 | 1.20 | 0.3086 | ||
| Heat-treated LPS (Intact control) | Absolute difference in % bees that left voluntarily | -5.87 | 8.33 | -30.99 | 0.48 | ||
| Log odds ratio | -1.07 | 0.66 | -2.38 | 0.25 | 0.0546 | ~ | |
| Ringers (Intact control) | Absolute difference in % bees that left voluntarily | -3.23 | 7.79 | -26.72 | 6.36 | ||
| Log odds ratio | -0.58 | 0.98 | -2.37 | 1.47 | 0.2559 | ||
| % bees forced out | |||||||
| LPS (Heat-treated LPS) | Absolute difference in % bees that were forced out | 4.62 | 6.30 | -4.80 | 20.93 | ||
| Log odds ratio | 0.39 | 0.39 | -0.36 | 1.19 | 0.1601 | ||
| LPS (Intact control) | Absolute difference in % bees that were forced out | -7.76 | 8.27 | -28.69 | 0.83 | ||
| Log odds ratio | -0.80 | 0.48 | -1.71 | 0.21 | 0.0535 | ~ | |
| LPS (Ringers) | Absolute difference in % bees that were forced out | -3.27 | 6.31 | -19.17 | 7.38 | ||
| Log odds ratio | -0.30 | 0.46 | -1.18 | 0.64 | 0.2446 | ||
| Heat-treated LPS (Ringers) | Absolute difference in % bees that were forced out | -7.88 | 7.87 | -27.16 | 1.34 | ||
| Log odds ratio | -0.69 | 0.44 | -1.53 | 0.18 | 0.0556 | ~ | |
| Heat-treated LPS (Intact control) | Absolute difference in % bees that were forced out | -12.38 | 10.49 | -36.95 | -0.32 | ||
| Log odds ratio | -1.18 | 0.44 | -2.03 | -0.29 | 0.0078 | ** | |
| Ringers (Intact control) | Absolute difference in % bees that were forced out | -3.23 | 7.79 | -26.72 | 6.36 | ||
| Log odds ratio | -0.58 | 0.98 | -2.37 | 1.47 | 0.2559 | ||
sessionInfo()R version 3.6.3 (2020-02-29)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS Catalina 10.15.4
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.6/Resources/lib/libRlapack.dylib
locale:
[1] en_AU.UTF-8/en_AU.UTF-8/en_AU.UTF-8/C/en_AU.UTF-8/en_AU.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] cowplot_1.0.0 tidybayes_2.0.1 bayestestR_0.5.1 kableExtra_1.1.0
[5] gridExtra_2.3 forcats_0.5.0 stringr_1.4.0 dplyr_0.8.5
[9] purrr_0.3.3 readr_1.3.1 tidyr_1.0.2 tibble_3.0.0
[13] ggplot2_3.3.0 tidyverse_1.3.0 bayesplot_1.7.1 brms_2.12.0
[17] Rcpp_1.0.3 showtext_0.7-1 showtextdb_2.0 sysfonts_0.8
[21] workflowr_1.6.0
loaded via a namespace (and not attached):
[1] colorspace_1.4-1 ellipsis_0.3.0 ggridges_0.5.2
[4] rsconnect_0.8.16 rprojroot_1.3-2 markdown_1.1
[7] base64enc_0.1-3 fs_1.3.1 rstudioapi_0.11
[10] farver_2.0.3 rstan_2.19.3 svUnit_0.7-12
[13] DT_0.13 fansi_0.4.1 mvtnorm_1.1-0
[16] lubridate_1.7.8 xml2_1.3.1 bridgesampling_1.0-0
[19] knitr_1.28 shinythemes_1.1.2 jsonlite_1.6.1
[22] broom_0.5.4 dbplyr_1.4.2 shiny_1.4.0
[25] compiler_3.6.3 httr_1.4.1 backports_1.1.6
[28] assertthat_0.2.1 Matrix_1.2-18 fastmap_1.0.1
[31] cli_2.0.2 later_1.0.0 htmltools_0.4.0
[34] prettyunits_1.1.1 tools_3.6.3 igraph_1.2.5
[37] coda_0.19-3 gtable_0.3.0 glue_1.4.0
[40] reshape2_1.4.4 cellranger_1.1.0 vctrs_0.2.4
[43] nlme_3.1-147 crosstalk_1.1.0.1 insight_0.8.1
[46] xfun_0.13 ps_1.3.0 rvest_0.3.5
[49] mime_0.9 miniUI_0.1.1.1 lifecycle_0.2.0
[52] gtools_3.8.2 zoo_1.8-7 scales_1.1.0
[55] colourpicker_1.0 hms_0.5.3 promises_1.1.0
[58] Brobdingnag_1.2-6 parallel_3.6.3 inline_0.3.15
[61] RColorBrewer_1.1-2 shinystan_2.5.0 curl_4.3
[64] yaml_2.2.1 loo_2.2.0 StanHeaders_2.19.2
[67] stringi_1.4.6 highr_0.8 dygraphs_1.1.1.6
[70] pkgbuild_1.0.6 rlang_0.4.5 pkgconfig_2.0.3
[73] matrixStats_0.56.0 evaluate_0.14 lattice_0.20-41
[76] labeling_0.3 rstantools_2.0.0 htmlwidgets_1.5.1
[79] processx_3.4.2 tidyselect_1.0.0 plyr_1.8.6
[82] magrittr_1.5 R6_2.4.1 generics_0.0.2
[85] DBI_1.1.0 pillar_1.4.3 haven_2.2.0
[88] whisker_0.4 withr_2.1.2 xts_0.12-0
[91] abind_1.4-5 modelr_0.1.5 crayon_1.3.4
[94] arrayhelpers_1.1-0 rmarkdown_2.1 grid_3.6.3
[97] readxl_1.3.1 callr_3.4.3 git2r_0.26.1
[100] threejs_0.3.3 reprex_0.3.0 digest_0.6.25
[103] webshot_0.5.2 xtable_1.8-4 httpuv_1.5.2
[106] stats4_3.6.3 munsell_0.5.0 viridisLite_0.3.0
[109] shinyjs_1.1